Translation of Pharmacodynamic Biomarkers of Antibiotic Efficacy in Specific Populations to Optimize Doses
Abstract
:1. Introduction
2. Exposure-Matching as a Surrogate for Efficacy
3. Clinical Trial Endpoints for Exposure–Response Analyses
4. Systemic Efficacy Biomarkers of Inflammation and Infection
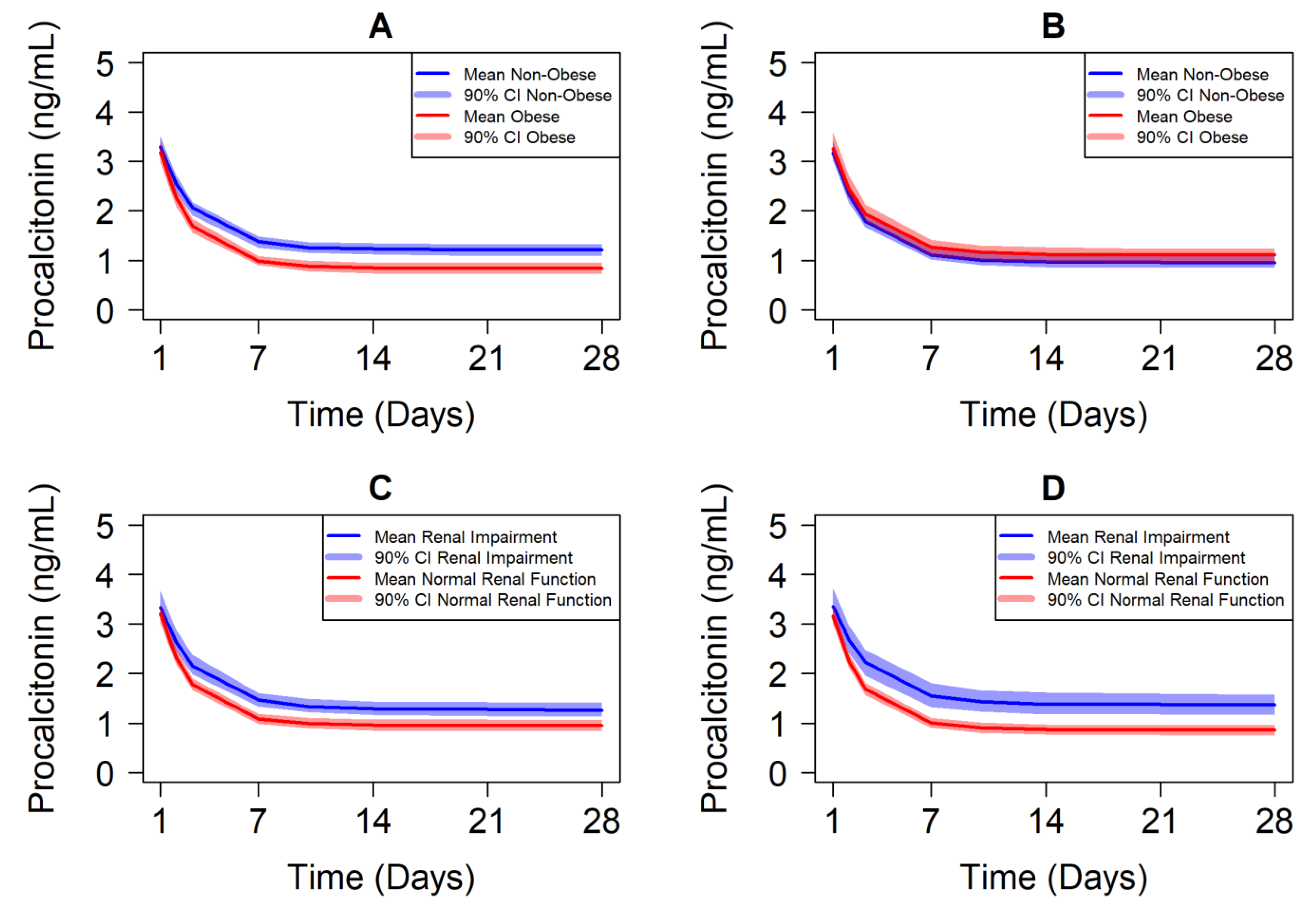
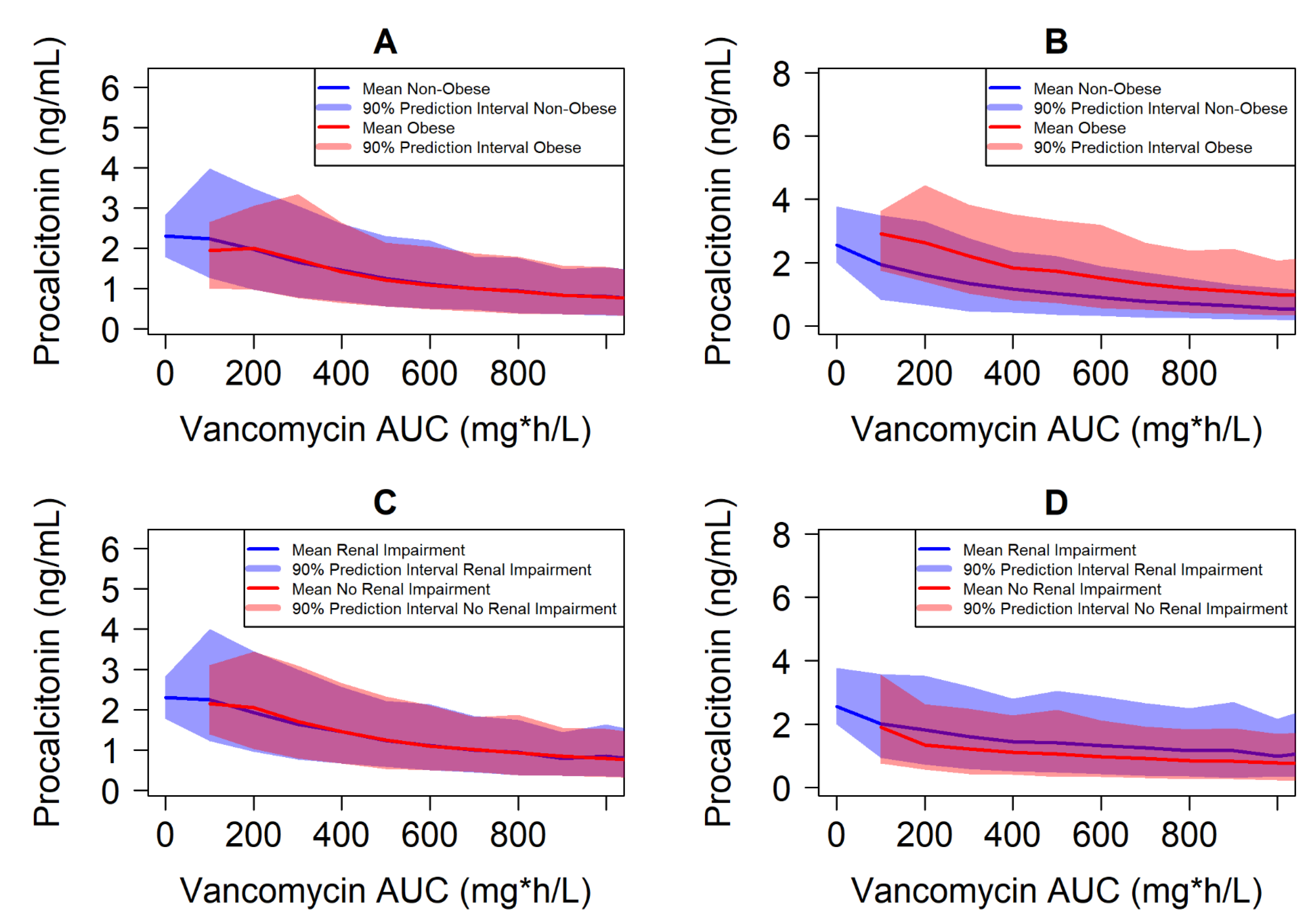
5. Case Study Illustrating Exposure–Response-Matching Using Biomarkers
6. Summary and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, P.S. Antibacterial Discovery: 21st Century Challenges. Antibiotics 2020, 9, 213. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef]
- Phillips, A.N.; Pocock, S.J. Sample size requirements for prospective studies, with examples for coronary heart disease. J. Clin. Epidemiol. 1989, 42, 639–648. [Google Scholar] [CrossRef]
- Boucher, H.W.; Ambrose, P.G.; Chambers, H.F.; Ebright, R.H.; Jezek, A.; Murray, B.E.; Newland, J.G.; Ostrowsky, B.; Rex, J.H.; on behalf of the Infectious Diseases Society of America. White Paper: Developing Antimicrobial Drugs for Resistant Pathogens, Narrow-Spectrum Indications, and Unmet Needs. J. Infect. Dis. 2017, 216, 228–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rex, J.H.; Eisenstein, B.I.; Alder, J.; Goldberger, M.; Meyer, R.; Dane, A.; Friedland, I.; Knirsch, C.; Sanhai, W.R.; Tomayko, J.; et al. A comprehensive regulatory framework to address the unmet need for new antibacterial treatments. Lancet Infect. Dis. 2013, 13, 269–275. [Google Scholar] [CrossRef]
- Echols, R.; Ariyasu, M.; Nagata, T.D. Pathogen-focused Clinical Development to Address Unmet Medical Need: Cefiderocol Targeting Carbapenem Resistance. Clin. Infect. Dis. 2019, 69, S559–S564. [Google Scholar] [CrossRef] [Green Version]
- Umscheid, C.A.; Margolis, D.J.; Grossman, C.E. Key concepts of clinical trials: A narrative review. Postgrad. Med. 2011, 123, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhu, H.; Madabushi, R.; Liu, Q.; Huang, S.M.; Zineh, I. Model-Informed Drug Development: Current US Regulatory Practice and Future Considerations. Clin. Pharmacol. Ther. 2019, 105, 899–911. [Google Scholar] [CrossRef]
- Grimsrud, K.N.; Sherwin, C.M.; Constance, J.E.; Tak, C.; Zuppa, A.F.; Spigarelli, M.G.; Mihalopoulos, N.L. Special population considerations and regulatory affairs for clinical research. Clin. Res. Regul. Aff. 2015, 32, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Mulugeta, Y.; Barrett, J.S.; Nelson, R.; Eshete, A.T.; Mushtaq, A.; Yao, L.; Glasgow, N.; Mulberg, A.E.; Gonzalez, D.; Green, D.; et al. Exposure Matching for Extrapolation of Efficacy in Pediatric Drug Development. J. Clin. Pharmacol. 2016, 56, 1326–1334. [Google Scholar] [CrossRef]
- Bidell, M.R.; Lodise, T.P. Suboptimal Clinical Response Rates with Newer Antibiotics among Patients with Moderate Renal Impairment: Review of the Literature and Potential Pharmacokinetic and Pharmacodynamic Considerations for Observed Findings. Pharmacotherapy 2018, 38, 1205–1215. [Google Scholar] [CrossRef]
- Crass, R.L.; Rodvold, K.A.; Mueller, B.A.; Pai, M.P. Renal Dosing of Antibiotics: Are We Jumping the Gun? Clin. Infect. Dis. 2019, 68, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Crass, R.L.; Pai, M.P. Estimating Renal Function in Drug Development: Time to Take the Fork in the Road. J. Clin. Pharmacol. 2019, 59, 159–167. [Google Scholar] [CrossRef]
- Shah, B.R.; Hux, J.E. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 2003, 26, 510–513. [Google Scholar] [CrossRef] [Green Version]
- Breslow, L.H. The Best Pharmaceuticals for Children Act of 2002: The rise of the voluntary incentive structure and congressional refusal to require pediatric testing. Harv. J. Legis. 2003, 40, 133–193. [Google Scholar]
- Roberts, R.; Rodriguez, W.; Murphy, D.; Crescenzi, T. Pediatric drug labeling: Improving the safety and efficacy of pediatric therapies. JAMA 2003, 290, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Wharton, G.T.; Murphy, M.D.; Avant, D.; Goldsmith, J.V.; Chai, G.; Rodriguez, W.J.; Eisenstein, E.L. Impact of pediatric exclusivity on drug labeling and demonstrations of efficacy. Pediatrics 2014, 134, e512–e518. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, K.; Putera, M.; Hornik, C.P.; Brian Smith, P.; Benjamin, D.K., Jr.; Mulugeta, Y.; Burckart, G.J.; Cohen-Wolkowiez, M.; Gonzalez, D. Exposure Matching of Pediatric Anti-infective Drugs: Review of Drugs Submitted to the Food and Drug Administration for Pediatric Approval. Clin. Ther. 2016, 38, 1995–2005. [Google Scholar] [CrossRef] [Green Version]
- Winter, S.S.; Page-Reeves, J.M.; Page, K.A.; Haozous, E.; Solares, A.; Nicole Cordova, C.; Larson, R.S. Inclusion of special populations in clinical research: Important considerations and guidelines. J. Clin. Transl. Res. 2018, 4, 56–69. [Google Scholar]
- Krekels, E.H.J.; van Hasselt, J.G.C.; van den Anker, J.N.; Allegaert, K.; Tibboel, D.; Knibbe, C.A.J. Evidence-based drug treatment for special patient populations through model-based approaches. Eur. J. Pharm. Sci. 2017, 109, S22–S26. [Google Scholar] [CrossRef]
- Ravenstijn, P.; Chetty, M.; Manchandani, P. Design and conduct considerations for studies in patients with impaired renal function. Clin. Transl. Sci. 2021, 14, 1689–1704. [Google Scholar] [CrossRef]
- Katsube, T.; Wajima, T.; Ishibashi, T.; Arjona Ferreira, J.C.; Echols, R. Pharmacokinetic/Pharmacodynamic Modeling and Simulation of Cefiderocol, a Parenteral Siderophore Cephalosporin, for Dose Adjustment Based on Renal Function. Antimicrob. Agents Chemother. 2017, 61, e01381-16. [Google Scholar] [CrossRef] [Green Version]
- CDER. Guidance for Industry Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Beltsville, MD, USA, 2013. [Google Scholar]
- CDER. Complicated Urinary Tract Infections: Developing Drugs for Treatment Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Beltsville, MD, USA, 2018. [Google Scholar]
- CDER. Community-Acquired Bacterial Pneumonia: Developing Drugs for Treatment Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Beltsville, MD, USA, 2020. [Google Scholar]
- CDER. Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia: Developing Drugs for Treatment Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Beltsville, MD, USA, 2020. [Google Scholar]
- Munoz-Price, L.S.; Frencken, J.F.; Tarima, S.; Bonten, M. Handling Time-dependent Variables: Antibiotics and Antibiotic Resistance. Clin. Infect. Dis. 2016, 62, 1558–1563. [Google Scholar] [CrossRef] [Green Version]
- Schurink, C.A.M.; Nieuwenhoven, C.A.V.; Jacobs, J.A.; Rozenberg-Arska, M.; Joore, H.C.A.; Buskens, E.; Hoepelman, A.I.M.; Bonten, M.J.M. Clinical pulmonary infection score for ventilator-associated pneumonia: Accuracy and inter-observer variability. Intensive Care Med. 2004, 30, 217–224. [Google Scholar] [CrossRef]
- Overcash, J.S.; Kim, C.; Keech, R.; Gumenchuk, I.; Ninov, B.; Gonzalez-Rojas, Y.; Waters, M.; Simeonov, S.; Engelhardt, M.; Saulay, M.; et al. Ceftobiprole Compared with Vancomycin Plus Aztreonam in the Treatment of Acute Bacterial Skin and Skin Structure Infections: Results of a Phase 3, Randomized, Double-blind Trial (TARGET). Clin. Infect. Dis. 2020, 73, e1507–e1517. [Google Scholar] [CrossRef]
- O’Riordan, W.; Green, S.; Overcash, J.S.; Puljiz, I.; Metallidis, S.; Gardovskis, J.; Garrity-Ryan, L.; Das, A.F.; Tzanis, E.; Eckburg, P.B.; et al. Omadacycline for Acute Bacterial Skin and Skin-Structure Infections. N. Engl. J. Med. 2019, 380, 528–538. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, W.; McManus, A.; Teras, J.; Poromanski, I.; Cruz-Saldariagga, M.; Quintas, M.; Lawrence, L.; Liang, S.; Cammarata, S.; Group, P.S. A Comparison of the Efficacy and Safety of Intravenous Followed by Oral Delafloxacin With Vancomycin Plus Aztreonam for the Treatment of Acute Bacterial Skin and Skin Structure Infections: A Phase 3, Multinational, Double-Blind, Randomized Study. Clin. Infect. Dis. 2018, 67, 657–666. [Google Scholar] [CrossRef]
- Huang, D.B.; O’Riordan, W.; Overcash, J.S.; Heller, B.; Amin, F.; File, T.M.; Wilcox, M.H.; Torres, A.; Dryden, M.; Holland, T.L.; et al. A Phase 3, Randomized, Double-Blind, Multicenter Study to Evaluate the Safety and Efficacy of Intravenous Iclaprim Vs Vancomycin for the Treatment of Acute Bacterial Skin and Skin Structure Infections Suspected or Confirmed to be Due to Gram-Positive Pathogens: REVIVE-1. Clin. Infect. Dis. 2018, 66, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Corey, G.R.; Kabler, H.; Mehra, P.; Gupta, S.; Overcash, J.S.; Porwal, A.; Giordano, P.; Lucasti, C.; Perez, A.; Good, S.; et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N. Engl. J. Med. 2014, 370, 2180–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucher, H.W.; Wilcox, M.; Talbot, G.H.; Puttagunta, S.; Das, A.F.; Dunne, M.W. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N. Engl. J. Med. 2014, 370, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Prokocimer, P.; De Anda, C.; Fang, E.; Mehra, P.; Das, A. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: The ESTABLISH-1 randomized trial. JAMA 2013, 309, 559–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaalian Dastjerdi, H.; Topfer, D.; Rupitsch, S.J.; Maier, A. Measuring Surface Area of Skin Lesions with 2D and 3D Algorithms. Int. J. Biomed. Imaging 2019, 2019, 4035148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Brubaker, L.; Wolfe, A.J.; Mueller, E.R.; Schreckenberger, P.C. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lortholary, O.; Lefort, A.; Tod, M.; Chomat, A.M.; Darras-Joly, C.; Cordonnier, C. Pharmacodynamics and pharmacokinetics of antibacterial drugs in the management of febrile neutropenia. Lancet Infect. Dis. 2008, 8, 612–620. [Google Scholar] [CrossRef]
- Hung, S.K.; Lan, H.M.; Han, S.T.; Wu, C.C.; Chen, K.F. Current Evidence and Limitation of Biomarkers for Detecting Sepsis and Systemic Infection. Biomedicines 2020, 8, 494. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Baghi, H.B. Presepsin: A promising biomarker for the detection of bacterial infections. Biomed. Pharmacother. 2019, 111, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, A.L.; Vanimaya; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R.; Manoj, G. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Povoa, P.; Almeida, E.; Moreira, P.; Fernandes, A.; Mealha, R.; Aragao, A.; Sabino, H. C-reactive protein as an indicator of sepsis. Intensive Care Med. 1998, 24, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Kataria, Y.; Remick, D. Sepsis Biomarkers. Methods Mol. Biol. 2021, 2321, 177–189. [Google Scholar] [CrossRef]
- Shehabi, Y.; Sterba, M.; Garrett, P.M.; Rachakonda, K.S.; Stephens, D.; Harrigan, P.; Walker, A.; Bailey, M.J.; Johnson, B.; Millis, D.; et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2014, 190, 1102–1110. [Google Scholar] [CrossRef]
- Dandona, P.; Nix, D.; Wilson, M.F.; Aljada, A.; Love, J.; Assicot, M.; Bohuon, C. Procalcitonin increase after endotoxin injection in normal subjects. J. Clin. Endocrinol. Metab. 1994, 79, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Grace, E.; Turner, R.M. Use of procalcitonin in patients with various degrees of chronic kidney disease including renal replacement therapy. Clin. Infect. Dis. 2014, 59, 1761–1767. [Google Scholar] [CrossRef] [Green Version]
- Heilmann, E.; Gregoriano, C.; Wirz, Y.; Luyt, C.E.; Wolff, M.; Chastre, J.; Tubach, F.; Christ-Crain, M.; Bouadma, L.; Annane, D.; et al. Association of kidney function with effectiveness of procalcitonin-guided antibiotic treatment: A patient-level meta-analysis from randomized controlled trials. Clin. Chem. Lab. Med. 2020, 59, 441–453. [Google Scholar] [CrossRef]
- Aulin, L.B.S.; de Lange, D.W.; Saleh, M.A.A.; van der Graaf, P.H.; Voller, S.; van Hasselt, J.G.C. Biomarker-Guided Individualization of Antibiotic Therapy. Clin. Pharmacol. Ther. 2021, 110, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G., Jr.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, [2017–2020]. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?Cycle=2017-2020 (accessed on 28 September 2021).
- Yamamoto, M.; Kuzuya, T.; Baba, H.; Yamada, K.; Nabeshima, T. Population pharmacokinetic analysis of vancomycin in patients with gram-positive infections and the influence of infectious disease type. J. Clin. Pharm. Ther. 2009, 34, 473–483. [Google Scholar] [CrossRef]
- Dayneka, N.L.; Garg, V.; Jusko, W.J. Comparison of four basic models of indirect pharmacodynamic responses. J. Pharmacokinet. Biopharm. 1993, 21, 457–478. [Google Scholar] [CrossRef]
- Leli, C.; Ferranti, M.; Moretti, A.; Al Dhahab, Z.S.; Cenci, E.; Mencacci, A. Procalcitonin levels in gram-positive, gram-negative, and fungal bloodstream infections. Dis. Markers 2015, 2015, 701480. [Google Scholar] [CrossRef] [Green Version]
- Overgaard, R.V.; Ingwersen, S.H.; Tornoe, C.W. Establishing Good Practices for Exposure-Response Analysis of Clinical Endpoints in Drug Development. CPT Pharmacomet. Syst. Pharmacol. 2015, 4, 565–575. [Google Scholar] [CrossRef]
- Marier, J.F.; Jomphe, C.; Peyret, T.; Wang, Y. Population pharmacokinetics and exposure-response analyses of teduglutide in adult and pediatric patients with short bowel syndrome. Clin. Transl. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gewitz, A.D.; Solans, B.P.; Mac Kenzie, W.R.; Heilig, C.; Whitworth, W.C.; Johnson, J.L.; Nsubuga, P.; Dorman, S.; Weiner, M.; Savic, R.M.; et al. Longitudinal Model-Based Biomarker Analysis of Exposure-Response Relationships in Adults with Pulmonary Tuberculosis. Antimicrob. Agents Chemother. 2021, 65, e0179420. [Google Scholar] [CrossRef] [PubMed]
- Sturm, S.; Lemenuel-Diot, A.; Patel, K.; Gibiansky, L.; Bhardwaj, R.; Smith, P.F.; Dang, S.; Zwanziger, E.; Nasmyth-Miller, C.; Ravva, P. Pharmacologic effects of oseltamivir in immunocompromised adult patients as assessed by population PK/PD analysis and drug-disease modelling for dosing regimen optimization. Br. J. Clin. Pharmacol. 2021, 87, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Snelder, N.; Heinig, R.; Drenth, H.J.; Joseph, A.; Kolkhof, P.; Lippert, J.; Garmann, D.; Ploeger, B.; Eissing, T. Population Pharmacokinetic and Exposure-Response Analysis of Finerenone: Insights Based on Phase IIb Data and Simulations to Support Dose Selection for Pivotal Trials in Type 2 Diabetes with Chronic Kidney Disease. Clin. Pharmacokinet. 2020, 59, 359–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Hu, C.; Zhu, Y.; Randazzo, B.; Song, M.; Sharma, A.; Xu, Z.; Zhou, H. Extrapolating Pharmacodynamic Effects from Adults to Pediatrics: A Case Study of Ustekinumab in Pediatric Patients With Moderate to Severe Plaque Psoriasis. Clin. Pharmacol. Ther. 2021, 109, 131–139. [Google Scholar] [CrossRef]
- Zhang, Q.; Travis, J.; Rothwell, R.; Jay, C.E.; Jahidur, R.; Zhang, Y.; Crentsil, V.; Altepeter, T.; Lee, J.J.; Burckart, G.J.; et al. Applying the Noninferiority Paradigm to Assess Exposure-Response Similarity and Dose Between Pediatric and Adult Patients. J. Clin. Pharmacol. 2021, 61 (Suppl. S1), S165–S174. [Google Scholar] [CrossRef]
| Therapeutic Indication | Primary Endpoint | Secondary Endpoint | Potential Continuous and Ordinal Endpoint Measurements |
|---|---|---|---|
| Acute Bacterial Skin and Skin Structure Infections (ABSSSI) | Percent reduction (≥20% typically) in lesion size at 48 to 72 h | Resolution of ABSSSI at 7 to 14 days after therapy completion | Lesion size surface area by serial image analysis Symptom scores (e.g., pain) |
| Community-Acquired Bacterial Pneumonia | Improvement in at least two symptoms (with no worsening) at day 4 All-cause mortality at 28 days if including severe cases | Improvement in at least two symptoms (with no worsening) at day 4 and vital signs Clinical outcome at end of therapy or at a fixed predefined time point | Change in systemic biomarkers such as C-reactive protein, procalcitonin, calprotectin, presepsin, etc. |
| Hospital-Acquired Bacterial Pneumonia (HABP) and Ventilator-Associated Bacterial Pneumonia (VABP) | All-cause mortality at any time between 14 and 28 days | (1) Resolution of signs and symptoms of HABP/VABP at approximately 7 to 14 days after the completion of antibacterial drug therapy, (2) days spent in the hospital, and (3) days spent on mechanical ventilation (for VABP and ventilated-HABP patients) | Clinical pulmonary infection scores, procalcitonin values |
| Nosocomial Pneumonia | All-cause mortality at 14 days | Clinical and microbiological outcomes at Test of Cure or early and later time points | SOFA scores Clinical pulmonary infection score |
| Complicated Urinary Tract Infections (cUTI) | Microbial Eradication Clinical Cure | Microbial eradication rate Clinical response at the Test of Cure | Reduction in urine bacterial colony forming units |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pai, M.P.; Crass, R.L. Translation of Pharmacodynamic Biomarkers of Antibiotic Efficacy in Specific Populations to Optimize Doses. Antibiotics 2021, 10, 1368. https://doi.org/10.3390/antibiotics10111368
Pai MP, Crass RL. Translation of Pharmacodynamic Biomarkers of Antibiotic Efficacy in Specific Populations to Optimize Doses. Antibiotics. 2021; 10(11):1368. https://doi.org/10.3390/antibiotics10111368
Chicago/Turabian StylePai, Manjunath P., and Ryan L. Crass. 2021. "Translation of Pharmacodynamic Biomarkers of Antibiotic Efficacy in Specific Populations to Optimize Doses" Antibiotics 10, no. 11: 1368. https://doi.org/10.3390/antibiotics10111368
APA StylePai, M. P., & Crass, R. L. (2021). Translation of Pharmacodynamic Biomarkers of Antibiotic Efficacy in Specific Populations to Optimize Doses. Antibiotics, 10(11), 1368. https://doi.org/10.3390/antibiotics10111368






