Genitourinary Tuberculosis: A Comprehensive Review of a Neglected Manifestation in Low-Endemic Countries
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Epidemiology
3.2. Aetiology and Risk Factors
3.3. Physiopathology
3.4. Clinical Presentation
3.5. Diagnosis and Differential Diagnosis
3.5.1. Smear Microscopy
3.5.2. Urine Culture
3.5.3. Nucleic Acid Amplification Tests
3.5.4. Whole-Genome Sequencing (WGS)
3.5.5. Histological Examination
3.5.6. Imaging
Plain X-ray
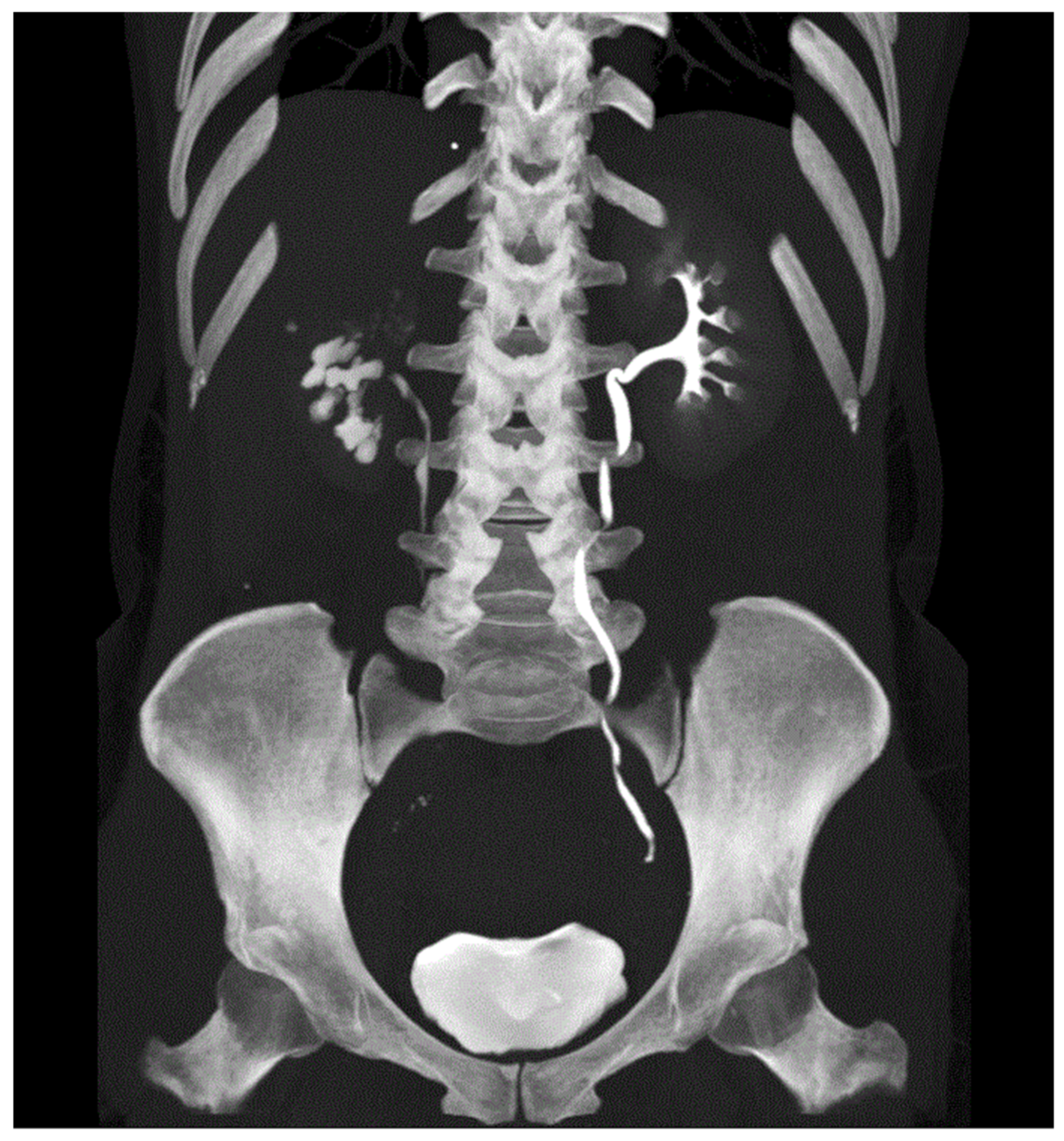
Intravenous Urography (IVU)
Ultrasonography (US)
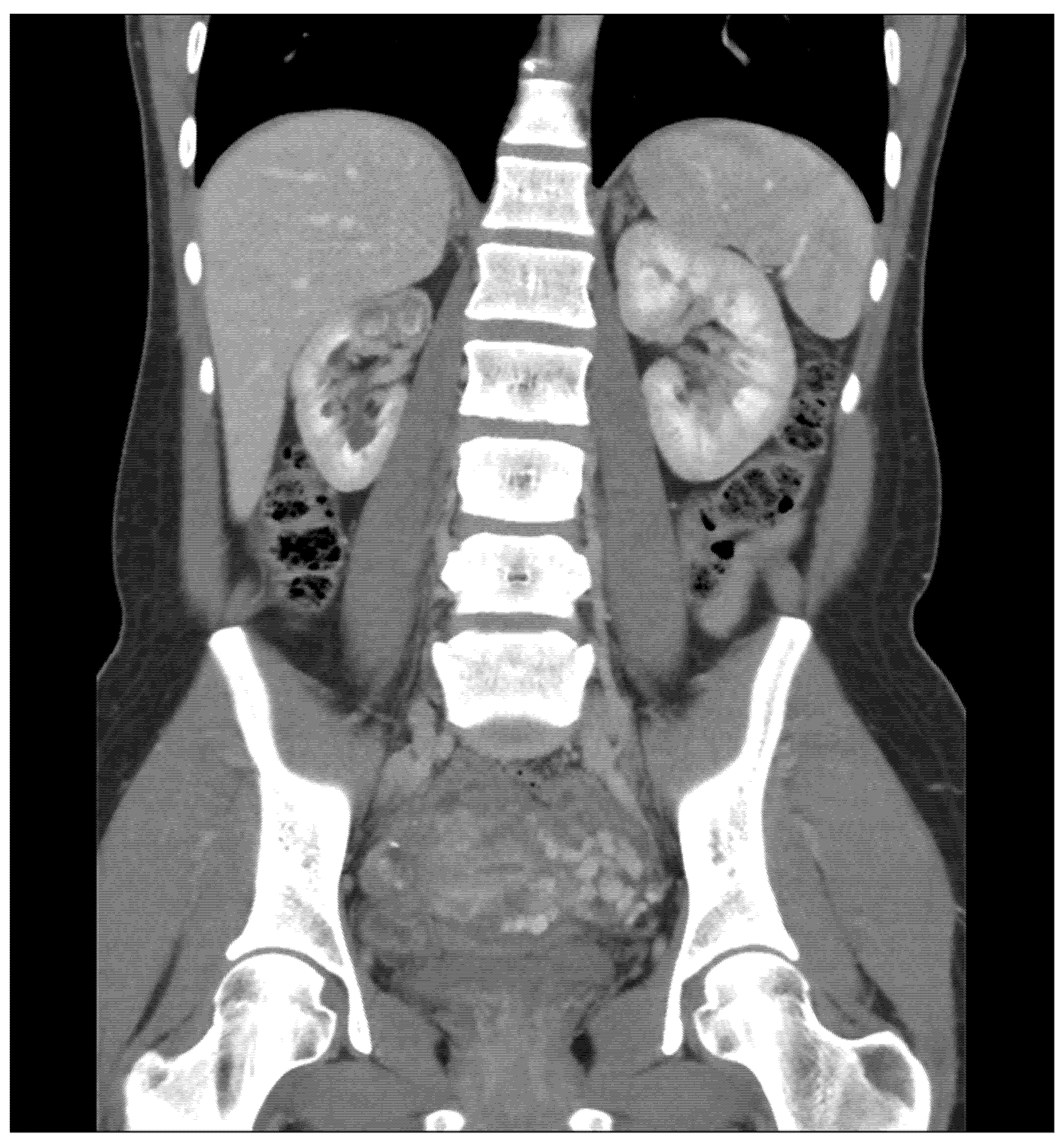
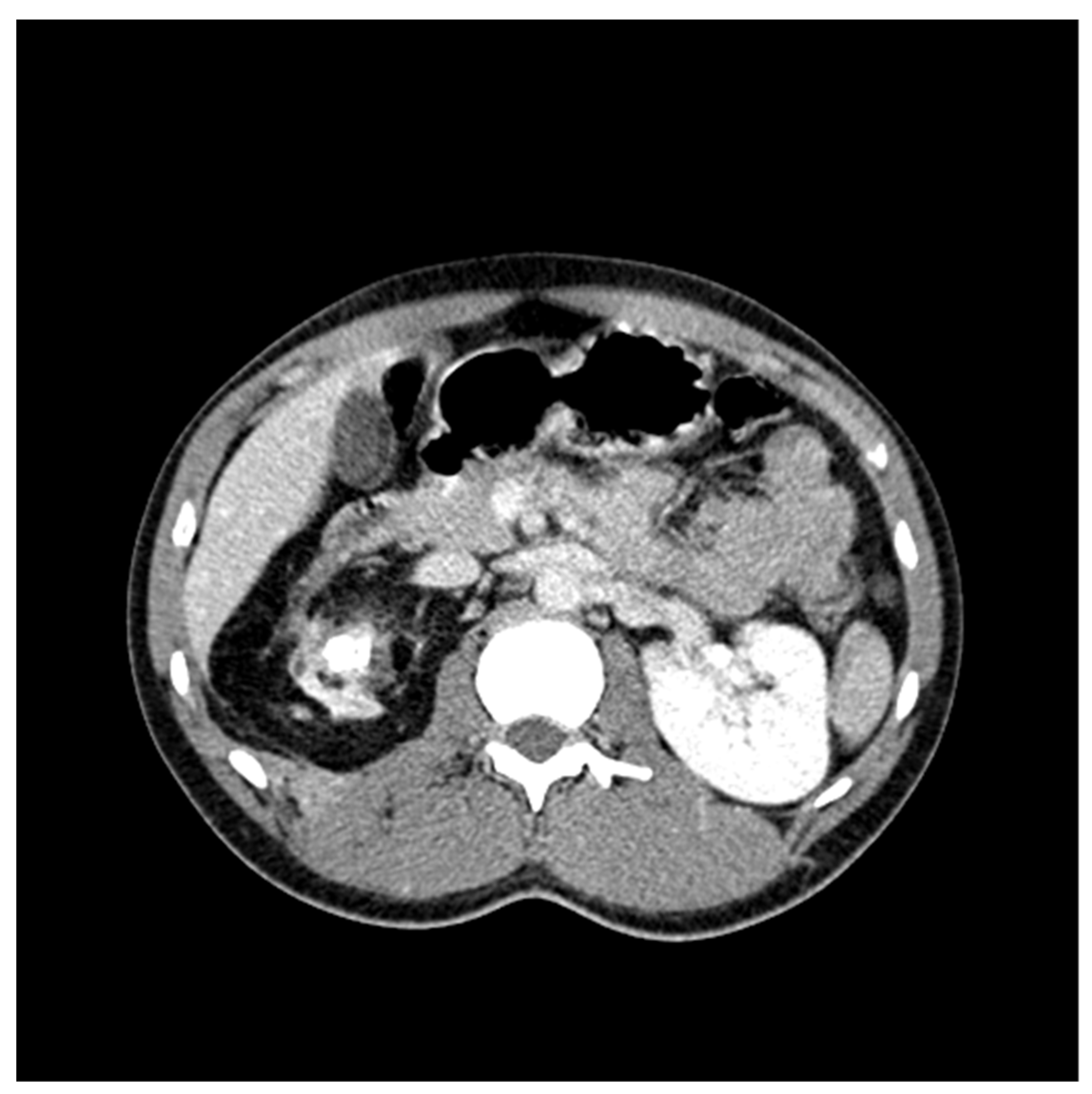
CT Scan and CT Urography
Positron Emission Tomography (PET)–CT Imaging
Magnetic Resonance Imaging (MRI)
Endoscopy–Laparoscopy
Hysterosalpingography (HSG)
3.6. Medical Treatment
3.7. Surgical Management
3.8. Follow-up
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Muneer, A.; Macrae, B.; Krishnamoorthy, S.; Zumla, A. Urogenital tuberculosis—Epidemiology, pathogenesis and clinical features. Nat. Rev. Urol. 2019, 16, 573–598. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, N.; Pontarelli, A.; Alagna, R.; Saderi, L.; Ferrarese, M.; Castellotti, P.; Viggiani, P.; Cirillo, D.; Besozzi, G.; Sotgiu, G.; et al. Epidemiology and treatment outcome of MDR and pre-XDR TB in international migrants at two reference centers in the North of Italy: A cross-sectional study coordinated by Stop TB Italia Onlus. Public Health 2020, 180, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Mert, A.; Guzelburc, V.; Guven, S. Urinary tuberculosis: Still a challenge. World J. Urol. 2020, 38, 1–6. [Google Scholar] [CrossRef]
- Mantica, G.; Van Der Merwe, A.; Terrone, C.; Gallo, F.; Zarrabi, A.D.; Vlok, A.L.; Ackermann, H.M.; Territo, A.; Esperto, F.; Olapade-Olapa, E.O.; et al. Awareness of European practitioners toward uncommon tropical diseases: Are we prepared to deal with mass migration? Results of an international survey. World J. Urol. 2019, 38, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Mantica, G.; Van Der Merwe, A.; Bonkat, G. Greetings from Africa: The Emergence of Tropical Urological Diseases in Europe. We Had Better Be Prepared! Eur. Urol. 2019, 76, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, F.; Gualano, G.; Timelli, L.; Vittozzi, P.; Di Bari, V.; Libertone, R.; Cerva, C.; Pinnarelli, L.; Nisii, C.; Ianniello, S.; et al. Increase in Tuberculosis Diagnostic Delay during First Wave of the COVID-19 Pandemic: Data from an Italian Infectious Disease Referral Hospital. Antibiotics 2021, 10, 272. [Google Scholar] [CrossRef]
- Grange, J.; Yates, M.; Ormerod, L. Factors determining ethnic differences in the incidence of bacteriologically confirmed genitourinary tuberculosis in South East England. J. Infect. 1995, 30, 37–40. [Google Scholar] [CrossRef]
- Tuberculosis Surveillance and Monitoring in Europe 2021–2019 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2021.
- Nerli, R.; Kamat, G.; Alur, S.; Koura, A.; Vikram, P.; Amarkhed, S. Genitourinary tuberculosis in pediatric urological practice. J. Pediatr. Urol. 2008, 4, 299–303. [Google Scholar] [CrossRef]
- Rodriguez-Takeuchi, S.Y.; Renjifo, M.E.; Medina, F.J. Extrapulmonary Tuberculosis: Pathophysiology and Imaging Findings. RadioGraphics 2019, 39, 2023–2037. [Google Scholar] [CrossRef]
- Yeboah-Manu, D.; Asare, P.; Asante-Poku, A.; Otchere, I.D.; Osei-Wusu, S.; Danso, E.; Forson, A.; Koram, K.A.; Gagneux, S. Spatio-Temporal Distribution of Mycobacterium tuberculosis Complex Strains in Ghana. PLoS ONE 2016, 11, e0161892. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, P.; Hemal, A.; Kumar, R. Genital tuberculosis: Current status of diagnosis and management. Transl. Androl. Urol. 2017, 6, 222–233. [Google Scholar] [CrossRef]
- Vynnycky, E.; Fine, P.E.M. The natural history of tuberculosis: The implications of age-dependent risks of disease and the role of reinfection. Epidemiol. Infect. 1997, 119, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 16076. [Google Scholar] [CrossRef]
- Walzl, G.; Ronacher, K.; Hanekom, W.A.; Scriba, T.; Zumla, P.S.A. Immunological biomarkers of tuberculosis. Nat. Rev. Immunol. 2011, 11, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Narayana, A.S. Overview of renal tuberculosis. Urology 1982, 19, 231–237. [Google Scholar] [CrossRef]
- Christensen, W.I. Genitourinary tuberculosis. Review of 102 cases. Medicine 1974, 53, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Venyo, A.K.-G. Tuberculosis of the Penis: A Review of the Literature. Scientifica 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Choi, S.H. Treatment experience for incidentally diagnosed asymptomatic prostate tuberculosis in a patient with history of BCG intravesical therapy. Urol. Case Rep. 2018, 17, 39–41. [Google Scholar] [CrossRef]
- Medlar, E.; Spain, D.; Holliday, R. Post-Mortem Compared with Clinical Diagnosis of Genito-Urinary Tuberculosis in Adult Males. J. Urol. 1949, 61, 1078–1088. [Google Scholar] [CrossRef]
- De Figueiredo, A.A.; Lucon, A.M.; Srougi, M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol. Urodyn. 2006, 25, 433–440. [Google Scholar] [CrossRef]
- Lattimer, J.K.; Kohen, R.J. Renal tuberculosis. Am. J. Med. 1954, 17, 533–539. [Google Scholar] [CrossRef]
- Kerr, W.K.; Gale, G.L.; Peterson, K.S. Reconstructive Surgery for Genitourinary Tuberculosis. J. Urol. 1969, 101, 254–266. [Google Scholar] [CrossRef]
- Lenk, S.; Schroeder, J. Genitourinary tuberculosis. Curr. Opin. Urol. 2001, 11, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, D.M.; Leonard, M.K.; LoBue, P.A.; Cohn, D.L.; Daley, C.L.; Desmond, E.; Keane, J.; Lewinsohn, D.A.; Loeffler, A.M.; Mazurek, G.H.; et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin. Infect. Dis. 2017, 64, 111–115. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Use of Next-Generation Sequencing Technologies for the Detection of Mutations Associated with Drug Resistance in Mycobacterium Tuberculosis Complex: Technical Guide. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/274443/WHO-CDS-TB-2018.19-eng.pdf (accessed on 3 August 2021).
- WHO. Automated Real-Time Nucleic Acid Amplifcation Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children: Policy Update; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Comas, I. Genomic Epidemiology of Tuberculosis. Adv. Exp. Med. Biol. 2017, 1019, 79–93. [Google Scholar] [PubMed]
- Kulchavenya, E.; Khomyakov, V. Male genital tuberculosis in Siberians. World J. Urol. 2006, 24, 74–78. [Google Scholar] [CrossRef]
- Zumla, A.; James, D.G. Granulomatous infections: Etiology and classification. Clin. Infect. Dis. 1996, 23, 146–158. [Google Scholar] [CrossRef]
- Hemal, A.; Gupta, N.; Rajeev, T.; Kumar, R.; Dar, L.; Seth, P. Polymerase chain reaction in clinically suspected genitourinary tuberculosis: Comparison with intravenous urography, bladder biopsy, and urine acid fast bacilli culture. Urology 2000, 56, 570–574. [Google Scholar] [CrossRef]
- Merchant, S.; Bharati, A.; Merchant, N. Tuberculosis of the uro-genital system—Urinary tract tuberculosis: Renal tuberculosis. I. Indian J. Radiol. Imaging 2013, 23, 46–63. [Google Scholar]
- Dyer, R.B.; Chen, M.Y.; Zagoria, R. Abnormal calcifications in the urinary tract. Radiographics 1998, 18, 1405–1424. [Google Scholar] [CrossRef]
- Kollins, S.A.; Hartman, G.W.; Carr, D.T.; Segura, J.W.; Hattery, R.R. Roentgenographic Findings in Urinary Tract Tuberculosis. Am. J. Roentgenol. 1974, 121, 487–499. [Google Scholar] [CrossRef]
- Rui, X.; Li, X.-D.; Cai, S.; Chen, G.; Cai, B. Ultrasonographic diagnosis and typing of renal tuberculosis. Int. J. Urol. 2007, 15, 135–139. [Google Scholar] [CrossRef]
- Engin, G.; Acunaş, B.; Acunaş, G.; Tunaci, M. Imaging of Extrapulmonary Tuberculosis. Radiographics 2000, 20, 471–488. [Google Scholar] [CrossRef] [PubMed]
- Segovis, C.M.; Dyer, R.B. The “watering can perineum”. Abdom. Radiol. 2016, 41, 1214. [Google Scholar] [CrossRef] [PubMed]
- Middleton, W.D.; Dahiya, N.; Naughton, C.K.; Teefey, S.A.; Siegel, C.A. High-Resolution Sonography of the Normal Extrapelvic Vas Deferens. J. Ultrasound Med. 2009, 28, 839–846. [Google Scholar] [CrossRef] [PubMed]
- GGrace, G.A.; Devaleenal, D.B.; Natrajan, M. Genital tuberculosis in females. Indian J. Med Res. 2017, 145, 425–436. [Google Scholar]
- Di Gennaro, F.; Pisani, L.; Veronese, N.; Pizzol, D.; Lippolis, V.; Saracino, A.; Monno, L.; Huson, M.A.; Copetti, R.; Putoto, G.; et al. Potential Diagnostic Properties of Chest Ultrasound in Thoracic Tuberculosis—A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 2235. [Google Scholar] [CrossRef]
- Wang, L.-J.; Wu, C.-F.; Wong, Y.-C.; Chuang, C.K.; Chu, S.-H.; Chen, C.-J. Imaging findings of urinary tuberculosis on excretory urography and computerized tomography. J. Urol. 2003, 169, 524–528. [Google Scholar] [CrossRef]
- Dyer, R.B.; Chen, M.Y.; Zagoria, R. Classic Signs in Uroradiology. Radiographics 2004, 24, S247–S280. [Google Scholar] [CrossRef]
- Roylance, J.; Penry, B.; Davies, E.R.; Roberts, M. Radiology in the Management of Urinary Tract Tuberculosis. BJU Int. 1970, 42, 679–687. [Google Scholar] [CrossRef]
- Prakash, J.; Goel, A.; Sankhwar, S.; Singh, B.P. Extensive renal and ureteral calcification due to tuberculosis: Rare images for an uncommon condition. BMJ Case Rep. 2013, 2013, 2012008508. [Google Scholar] [CrossRef]
- Reddy, M.N.; Verma, S. Lesions of the Seminal Vesicles and their MRI Characteristics. J. Clin. Imaging Sci. 2014, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.B.; Sharma, E.; Sharma, S.; Dharmendra, S. Female genital tuberculosis: Revisited. Indian J. Med Res. 2018, 148, S71–S83. [Google Scholar]
- Martin, C.; Castaigne, C.; Vierasu, I.; Garcia, C.; Wyndham-Thomas, C.; de Wit, S. Prospective Serial FDG PET/CT During Treatment of Extrapulmonary Tuberculosis in HIV-Infected Patients. Clin. Nucl. Med. 2018, 43, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.L.; Pedrassa, B.C.; Bormann, R.L.; Kierszenbaum, M.L.; Torres, L.R.; D’Ippolito, G. Abdominal tuberculosis: A radiological review with emphasis on computed tomography and magnetic resonance imaging findings. Radiol. Bras. 2015, 48, 181–191. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, L.; Zhang, X.; Ji, Q.; Shen, W. Multiparametric Magnetic Resonance Imaging Characteristics of Prostate Tuberculosis. Korean J. Radiol. 2015, 16, 846–852. [Google Scholar] [CrossRef]
- Bour, L.; Schull, A.; Delongchamps, N.-B.; Beuvon, F.; Muradyan, N.; Legmann, P.; Cornud, F. Multiparametric MRI features of granulomatous prostatitis and tubercular prostate abscess. Diagn. Interv. Imaging 2013, 94, 84–90. [Google Scholar] [CrossRef]
- Prakash, G.; Singh, V.; Sinha, R.J.; Babu, S.; Jhanwar, A.; Mehrotra, C.N. Primary tuberculosis of urethra presenting as stricture urethra and watering can perineum: A rarity. Urol. Ann. 2016, 8, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.B.; Roy, K.K.; Pushparaj, M.; Gupta, N.; Jain, S.K.; Malhotra, N.; Mittal, S. Genital tuberculosis: An important cause of Asherman’s syndrome in India. Arch. Gynecol. Obstet. 2008, 277, 37–41. [Google Scholar] [CrossRef]
- Ahmadi, F.; Zafarani, F.; Shahrzad, G. Hysterosalpingographic Appearances of Female Genital Tract Tuberculosis: Part I. Fallopian Tube. Int. J. Fertil. Steril. 2013, 7, 245–252. [Google Scholar] [PubMed]
- Cox, H.S.; Morrow, M.; Deutschmann, P.W. Long term efficacy of DOTS regimens for tuberculosis: Systematic review. BMJ 2008, 336, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Dorman, S.E.; Nahid, P.; Kurbatova, E.V.; Phillips, P.P.; Bryant, K.; Dooley, K.E.; Engle, M.; Goldberg, S.V.; Phan, H.T.; Hakim, J.; et al. Four-Month Rifapentine Regimens with or without Moxifloxacin for Tuberculosis. N. Engl. J. Med. 2021, 384, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Ammerman, N.C.; Chang, Y.S.; Tasneen, R.; Chaisson, R.E.; Jain, S.; Nuermberger, E.; Grosset, J.H. Treatment-Shortening Effect of a Novel Regimen Combining Clofazimine and High-Dose Rifapentine in Pathologically Distinct Mouse Models of Tuberculosis. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Lee, J.-K.; Lee, J.Y.; Kim, D.K.; Yoon, H.I.; Jeong, I.; Heo, E.Y.; Park, Y.S.; Jo, Y.S.; Lee, J.H.; Park, S.S.; et al. Substitution of ethambutol with linezolid during the intensive phase of treatment of pulmonary tuberculosis: A prospective, multicentre, randomised, open-label, phase 2 trial. Lancet Infect. Dis. 2019, 19, 46–55. [Google Scholar] [CrossRef]
- Seon, H.J.; Kim, Y.I.; Lim, S.C.; Kwon, Y.S. Clinical significance of residual lesions in chest computed tomography after anti-tuberculosis treatment. Int. J. Tuberc. Lung Dis. 2014, 18, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Canetti, D.; Riccardi, N.; Martini, M.; Villa, S.; Di Biagio, A.; Codecasa, L.; Castagna, A.; Barberis, I.; Gazzaniga, V.; Besozzi, G. HIV and tuberculosis: The paradox of dual illnesses and the challenges of their fighting in the history. Tuberculosis 2020, 122, 101921. [Google Scholar] [CrossRef]
- Friedrich, S.O.; Rachow, A.; Saathoff, E.; Singh, K.; Mangu, C.D.; Dawson, R.; Phillips, P.P.; Venter, A.; Bates, M.; Boehme, C.C.; et al. Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir. Med. 2013, 1, 462–470. [Google Scholar] [CrossRef]
- Patel, S.V.; Nimavat, K.B.; Alpesh, P.B.; Shukla, L.K.; Shringarpure, K.S.; Mehta, K.G.; Joshi, C.C. Treatment outcome among cases of multidrug-resistant tuberculosis (MDR TB) in Western India: A prospective study. J. Infect. Public Health 2015, 9, 478–484. [Google Scholar] [CrossRef]
- Riccardi, N.; Del Puente, F.; Magnè, F.; Taramasso, L.; Di Biagio, A. Bedaquiline: A New Hope for Shorter and Better Anti-Tuberculosis Regimens. Recent Pat. Anti-Infect. Drug Discov. 2018, 13, 3–11. [Google Scholar] [CrossRef]
- Riccardi, N.; Villa, S.; Alagna, R.; Giacomelli, A.; Saderi, L.; Cirillo, D.M.; Besozzi, G.; Sotgiu, G.; Codecasa, L. Advantages and Challenges of Tailored Regimens for Drug-Resistant Tuberculosis: A StopTB Italia Look into the Future. Infect. Drug Resist. 2020, ume 13, 2795–2800. [Google Scholar] [CrossRef]
- Matsui, K.; Furumoto, A.; Ohba, K.; Mochizuki, K.; Tanaka, T.; Takaki, M.; Morimoto, K.; Ariyoshi, K. Use of Corticosteroids for Urinary Tuberculosis Patients at Risk of Developing Ureteral Obstruction. Intern. Med. 2016, 55, 3539–3542. [Google Scholar] [CrossRef][Green Version]
- Figueiredo, A.A.; Lucon, A.M.; Junior, R.F.; Srougi, M. Epidemiology of urogenital tuberculosis worldwide. Int. J. Urol. 2008, 15, 827–832. [Google Scholar] [CrossRef]
- Kulchavenya, E. Surgery. In Urogenital Tuberculosis: Epidemiology, Diagnosis, Therapy; Springer: Cham, Switzerland; London, UK, 2019; pp. 109–110. [Google Scholar]
- Mittal, A.; Ranjan, S.K.; Narain, T.A.; Panwar, V.K. Surgical Management of Genitourinary Tuberculosis: Our Experience and review of literature. Pol. Przegl. Chir. 2020, 92, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, G.; Krishnamoorthy, S. Surgical management of renal tuberculosis. Indian J. Urol. 2008, 24, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Modi, P.R.; Rizvi, S.J. Retroperitoneoscopic Nephrectomy for Nephrocolonic Fistula Due to Tuberculous Nonfunctioning Kidney. J. Laparoendosc. Adv. Surg. Tech. 2008, 18, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Park, Y.H.; Jeon, H.G.; Jeong, W.; Rha, K.H.; Choi, H.; Kim, H.H. The Feasibility of Laparoendoscopic Single-Site Nephrectomy: Initial Experience Using Home-made Single-port Device. Urology 2010, 76, 862–865. [Google Scholar] [CrossRef]
- Hemal, A.K.; Gupta, N.P.; Kumar, R. Comparison of retroperitoneoscopic nephrectomy with open surgery for tuberculous nonfunctioning kidneys. J. Urol. 2000, 164, 32–35. [Google Scholar] [CrossRef]
- Par, K.D.; Ray, R.P.; Ghosh, B. Role of surgical intervention in genitourinary tuberculosis in the era of modern antitubercular chemotherapy. Pain 2015, 5, 23–80. [Google Scholar]
- Hemal, A.; Aron, M. Orthotopic neobladder in management of tubercular thimble bladders: Initial experience and long-term results. Urology 1999, 53, 298–301. [Google Scholar] [CrossRef]
- Figueiredom, A.; Luconm, A. Urogenital tuberculosis: Update and review of 8961 cases from the world literature. Rev. Urol. 2008, 10, 207–217. [Google Scholar]
- Gokce, G.; Kilicarslan, H.; Ayan, S.; Tas, F.; Akar, R.; Kaya, K.; Gultekin, E.Y. Genitourinary Tuberculosis: A Review of 174 Cases. Scand. J. Infect. Dis. 2002, 34, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Bellinzoni, P.; Broglia, L.; Colombo, R.; De Marchi, D.; Falcone, L.; Giusti, G.; Grasso, V.; Mantica, G.; Passaretti, G.; et al. Hospital care in Departments defined as COVID-free: A proposal for a safe hospitalization protecting healthcare professionals and patients not affected by COVID-19. Arch. Ital. Urol. Androl. 2020, 92. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, F.; Di Stasio, A.; Mantica, G.; Cavallone, B.; Serao, A. COVID-19 pandemic and uro-oncology follow-up: A “virtual” multidisciplinary team strategy and patients’ satisfaction assessment. Arch. Ital. Urol. Androl. 2020, 92. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantica, G.; Ambrosini, F.; Riccardi, N.; Vecchio, E.; Rigatti, L.; De Rose, A.F.; Van der Merwe, A.; Terrone, C.; Bartoletti, R.; Bonkat, G. Genitourinary Tuberculosis: A Comprehensive Review of a Neglected Manifestation in Low-Endemic Countries. Antibiotics 2021, 10, 1399. https://doi.org/10.3390/antibiotics10111399
Mantica G, Ambrosini F, Riccardi N, Vecchio E, Rigatti L, De Rose AF, Van der Merwe A, Terrone C, Bartoletti R, Bonkat G. Genitourinary Tuberculosis: A Comprehensive Review of a Neglected Manifestation in Low-Endemic Countries. Antibiotics. 2021; 10(11):1399. https://doi.org/10.3390/antibiotics10111399
Chicago/Turabian StyleMantica, Guglielmo, Francesca Ambrosini, Niccolò Riccardi, Enrico Vecchio, Lorenzo Rigatti, Aldo Franco De Rose, André Van der Merwe, Carlo Terrone, Riccardo Bartoletti, and Gernot Bonkat. 2021. "Genitourinary Tuberculosis: A Comprehensive Review of a Neglected Manifestation in Low-Endemic Countries" Antibiotics 10, no. 11: 1399. https://doi.org/10.3390/antibiotics10111399
APA StyleMantica, G., Ambrosini, F., Riccardi, N., Vecchio, E., Rigatti, L., De Rose, A. F., Van der Merwe, A., Terrone, C., Bartoletti, R., & Bonkat, G. (2021). Genitourinary Tuberculosis: A Comprehensive Review of a Neglected Manifestation in Low-Endemic Countries. Antibiotics, 10(11), 1399. https://doi.org/10.3390/antibiotics10111399






