Containment of Phytoplasma-Associated Plant Diseases by Antibiotics and Other Antimicrobial Molecules
Abstract
1. Introduction
2. Antibiotics
2.1. Field Application
2.2. In Vitro Applications
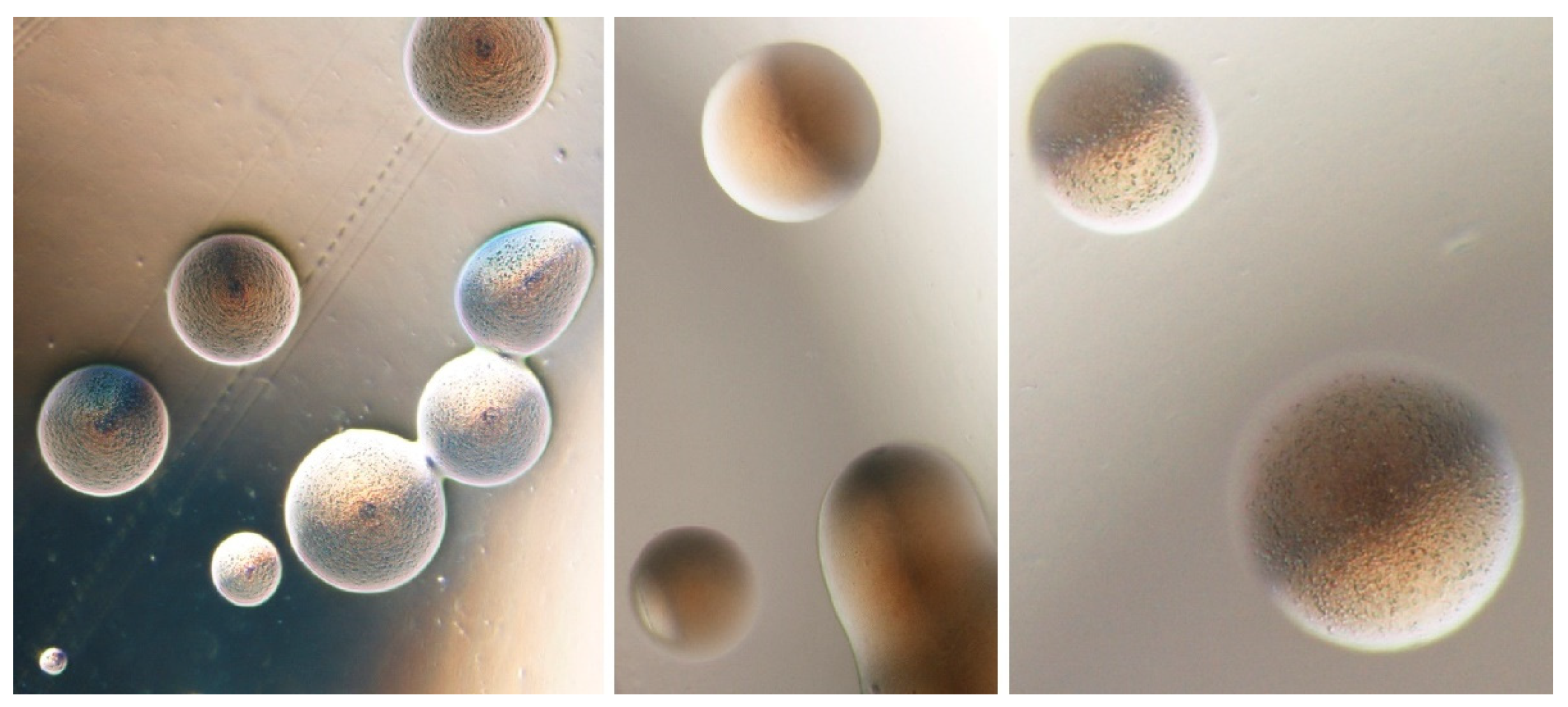
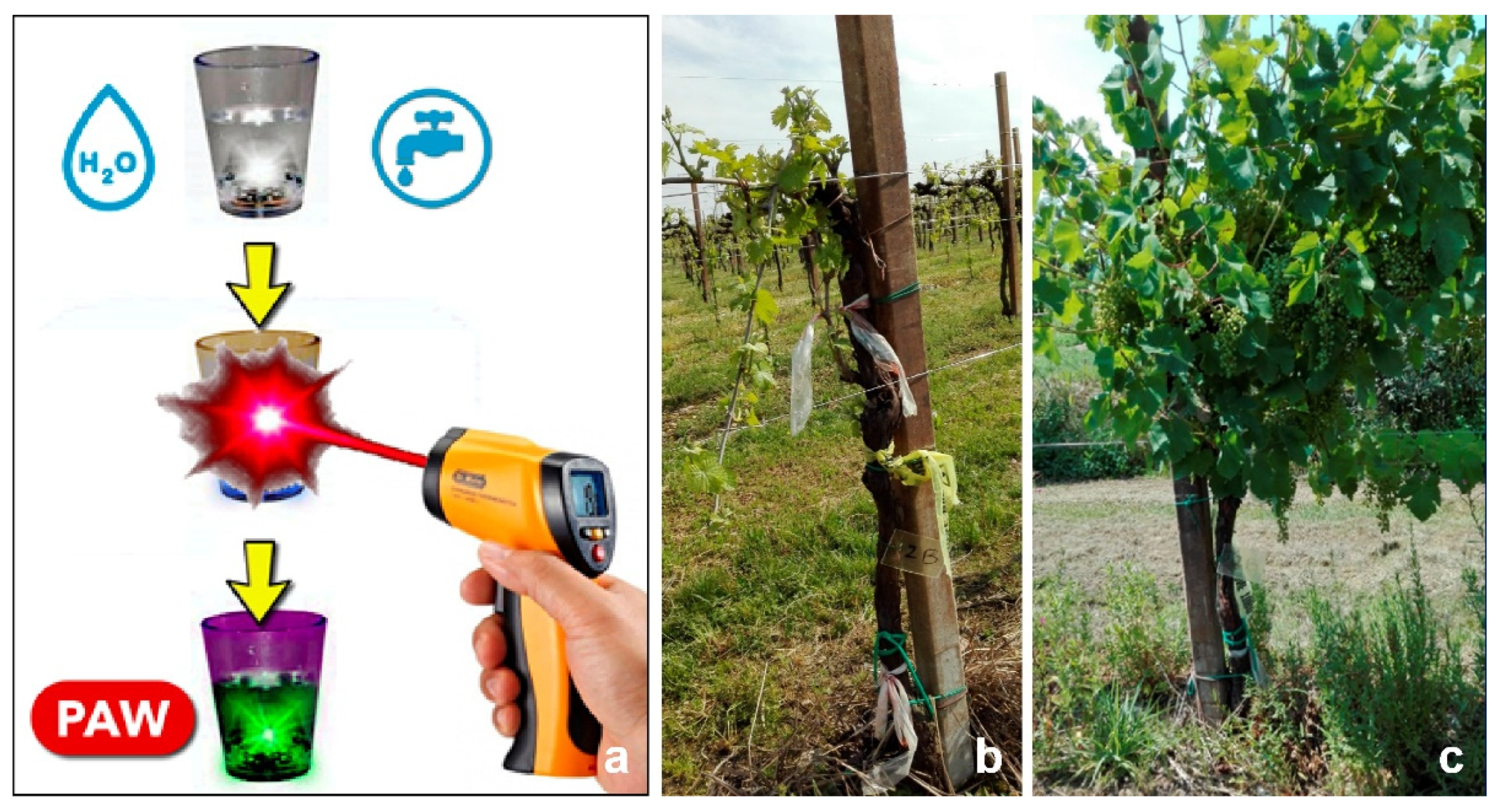
3. Antimicrobial Molecules
4. Plant Resistance Inducers
5. Discussion and Conclusions
Funding
Conflicts of Interest
References
- Bertaccini, A.; Duduk, B.; Paltrinieri, S.; Contaldo, N. Phytoplasmas and phytoplasma diseases: A severe threat to agriculture. Am. J. Plant Sci. 2014, 5, 1763–1788. [Google Scholar] [CrossRef]
- Namba, S. Molecular and biological properties of phytoplasmas. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Ishiie, T.; Doi, Y.; Yora, K.; Asuyama, H. Suppressive effects of antibiotics of the tetracycline group on symptom development in mulberry dwarf disease. Jpn. J. Phytopathol. 1967, 33, 267–275. [Google Scholar] [CrossRef]
- Bertaccini, A.; Duduk, B. Phytoplasma and phytoplasma diseases: A review of recent research. Phytopathol. Mediterr. 2010, 48, 355–378. [Google Scholar]
- Chalak, L.; Elbitar, A.; Mourad, N.; Mortada, C.; Choueiri, E. Elimination of grapevine “bois noir” phytoplasma by tissue culture coupled or not with heat therapy or hot water treatment. Adv. Crop Sci. Tech. 2013, 1, 107. [Google Scholar]
- Parmessur, Y.; Aljanabi, S.; Saumtally, S.; Dookun-Saumtally, A. Sugarcane yellow leaf virus and sugarcane yellows phytoplasma: Elimination by tissue culture. Plant Pathol. 2002, 51, 561–566. [Google Scholar] [CrossRef]
- Wongkaew, P.; Fletcher, J. Sugarcane white leaf phytoplasma in tissue culture: Long-term maintenance, transmission, and oxytetracycline remission. Plant Cell Rep. 2004, 23, 426–434. [Google Scholar] [CrossRef]
- Möllers, C.; Sarkar, S. Regeneration of healthy plants from Catharanthus roseus infected with mycoplasma-like organisms through callus culture. Plant Sci. 1989, 60, 83–89. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Duffy, B. Use of antibiotics in plant agriculture. Rev. Sci. Tech. 2012, 31, 199–210. [Google Scholar] [CrossRef] [PubMed]
- McCoy, R.E. Use of tetracycline antibiotics to control yellows diseases. Plant Dis. 1982, 66, 539–542. [Google Scholar] [CrossRef]
- Nyland, G.; Moller, W.J. Control of pear decline with a tetracycline. Plant Dis. Rep. 1973, 57, 634–637. [Google Scholar]
- Kirkpatrick, H.C.; Lowe, S.K.; Nyland, G. Peach rosette: The morphology of an associated mycoplasma-like organism and the chemotherapy of the disease. Phytopathology 1975, 65, 864–870. [Google Scholar] [CrossRef]
- Asuyama, H.; Iida, T.T. Effects of tetracycline compounds on plant diseases caused by mycoplasma-like agents. Ann. N. Y. Acad. Sci. 1973, 225, 509–521. [Google Scholar] [CrossRef]
- Seidl, V. Some results of several years’ study on apple proliferation disease. Acta Phytopathol. Acad. Sci. Hung. 1980, 15, 241–245. [Google Scholar] [CrossRef]
- Bindra, O.S.; Sohi, A.S.; Khatri, H.I.; Doel, G.S. Effect of acromycin (tetracycline hydrochloride) on brinjal little leaf pathogen. Curr. Sci. 1972, 41, 819–820. [Google Scholar]
- Anjaneyulu, A.; Ramakrishnan, K. Therapy of eggplant little leaf disease with tetracyclines. Curr. Sci. 1973, 38, 271–272. [Google Scholar]
- Tanaka, K.; Nonaka, F. Studies on onion yellows caused by a mycoplasma like organism. Effect of tetracycline on the development of onion yellows symptoms. Bull. Fac. Agric. Saga Univ. 1984, 56, 73–78. [Google Scholar]
- Giunchedi, L.; Poggi Pollini, C. Mycoplasma-like organisms associated with false dragon head (Physostegia virginiana) flower virescence and proliferation and remission of symptoms following tetracycline treatment. Phytopathol. Mediterr. 1986, 25, 151–154. [Google Scholar]
- Bertaccini, A.; Marani, F.; Rapetti, S. Phyllody and virescence in ranunculus hybrids. Acta Hortic. 1988, 234, 123–128. [Google Scholar] [CrossRef]
- Upadhyay, R. Varietal susceptibility and effect of antibiotics on little leaf phytoplasma of brinjal (Solanum melongena L). Int. J. Emer. Trends Sci. Technol. 2016, 3, 3911–3914. [Google Scholar]
- Davies, D.L.; Clark, M.F. Maintenance of mycoplasma-like organisms occurring in Pyrus species by micropropagation and their elimination by tetracycline therapy. Plant Pathol. 1994, 43, 819–823. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Laimer, M.; Bertaccini, A. Phytoplasma elimination from perennial horticultural crops. In Phytoplasmas: Plant Pathogenic Bacteria-II Transmission and Management of Phytoplasma Associated Diseases; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; pp. 185–206. [Google Scholar]
- Pereira, J.E.S.; Fortes, G.R.D.L. Antibiotics toxicity on the in vitro potato cultivation in semi-solid and liquid media. Pesqui. Agropecu. Bras. 2003, 38, 1273–1279. [Google Scholar] [CrossRef]
- Chalak, L.; Elbitar, A.; Rizk, R.; Choueiri, E.; Salar, P.; Bovè, J.-M. Attempts to eliminate ‘Candidatus Phytoplasma phoenicium’ from infected Lebanese almond varieties by tissue culture techniques combined or not with thermotherapy. Eur. J. Plant Pathol. 2005, 1, 85–89. [Google Scholar] [CrossRef]
- Gribaudo, I.; Ruffa, P.; Cuozzo, D.; Gambino, G.; Marzachì, C. Attempts to eliminate phytoplasmas from grapevine clones by tissue culture techniques. Bull. Insectol. 2007, 60, 315–316. [Google Scholar]
- Carvalho, M.J.S.; Oliveira, E.J.; Souza, A.S.; Pereira, J.S.; Diamantino, M.S.A.S.; Oliveira, S.A.S. Cleaning cassava genotypes infected with cassava frogskin disease via in vitro shoot tip culture. Genet. Mol. Res. 2017, 16, gmr16029556. [Google Scholar] [CrossRef] [PubMed]
- Bertaccini, A.; Davis, R.E.; Lee, I.-M. In vitro micropropagation for maintenance of mycoplasma-like organisms in infected plant tissues. Hortic. Sci. 1992, 27, 1041–1043. [Google Scholar] [CrossRef]
- Cantagallo, B.; Bertaccini, A. Impiego di RIP (“Ribosome-Inactivating Proteins”) e tetracicline per l’eliminazione di fitoplasmi da materiale vegetale micropropagato. Master’s Thesis, University of Bologna, Bologna, Italy, 2002. [Google Scholar]
- Tanno, K.; Maejima, K.; Miyazaki, A.; Koinuma, H.; Iwabuchi, N.; Kitazawa, Y.; Nijo, T.; Hashimoto, M.; Yamaji, Y.; Namba, S. Comprehensive screening of antimicrobials to control phytoplasma diseases using an in vitro plant-phytoplasma co-culture system. Microbiology 2018, 164, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, N.; Satta, E.; Zambon, Y.; Paltrinieri, S.; Bertaccini, A. Development and evaluation of different complex media for phytoplasma isolation and growth. J. Microbiol. Methods 2016, 127, 105–110. [Google Scholar] [CrossRef]
- Contaldo, N.; D’Amico, G.; Paltrinieri, S.; Diallo, H.A.; Bertaccini, A.; Arocha Rosete, Y. Molecular and biological characterization of phytoplasmas from coconut palms affected by the lethal yellowing disease in Africa. Microbiol. Res. 2019, 223–225, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Plavsic, B.; Krivokapic, K.; Eric, Z.; Buturovic, D. Kinetin treatment of “stolbur” diseased tomato plants (Lycopersicum esculentum L.) and the possibility of its application in chemotherapy. Acta Bot. Croat. 1986, 45, 27–32. [Google Scholar]
- Plavsic, B.; Krivokapic, K.; Eric, Z. Kinetin treatment of “stolbur” diseased plants and possibility of its application in chemotherapy. In Mycoplasma Diseases of Crops. Basic and Applied Aspects; Maramorosch, K., Raychaudhuri, S.P., Eds.; Springer: New York, NY, USA, 1988; pp. 417–430. [Google Scholar]
- Curković-Perica, M.; Lepedus, H.; Seruga Musić, M. Effect of indole-3-butyric acid on phytoplasmas in infected Catharanthus roseus shoots grown in vitro. FEMS Microb. Lett. 2007, 268, 171–177. [Google Scholar] [CrossRef]
- Musetti, R.; Scaramagli, S.; Vighi, C.; Pressacco, L.; Torrigiani, P.; Favali, M.A. The role of polyamines in phytoplasma-infected periwinkle plants. Plant Biosyst. 1999, 133, 37–45. [Google Scholar] [CrossRef]
- Chang, C.J. Pathogenicity of aster yellows phytoplasma and Spiroplasma citri on periwinkle. Phytopathology 1998, 88, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Pertot, I.; Musetti, R.; Pressacco, L.; Osler, R. Changes in indole-3-acetic acid level in micropropagated tissues of Catharanthus roseus infected by the agent of the clover phyllody and effect of exogenous auxins on phytoplasma morphology. Cytobios 1998, 95, 13–23. [Google Scholar]
- Curkovic-Perica, M. Auxin-treatment induces recovery of phytoplasma-infected periwinkle. J. Appl. Microbiol. 2008, 105, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Aldaghi, M.; Massart, S.; Druart, P.; Bertaccini, A.; Jijakli, M.H.; Lepoivre, P. Preliminary in vitro evaluation of antimicrobial activity of some chemicals and essential oils on apple proliferation disease. Commun. Appl. Biol. Sci. Ghent Univ. 2008, 73, 335–341. [Google Scholar]
- Houston, L.L.; Ramakrishnan, S.; Hermodson, M.A. Seasonal variation in different forms of pokeweed antiviral protein, a potent inactivator of ribosomes. J. Biol. Chem. 1983, 258, 9601–9604. [Google Scholar] [CrossRef]
- Veronesi, F.; Bertaccini, A.; Parente, A.; Mastronicola, M.; Pastore, M. PCR indexing of phytoplasma-infected micropropagated periwinkle treated with PAP-II, a ribosome inactivating protein from Phytolacca americana leaves. Acta Hortic. 2000, 530, 113–120. [Google Scholar] [CrossRef]
- Bertaccini, A.; Carraro, L.; Davies, D.; Laimer da Câmara Machado, M.; Martini, M.; Paltrinieri, S.; Seemüller, E. Micropropagation of a collection of phytoplasma strains in periwinkle and other host plants. In Proceedings of the 13th Congress of IOM, ACROS, Fukuoka, Japan, 14–19 July 2000; p. 101. [Google Scholar]
- Bertaccini, A. Phytoplasma Collection. Available online: https://www.ipwgnet.org/collection (accessed on 20 August 2020).
- Perez, S.; Biondi, E.; Laurita, R.; Proto, M.; Sarti, F.; Gherardi, M.; Bertaccini, A.; Colombo, V. Plasma activated water as resistance inducer against bacterial leaf spot of tomato. PLoS ONE 2019, 14, e0217788. [Google Scholar] [CrossRef] [PubMed]
- Laurita, R.; Contaldo, N.; Zambon, Y.; Bisag, A.; Canel, A.; Gherardi, M.; Laghi, G.; Bertaccini, A.; Colombo, V. On the use of plasma activated water in viticulture: Induction of resistance and agronomic performance in greenhouse and open field. Plasma Proc. Polym. 2021, 18, e2000206. [Google Scholar] [CrossRef]
- Zambon, Y.; Contaldo, N.; Canel, A.; Laurita, R.; Gherardi, M.; Colombo, V.; Bertaccini, A. Plasma atmosferico freddo: Energia per una viticoltura eco-sostenibile. Conegliano Valdobbiadene 2017, 4, 79–82. [Google Scholar]
- Zambon, Y.; Contaldo, N.; Canel, A.; Laurita, R.; Beltrami, M.; Gherardi, M.; Colombo, V.; Bertaccini, A. Controllo e sostenibilità dei giallumi della vite con il plasma. Vite Vino 2018, 2, 66–71. [Google Scholar]
- Zambon, Y.; Contaldo, N.; Laurita, R.; Várallyay, E.; Canel, A.; Gherardi, M.; Colombo, V.; Bertaccini, A. Plasma activated water triggers plant defence responses. Sci. Rep. 2020, 10, 19211. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hiruki, C. PCR (Polymerase Chain Reaction)-based selection of phytoplasma-free clones of paulownia tissue culture after heat treatment of witches’ broom. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 1996, 72, 44–47. [Google Scholar] [CrossRef][Green Version]
- Heinrich, M.; Botti, S.; Caprara, l.; Arthofer, W.; Strommer, S.; Hanzer, V.; Katinger, H.; Bertaccini, A.; Laimer da Camara Machado, M. Improved detection methods for fruit tree phytoplasmas. Plant Mol. Biol. Rep. 2001, 19, 169–179. [Google Scholar] [CrossRef]
- Bertaccini, A.; Paltrinieri, S.; Contaldo, N. Standard detection protocol: PCR and RFLP analyses based on 16S rRNA gene. Methods Mol. Biol. 2019, 1875, 83–95. [Google Scholar] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertaccini, A. Containment of Phytoplasma-Associated Plant Diseases by Antibiotics and Other Antimicrobial Molecules. Antibiotics 2021, 10, 1398. https://doi.org/10.3390/antibiotics10111398
Bertaccini A. Containment of Phytoplasma-Associated Plant Diseases by Antibiotics and Other Antimicrobial Molecules. Antibiotics. 2021; 10(11):1398. https://doi.org/10.3390/antibiotics10111398
Chicago/Turabian StyleBertaccini, Assunta. 2021. "Containment of Phytoplasma-Associated Plant Diseases by Antibiotics and Other Antimicrobial Molecules" Antibiotics 10, no. 11: 1398. https://doi.org/10.3390/antibiotics10111398
APA StyleBertaccini, A. (2021). Containment of Phytoplasma-Associated Plant Diseases by Antibiotics and Other Antimicrobial Molecules. Antibiotics, 10(11), 1398. https://doi.org/10.3390/antibiotics10111398





