Antimicrobially Active Semen Extenders Allow the Reduction of Antibiotic Use in Pig Insemination
Abstract
1. Introduction
2. Results
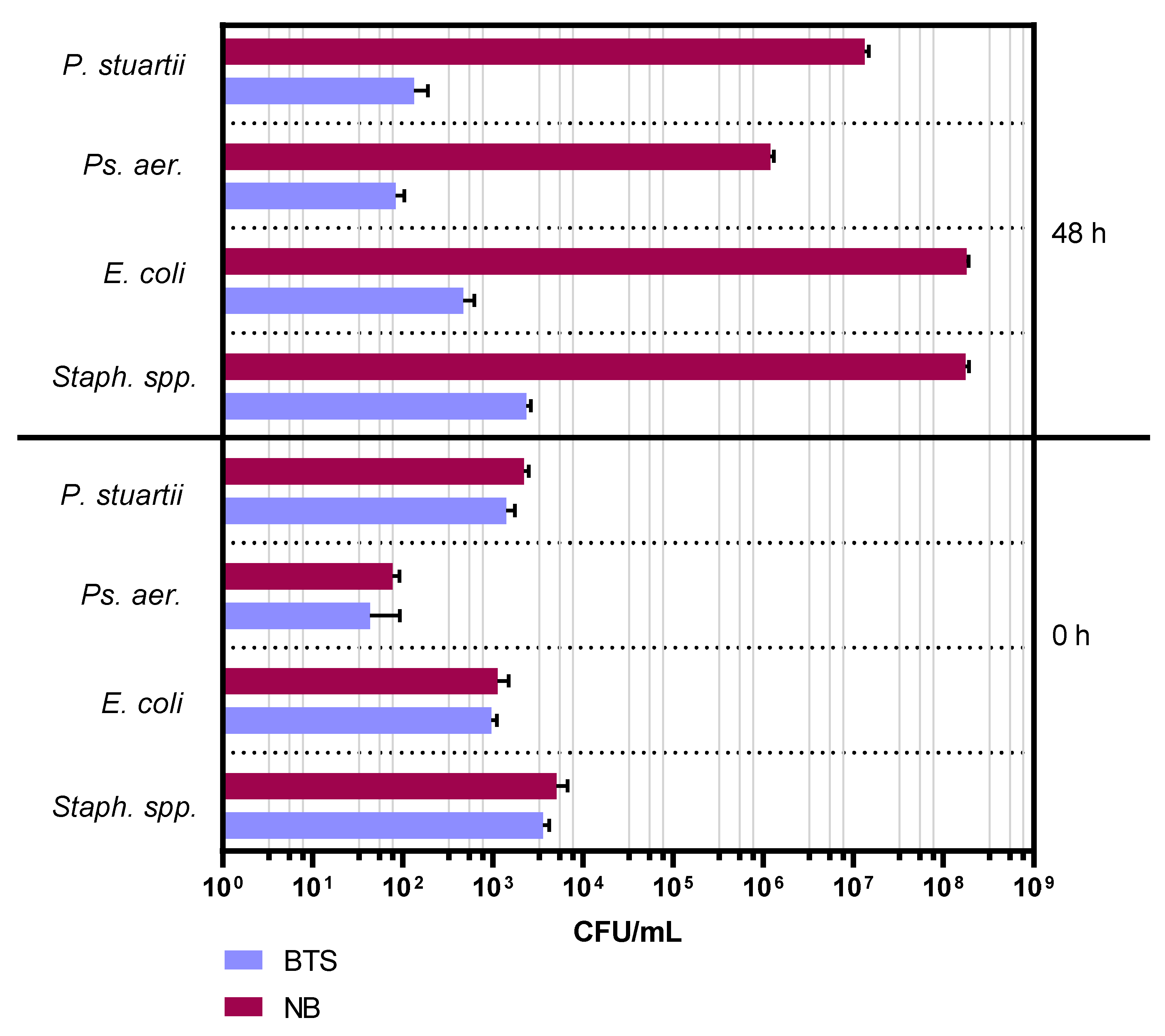
2.1. Experiment 1: Comparison of Bacterial Growth in Nutrient Broth and Antibiotic-Free BTS Extender
2.2. Experiment 2: Comparison of Bacterial Growth in Two Different Types of Antibiotic-Free Extenders
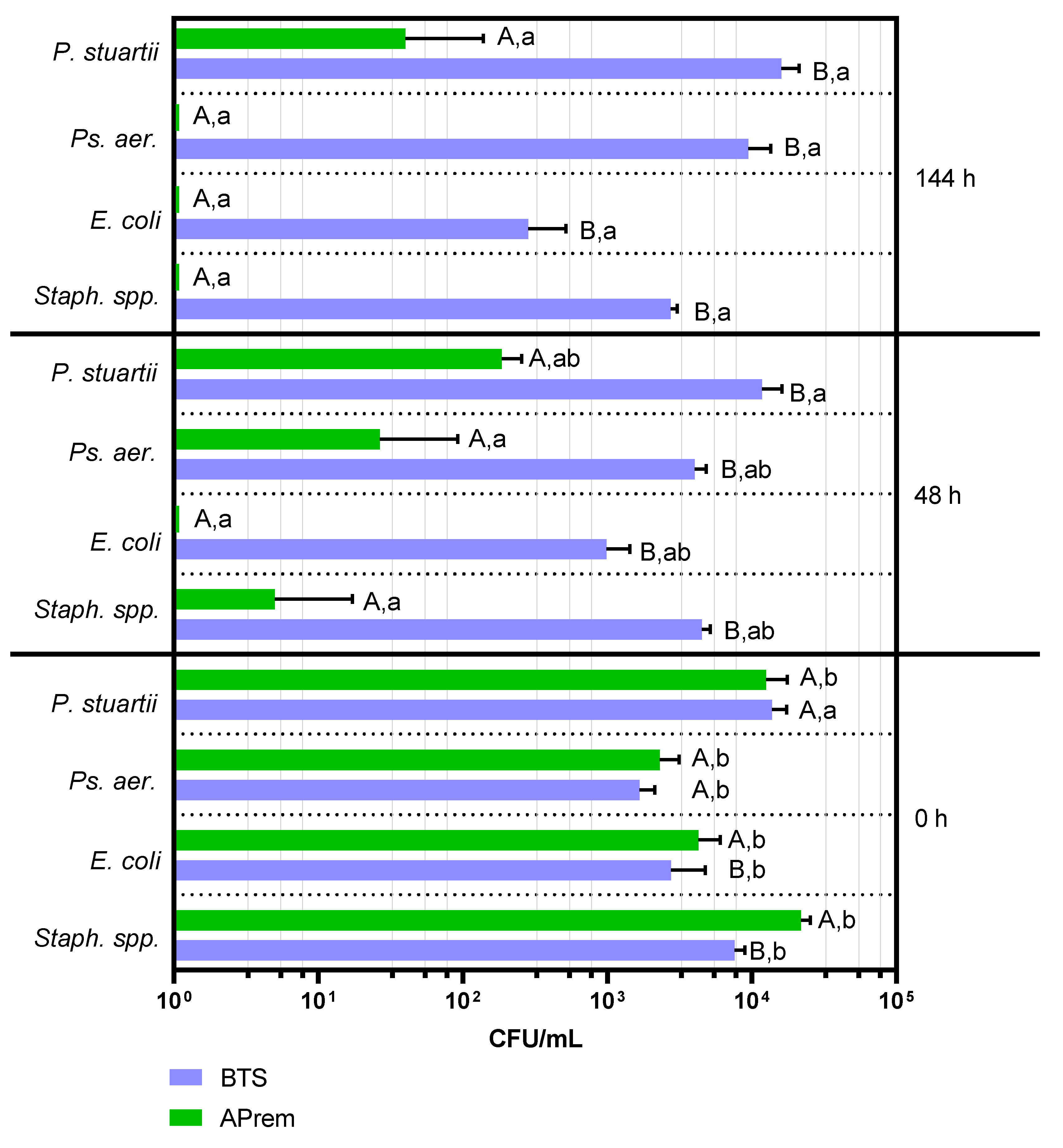
2.3. Experiment 3: Comparison of Bacterial Growth in Semen Diluted with Two Different Types of Antibiotic-Free Extenders
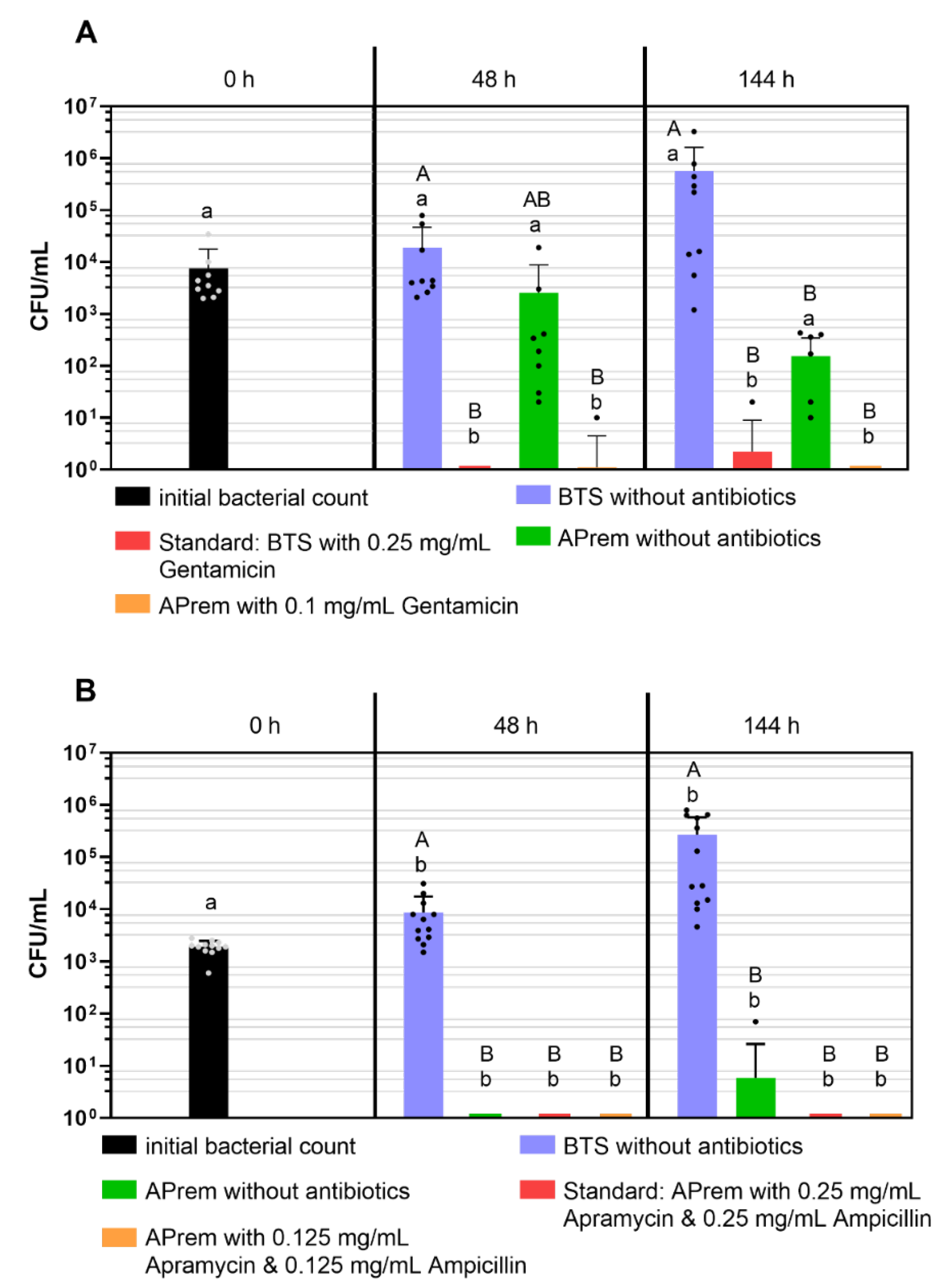
2.4. Experiment 4a: Comparison of Bacterial Growth in Semen Diluted with Two Different Types of Extenders Containing Different Concentrations of Gentamicin
2.5. Experiment 4b: Comparison of Bacterial Growth in Semen Diluted with Androstar Premium Containing Different Concentrations of Apramycin and Ampicillin
3. Discussion
4. Materials and Methods
4.1. Chemicals and Media
4.2. Microbiology
4.2.1. Bacterial Count
4.2.2. Bacterial Inoculation of Samples
4.3. Spermatology
4.3.1. Semen Collection and Processing
4.3.2. Assessment of Sperm Motility
4.3.3. Assessment of Sperm Membrane Integrity
4.3.4. Assessment of Sperm Agglutinations
4.4. Experimental Design
4.4.1. Experiment 1: Comparison of Bacterial Growth in Nutrient Broth and Antibiotic-Free BTS Extender
4.4.2. Experiment 2: Comparison of Bacterial Growth in two different Types of Antibiotic-Free Extenders
4.4.3. Experiment 3: Comparison of Bacterial Growth in Semen Diluted with Two Different Types of Antibiotic-Free Extenders
4.4.4. Experiment 4a: Comparison of Bacterial Growth in Semen Diluted with Two Different Types of Extenders Containing Different Concentrations of Gentamicin
4.4.5. Experiment 4b: Comparison of Bacterial Growth in Semen Diluted with Two Different Types of Extenders Containing Different Concentrations of Apramycin and Ampicillin
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waberski, D.; Riesenbeck, A.; Schulze, M.; Weitze, K.F.; Johnson, L. Application of preserved boar semen for artificial insemination: Past, present and future challenges. Theriogenology 2019, 137, 2–7. [Google Scholar] [CrossRef]
- Steverink, D.W.; Soede, N.M.; Bouwman, E.G.; Kemp, B. Semen backflow after insemination and its effect on fertilisation results in sows. Anim. Reprod. Sci. 1998, 54, 109–119. [Google Scholar] [CrossRef]
- Araújo, É.B.; Costa, E.P.D.; Costa, A.H.A.D.; Lopes, F.G.; Macedo, G.G.; Paula, T.A.R.D. Reproductive performance of sows submitted to intrauterine insemination. Rev. Bras. Zootec. 2009, 38, 1460–1467. [Google Scholar] [CrossRef][Green Version]
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; de Schaetzen, M.A.; Van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial resistance in the food chain: A review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, A.; Trevisi, P.; Luise, D.; Modesto, M.; Blasioli, S.; Braschi, I.; Mattarelli, P. Exploring the Animal Waste Resistome: The Spread of Antimicrobial Resistance Genes Through the Use of Livestock Manure. Front. Microbiol. 2020, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Bussalleu, E.; Sancho, S.; Briz, M.D.; Yeste, M.; Bonet, S. Do antimicrobial peptides PR-39, PMAP-36 and PMAP-37 have any effect on bacterial growth and quality of liquid-stored boar semen? Theriogenology 2017, 89, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Sancho, S.; Briz, M.; Yeste, M.; Bonet, S.; Bussalleu, E. Effects of the antimicrobial peptide protegrine 1 on sperm viability and bacterial load of boar seminal doses. Reprod. Domest. Anim. 2017, 52 (Suppl. 4), 69–71. [Google Scholar] [CrossRef]
- Schulze, M.; Junkes, C.; Mueller, P.; Speck, S.; Ruediger, K.; Dathe, M.; Mueller, K. Effects of cationic antimicrobial peptides on liquid-preserved boar spermatozoa. PLoS ONE 2014, 9, e100490. [Google Scholar] [CrossRef]
- Puig-Timonet, A.; Castillo-Martín, M.; Pereira, B.A.; Pinart, E.; Bonet, S.; Yeste, M. Evaluation of porcine beta defensins-1 and -2 as antimicrobial peptides for liquid-stored boar semen: Effects on bacterial growth and sperm quality. Theriogenology 2018, 111, 9–18. [Google Scholar] [CrossRef]
- Hensel, B.; Jakop, U.; Scheinpflug, K.; Schröter, F.; Sandmann, M.; Mühldorfer, K.; Schulze, M. Low temperature preservation: Influence of putative bioactive microalgae and hop extracts on sperm quality and bacterial load in porcine semen. Sustain. Chem. Pharm. 2021, 19, 100359. [Google Scholar] [CrossRef]
- Elmi, A.; Prosperi, A.; Zannoni, A.; Bertocchi, M.; Scorpio, D.G.; Forni, M.; Foni, E.; Bacci, M.L.; Ventrella, D. Antimicrobial capabilities of non-spermicidal concentrations of tea tree (Melaleuca alternifolia) and rosemary (Rosmarinus officinalis) essential oils on the liquid phase of refrigerated swine seminal doses. Res. Vet. Sci. 2019, 127, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Duran, F.; Acosta-Torres, L.S.; Serrano-Díaz, P.N.; Toscano-Torres, I.A.; Olivo-Zepeda, I.B.; García-Caxin, E.; Nuñez-Anita, R.E. Toxicity and antimicrobial effect of silver nanoparticles in swine sperms. Syst. Biol. Reprod. Med. 2020, 66, 281–289. [Google Scholar] [CrossRef]
- Morrell, J.M.; Wallgren, M. Removal of bacteria from boar ejaculates by Single Layer Centrifugation can reduce the use of antibiotics in semen extenders. Anim. Reprod. Sci. 2011, 123, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pastor, F.; Lacalle, E.; Martínez-Martínez, S.; Fernández-Alegre, E.; Álvarez-Fernández, L.; Martinez-Alborcia, M.J.; Bolarin, A.; Morrell, J.M. Low density Porcicoll separates spermatozoa from bacteria and retains sperm quality. Theriogenology 2021, 165, 28–36. [Google Scholar] [CrossRef]
- Barone, F.; Ventrella, D.; Zannoni, A.; Forni, M.; Bacci, M. Can microfiltered seminal plasma preserve the morphofunctional characteristics of porcine spermatozoa in the absence of antibiotics? A preliminary study. Reprod. Domest. Anim. 2016, 51, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Waberski, D.; Luther, A.-M.; Grünther, B.; Jäkel, H.; Henning, H.; Vogel, C.; Peralta, W.; Weitze, K.F. Sperm function in vitro and fertility after antibiotic-free, hypothermic storage of liquid preserved boar semen. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Jäkel, H.; Scheinpflug, K.; Mühldorfer, K.; Gianluppi, R.; Lucca, M.S.; Mellagi, A.P.G.; Bortolozzo, F.P.; Waberski, D. In vitro performance and in vivo fertility of antibiotic-free preserved boar semen stored at 5 °C. J. Anim. Sci. Biotechnol. 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Aalbers, J.; Grooten, H.G. Artificial Insemination of Swine: Fecundity of Boar Semen Stored in Beltsville TS (BTS), Modified Modena (MM), or MR-A and Inseminated on One, Three and Four Days After Collection 1 2. Reprod. Domest. Anim. 1988, 23, 49–55. [Google Scholar] [CrossRef]
- Riesenbeck, A.; Schulze, M.; Rüdiger, K.; Henning, H.; Waberski, D. Quality Control of Boar Sperm Processing: Implications from European AI Centres and Two Spermatology Reference Laboratories. Reprod. Domest. Anim. 2015, 50 (Suppl. 2), 1–4. [Google Scholar] [CrossRef]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M.C. Storage of boar semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Wooley, R.E.; Jones, M.S. Action of EDTA-Tris and antimicrobial agent combinations on selected pathogenic bacteria. Vet. Microbiol. 1983, 8, 271–280. [Google Scholar] [CrossRef]
- Corral, L.G.; Post, L.S.; Montville, T.J. Antimicrobial activity of sodium bicarbonate: A research note. J. Food Sci. 1988, 53, 981–982. [Google Scholar] [CrossRef]
- Schulze, M.; Nitsche-Melkus, E.; Hensel, B.; Jung, M.; Jakop, U. Antibiotics and their alternatives in Artificial Breeding in livestock. Anim. Reprod. Sci. 2020, 220, 106284. [Google Scholar] [CrossRef]
- Althouse, G.C.; Kuster, C.E.; Clark, S.G.; Weisiger, R.M. Field investigations of bacterial contaminants and their effects on extended porcine semen. Theriogenology 2000, 53, 1167–1176. [Google Scholar] [CrossRef]
- Althouse, G.C.; Lu, K.G. Bacteriospermia in extended porcine semen. Theriogenology 2005, 63, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Maroto Martín, L.O.; Muñoz, E.C.; De Cupere, F.; Van Driessche, E.; Echemendia-Blanco, D.; Rodríguez, J.M.; Beeckmans, S. Bacterial contamination of boar semen affects the litter size. Anim. Reprod. Sci. 2010, 120, 95–104. [Google Scholar] [CrossRef]
- Schulze, M.; Ammon, C.; Rüdiger, K.; Jung, M.; Grobbel, M. Analysis of hygienic critical control points in boar semen production. Theriogenology 2015, 83, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Gączarzewicz, D.; Udała, J.; Piasecka, M.; Błaszczyk, B.; Stankiewicz, T. Bacterial contamination of boar semen and its relationship to sperm quality preserved in commercial extender containing gentamicin sulfate. Pol. J. Vet. Sci. 2016, 19, 451–459. [Google Scholar] [CrossRef]
- Yakandawala, N.; Gawande, P.V.; Lovetri, K.; Madhyastha, S. Effect of ovotransferrin, protamine sulfate and EDTA combination on biofilm formation by catheter-associated bacteria. J. Appl. Microbiol. 2007, 102, 722–727. [Google Scholar] [CrossRef]
- Viudes-de-Castro, M.P.; Marco-Jimenez, F.; Vicente, J.S.; Marin, C. Antibacterial Activity of Some Molecules Added to Rabbit Semen Extender as Alternative to Antibiotics. Animals 2021, 11, 1178. [Google Scholar] [CrossRef]
- Strzeżek, J.; Hopfer, E.; Zaborniak, A. Zinc ion-dependent protein in boar semen. II. Effects on sperm motility and antibacterial properties. Anim. Reprod. Sci. 1987, 13, 133–142. [Google Scholar] [CrossRef]
- Schulze, M.; Czirják, G.Á.; Müller, K.; Bortfeldt, R.; Jung, M.; Jakop, U. Antibacterial defense and sperm quality in boar ejaculates. J. Reprod. Immunol. 2019, 131, 13–20. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Bussalleu, E.; Yeste, M.; Torner, E.; Bonet, S. How do different concentrations of Clostridium perfringens affect the quality of extended boar spermatozoa? Anim. Reprod. Sci. 2013, 140, 83–91. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Bussalleu, E.; Yeste, M.; Bonet, S. Effect of Pseudomonas aeruginosa on sperm capacitation and protein phosphorylation of boar spermatozoa. Theriogenology 2016, 85, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Pinart, E.; Domènech, E.; Bussalleu, E.; Yeste, M.; Bonet, S. A comparative study of the effects of Escherichia coli and Clostridium perfringens upon boar semen preserved in liquid storage. Anim. Reprod. Sci. 2017, 177, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Bermúdez, A.; Bonet, S.; Yeste, M.; Pinart, E. Long-term storage of boar seminal doses contaminated with Proteus vulgaris: A dose-dependent effect on sperm motility and sperm-bacteria interaction. Anim. Reprod. Sci. 2020, 216, 106349. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Martínez, N.; Bussalleu, E.; Garcia-Bonavila, E.; Bonet, S.; Yeste, M. Effects of Enterobacter cloacae on boar sperm quality during liquid storage at 17 C. Anim. Reprod. Sci. 2014, 148, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Jasko, D.J.; Bedford, S.J.; Cook, N.L.; Mumford, E.L.; Squires, E.L.; Pickett, B.W. Effect of antibiotics on motion characteristics of cooled stallion spermatozoa. Theriogenology 1993, 40, 885–893. [Google Scholar] [CrossRef]
- Anel-Lopez, L.; Riesco, M.F.; Montes-Garrido, R.; Neila-Montero, M.; Boixo, J.C.; Chamorro, C.; Ortega-Ferrusola, C.; Carvajal, A.; Altonaga, J.R.; de Paz, P.; et al. Comparing the Effect of Different Antibiotics in Frozen-Thawed Ram Sperm: Is It Possible to Avoid Their Addition? Front. Vet. Sci. 2021, 8, 656937. [Google Scholar] [CrossRef] [PubMed]
- Althouse, G.; Rossow, K. The potential risk of infectious disease dissemination via artificial insemination in swine. Reprod. Domest. Anim. 2011, 46, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Höfner, L.; Luther, A.-M.; Palladini, A.; Fröhlich, T.; Waberski, D. Tolerance of Stored Boar Spermatozoa to Autologous Seminal Plasma: A Proteomic and Lipidomic Approach. Int. J. Mol. Sci. 2020, 21, 6474. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Extender | Antibiotics (Each) | 48 h Storage | 144 h Storage |
|---|---|---|---|---|
| Membrane intact (propidium iodide and FITC-PNA negative) sperm (%) | BTS | none | 85.4 ± 7.8 a,A | 84.4 ± 7.7 a,B |
| APrem | none | 86.7 ± 7.0 b,A | 85.8 ± 8.0 b,B | |
| APrem | 0.25 mg/mL AA | 86.4 ± 7.6 b,A | 85.5 ± 7.7 b,B | |
| APrem | 0.125 mg/mL AA | 86.5 ± 7.4 b,A | 85.3 ± 7.6 a,b,B | |
| Degree of agglutinations | BTS | none | 1.1 ± 0.3 A | 2.1 ± 0.3 B |
| APrem | none | 1.1 ± 0.3 A | 2.1 ± 0.3 B | |
| APrem | 0.25 mg/mL AA | 1.1 ± 0.3 A | 2.1 ± 0.3 B | |
| APrem | 0.125 mg/mL AA | 1.1 ± 0.3 A | 2.1 ± 0.3 B | |
| Total motility (%) | BTS | none | 82.9 ± 8.7 a,A | 81.4 ± 9.7 a,A |
| APrem | none | 85.3 ± 6.3 a,A | 84.6 ± 7.5 a,A | |
| APrem | 0.25 mg/mL AA | 83.6 ± 8.1 a,A | 81.0 ± 10.2 a,B | |
| APrem | 0.125 mg/mL AA | 83.2 ± 8.8 a,A | 84.1 ± 7.5 a,A | |
| Progressive motility (%) | BTS | none | 76.9 ± 9.1 a,A | 73.9 ± 9.2 ab,B |
| APrem | none | 79.5 ± 6.8 a,A | 78.2 ± 7.9 b,A | |
| APrem | 0.25 mg/mL AA | 76.7 ± 8.2 a,A | 73.4 ± 9.8 a,B | |
| APrem | 0.125 mg/mL AA | 76.6 ± 9.3 a,A | 76.7 ± 7.4 ab,A | |
| Straight line velocity (µm/s) | BTS | none | 63.8 ± 7.4 a,A | 66.7 ± 9.4 a,A |
| APrem | none | 73.9 ± 8.7 a,b,A | 73.7 ± 8.1 a,A | |
| APrem | 0.25 mg/mL AA | 77.2 ± 11.6 b,A | 73.1 ± 7.6 a,A | |
| APrem | 0.125 mg/mL AA | 77.3 ± 9.1 b,A | 73.2 ± 8.7 a,A | |
| Linearity | BTS | none | 0.41 ± 0.09 A | 0.36 ± 0.05 a,A |
| APrem | none | 0.38 ± 0.07 A | 0.35 ± 0.06 a,A | |
| APrem | 0.25 mg/mL AA | 0.38 ± 0.08 A | 0.34 ± 0.05 a,A | |
| APrem | 0.125 mg/mL AA | 0.39 ± 0.08 A | 0.34 ± 0.05 a,A | |
| Amplitude of lateralhead displacement (µm) | BTS | none | 1.46 ± 0.47 a,A | 1.63 ± 0.29 a,A |
| APrem | none | 1.73 ± 0.35 b,A | 1.91 ± 0.25 b,B | |
| APrem | 0.25 mg/mL AA | 1.73 ± 0.31 b,A | 1.91 ± 0.23 b,A | |
| APrem | 0.125 mg/mL AA | 1.75 ± 0.35 b,A | 1.94 ± 0.29 b,B | |
| BCF (Hz) | BTS | none | 30.0 ± 4.4 a,A | 26.7 ± 2.2 B |
| APrem | none | 28.4 ± 4.1 a,b,A | 24.9 ± 2.6 B | |
| APrem | 0.25 mg/mL AA | 27.6 ± 3.1 b,A | 25.1 ± 2.5 B | |
| APrem | 0.125 mg/mL AA | 27.5 ± 3.6 a,b,A | 25.4 ± 2.8 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luther, A.-M.; Nguyen, T.Q.; Verspohl, J.; Waberski, D. Antimicrobially Active Semen Extenders Allow the Reduction of Antibiotic Use in Pig Insemination. Antibiotics 2021, 10, 1319. https://doi.org/10.3390/antibiotics10111319
Luther A-M, Nguyen TQ, Verspohl J, Waberski D. Antimicrobially Active Semen Extenders Allow the Reduction of Antibiotic Use in Pig Insemination. Antibiotics. 2021; 10(11):1319. https://doi.org/10.3390/antibiotics10111319
Chicago/Turabian StyleLuther, Anne-Marie, Thu Quynh Nguyen, Jutta Verspohl, and Dagmar Waberski. 2021. "Antimicrobially Active Semen Extenders Allow the Reduction of Antibiotic Use in Pig Insemination" Antibiotics 10, no. 11: 1319. https://doi.org/10.3390/antibiotics10111319
APA StyleLuther, A.-M., Nguyen, T. Q., Verspohl, J., & Waberski, D. (2021). Antimicrobially Active Semen Extenders Allow the Reduction of Antibiotic Use in Pig Insemination. Antibiotics, 10(11), 1319. https://doi.org/10.3390/antibiotics10111319






