Photofungizides Based on Curcumin and Derivates Thereof against Candida albicans and Aspergillus niger
Abstract
:1. Introduction
2. Results
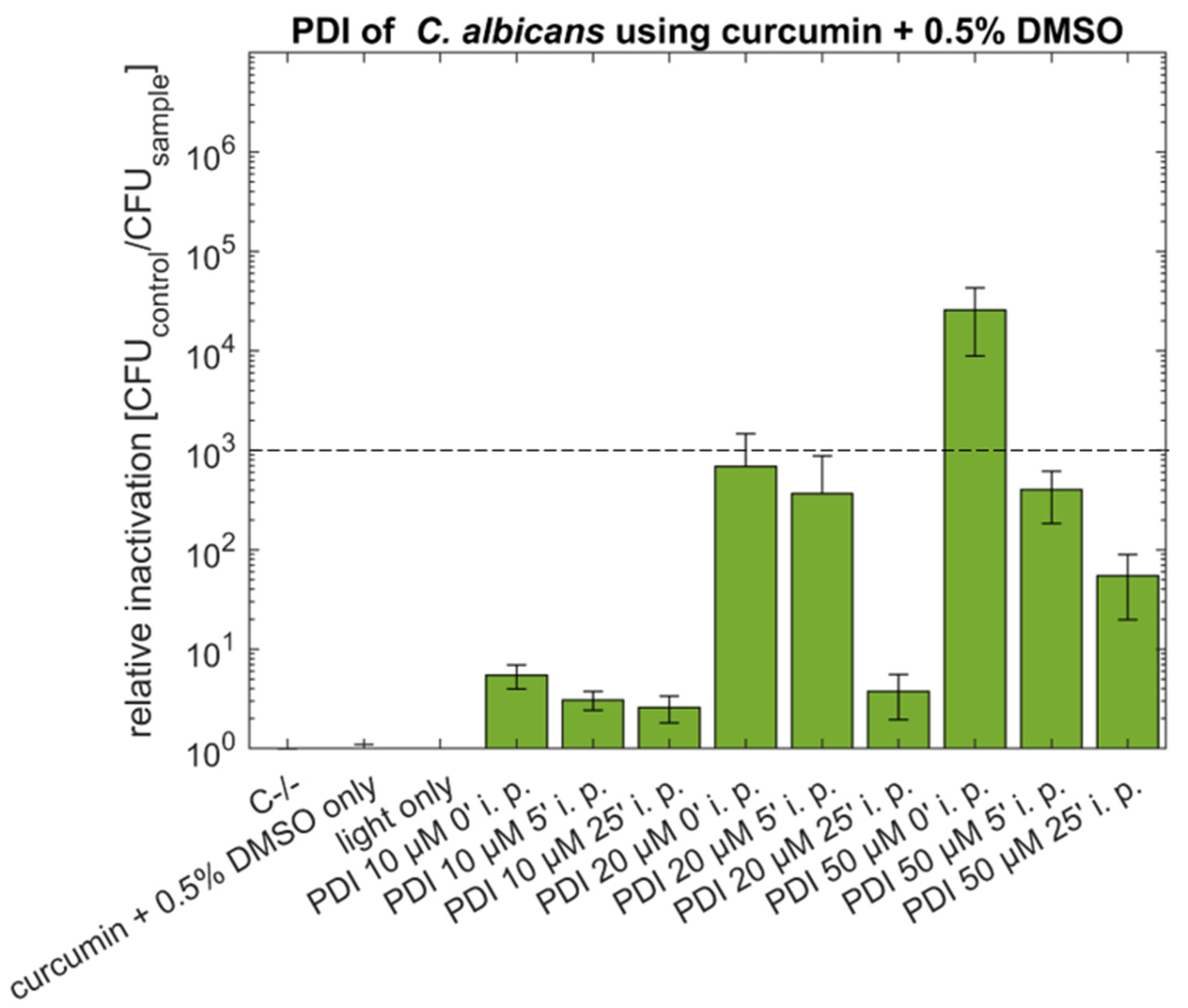
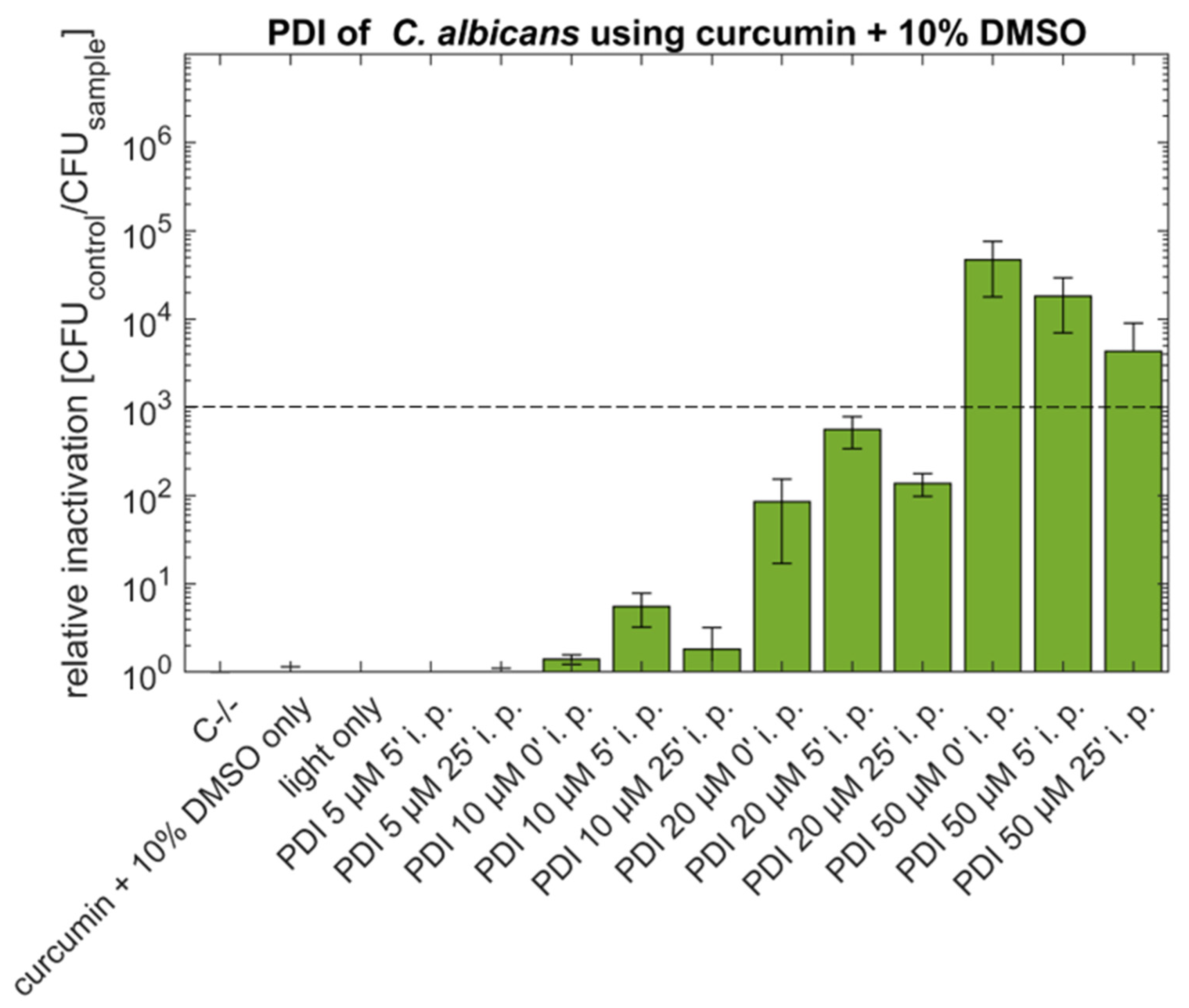
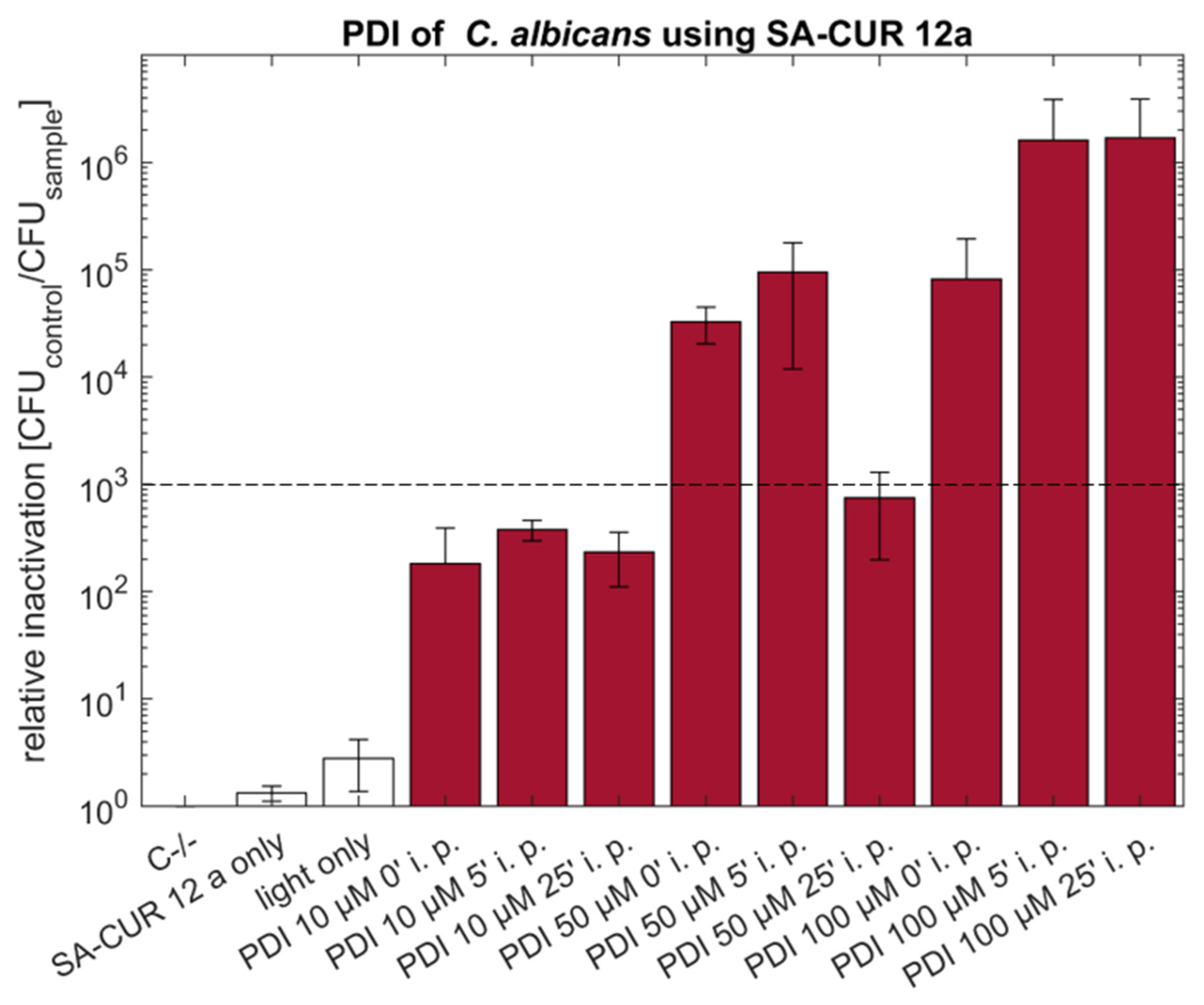
2.1. PDI against Candida albicans with Curcumin or SA-CUR 12a
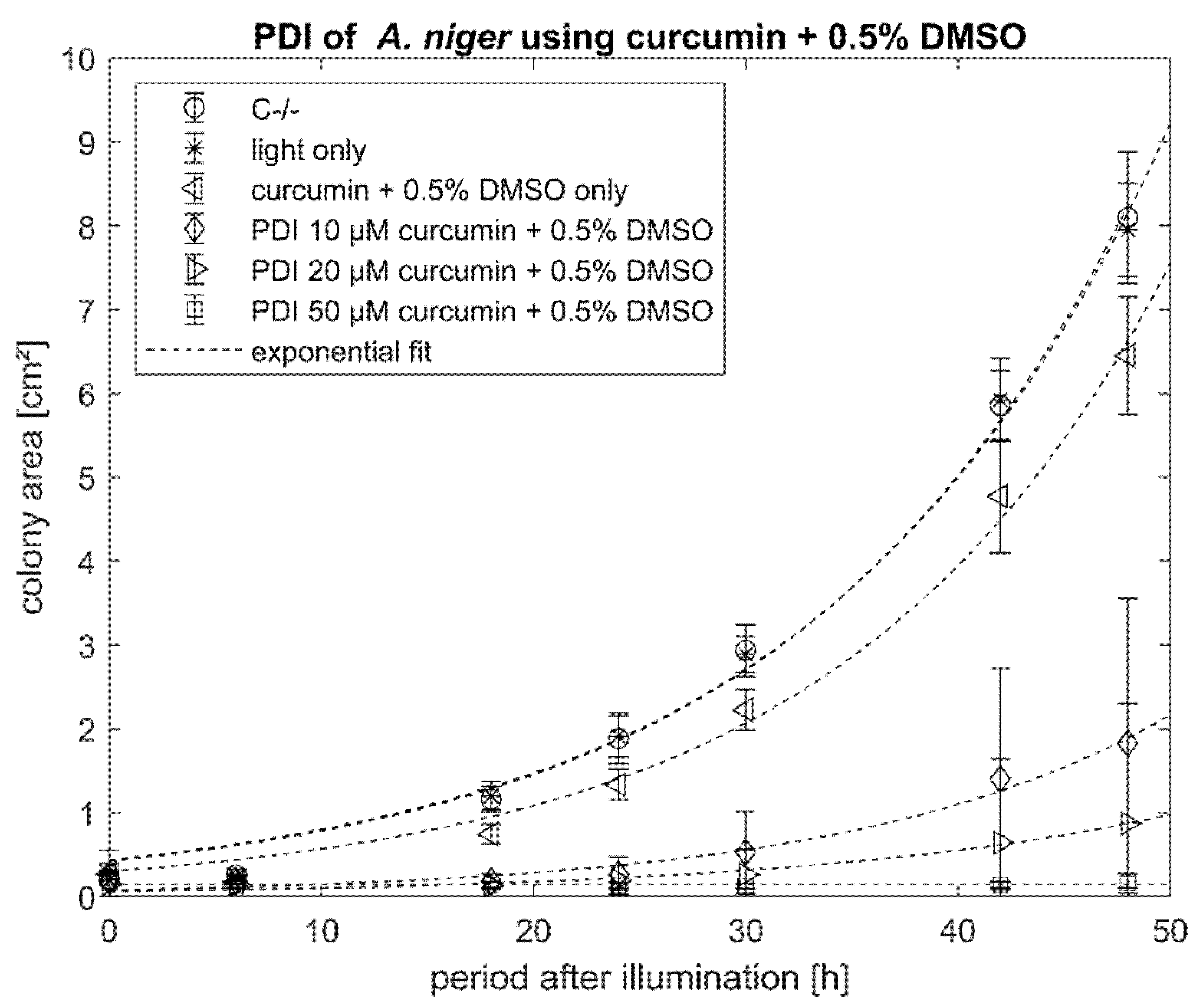
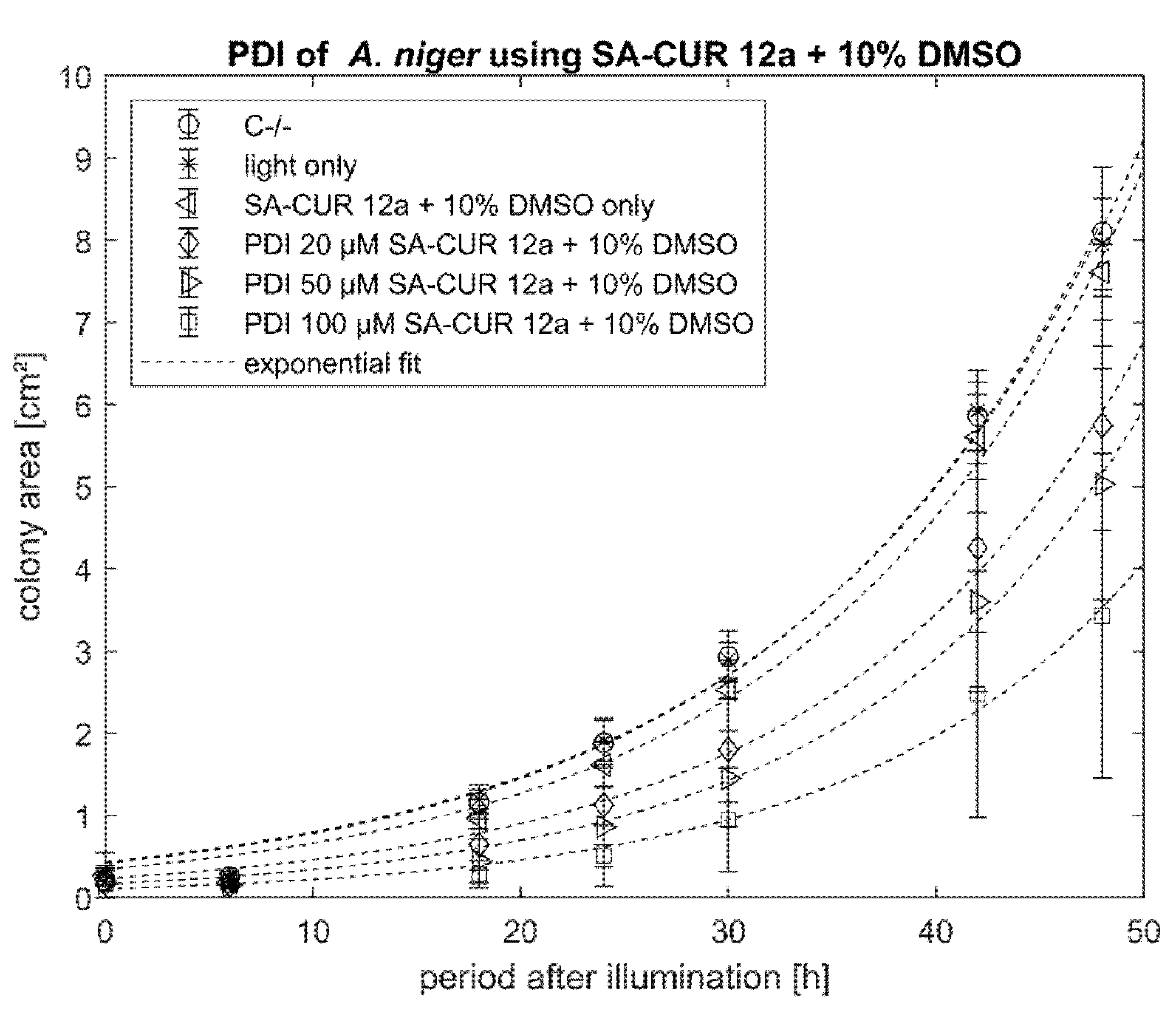
2.2. PDI against Aspergillus niger with Curcumin or SA-CUR 12a
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of PS Solutions
5.2. Culture of C. albicans
5.3. PDI against C. albicans
5.4. Culture of A. niger
5.5. PDI against A. niger
5.6. Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A. niger | Aspergillus niger |
| C. albicans | Candida albicans |
| CFU | Colony forming unit(s) |
| DPBS | Dulbecco’s Phosphate Buffered Saline |
| i.p. | incubation period |
| PDI | Photodynamic Inactivation |
| PS | Photosensitiser |
| ROS | Reactive oxygen species |
| SB | Sabouraud Broth |
Appendix A
| Control before and after Illumination | Treatment before and after Illumination |
| C−/− [n = 6] | PDI 10 µM curcumin + 0.5% DMSO [n = 9] |
| Light only [n = 6] | PDI 20 µM curcumin + 0.5% DMSO [n = 6] |
| Curcumin + 0.5% DMSO only [n = 9] | PDI 50 µM curcumin + 0.5% DMSO [n = 9] |
| SA-CUR 12a only [n = 6] | PDI 20 µM SA-CUR 12a [n = 6] |
| SA-CUR 12a + 10% DMSO only [n = 6] | PDI 50 µM SA-CUR 12a [n = 6] |
| PDI 100 µM SA-CUR 12a [n = 6] | |
| PDI 20 µM SA-CUR 12a + 10% DMSO [n = 6] | |
| PDI 50 µM SA-CUR 12a + 10% DMSO [n = 6] | |
| PDI 100 µM SA-CUR 12a + 10% DMSO [n = 6] |
References
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Richardson, M.; Lass-Flörl, C. Changing epidemiology of systemic fungal infections. Clin. Microbiol. Infect. 2008, 14, 5–24. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, S.R.; Guarner, J. Emerging and reemerging fungal infections. Semin. Diagn. Pathol. 2019, 36, 177–181. [Google Scholar] [CrossRef]
- Friedman, D.Z.P.; Schwartz, I.S. Emerging Fungal Infections: New Patients, New Patterns, and New Pathogens. J. Fungi 2019, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Pianalto, K.M.; Alspaugh, J.A. New Horizons in Antifungal Therapy. J. Fungi 2016, 2, 26. [Google Scholar] [CrossRef]
- Awada, M.; Becerik-Gerber, B.; Hoque, S.; O’Neill, Z.; Pedrielli, G.; Wen, J.; Wu, T. Ten questions concerning occupant health in buildings during normal operations and extreme events including the COVID-19 pandemic. Build. Environ. 2021, 188, 107480. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagnosis Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [Green Version]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Anti Infect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Jori, G. Photodynamic Therapy of Microbial Infections: State of the Art and Perspectives. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 505–520. [Google Scholar] [CrossRef]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.-Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Picco, D.d.C.R.; Cavalcante, L.L.R.; Trevisan, R.L.B.; Souza-Gabriel, A.E.; Borsatto, M.C.; Corona, S.A.M. Effect of curcumin-mediated photodynamic therapy on Streptococcus mutans and Candida albicans: A systematic review of in vitro studies. Photodiagnosis Photodyn. Ther. 2019, 27, 455–461. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisch, T. Resistance in antimicrobial photodynamic inactivation of bacteria. Photochem. Photobiol. Sci. 2015, 14, 1518–1526. [Google Scholar] [CrossRef] [Green Version]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updates 2017, 31, 31–42. [Google Scholar] [CrossRef]
- Toda, M.; Williams, S.R.; Berkow, E.L.; Farley, M.M.; Harrison, L.H.; Bonner, L.; Marceaux, K.M.; Hollick, R.; Zhang, A.Y.; Schaffner, W.; et al. Population-Based Active Surveillance for Culture-Confirmed Candidemia—Four Sites, United States, 2012–2016. MMWR Surveill Summ. 2019, 68, 1–15. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2016, 64, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Grimm, L.H.; Kelly, S.; Krull, R.; Hempel, D.C. Morphology and productivity of filamentous fungi. Appl. Microbiol. Biotechnol. 2005, 69, 375–384. [Google Scholar] [CrossRef]
- Jenks, J.D.; Hoenigl, M. Treatment of Aspergillosis. J. Fungi 2018, 4, 98. [Google Scholar] [CrossRef] [Green Version]
- Brambilla, A.; Sangiorgio, A. Mould growth in energy efficient buildings: Causes, health implications and strategies to mitigate the risk. Renew. Sustain. Energy Rev. 2020, 132, 110093. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [Green Version]
- U.S. Environmental Protection Agency Indoor Environments Devision. Mold Remediation in Schools and Commercial Buildings. EPA 402-K-01-001; U.S. EPA, 2008. Available online: https://www.epa.gov/mold/printable-version-mold-remediation-schools-and-commercial-buildings (accessed on 21 October 2021).
- CDC. Antibiotic Resistance Threats in the United States, 2019; US Department of Health and Human Services, Centres for Disease Control Prevention: Atlanta, GA, USA, 2019. [CrossRef] [Green Version]
- Winter, S.; Tortik, N.; Kubin, A.; Krammer, B.; Plaetzer, K. Back to the roots: Photodynamic inactivation of bacteria based on water-soluble curcumin bound to polyvinylpyrrolidone as a photosensitizer. Photochem. Photobiol. Sci. 2013, 12, 1795–1802. [Google Scholar] [CrossRef]
- Chignell, C.F.; Bilskj, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. Spectral and photochemical properties of curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; Pavarina, A.C.; Dovigo, L.N.; Brunetti, I.L.; Bagnato, V.S.; Vergani, C.E.; Costa, C.A. Phototoxic effect of curcumin on methicillin-resistant Staphylococcus aureus and L929 fibroblasts. Lasers Med. Sci. 2013, 28, 391–398. [Google Scholar] [CrossRef]
- Al-Asmari, F.; Mereddy, R.; Sultanbawa, Y. A novel photosensitization treatment for the inactivation of fungal spores and cells mediated by curcumin. J. Photochem. Photobiol. B Biol. 2017, 173, 301–306. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.; Brunetti, I.L.; Costa, C.A.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef]
- Glueck, M.; Schamberger, B.; Eckl, P.; Plaetzer, K.J.P.; Sciences, P. New horizons in microbiological food safety: Photodynamic Decontamination based on a curcumin derivative. Photochem. Photobiol. Sci. 2017, 16, 1784–1791. [Google Scholar] [CrossRef] [Green Version]
- Spaeth, A.; Graeler, A.; Maisch, T.; Plaetzer, K. CureCuma–cationic curcuminoids with improved properties and enhanced antimicrobial photodynamic activity. Eur. J. Med. Chem. 2018, 159, 423–440. [Google Scholar] [CrossRef]
- Tortik, N.; Steinbacher, P.; Maisch, T.; Spaeth, A.; Plaetzer, K. A comparative study on the antibacterial photodynamic efficiency of a curcumin derivative and a formulation on a porcine skin model. Photochem. Photobiol. Sci. 2016, 15, 187–195. [Google Scholar] [CrossRef]
- Jeong, R.-D.; Shin, E.-J.; Chu, E.-H.; Park, H.-J. Effects of Ionizing Radiation on Postharvest Fungal Pathogens. Plant Pathol. J. 2015, 31, 176–180. [Google Scholar] [CrossRef] [Green Version]
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Manuel, R.; Brown, C.S. Candida auris: A Review of the Literature. Clin. Microbiol. Rev. 2018, 31, e00029-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Annex, G Use of Disinfectants: Alcohol and Bleach. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care; World Health Organization: Geneva, Switzerland, 2014; pp. 65–66. [Google Scholar]
- Preuß, A.; Saltsman, I.; Mahammed, A.; Pfitzner, M.; Goldberg, I.; Gross, Z.; Röder, B. Photodynamic inactivation of mold fungi spores by newly developed charged corroles. J. Photochem. Photobiol. B Biol. 2014, 133, 39–46. [Google Scholar] [CrossRef]
- Xing, C.; Yang, G.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Conjugated Polymers for Light-Activated Antifungal Activity. Small 2012, 8, 525–529. [Google Scholar] [CrossRef]
- Alves, A.D.; Gurgel-Juarez, N.; Vieira, A.C.; Proietti, A.A.; Pinheiro Barcessat, A.R. PDT technology: An alternative to control oral candidiasis in critically ill patients. Lasers Dent. Sci. 2021, 5, 193–197. [Google Scholar] [CrossRef]
- Martín Santiago, M.P.; Gutknecht, N.; Martín-Carrillo, N.; Foronda, P.; Valladares, B.; Montero Gómez, N. In vitro study of photodynamic therapy with visible laser systems applied to fungal infections. Lasers Dent. Sci. 2020, 4, 103–110. [Google Scholar] [CrossRef]
- Huang, S.-H.; Wu, C.-H.; Chen, S.-J.; Sytwu, H.-K.; Lin, G.-J. Immunomodulatory effects and potential clinical applications of dimethyl sulfoxide. Immunobiology 2020, 225, 151906. [Google Scholar] [CrossRef]
- Santos, N.C.; Figueira-Coelho, J.; Martins-Silva, J.; Saldanha, C. Multidisciplinary utilization of dimethyl sulfoxide: Pharmacological, cellular, and molecular aspects. Biochem. Pharmacol. 2003, 65, 1035–1041. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Carmello, J.C.; de Souza Costa, C.A.; Vergani, C.E.; Brunetti, I.L.; Bagnato, V.S.; Pavarina, A.C. Curcumin-mediated photodynamic inactivation of Candida albicans in a murine model of oral candidiasis. Med. Mycol. 2013, 51, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Spaeth, A.; Plaetzer, K.; Maisch, T.; Eichner, A. 1,7-Diaryl-1,6-Heptadien-3,5-Dion-Derivate, Verfahren zur Herstellung und Verwendung Derselben; E.P. Office: Munich, Germany, 2017. [Google Scholar]
- Heinlin, J.; Maisch, T.; Zimmermann, J.L.; Shimizu, T.; Holzmann, T.; Simon, M.; Heider, J.; Landthaler, M.; Morfill, G.; Karrer, S. Contact-free inactivation of Trichophyton rubrum and Microsporum canis by cold atmospheric plasma treatment. Future Microbiol. 2013, 8, 1097–1106. [Google Scholar] [CrossRef]
- NIH. ImageJ. Available online: http://imagej.nih.gov/ij/ (accessed on 13 September 2015).





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schamberger, B.; Plaetzer, K. Photofungizides Based on Curcumin and Derivates Thereof against Candida albicans and Aspergillus niger. Antibiotics 2021, 10, 1315. https://doi.org/10.3390/antibiotics10111315
Schamberger B, Plaetzer K. Photofungizides Based on Curcumin and Derivates Thereof against Candida albicans and Aspergillus niger. Antibiotics. 2021; 10(11):1315. https://doi.org/10.3390/antibiotics10111315
Chicago/Turabian StyleSchamberger, Barbara, and Kristjan Plaetzer. 2021. "Photofungizides Based on Curcumin and Derivates Thereof against Candida albicans and Aspergillus niger" Antibiotics 10, no. 11: 1315. https://doi.org/10.3390/antibiotics10111315
APA StyleSchamberger, B., & Plaetzer, K. (2021). Photofungizides Based on Curcumin and Derivates Thereof against Candida albicans and Aspergillus niger. Antibiotics, 10(11), 1315. https://doi.org/10.3390/antibiotics10111315






