Use of Antimicrobials in a French Veterinary Teaching Hospital: A Retrospective Study
Abstract
:1. Introduction
2. Results
2.1. Overall Antimicrobials Prescriptions
2.2. Data on Critically Important Antibiotics
2.3. Sales of Antimicrobials
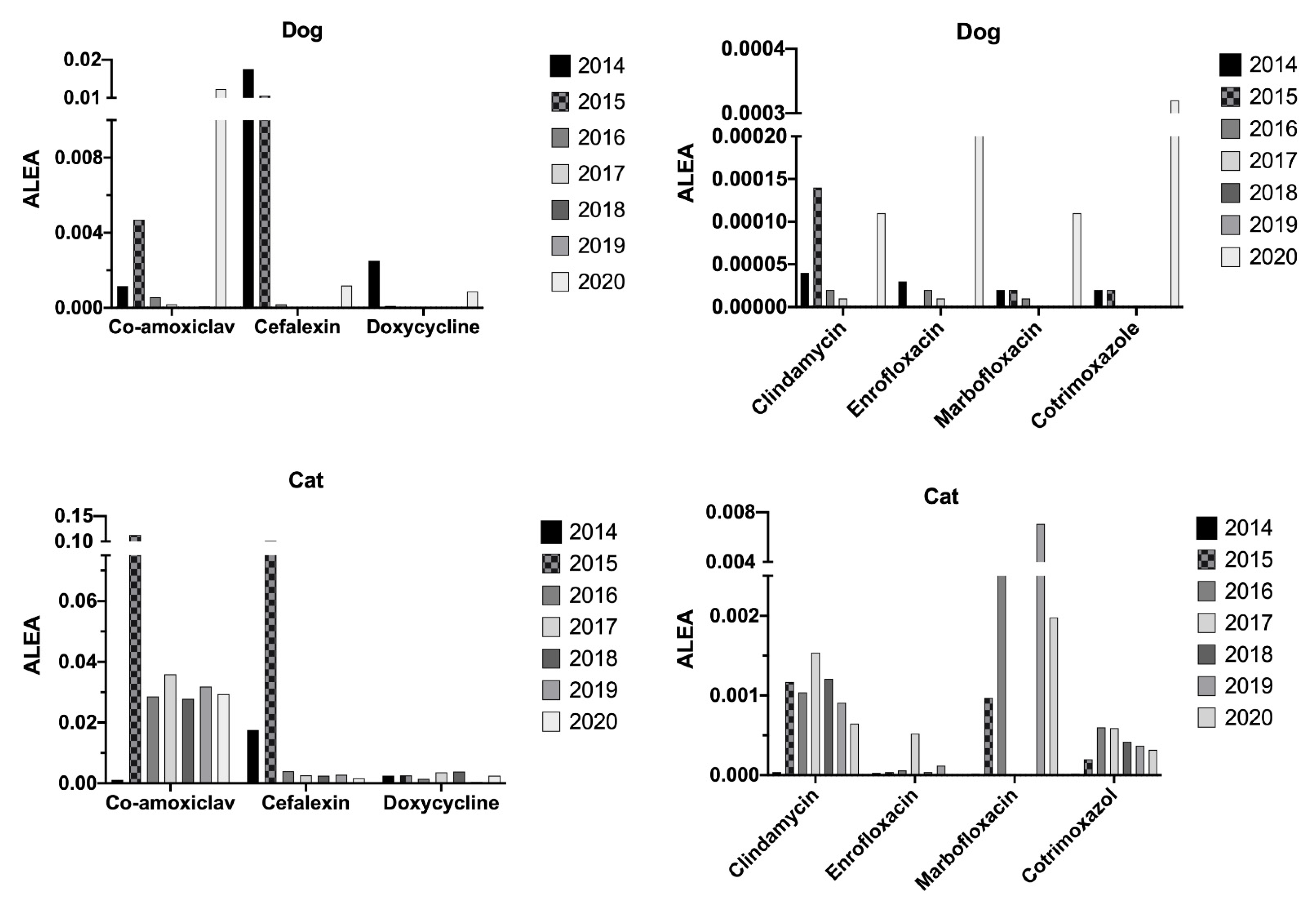
2.4. Animals’ Exposure Levels
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Data Collection of Dispensation and Prescription of Antimicrobials
4.3. Prescription Analysis
4.4. Calculation
- -
- Antibiotics dispensed by the pharmacy
- -
- Animal level of exposure to antimicrobials (ALEA)
4.5. Data Organization
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Pokharel, S.; Shrestha, P.; Adhikari, B. Antimicrobial use in food animals and human health: Time to implement ‘One Health’ approach. Antimicrob. Resist Infect. Control 2020, 9, 181. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance. 2016. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 8 November 2021).
- World Organisation for Animal Health (OIE). Terrestrial Animal Health Code. 2021. Chapter 6.7 to 6.11. Available online: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/ (accessed on 8 November 2021).
- Food and Agriculture Organization of the United Nations (FAO). The FAO Action Plan on Antimicrobial Resistance 2016–2020. 2016. Available online: https://www.fao.org/3/i5996e/i5996e.pdf (accessed on 8 November 2021).
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; de Schaetzen, M.A.; Van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial resistance in the food chain: A review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef] [Green Version]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Antunes, P.; Novais, C.; Peixe, L. Food-to-Humans Bacterial Transmission. Microbiol. Spectr. 2020, 8, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Warren, A.; Townsend, K.; King, T.; Moss, S.; Yates, R.M.; Trott, D.J. Multi-drug resistant Escherichia coli with extended spectrum β-lactamase activity and fluoroquinolone resistance isolated from clinical infections in dogs. Aus. Vet. J. 2001, 79, 621–623. [Google Scholar] [CrossRef]
- Prescott, J.F.; Hanna, W.J.; Reid-Smith, R.; Drost, K. Antimicrobial drug use and resistance in dogs. Can. Vet. J. 2002, 43, 107–116. [Google Scholar]
- Faires, M.C.; Tater, K.C.; Weese, J.S. Methicillin-resistant Staphylococcus aureus colonization in people and pets. JAVMA 2009, 235, 540–543. [Google Scholar] [CrossRef] [Green Version]
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 2004, 54, 321–332. [Google Scholar] [CrossRef]
- Pomba, C.; Rantala, M.; Greko, C.; Baptiste, K.E.; Catry, B.; van Duijkeren, E.; Mateus, A. Public health risk of antimicrobial resistance transfer from companion animals. J. Antimicrob. Chemother. 2017, 72, 957–968. [Google Scholar] [CrossRef]
- Zhang, X.; Doi, Y.; Huang, X.; Li, H.Y.; Zhong, L.L.; Zeng, K.J.; Zhang, Y.F.; Patil, S.; Tian, G.B. Possible transmission of mcr-1-harboring Escherichia coli between companion animals and human. Emerg. Infect. Dis. 2016, 22, 1679–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoons-Smit, A.M.; Savelkoul, P.H.M.; Stoof, J.; Starink, T.M.; Vandenbroucke-Grauls, C.M. Transmission of Staphylococcus aureus between humans and domestic animals. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 24, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Van Duijkeren, E.; Wolfhagen, M.J.; Box, A.T.; Keck, M.E.O.C.; Wannet, W.J.B.; Fluit, A.C. Human-to-dog transmission of methicillin-resistant Staphylococcus aureus. Emerg. Infect. Dis. 2004, 10, 2235–2237. [Google Scholar] [CrossRef]
- Loeffler, A.; Boag, A.K.; Sung, J.; Lindsay, J.A.; Guardabassi, L.; Dalsgaard, A.; Smith, H.; Stevens, K.B.; Lloyd, D.H. Prevalence of methicillin-resistant Staphylococcus aureus among staff and pets in a small animal referral hospital in the UK. J. Antimicrob. Chemother. 2005, 56, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Ministère de la Santé. Plan de Lutte Contre les Infections Nosocomiales; Ministère de la Santé: Paris, France, 1994.
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial Resistance in Bacteria: Mechanisms, Evolution, and Persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef] [PubMed]
- AFVAC. Guide de Bonnes Pratiques—Fiches de Recommandations Pour un bon Usage des Antibiotiques. Filière Animaux de Compagnie; AFVAC: Paris, France, 2017. [Google Scholar]
- Agence Nationale D’Accréditation et d’Evaluation de la Santé. Le bon Usage des Antibiotiques à L’hôpital. Recommandations Pour Maitriser le Développement de la Résistance Bactérienne. 1996. Available online: https://urgences-serveur.fr/IMG/pdf/antibio-2.pdf (accessed on 8 November 2021).
- Ministère de la Santé. Plan National Pour Maitriser L’activité des Antibiotiques 2001–2005; Ministère de la Santé: Paris, France, 2005.
- Ministère de la Santé. Plan National Pour Préserver L’efficacité des Antibiotiques. Available online: https://solidarites-sante.gouv.fr/IMG/pdf/plan_antibiotiques_2011-2016_.pdf (accessed on 8 November 2021).
- Feuille Interministérielle de Maîtrise de L’antibiorésistance. Comité Interministériel Pour la Santé. 2016. Available online: https://solidarites-sante.gouv.fr/IMG/pdf/feuille_de_route_antibioresistance_nov_2016.pdf (accessed on 8 November 2021).
- French Ministry of Agriculture, Agrifood and Forestry. Ecoantibio: Reducing Antibiotic Use in Veterinary Medicine (2012–2016). Available online: https://agriculture.gouv.fr/plan-ecoantibio-2012-2017-lutte-contre-lantibioresistance (accessed on 8 November 2021).
- French Ministry of Agriculture, Agrifood and Forestry. Ecoantibio 2: The French National Plan for the Reduction of the Risks of Antimicrobial Resistance in Veterinary Medicine (2017–2021). Available online: https://agriculture.gouv.fr/le-plan-ecoantibio-2-2017-2021 (accessed on 8 November 2021).
- Arrêté du 22 Juillet 2015 Relatif aux Bonnes Pratiques D’emploi des Médicaments Contenant une ou Plusieurs Substances Antibiotiques en Médecine Vétérinaire. 2005. Available online: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000031142007/ (accessed on 8 November 2021).
- WHO. Critically Important Antimicrobials for Human Medicine: 6th Revision. 2019. Available online: https://www.who.int/publications/i/item/9789241515528 (accessed on 8 November 2021).
- OIE. List of Antimicrobial Agents of Veterinary Importance. 2018. Available online: https://www.oie.int/app/uploads/2021/03/a-oie-list-antimicrobials-may2018.pdf (accessed on 8 November 2021).
- Décret n 2016-317 du 16 Mars 2016 Relatif à la Prescription et à la Delivrance des Medicaments Utilisés en Médecine Vétérinaire Contenant une ou Plusieurs Substances Antibiotiques D’importance Critique. 2016. Available online: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000032251629/ (accessed on 8 November 2021).
- Arrêté du 18 Mars 2016 Fixant la Liste des Substances Antibiotiques D’importance Critique Prévue à L’article L. 5144-1-1 du Code de la Santé Publique. 2016. Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000032291325 (accessed on 8 November 2021).
- Vercelli, C.; Della Ricca, M.; Re, M.; Gambino, G.; Re, G. Antibiotic Stewardship for Canine and Feline Acute Urinary Tract Infection: An Observational Study in a Small Animal Hospital in Northwest Italy. Antibiotics 2021, 10, 562. [Google Scholar] [CrossRef]
- Hubbuch, A.; Schmitt, K.; Lehner, C.; Hartnack, S.; Schuller, S.; Schüpbach-Regula, G.; Mevissen, M.; Peter, R.; Müntener, C.; Naegeli, H.; et al. Antimicrobial prescriptions in cats in Switzerland before and after the introduction of an online antimicrobial stewardship tool. BMC Vet. Res. 2020, 16, 229. [Google Scholar] [CrossRef] [PubMed]
- Lehner, C.; Hubbuch, A.; Schmitt, K.; Schuepbach-Regula, G.; Willi, B.; Mevissen, M.; Peter, R.; Muentener, C.R.; Naegeli, H.; Schuller, S. Effect of antimicrobial stewardship on antimicrobial prescriptions for selected diseases of dogs in Switzerland. Vet. Intern. Med. 2020, 34, 2418–2431. [Google Scholar] [CrossRef]
- Weese, J.S. Investigation of antimicrobial use and the impact of antimicrobial use guidelines in a small animal veterinary teaching hospital: 1995–2004. J. Am. Vet. Med. Assoc. 2006, 228, 553–558. [Google Scholar] [CrossRef]
- Ihedioha, T.E.; Asuzu, I.U.; Nwanta, J.A. Trends in the clinical use of antibiotics in a veterinary hospital in Nigeria, 2013–2017. Thai J. Vet. Med. 2020, 50, 487–494. [Google Scholar]
- Chirollo, C.; Nocera, F.P.; Piantedosi, D.; Fatone, G.; Della Valle, G.; De Martino, L.; Cortese, L. Data on before and after the Traceability System of Veterinary Antimicrobial Prescriptions in Small Animals at the University Veterinary Teaching Hospital of Naples. Animals 2021, 11, 913. [Google Scholar] [CrossRef]
- Escher, M.; Vanni, M.; Intorre, L.; Caprioli, A.; Tognetti, R.; Scavia, G. Use of antimicrobials in companion animal practice: A retrospective study in a veterinary teaching hospital in Italy. J. Antimicrob. Chem. 2011, 66, 920–927. [Google Scholar] [CrossRef] [Green Version]
- Peter, R.; Müntener, C.; Demuth, D.; Heim, D.; Mevissen, M.; Schüpbach-Regula, G. AntibioticScout: Online tool for antimicrobial stewardship in veterinary medicine. Schweiz Arch. Tierheilkd. 2016, 158, 805–810. [Google Scholar] [CrossRef]
- Peter, R.; Demuth, D.; Müntener, C.; Lampart, M.; Heim, D.; Mevissen, M. AntibioticScout.Ch: A decision supporting tool for antimicrobial stewardship: Application to companion animal medicine. Schweiz Arch. Tierheilkd. 2017, 159, 525–533. [Google Scholar] [CrossRef]
- ANSES. Sales Survey of Veterinary Medicinal Products Containing Antimicrobials in France in 2016; Annual Report; ANSES: Paris, France, 2017. [Google Scholar]






| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Mean | SD | |
|---|---|---|---|---|---|---|---|---|---|
| Number of medical visits 1 | 20,270 | 20,300 | 20,144 | 18,117 | 18,827 | 18,795 | 14,676 | 18,732.7 | 1986 |
| Number of animals | 8599 | 8508 | 8569 | 8147 | 8372 | 7900 | 6383 | 8068.29 | 784.5 |
| Number of animals for which at least one prescription has been established (all drugs) 2 | 3255 (37.8%) | 5223 (61.4%) | 5529 (64.5%) | 5497 (67.5%) | 5840 (69.7%) | 5859 (74.2%) | 4441 (69.6%) | 5092 (63.5%) | 941 (12.1%) |
| Number of animals with at least one antibiotic prescription 2 | 1264 (14.7%) a | 1438 (16.9%) a | 1374 (16.1%) a | 1347 (16.5%) a | 1580 (18.9%) a | 1814 (22.9%) a | 1162 (16.9%) a | 1426 (17.6%) | 216 (2.7%) |
| Total number of prescriptions (all drugs) | 5033 | 8296 | 8733 | 8894 | 10,725 | 11,107 | 8180 | 8710 | 1992 |
| Number of prescriptions with at least one antibiotic 3 | 1572 (31.2%) | 1995 (24.0%) a | 1855 (21.2%) a | 1944 (21.9%) a | 2484 (23.2%) a | 2637 (23.7%) a | 1883 (23.0%) a | 2053 (24.0%) | 375 (3.3%) |
| Number of prescriptions with a single antibiotic prescribed 4 | 1390 (88.4%) | 1669 (83.7%) | 1598 (86.1%) | 1656 (85.2%) | 2029 (81.7%) | 2146 (81.4%) | 1516 (80.5%) | 1715 (83.9%) | 273 (2.9%) |
| Number of prescriptions with multiple antibiotics prescribed 4 | 182 (11.6%) | 326 (16.3%) a | 257 (13.8%) | 288 (14.8%) a | 457 (18.4%) a | 491 (18.6%) a | 367 (19.5%) a | 338 (21.8%) | 110 (16.4%) |
| Number of prescriptions with at least one CIA 4 | 132 (8.4%) | 191 (9.6%) | 109 (5.9%) a | 152 (7.8%) | 191 (7.7%) | 160 (6.1%) a | 159 (8.4%) | 156 (7.6%) | 32 (1.53%) |
| Number of prescriptions with only one CIA (without other antibiotics) 5 | 75 (56.8%) | 83 (43.5%) | 83 (76.1%) | 78 (51.3%) | 176 (92.1%) a | 69 (43.1%) | 133 (83.7%) a | 98 (63.7%) | 38.6 (19.8%) |
| Number of prescriptions with one CIA and other antibiotics 5 | 57 (43.2%) | 108 (56.5%) | 26 (23.9%) a | 74 (48.7%) | 15 (7.9%) a | 91 (56.9%) | 26 (16.3%) a | 57 (36.5%) | 35 (19.8%) |
| Number of prescriptions per animals | 1.5 | 1.6 | 1.6 | 1.6 | 1.8 | 1.9 | 1.8 | 1.7 | 0.1 |
| Number of antimicrobial prescriptions per animals | 1.2 | 1.4 | 1.4 | 1.4 | 1.6 | 1.5 | 1.6 | 1.4 | 0.1 |
| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Mean | |
|---|---|---|---|---|---|---|---|---|
| Dogs | 2113 (830) | 3064 (1020) | 3016 (966) | 2783 (791) | 2855 (810) | 2840 (885) | 2277 (646) | 31.57 |
| 39.3% | 33.3% a | 32.0% a | 28.4% a | 28.4% a | 31.2% a | 28.4% a | ||
| Cats | 1089 (399) | 2065 (378) | 2637 (352) | 2240 (290) | 2236 (352) | 2174 (358) | 1753 (275) | 18.43 |
| 36.6% | 18.3% a | 13.3% a | 12.9% a | 15.7% a | 16.5% a | 15.7% a | ||
| Horses | ND | 7 (0) | 60 (24) | 403 (242) | 653 (390) | 746 (543) | 333 (208) | 59.00 |
| ND | 0.0% | 40.0% a | 60.0% a | 59.7% a | 72.8% a | 62.5% a | ||
| Uncommon pets | 41 (29) | 84 (13) | 85 (31) | 68 (23) | 86 (22) | 90 (25) | 75 (28) | 32.29 |
| 70.7% | 15.5% a | 15.3% a | 33.8% a | 25.6% a | 27.8% a | 37.3% a |
| 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|
| Number of completed questionnaires | 25 | 94 | 168 | 145 | 96 |
| 22.9% | 61.8% | 57.1% | 90.6% | 60.4% | |
| Number of “susceptibility test pending results” responses | 10 | 33 | 47 | 41 | 38 |
| 9.2% | 21.7% | 24.6% | 25.6% | 23.9% | |
| Number of “sampling not feasible” responses | 2 | 13 | 36 | 26 | 45 |
| 1.8% | 8.6% | 18.8% | 16.3% | 28.3% | |
| Number “susceptibility test results less than 3 months” responses | 2 | 58 | 104 | 104 | 74 |
| 1.8% | 38.2% | 54.5% | 65.0% | 46.5% | |
| Number “probabilistic use of antibiotic” responses | 6 | 22 | 59 | 59 | 61 |
| 5.5% | 14.5% | 30.9% | 36.9% | 38.4% | |
| Number “no adapted antibiotic available” responses | 19 | 81 | 128 | 112 | 96 |
| 17.4% | 53.3% | 67.0% | 70.0% | 60.4% |
| 2016 | 2017 | 2018 | 2019 | 2020 | ||
|---|---|---|---|---|---|---|
| Gram − | Pseudomonas aeruginosa | 9 | 17 | 34 | 32 | 19 |
| Pseudomonas fluorescens | 2 | 2 | 2 | 1 | ||
| Escherichia coli | 2 | 2 | 15 | 8 | 3 | |
| Morganella morganii | 1 | 1 | ||||
| Klebsielle pneumoniae | 1 | 7 | 1 | |||
| Proteus mirabilis | 3 | 5 | 4 | 5 | 1 | |
| Chryseobacterium indologenes | 2 | |||||
| Pasteurella pneumotropica | 1 | |||||
| Serratia marcescens | 1 | 2 | ||||
| Acinetobacter baumanii | 2 | 2 | ||||
| Gram + | Staphylococcus spp. (coagulase positive) | 5 | 18 | 23 | 19 | 5 |
| Staphylococcus spp. (coagulase negative) | 2 | 5 | ||||
| Streptococcus equiissp.equii | 2 | 1 | ||||
| Streptococcus equiissp.zooepidemicus | 9 | 2 | 1 | |||
| Streptococcus dysglactiae | 1 | |||||
| Streptococcus canis | 1 | |||||
| Enterococcus cloacae | 1 | 1 | 1 | 5 | ||
| Enterococcus faecalis | 1 | 1 | ||||
| Clostridium perfringens | 1 | |||||
| Rhodococcus equi | 3 | |||||
| Aerococcus viridans | 1 |
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|---|
| Fusidic acid | ↓89.3% | ↓5.2% | ↓8.9% | ↓14.5% | ↑65.7% | ↓5.5% |
| Aminoglycosides | ↓53.7% | ↑2.2% | ↑402.6% | ↓0.1% | ↑189.1% | ↑23.7% |
| Penicillins | ↑6.8% | ↑7.7% | ↑53.5% | ↓12.5% | ↑17.1% | ↑4.1% |
| Cephalosporins 1G | ↓35.7% | ↓16.5% | ↓25.9% | ↓52.1% | ↓46.8% | ↓67.6% |
| Fluoroquinolones | ↑81.8% | ↑12.3% | ↑64.5% | ↓22.3% | ↓3.2% | ↓67.0% |
| Lincosamides | ↑125.3% | ↑103.5% | ↑10.1% | ↑43.4% | ↓56.4% | ↓69.6% |
| Macrolides | ↓8.6% | ↓30% | ↓67.6% | ↓41.3% | ↓64.8% | ↓88.4% |
| Phenicols | ↑47.7% | ↑9.2% | =0% | ↑42.5% | ↑13.8% | ↓20% |
| Polymixins | ↓13.7% | ↑68.3% | ↑512.1% | ↑387.3% | ↑247.7% | ↓94.4% |
| Sulfamide | ↑116.7% | ↓83.3% | ↓100% | ↑33.3% | ↑600% | ↑666.7% |
| Cotrimoxazole | ↑38.0% | ↓24.1% | ↑136.9% | ↑26.7% | ↑44.7% | ↑113.3% |
| Tetracyclines | ↓95.3% | ↓97.2% | ↓93.9% | ↓93.2% | ↓99.6% | ↓95.5% |
| Total of antibiotic per year (mg) and% of variation since 2014 | ↓39.5% | ↓34.9% | ↓26.7% | ↓53.2% | ↓47.3% | ↓58.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prouillac, C. Use of Antimicrobials in a French Veterinary Teaching Hospital: A Retrospective Study. Antibiotics 2021, 10, 1369. https://doi.org/10.3390/antibiotics10111369
Prouillac C. Use of Antimicrobials in a French Veterinary Teaching Hospital: A Retrospective Study. Antibiotics. 2021; 10(11):1369. https://doi.org/10.3390/antibiotics10111369
Chicago/Turabian StyleProuillac, Caroline. 2021. "Use of Antimicrobials in a French Veterinary Teaching Hospital: A Retrospective Study" Antibiotics 10, no. 11: 1369. https://doi.org/10.3390/antibiotics10111369
APA StyleProuillac, C. (2021). Use of Antimicrobials in a French Veterinary Teaching Hospital: A Retrospective Study. Antibiotics, 10(11), 1369. https://doi.org/10.3390/antibiotics10111369






