Susceptibility of Commensal E. coli Isolated from Conventional, Antibiotic-Free, and Organic Meat Chickens on Farms and at Slaughter toward Antimicrobials with Public Health Relevance
Abstract
1. Introduction
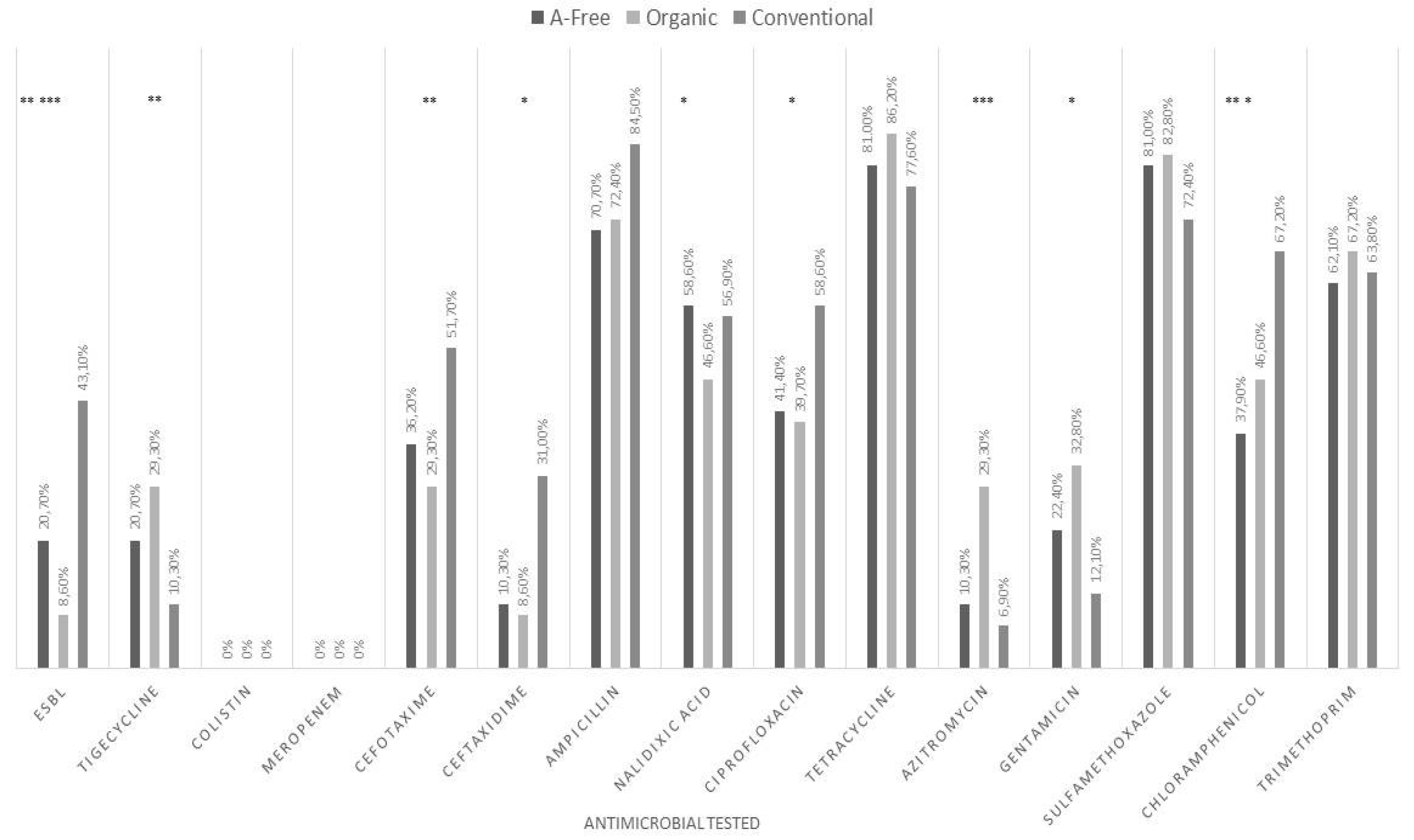
2. Results
3. Discussion
4. Materials and Methods
4.1. Sampling
4.2. Isolation and Identification of E. coli
4.3. Antibiotic Susceptibility Testing and ESBL E. coli Detection
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ilea, R.C. Intensive Livestock Farming: Global Trends, Increased Environmental Concerns, and Ethical Solutions. J. Agric. Environ. Ethic. 2008, 22, 153–167. [Google Scholar] [CrossRef]
- Alonso, C.A.; Zarazaga, M.; Ben Sallem, R.; Jouini, A.; BEN Slama, K.; Torres, C. Antibiotic resistance inEscherichia coliin husbandry animals: The African perspective. Lett. Appl. Microbiol. 2017, 64, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Chimera, E.T.; Fosgate, G.T.; Etter, E.M.; Boulangé, A.; Vorster, I.; Neves, L. A one health investigation of pathogenic trypanosomes of cattle in Malawi. Prev. Veter. Med. 2021, 188, 105255. [Google Scholar] [CrossRef]
- Castanon, J. History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Drug-Resistant Infections: A Threat to Our Economic Future; World Bank: Washington, DC, USA, 2017.
- Roila, R.; Ranucci, D.; Valiani, A.; Galarini, R.; Servili, M.; Branciari, R. Antimicrobial and anti-biofilm activity of olive oil by-products against Campylobacter spp. isolated from chicken meat. Acta Sci. Pol. Technol. Aliment. 2019, 18, 43–52. [Google Scholar]
- Grace, D. Review of evidence on antimicrobial resistance and animal agriculture in developing countries. Evid. Demand Int. Livest. Res. Inst. 2015. [Google Scholar] [CrossRef]
- Young, A.L.; Nicol, M.P.; Moodley, C.; Bamford, C.M. The accuracy of extended-spectrum beta-lactamase detection in Escherichia coli and Klebsiella pneumoniae in South African laboratories using the Vitek 2 Gram-negative susceptibility card AST-N255. South. Afr. J. Infect. Dis. 2019, 34, 1–6. [Google Scholar] [CrossRef]
- Agyare, C.; Boamah, V.E.; Zumbi, C.N.; Osei, F.B. Antibiotic Use in Poultry Production and Its Effects on Bacterial Resistance. Antimicrob Resist. A Glob. Threat. 2018, 33–50. [Google Scholar] [CrossRef]
- Li, J.; Fu, Y.; Zhang, J.; Wang, Y.; Zhao, Y.; Fan, X.; Yu, L.; Wang, Y.; Zhang, X.; Li, C. Efficacy of tigecycline monotherapy versus combination therapy with other antimicrobials against carbapenem-resistant Acinetobacter baumannii sequence type 2 in Heilongjiang Province. Ann. Palliat. Med. 2019, 8, 651–659. [Google Scholar] [CrossRef]
- Giamarellou, H. Epidemiology of infections caused by polymyxin-resistant pathogens. Int. J. Antimicrob. Agents 2016, 48, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, R.; Liu, D.; Walsh, T.; Zhang, R.; Lv, Y.; Ke, Y.; Ji, Q.; Wei, R.; Liu, Z.; et al. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019, 4, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, C.; Cui, C.-Y.; Zhang, Y.; Liu, X.; Cui, Z.-H.; Ma, X.-Y.; Feng, Y.-J.; Fang, L.-X.; Lian, X.-L.; et al. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat. Microbiol. 2019, 4, 1457–1464. [Google Scholar] [CrossRef]
- Skov, R.L.; Monnet, D.L. Plasmid-mediated colistin resistance (mcr-1 gene): Three months later, the story unfolds. Eurosurveillance 2016, 21, 30155. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Johnson, A.P. Transferable resistance to colistin: A new but old threat: Table 1. J. Antimicrob. Chemother. 2016, 71, 2066–2070. [Google Scholar] [CrossRef]
- Countries should Reduce Use of Colistin in Animals to Decrease the Risk of Antimicrobial Resistance|European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/countries-should-reduce-use-colistin-animals-decrease-risk-antimicrobial-resistance (accessed on 23 August 2021).
- Rhouma, M.; Beaudry, F.; Letellier, A. Resistance to colistin: What is the fate for this antibiotic in pig production? Int. J. Antimicrob. Agents 2016, 48, 119–126. [Google Scholar] [CrossRef]
- Kempf, I.; Jouy, E.; Chauvin, C. Colistin use and colistin resistance in bacteria from animals. Int. J. Antimicrob. Agents 2016, 48, 598–606. [Google Scholar] [CrossRef]
- Jeannot, K.; Bolard, A.; Plésiat, P. Resistance to polymyxins in Gram-negative organisms. Int. J. Antimicrob. Agents 2017, 49, 526–535. [Google Scholar] [CrossRef]
- Webb, H.E.; Angulo, F.J.; Granier, S.A.; Scott, H.M.; Loneragan, G.H. Illustrative examples of probable transfer of resistance determinants from food animals to humans: Streptothricins, glycopeptides, and colistin. F1000Research 2017, 6, 1805. [Google Scholar] [CrossRef]
- Webster, P. The perils of poultry. Can. Med. Assoc. J. 2009, 181, 21–24. [Google Scholar] [CrossRef]
- Dutil, L.; Irwin, R.; Finley, R.; Ng, L.K.; Avery, B.; Boerlin, P.; Bourgault, A.-M.; Cole, L.; Daignault, D.; Desruisseau, A.; et al. Ceftiofur Resistance inSalmonella entericaSerovar Heidelberg from Chicken Meat and Humans, Canada. Emerg. Infect. Dis. 2010, 16, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance|FDA. Available online: https://www.fda.gov/animal-veterinary/safety-health/antimicrobial-resistance (accessed on 1 September 2021).
- Dierikx, C.M.; Van Der Goot, J.A.; Smith, H.E.; Kant, A.; Mevius, D.J. Presence of ESBL/AmpC -Producing Escherichia coli in the Broiler Production Pyramid: A Descriptive Study. PLoS ONE 2013, 8, e79005. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Corsello, G.; Cricelli, C.; Ferrara, N.; Ghiselli, A.; Lucchin, L.; Poli, A. Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing: An Italian consensus document. Food Nutr. Res. 2015, 59, 27606. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Endimiani, A.; Hujer, K.M.; Bonomo, R.A. The continuing challenge of ESBLs. Curr. Opin. Pharmacol. 2007, 7, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Black, S.R.; Weaver, K.N.; Weinstein, R.A.; Hayden, M.K.; Lin, M.Y.; Lavin, M.A.; Gerber, S.I. Regional Infection Control Assessment of Antibiotic Resistance Knowledge and Practice. Infect. Control. Hosp. Epidemiol. 2015, 36, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Pesciaroli, M.; Magistrali, C.F.; Filippini, G.; Epifanio, E.M.; Lovito, C.; Marchi, L.; Maresca, C.; Massacci, F.R.; Orsini, S.; Scoccia, E.; et al. Antibiotic-resistant commensal Escherichia coli are less frequently isolated from poultry raised using non-conventional management systems than from conventional broiler. Int. J. Food Microbiol. 2019, 314, 108391. [Google Scholar] [CrossRef]
- EUCAST: New S, I and R definitions. Available online: https://www.eucast.org/newsiandr/ (accessed on 23 August 2021).
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Veter. Sci. 2018, 5, 254. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Homenuik, K.; Nichol, K.; Noreddin, A.; Vercaigne, L.; Embil, J.; Gin, A.; Karlowsky, J.A.; Hoban, D.J. The Glycylcyclines. Drugs 2004, 64, 63–88. [Google Scholar] [CrossRef]
- Rose, W.E.; Rybak, M.J. Tigecycline: First of a New Class of Antimicrobial Agents. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2006, 26, 1099–1110. [Google Scholar] [CrossRef]
- Sun, H.; Wan, Y.; Du, P.; Liu, D.; Li, R.; Zhang, P.; Wu, Y.; Fanning, S.; Wang, Y.; Bai, L. Investigation of tigecycline resistant Escherichia coli from raw meat reveals potential transmission among food-producing animals. Food Control. 2020, 121, 107633. [Google Scholar] [CrossRef]
- Huang, X.; Yu, L.; Chen, X.; Zhi, C.; Yao, X.; Liu, Y.; Wu, S.; Guo, Z.; Yi, L.; Zeng, Z.; et al. High Prevalence of Colistin Resistance and mcr-1 Gene in Escherichia coli Isolated from Food Animals in China. Front. Microbiol. 2017, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Das, T.; Islam, Z.; Herrero-Fresno, A.; Biswas, P.K.; Olsen, J.E. High prevalence of mcr-1-encoded colistin resistance in commensal Escherichia coli from broiler chicken in Bangladesh. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Majewski, M.; Łukomska, A.; Wilczyński, J.; Wystalska, D.; Racewicz, P.; Nowacka-Woszuk, J.; Pszczola, M.; Anusz, K. Colistin resistance of non-pathogenic strains of Escherichia coli occurring as natural intestinal flora in broiler chickens treated and not treated with colistin sulphate. J. Veter. Res. 2020, 64, 399–405. [Google Scholar] [CrossRef]
- Botsoglou, N. Drug Residues in Foods: Pharmacology, Food Safety, and Analysis; Marcel Dekker: New York , NY, USA, 2001; ISBN 9780824745271. [Google Scholar]
- Goetting, V.; Lee, K.A.; Tell, L.A. Pharmacokinetics of veterinary drugs in laying hens and residues in eggs: A review of the literature. J. Veter. Pharmacol. Ther. 2011, 34, 521–556. [Google Scholar] [CrossRef]
- Lim, L.M.; Ly, N.; Anderson, D.; Yang, J.C.; Macander, L.; Jarkowski, A.; Forrest, A.; Bulitta, J.; Tsuji, B.T. Resurgence of Colistin: A Review of Resistance, Toxicity, Pharmacodynamics, and Dosing. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010, 30, 1279–1291. [Google Scholar] [CrossRef]
- Plaza-Rodríguez, C.; Alt, K.; Grobbel, M.; Hammerl, J.A.; Irrgang, A.; Szabo, I.; Stingl, K.; Schuh, E.; Wiehle, L.; Pfefferkorn, B.; et al. Wildlife as Sentinels of Antimicrobial Resistance in Germany? Front. Veter. Sci. 2021, 7. [Google Scholar] [CrossRef]
- Swift, B.M.; Bennett, M.; Waller, K.; Dodd, C.; Murray, A.; Gomes, R.L.; Humphreys, B.; Hobman, J.L.; Jones, M.A.; Whitlock, S.E.; et al. Anthropogenic environmental drivers of antimicrobial resistance in wildlife. Sci. Total. Environ. 2018, 649, 12–20. [Google Scholar] [CrossRef]
- Ibekwe, A.M.; Murinda, S.E.; Debroy, C.; Reddy, G.B. Potential pathogens, antimicrobial patterns and genotypic diversity ofEscherichia coliisolates in constructed wetlands treating swine wastewater. FEMS Microbiol. Ecol. 2016, 92, fiw006. [Google Scholar] [CrossRef]
- Alhendi, A.B.; Musa Homeida, A.-A.; Gaili, E.-S.; Alhendi, A.B.; Homeida, A.-A.M. Drug residues in broiler chickens fed with antibiotics in ration GAILI: Drug residues in broiler chickens fed with antibiotics in ration. Vet. Arh. 2000, 70, 199–205. [Google Scholar]
- Zakeri, B.; Wright, G.D. Chemical biology of tetracycline antibioticsThis paper is one of a selection of papers published in this Special Issue, entitled CSBMCB—Systems and Chemical Biology, and has undergone the Journal's usual peer review process. Biochem. Cell Biol. 2008, 86, 124–136. [Google Scholar] [CrossRef]
- Kabir, J.; Umoh, V.; Audu-Okoh, E.; Umoh, J.; Kwaga, J. Veterinary drug use in poultry farms and determination of antimicrobial drug residues in commercial eggs and slaughtered chicken in Kaduna State, Nigeria. Food Control. 2004, 15, 99–105. [Google Scholar] [CrossRef]
- Carattoli, A. Animal reservoirs for extended spectrum β-lactamase producers. Clin. Microbiol. Infect. 2008, 14, 117–123. [Google Scholar] [CrossRef]
- Musa, L.; Proietti, P.C.; Branciari, R.; Menchetti, L.; Bellucci, S.; Ranucci, D.; Marenzoni, M.L.; Franciosini, M.P. Antimicrobial Susceptibility of Escherichia coli and ESBL-Producing Escherichia coli Diffusion in Conventional, Organic and Antibiotic-Free Meat Chickens at Slaughter. Animals 2020, 10, 1215. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.M. Enteric Diseases of Poultry with Special Attention to Clostridium perfringens. Pak. Vet. J. 2011, 31, 175–184. [Google Scholar]
- Cooper, K.; Songer, J.G.; Uzal, F.A. Diagnosing clostridial enteric disease in poultry. J. Veter. Diagn. Investig. 2013, 25, 314–327. [Google Scholar] [CrossRef] [PubMed]
- The European Union. Ban on antibiotics as growth promoters in animal feed enters into effect. In Proceedings of the European Commission. Regulation; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- Jiménez-Belenguer, A.; Doménech, E.; Villagrá, A.; Fenollar, A.; Ferrús, M.A. Antimicrobial resistance of Escherichia coli isolated in newly-hatched chickens and effect of amoxicillin treatment during their growth. Avian Pathol. 2016, 45, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Burow, E.; Grobbel, M.; Tenhagen, B.-A.; Simoneit, C.; Szabó, I.; Wendt, D.; Kürbis, C.; Ladwig-Wiegard, M.; Banneke, S.; Käsbohrer, A. Antibiotic Resistance in Escherichia coli from Broiler Chickens After Amoxicillin Treatment in an Experimental Environment. Microb. Drug Resist. 2020, 26, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, W.; VAN Gompel, L.; Schmitt, H.; Liakopoulos, A.; Heres, L.; Urlings, B.A.; Mevius, D.; Bonten, M.J.M.; Heederik, D.J.J. ESBL carriage in pig slaughterhouse workers is associated with occupational exposure. Epidemiol. Infect. 2017, 145, 2003–2010. [Google Scholar] [CrossRef]
- Cui, S.; Ge, B.; Zheng, J.; Meng, J. Prevalence and Antimicrobial Resistance of Campylobacter spp. and Salmonella Serovars in Organic Chickens from Maryland Retail Stores. Appl. Environ. Microbiol. 2005, 71, 4108–4111. [Google Scholar] [CrossRef]
- Miranda, J.M.; Vázquez, B.I.; Fente, C.A.; Calo-Mata, P.; Cepeda, A.; Franco, C.M. Comparison of Antimicrobial Resistance in Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes Strains Isolated from Organic and Conventional Poultry Meat. J. Food Prot. 2008, 71, 2537–2542. [Google Scholar] [CrossRef]
- Millman, J.M.; Waits, K.; Grande, H.; Marks, A.R.; Marks, J.C.; Price, L.B.; Hungate, B.A. Prevalence of antibiotic-resistant E. coli in retail chicken: Comparing conventional, organic, kosher, and raised without antibiotics. F1000Research 2013, 2, 155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mollenkopf, D.F.; Cenera, J.K.; Bryant, E.M.; King, C.A.; Kashoma, I.; Kumar, A.; Funk, J.A.; Rajashekara, G.; Wittum, T.E. Organic or Antibiotic-Free Labeling Does Not Impact the Recovery of Enteric Pathogens and Antimicrobial-ResistantEscherichia colifrom Fresh Retail Chicken. Foodborne Pathog. Dis. 2014, 11, 920–929. [Google Scholar] [CrossRef]
- ISO. ISO 16649-1:2018—Microbiology of the food chain—Horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli—Part 1: Colony-count technique at 44 degrees C using membranes and 5-bromo-4-chloro-3-indolyl beta-D-glucuronide. Available online: https://www.iso.org/standard/64951.html (accessed on 1 September 2021).
- European Committe on Antimicrobial Susceptability Testing Clinical Breakpoints and Dosing of Antibiotics. Available online: https://eucast.org/clinical_breakpoints/ (accessed on 1 August 2020).
- Weinstein, M.P.; Patel, J.B.; Bobenchik, A.M.; Campeau, S.; Cullen, S.K.; Galas, M.F.; Gold, H.; Humphries, R.M.; Kirn, T.J.; Lewis Ii, J.S.; et al. M100 Performance Standards for Antimicrobial Susceptibility Testing A CLSI supplement for global application. In Performance Standards for Antimicrobial Susceptibility Testing Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Bonnedahl, J.; Järhult, J.D. Antibiotic resistance in wild birds. Upsala J. Med. Sci. 2014, 119, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Boiocchi, F.; Davies, M.P.; Hilton, A.C. An Examination of Flying Insects in Seven Hospitals in the United Kingdom and Carriage of Bacteria by True Flies (Diptera: Calliphoridae, Dolichopodidae, Fanniidae, Muscidae, Phoridae, Psychodidae, Sphaeroceridae). J. Med. Èntomol. 2019, 56, 1684–1697. [Google Scholar] [CrossRef] [PubMed]
- Bouki, C.; Venieri, D.; Diamadopoulos, E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: A review. Ecotoxicol. Environ. Saf. 2013, 91, 1–9. [Google Scholar] [CrossRef]
| Outcome | Explanatory Variable | Level | OR * | 95 % Confidence Interval | p-Value | ||
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||
| ESBL strains | Type of rearing | Conventional | ref a | - | - | - | |
| Organic | 0.13 | 0.04 | 0.37 | <0.001 | |||
| AF | 0.33 | 0.14 | 0.76 | 0.009 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | 2.72 | 1.23 | 6.02 | 0.01 | |||
| MDR strains | Type of rearing | Conventional | - | - | - | ||
| Organic | - | - | - | 0.17 | |||
| AF | - | - | - | 0.76 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.85 | |||
| Tigecycline | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 3.59 | 1.3 | 9.93 | 0.01 | |||
| AF | - | - | - | 0.13 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.15 | |||
| Colistin | Type of rearing | Conventional 1 | ref | - | - | - | |
| Organic | - | - | - | 1.00 | |||
| AF | - | - | - | 1.00 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 1.00 | |||
| Meropenem | Type of rearing | Conventional | ref | - | - | - | |
| Organic | - | - | - | 1.00 | |||
| AF | - | - | - | 0.98 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.61 | |||
| Cefotaxime | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 0.39 | 0.18 | 0.83 | 0.01 | |||
| AF | - | - | - | 0.09 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.55 | |||
| Ceftaxidime | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 0.36 | 0.15 | 0.83 | 0.02 | |||
| AF | - | - | - | 0.44 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.85 | |||
| Ampicillin | Type of rearing | Conventional | ref | - | - | - | |
| Organic | - | - | - | 0.14 | |||
| AF | - | - | - | 0.16 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.60 | |||
| Nalidixic acid | Type of rearing | Conventional | ref | - | - | - | |
| Organic | - | - | - | ||||
| AF | 2.32 | 1.07 | 5.07 | 0.03 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.48 | |||
| Ciprofloxacin | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 0.46 | 0.22 | 0.97 | 0.04 | |||
| AF | - | - | - | 0.06 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.17 | |||
| Tetracycline | Type of rearing | Conventional | ref | - | - | - | |
| Organic | - | - | - | 0.07 | |||
| AF | - | - | - | 0.23 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.45 | |||
| Azitromycin | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 4.39 | 1.86 | 10.39 | 0.001 | |||
| AF | - | - | - | 0.99 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.05 | |||
| Gentamicin | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 2.34 | 1.13 | 4.85 | 0.02 | |||
| AF | - | - | - | 0.13 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.89 | |||
| Sulfamethoxazole | Type of rearing | Conventional | ref | - | - | - | |
| Organic | - | - | - | 0.2 | |||
| AF | - | - | - | 0.24 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.94 | |||
| Chloramphenicol | Type of rearing | Conventional | ref | - | - | - | |
| Organic | 0.42 | 0.2 | 0.9 | 0.03 | |||
| AF | 0.3 | 0.14 | 0.64 | 0.002 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.22 | |||
| Trimethoprim | Type of rearing | Conventional | ref | - | - | - | |
| Organic | - | - | - | 0.38 | |||
| AF | - | - | - | 0.85 | |||
| Site of sampling | Farm | ref | - | - | - | ||
| Slaughterhouse | - | - | - | 0.36 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, L.; Proietti, P.C.; Marenzoni, M.L.; Stefanetti, V.; Kika, T.S.; Blasi, F.; Magistrali, C.F.; Toppi, V.; Ranucci, D.; Branciari, R.; et al. Susceptibility of Commensal E. coli Isolated from Conventional, Antibiotic-Free, and Organic Meat Chickens on Farms and at Slaughter toward Antimicrobials with Public Health Relevance. Antibiotics 2021, 10, 1321. https://doi.org/10.3390/antibiotics10111321
Musa L, Proietti PC, Marenzoni ML, Stefanetti V, Kika TS, Blasi F, Magistrali CF, Toppi V, Ranucci D, Branciari R, et al. Susceptibility of Commensal E. coli Isolated from Conventional, Antibiotic-Free, and Organic Meat Chickens on Farms and at Slaughter toward Antimicrobials with Public Health Relevance. Antibiotics. 2021; 10(11):1321. https://doi.org/10.3390/antibiotics10111321
Chicago/Turabian StyleMusa, Laura, Patrizia Casagrande Proietti, Maria Luisa Marenzoni, Valentina Stefanetti, Tana Shtylla Kika, Francesca Blasi, Chiara Francesca Magistrali, Valeria Toppi, David Ranucci, Raffaella Branciari, and et al. 2021. "Susceptibility of Commensal E. coli Isolated from Conventional, Antibiotic-Free, and Organic Meat Chickens on Farms and at Slaughter toward Antimicrobials with Public Health Relevance" Antibiotics 10, no. 11: 1321. https://doi.org/10.3390/antibiotics10111321
APA StyleMusa, L., Proietti, P. C., Marenzoni, M. L., Stefanetti, V., Kika, T. S., Blasi, F., Magistrali, C. F., Toppi, V., Ranucci, D., Branciari, R., & Franciosini, M. P. (2021). Susceptibility of Commensal E. coli Isolated from Conventional, Antibiotic-Free, and Organic Meat Chickens on Farms and at Slaughter toward Antimicrobials with Public Health Relevance. Antibiotics, 10(11), 1321. https://doi.org/10.3390/antibiotics10111321









