Effects of Growth Medium and Inoculum Size on Pharmacodynamics Activity of Marbofloxacin against Staphylococcus aureus Isolated from Caprine Clinical Mastitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates, Culture Medium and Antimicrobials
2.2. MIC and MBC Measurements
2.3. Time Kill Curves
2.4. Drug Stability
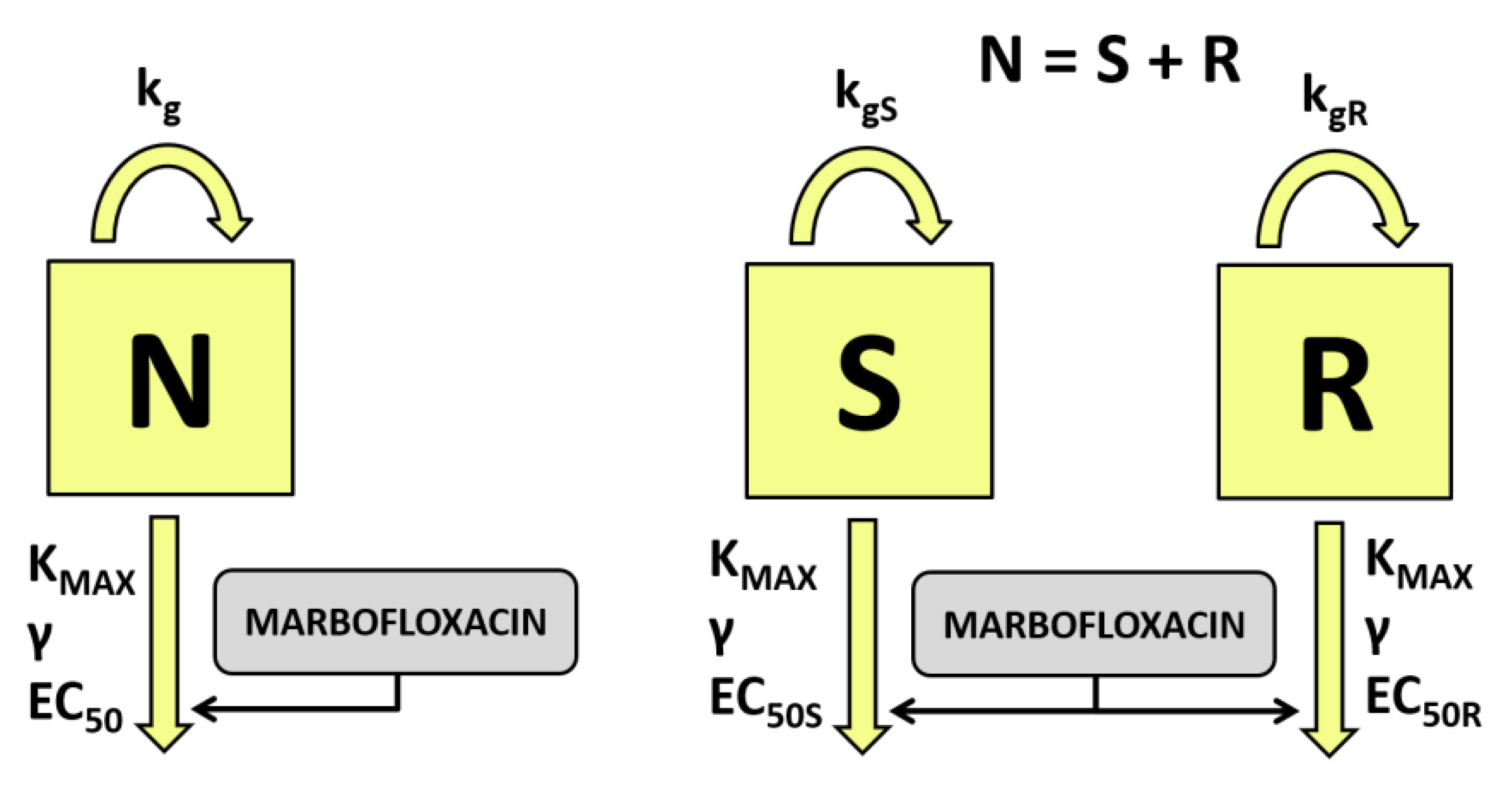
2.5. Pharmacodynamic Modeling
2.6. Simulations and PK/PD Relationships
2.7. Statistical Analysis
3. Results
3.1. MIC and MBC Determination
3.2. Time Kill Curves Modeling
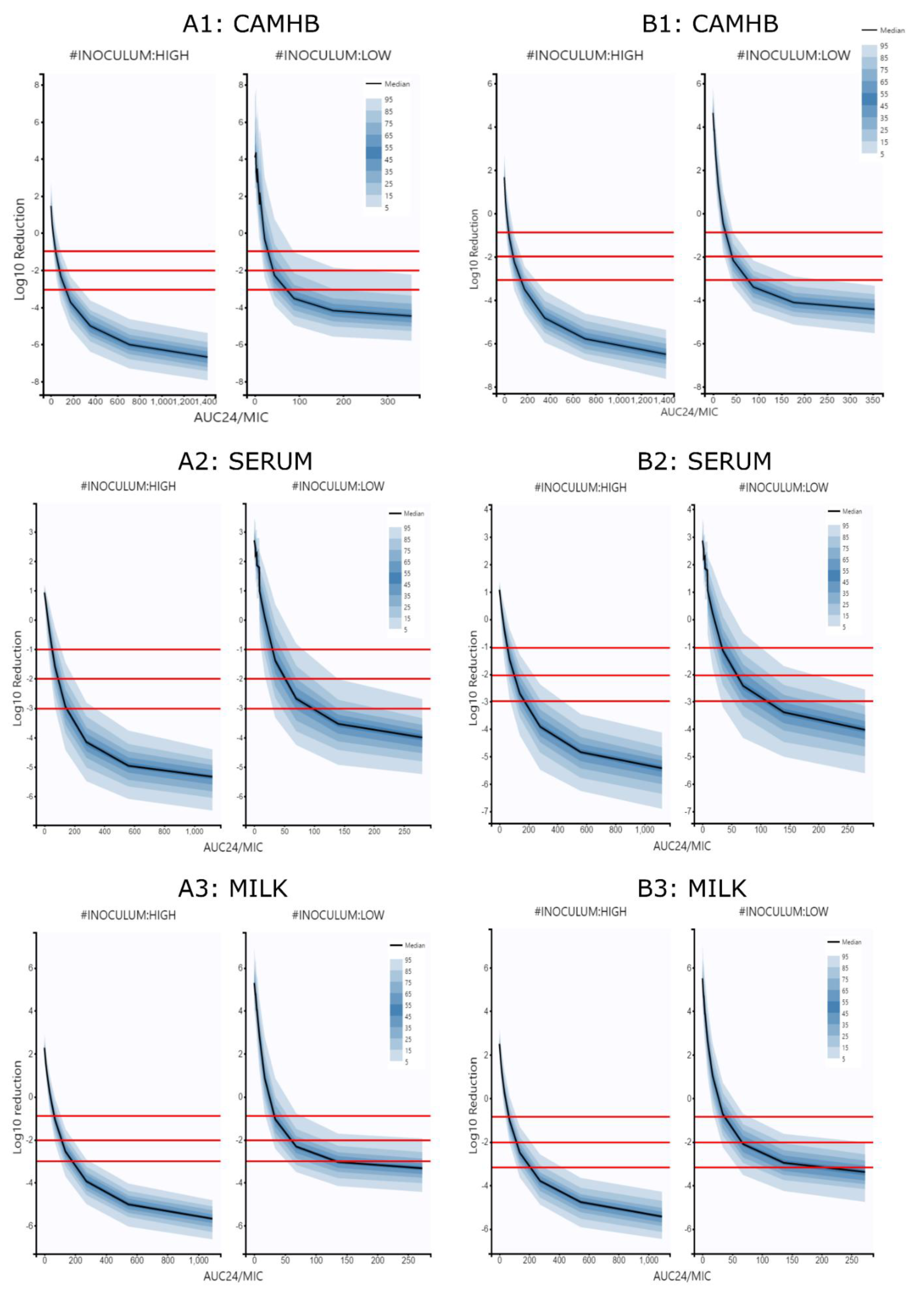
3.3. Simulations and PK/PD Relationships
4. Discussion
4.1. MIC and MBC Determination
4.2. Drug Effect Modeling
4.3. Simulations and PK/PD Cuttoff Points
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peton, V.; Le Loir, Y. Staphylococcus aureus in veterinary medicine. Infect. Genet. Evol. 2014, 21, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Mavrogianni, V.S.; Menzies, P.I.; Fragkou, I.A.; Fthenakis, G.C. Principles of Mastitis Treatment in Sheep and Goats. Veter. Clin. N. Am. Food Anim. Pr. 2011, 27, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Sierra, D.; Sánchez, A.; Corrales, J.; Marco, J.; Paape, M.; Gonzalo, C. Mastitis in small ruminants. Small Rumin. Res. 2007, 68, 145–153. [Google Scholar] [CrossRef]
- Asperger, H. Staphylococcus aureus. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA; Mississippi State University: Mississippi State, MS, USA, 2011; pp. 111–117. [Google Scholar]
- Constable, P.D.; Morin, D.E. Treatment of clinical mastitis: Using antimicrobial susceptibility profiles for treatment decisions. Veter. Clin. N. Am. Food Anim. Pr. 2003, 19, 139–155. [Google Scholar] [CrossRef]
- Pyörälä, S. Treatment of mastitis during lactation. Ir. Veter. J. 2009, 62, S40–S44. [Google Scholar] [CrossRef] [PubMed]
- Schrickx, J.A.; Fink-Gremmels, J. Implications of ABC transporters on the disposition of typical veterinary medicinal products. Eur. J. Pharmacol. 2008, 585, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Suojala, L.; Kaartinen, L.; Pyörälä, S. Treatment for bovine Escherichia coli mastitis—An evidence-based approach. J. Veter. Pharmacol. Ther. 2013, 36, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Papich, M.G. Pharmacokinetic–Pharmacodynamic (PK–PD) modeling and the rational selection of dosage regimes for the prudent use of antimicrobial drugs. Veter. Microbiol. 2014, 171, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Spreng, M.; Deleforge, J.; Thomas, V.; Boisramé, B.; Drugeon, H. Antibacterial activity of marbofloxacin. A new fluoroquinolone for veterinary use against canine and feline isolates. J. Veter. Pharmacol. Ther. 1995, 18, 284–289. [Google Scholar] [CrossRef] [PubMed]
- EMA. Committee for Veterinary Medicinal Products. Marbofloxacin (Extension to All Food Producing Species). Summary Report. 2009. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500014864.pdf. (accessed on 30 May 2021).
- EMA, Committee for Medicinal Products for Veterinary Use (CVMP). Categorisation of Antibiotics in the Europena Union; European Medicines Agency: Amsterdam, The Netherlands, 2020; EMA/CVMP/CHMP/682198/2017. [Google Scholar]
- Illambas, J.; Potter, T.; Cheng, Z.; Rycroft, A.; Fishwick, J.; Lees, P. Pharmacodynamics of marbofloxacin for calf pneumonia pathogens. Res. Veter. Sci. 2013, 94, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Gehring, R.; Riviere, J.E. Limitations of MIC as the sole criterion in antimicrobial drug dosage regimen design: The need for full characterization of antimicrobial pharmacodynamic profile especially for drug-resistant organisms. Veter. J. 2013, 198, 15–18. [Google Scholar] [CrossRef][Green Version]
- Pelligand, L.; Lees, P.; Sidhu, P.K.; Toutain, P.-L. Semi-Mechanistic Modeling of Florfenicol Time-Kill Curves and in silico Dose Fractionation for Calf Respiratory Pathogens. Front. Microbiol. 2019, 10, 1237. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.-L.; Sidhu, P.K.; Lees, P.; Rassouli, A.; Pelligand, L. VetCAST Method for Determination of the Pharmacokinetic-Pharmacodynamic Cut-Off Values of a Long-Acting Formulation of Florfenicol to Support Clinical Breakpoints for Florfenicol Antimicrobial Susceptibility Testing in Cattle. Front. Microbiol. 2019, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.I.; Friberg, L.E. Pharmacokinetic-Pharmacodynamic Modeling of Antibacterial Drugs. Pharmacol. Rev. 2013, 65, 1053–1090. [Google Scholar] [CrossRef]
- Serrano-Rodríguez, J.M.; Cárceles-García, C.; Cárceles-Rodríguez, C.M.; Gabarda, M.L.; Serrano-Caballero, J.M.; Fernández-Varón, E. Susceptibility and PK/PD relationships of Staphylococcus aureus strains isolated from the milk of sheep and goats with clinical mastitis to five veterinary fluoroquinolones. Veter. Rec. 2017, 180, 376. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Seven Informational Supplement; CLSI Document M100-Swayne; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Udekwu, K.I.; Parrish, N.; Ankomah, P.; Baquero, F.; Levin, B.R. Functional relationship between bacterial cell density and the efficacy of antibiotics. J. Antimicrob. Chemother. 2009, 63, 745–757. [Google Scholar] [CrossRef]
- Ganière, J.P.; Mangion, C.; Péridy, M. In Vitro determination in milk of the Minimal Inhibitory and Bactericidal Concentrations of celquinone, marbofloxacin, tylosin and spiramycin for bovine mastitis pathogens. Rev. Méd. Vét. 2004, 155, 411–416. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Determining Bactericidal Activity of Antimicrobial Agents; CLSI Document M26-A; CLSI: Wayne, PA, USA, 1999. [Google Scholar]
- Fernández-Varón, E.; García-Romero, E.; Serrano-Rodríguez, J.; Cárceles, C.; García-Galán, A.; Cárceles-García, C.; Fernández, R.; Muñoz, C.; de la Fe, C. PK/PD Analysis of Marbofloxacin by Monte Carlo Simulation against Mycoplasmaagalactiae in Plasma and Milk of Lactating Goats after IV, SC and SC-Long Acting Formulations Administration. Animals 2021, 11, 1104. [Google Scholar] [CrossRef] [PubMed]
- Melchior, M.B.; Fink-Gremmels, J.; Gaastra, W. Comparative Assessment of the Antimicrobial Susceptibility of Staphylococcus aureus Isolates from Bovine Mastitis in Biofilm Versus Planktonic Culture. J. Veter. Med. Ser. B 2006, 53, 326–332. [Google Scholar] [CrossRef]
- Kuang, Y.; Jia, H.; Miyanaga, K.; Tanji, Y. Effect of milk on antibacterial activity of tetracycline against Escherichia coli and Staphylococcus aureus isolated from bovine mastitis. Appl. Microbiol. Biotechnol. 2009, 84, 135–142. [Google Scholar] [CrossRef]
- Lemaire, S.; Tulkens, P.M.; Van Bambeke, F. Contrasting Effects of Acidic pH on the Extracellular and Intracellular Activities of the Anti-Gram-Positive Fluoroquinolones Moxifloxacin and Delafloxacin against Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 649–658. [Google Scholar] [CrossRef]
- Mould, D.R.; Upton, R. Basic Concepts in Population Modeling, Simulation, and Model-Based Drug Development-Part 2: Introduction to Pharmacokinetic Modeling Methods. CPT Pharmacometrics Syst. Pharmacol. 2013, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Treyaprasert, W.; Schmidt, S.; Rand, K.H.; Suvanakoot, U.; Derendorf, H. Pharmacokinetic/pharmacodynamic modeling of in vitro activity of azithromycin against four different bacterial strains. Int. J. Antimicrob. Agents 2007, 29, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.; Grégoire, N.; Couet, W.; Bulitta, J. Distinguishing Antimicrobial Models with Different Resistance Mechanisms via Population Pharmacodynamic Modeling. PLoS Comput. Biol. 2016, 12, e1004782. [Google Scholar] [CrossRef]
- Zhi, J.; Nightingale, C.H.; Quintiliani, R. Microbial pharmacodynamics of piperacillin in neutropenic mice of systematic infection due toPseudomonas aeruginosa. J. Pharmacokinet. Biopharm. 1988, 16, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Bhagunde, P.; Chang, K.-T.; Singh, R.; Singh, V.; Garey, K.; Nikolaou, M.; Tam, V.H. Mathematical Modeling To Characterize the Inoculum Effect. Antimicrob. Agents Chemother. 2010, 54, 4739–4743. [Google Scholar] [CrossRef]
- Nielsen, E.I.; Viberg, A.; Löwdin, E.; Cars, O.; Karlsson, M.O.; Sandström, M. Semimechanistic Pharmacokinetic/Pharmacodynamic Model for Assessment of Activity of Antibacterial Agents from Time-Kill Curve Experiments. Antimicrob. Agents Chemother. 2007, 51, 128–136. [Google Scholar] [CrossRef]
- Campion, J.J.; McNamara, P.J.; Evans, M.E. Pharmacodynamic Modeling of Ciprofloxacin Resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 209–219. [Google Scholar] [CrossRef]
- Andraud, M.; Chauvin, C.; Sanders, P.; Laurentie, M. Pharmacodynamic Modeling ofIn VitroActivity of Marbofloxacin againstEscherichia coliStrains. Antimicrob. Agents Chemother. 2010, 55, 756–761. [Google Scholar] [CrossRef]
- Ferran, A.A.; Bibbal, D.; Pellet, T.; Laurentie, M.; Gicquel-Bruneau, M.; Sanders, P.; Schneider, M.; Toutain, P.-L.; Bousquet-Melou, A. Pharmacokinetic/pharmacodynamic assessment of the effects of parenteral administration of a fluoroquinolone on the intestinal microbiota: Comparison of bactericidal activity at the gut versus the systemic level in a pig model. Int. J. Antimicrob. Agents 2013, 42, 429–435. [Google Scholar] [CrossRef]
- Shan, Q.; Zheng, G.; Liu, S.; Bai, Y.; Li, L.; Yin, Y.; Ma, L.; Zhu, X. Pharmacokinetic/pharmacodynamic relationship of marbofloxacin against Aeromonas hydrophila in Chinese soft-shelled turtles (Trionyx sinensis). J. Veter. Pharmacol. Ther. 2015, 38, 537–542. [Google Scholar] [CrossRef]
- Aliabadi, F.S.; Lees, P. Pharmacokinetics and pharmacodynamics of danofloxacin in serum and tissue fluids of goats following intravenous and intramuscular administration. Am. J. Veter. Res. 2001, 62, 1979–1989. [Google Scholar] [CrossRef]
- Ganière, J.; Denuault, L. Synergistic interactions between cefalexin and kanamycin in Mueller-Hinton broth medium and in milk. J. Appl. Microbiol. 2009, 107, 117–125. [Google Scholar] [CrossRef]
- Fang, W.; Pyörälä, S. Mastitis-Causing Escherichia coli: Serum Sensitivity and Susceptibility to Selected Antibacterials in Milk. J. Dairy Sci. 1996, 79, 76–82. [Google Scholar] [CrossRef]
- Konig, C.; Simmen, H.P.; Blaser, J. Bacterial concentrations in pus and infected peritoneal fluid-- implications for bactericidal activity of antibiotics. J. Antimicrob. Chemother. 1998, 42, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Mizunaga, S.; Kamiyama, T.; Fukuda, Y.; Takahata, M.; Mitsuyama, J. Influence of inoculum size of Staphylococcus aureus and Pseudomonas aeruginosa on in vitro activities and in vivo efficacy of fluoroquinolones and carbapenems. J. Antimicrob. Chemother. 2005, 56, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.; Podkowik, M.; Bystroń, J.; Bania, J. Production of Staphylococcal Enterotoxins D and R in Milk and Meat Juice by Staphylococcus aureus Strains. Foodborne Pathog. Dis. 2017, 14, 223–230. [Google Scholar] [CrossRef]
- Johnson, P.J.T.; Levin, B.R. Pharmacodynamics, Population Dynamics, and the Evolution of Persistence in Staphylococcus aureus. PLoS Genet. 2013, 9, e1003123. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Levin, B.R. Proximate and ultimate causes of the bactericidal action of antibiotics. Nat. Rev. Genet. 2020, 19, 123–132. [Google Scholar] [CrossRef]
- Drusano, G.L.; Hope, W.; MacGowan, A.; Louie, A. Suppression of Emergence of Resistance in Pathogenic Bacteria: Keeping Our Powder Dry, Part 2. Antimicrob. Agents Chemother. 2016, 60, 1194–1201. [Google Scholar] [CrossRef]
- Toutain, P.-L.; Bousquet-Melou, A.; Damborg, P.; Ferran, A.A.; Mevius, D.; Pelligand, L.; Veldman, K.T.; Lees, P. En Route towards European Clinical Breakpoints for Veterinary Antimicrobial Susceptibility Testing: A Position Paper Explaining the VetCAST Approach. Front. Microbiol. 2017, 8, 2344. [Google Scholar] [CrossRef]
- Martinez, M.N.; Toutain, P.-L.; Turnidge, J. The pharmacodynamics of antimicrobial agents. In Antimicrobial Therapy in Veterinary Medicine; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 79–103. [Google Scholar]
- Walker, R.D. Fluoroquinolones. In Antimicrobial Therapy in Veterinary Medicine; Iowa State University Press: Ames, IA, USA, 2000; pp. 315–338. [Google Scholar]
- McKellar, Q.; Bruni, S.F.S.; Jones, D.G. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J. Veter. Pharmacol. Ther. 2004, 27, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L.; Liu, W.; Fikes, S.; Cirz, R.; Robbins, N.; Kurhanewicz, S.; Rodriquez, J.; Brown, D.; Baluya, D.; Louie, A. Interaction of Drug- and Granulocyte-Mediated Killing of Pseudomonas aeruginosa in a Murine Pneumonia Model. J. Infect. Dis. 2014, 210, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L.; Vanscoy, B.; Liu, W.; Fikes, S.; Brown, D.; Louie, A. Saturability of Granulocyte Kill of Pseudomonas aeruginosa in a Murine Model of Pneumonia. Antimicrob. Agents Chemother. 2011, 55, 2693–2695. [Google Scholar] [CrossRef]
- Drusano, G.L.; Fregeau, C.; Liu, W.; Brown, D.L.; Louie, A. Impact of Burden on Granulocyte Clearance of Bacteria in a Mouse Thigh Infection Model. Antimicrob. Agents Chemother. 2010, 54, 4368–4372. [Google Scholar] [CrossRef] [PubMed]
- Licata, L.; Smith, C.E.; Goldschmidt, R.M.; Barrett, J.F.; Frosco, M. Comparison of the postantibiotic and postantibiotic sub-MIC effects of levofloxacin and ciprofloxacin on Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob. Agents Chemother. 1997, 41, 950–955. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coulet, M.; Waalkes, M.V.B.; Cox, P.; Lohuis, J. In Vitro and in vivo pharmacodynamic properties of the fluoroquinolone ibafloxacin. J. Veter. Pharmacol. Ther. 2002, 25, 401–411. [Google Scholar] [CrossRef] [PubMed]

| MEDIUM | CAMHB | Serum | Milk | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INOCULUM | LOW | HIGH | LOW | HIGH | LOW | HIGH | ||||||
| Drug (mg/L) | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| 0.125 | 5 | 2 | ||||||||||
| 0.25 | 4 | 1 | 1 | 2 | ||||||||
| 0.5 | 3 | 6 | 7 | 2 | 6 | 5 | 5 | 3 | ||||
| 1 | 2 | 3 | 5 | 5 | 3 | 5 | 4 | 7 | 1 | 9 | ||
| 2 | 3 | 3 | 3 | 5 | 3 | 2 | ||||||
| 4 | 2 | 6 | 3 | 8 | 8 | |||||||
| 8 | 2 | |||||||||||
| CAMHB | Serum | Milk | ||||
|---|---|---|---|---|---|---|
| Parameters | Estimates | IIV | Estimates | IIV | Estimates | IIV |
| NMAX (log CFU/mL) | 10.1 (0.85) | 0.04 (17.1) | 9.48 (0.62) | 0.01 (64.4) | 11.2 (0.97) | 0.033 (21.2) |
| kg (1/h) | 0.39 (3.13) | 0.06 (57.0) | 0.56 (2.96) | 0.06 (50.6) | 0.32 (3.26) | 0.07 (51.4) |
| dkg (1/h) | 2.41 (17.3) | 0.79 (8.47) | 2.37 (17.6) | 1.26 (7.01) | ||
| N0 (log CFU/mL) | 8.66 (0.50) | 0.014 (81.8) | 8.58 (0.48) | 0.02 (31.2) | 8.7 (0.5) | 0.02 (39.0) |
| γ | 0.92 (2.69) | 0.13 (16.2) | 1.23 (2.72) | 0.18 (14.7) | 1.13 (2.86) | 0.15 (16.8) |
| KMAX (1/h) | 0.36 (3.1) | 0.04 (110) | 0.39 (3.59) | 0.06(49.8) | 0.26 (3.04) | 0.05 (82.2) |
| EC50 (mg/L) | 0.81 (12.8) | 0.29 (14.7) | 1.45 (12.0) | 0.25 (15.4) | 0.77 (14.9) | 0.26 (17.5) |
| Covariate estimates | ||||||
| β_ NMAX_INOCULUM_LOW | −0.13 (7.26) | |||||
| β_dkg_INOCULUM_LOW | −1.11 (17.5) | −0.75 (30.2) | ||||
| β_N0_INOCULUM_LOW | −0.424 (1.96) | −0.42 (1.89) | −0.42 (2.07) | |||
| β_γ_INOCULUM_LOW | 0.315 (15.2) | |||||
| β_KMAX_INOCULUM_LOW | 0.20 (16.5) | |||||
| β_EC50_INOCULUM_LOW | −1.89 (5.28) | −1.70 (5.97) | −1.82 (5.19) | |||
| β_EC50_MIC | 2.25 (6.88) | 1.07 (11.3) | 1.74 (8.32) | |||
| CAMHB | Serum | Milk | ||||
|---|---|---|---|---|---|---|
| Parameters | Estimates | IIV | Estimates | IIV | Estimates | IIV |
| NMAX (log CFU/mL) | 10.3 (1.01) | 0.05 (15.9) | 9.57 (0.69) | 0.02 (48.1) | 11.1 (0.92) | 0.04 (19.1) |
| kgS (1/h) | 0.40 (3.33) | 0.04 (138.0) | 0.55 (3.11) | 0.09 (28.2) | 0.31 (3.28) | 0.08 (50.6) |
| fg | 0.62 (0.95) | 1.07 (82.3) | 0.60 (0.32) | 1.67 (106) | 0.61 (3.29) | 1.99 (72) |
| dkg (1/h) | 2.52 (18.0) | 0.885 (8.77) | 1.98 (18.6) | 1.11 (7.95) | ||
| N0 (log CFU/mL) | 6.84 (0.32) | 0.01 (65.1) | 6.78 (0.30) | 0.01 (24.7) | 6.84 (0.32) | 0.01 (27.8) |
| γ | 0.79 (4.60) | 0.13 (45.9) | 1.07 (3.20) | 0.18 (14.3) | 1.06 (5.74) | 0.15 (46.9) |
| KMAX (1/h) | 0.38 (3.63) | 0.04 (117) | 0.39 (4.16) | 0.06 (59.3) | 0.24 (4.19) | 0.05 (95.1) |
| EC50S (mg/L) | 0.73 (14.6) | 0.315 (15) | 1.29 (13.6) | 0.22 (24) | 0.62 (15.7) | 0.1 (28.4) |
| fs | 4.02 (0.74) | 2.28 (150) | 3.51 (0.7) | 2.67 (155) | 4.6 (5.59) | 1.54 (66.3) |
| Secondary parameters | ||||||
| EC50R | 8.85 (8.43) | 6.74 (3.97) | 8.01 (4.96) | |||
| kgR | 0.25 (0.08) | 0.33 (0.38) | 0.19 (0.20) | |||
| Covariate estimates | ||||||
| β_ NMAX_INOCULUM_LOW | −0.127(8.3) | |||||
| β_dkg_INOCULUM_LOW | −1.02 (20.6) | −0.62 (37) | ||||
| β_N0_INOCULUM_LOW | −0.246 (1.97) | −0.243 (1.9) | −0.25 (1.98) | |||
| β_γ_INOCULUM_LOW | 0.417 (14.9) | |||||
| β_KMAX_INOCULUM_LOW | −1.94 (5.47) | 0.23 (18.1) | 0.17 (20.3) | |||
| β_EC50_INOCULUM_LOW | 2.24 (7.33) | −1.54 (8.21) | −1.46 (7.61) | |||
| β_EC50_MIC | 1.08 (11.7) | 1.71 (7.96) | ||||
| CAMHB | Serum | Milk | ||||
|---|---|---|---|---|---|---|
| Parameters | Estimates | IIV | Estimates | IIV | Estimates | IIV |
| E0 | 0.92 (4.16) | 0.33 (4.10) | 0.94 (1.51) | 0.15 (4.51) | 2.29 (1.38) | 0.16 (4.52) |
| IMAX | 8.57 (1.06) | 0.11 (4.13) | 6.75 (0.92) | 0.09 (4.61) | 8.88 (0.50) | 0.07 (4.70) |
| γ | 0.88 (0.75) | 0.10 (4.24) | 1.23 (1.31) | 0.21 (4.51) | 0.97 (0.73) | 0.08 (4.70) |
| EC50 (mg/L) | 172.39 (6.03) | 0.38 (4.10) | 235.95 (6.24) | 0.35 (4.50) | 113.65 (2.76) | 0.27 (4.51) |
| Covariate estimates | ||||||
| β_E0_INOCULUM_LOW | 1.29 (2.07) | 1.05 (1.85) | 0.83 (1.90) | |||
| β_E0_MIC | 0.79 (8.60) | |||||
| β_γ_INOCULUM_LOW | 0.46 (2.15) | 0.31(2.99) | ||||
| β_Imax_MIC | 0.12 (15.8) | |||||
| β_EC50_INOCULUM_LOW | −1.93 (2.63) | −1.59 (2.96) | −1.91 (1.86) | |||
| β_EC50_MIC | −0.55 (16.3) | −1.05 (6.59) | ||||
| CAMHB | Serum | Milk | ||||
|---|---|---|---|---|---|---|
| Parameters | Estimates | IIV | Estimates | IIV | Estimates | IIV |
| E0 | 1.76 (3.31) | 0.02 (98.1) | 1.08 (1.53) | 0.15 (4.61) | 2.51 (1.46) | 0.08 (4.66) |
| Imax | 9.4 (0.79) | 0.09 (155) | 7.39 (1.06) | 0.10 (4.97) | 8.72 (0.76) | 0.07 (4.66) |
| γ | 0.81 (1.58) | 0.04 (69.2) | 1.01 (1.44) | 0.14 (4.79) | 0.97 (1.33) | 0.13 (4.59) |
| EC50 (mg/L) | 119 (5.93) | 0.20 (6.88) | 305.45 (6.64) | 0.37 (4.58) | 102.68 (2.42) | 0.24 (4.53) |
| Covariate estimates | ||||||
| β_E0_INOCULUM_LOW | 1.0 (2.75) | 0.97 (2.04) | 0.78 (2.41) | |||
| β_E0_MIC | −0.112 (33) | |||||
| β_γ_INOCULUM_LOW | 0.40 (4.27) | |||||
| β_EC50_INOCULUM_LOW | −1.88 (2.65) | −1.68 (2.98) | −1.74 (1.79) | |||
| β_EC50_MIC | 0.22 (35.2) | −1.07 (6.83) | ||||
| One Population Model | Two Populations Model | |||
|---|---|---|---|---|
| Medium | Inoculum | log10 Reduction | fAUC24/MIC | fAUC24/MIC |
| CAMHB | HIGH | −1 | 42.68 (21.57–87.97) | 43.15 (29.57–58.53) |
| CAMHB | HIGH | −2 | 74.49 (39.34–150.4) | 78.04 (54.17–106.54) |
| CAMHB | HIGH | −3 | 123.96 (64.69–242.95) | 133.53 (92.98–184.09) |
| CAMHB | LOW | −1 | 27.76 (13.19–85.61) | 27.17 (20.08–38.9) |
| CAMHB | LOW | −2 | 39.42 (18.33–127.66) | 40.62 (29.92–58.91) |
| CAMHB | LOW | −3 | 59.84 (27.39–194.15) | 66.65 (48.04–97.65) |
| SERUM | HIGH | −1 | 50.25 (24.9–109.51) | 52.65 (23.76–114.93) |
| SERUM | HIGH | −2 | 85.52 (44.91–187.63) | 97.31 (43.89–222.42) |
| SERUM | HIGH | −3 | 144.65 (71.15–316.14) | 166.2 (73.71–409.32) |
| SERUM | LOW | −1 | 29.43 (11.95–76.64) | 33.03 (12.57–91.57) |
| SERUM | LOW | −2 | 47.3 (18.68–139.08) | 54.38 (19.7–172.83) |
| SERUM | LOW | −3 | 86.77 (30.22–377.42) | 101.91 (31.7–449.27) |
| MILK | HIGH | −1 | 66.27 (40.93–107.98) | 67.67 (41.49–116.83) |
| MILK | HIGH | −2 | 105.47 (65.26–172.06) | 108.01 (66.71–193.43) |
| MILK | HIGH | −3 | 168.7 (100.16–282.9) | 175.58 (103.17–344.51) |
| MILK | LOW | −1 | 33.21 (18.38–76.76) | 38.46 (20.41–91.36) |
| MILK | LOW | −2 | 54.68 (27.69–160.3) | 63.81 (31.06–198.05) |
| MILK | LOW | −3 | 106.65 (45.01–527.62) | 123.31 (48.98–971.07) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzutti, A.M.; San Andrés-Larrea, M.I.; Fernández-Varón, E.; Zarazaga, M.d.P.; Molina-López, A.M.; Serrano-Rodríguez, J.M. Effects of Growth Medium and Inoculum Size on Pharmacodynamics Activity of Marbofloxacin against Staphylococcus aureus Isolated from Caprine Clinical Mastitis. Antibiotics 2021, 10, 1290. https://doi.org/10.3390/antibiotics10111290
Lorenzutti AM, San Andrés-Larrea MI, Fernández-Varón E, Zarazaga MdP, Molina-López AM, Serrano-Rodríguez JM. Effects of Growth Medium and Inoculum Size on Pharmacodynamics Activity of Marbofloxacin against Staphylococcus aureus Isolated from Caprine Clinical Mastitis. Antibiotics. 2021; 10(11):1290. https://doi.org/10.3390/antibiotics10111290
Chicago/Turabian StyleLorenzutti, Augusto Matías, Manuel Ignacio San Andrés-Larrea, Emilio Fernández-Varón, María del Pilar Zarazaga, Ana María Molina-López, and Juan Manuel Serrano-Rodríguez. 2021. "Effects of Growth Medium and Inoculum Size on Pharmacodynamics Activity of Marbofloxacin against Staphylococcus aureus Isolated from Caprine Clinical Mastitis" Antibiotics 10, no. 11: 1290. https://doi.org/10.3390/antibiotics10111290
APA StyleLorenzutti, A. M., San Andrés-Larrea, M. I., Fernández-Varón, E., Zarazaga, M. d. P., Molina-López, A. M., & Serrano-Rodríguez, J. M. (2021). Effects of Growth Medium and Inoculum Size on Pharmacodynamics Activity of Marbofloxacin against Staphylococcus aureus Isolated from Caprine Clinical Mastitis. Antibiotics, 10(11), 1290. https://doi.org/10.3390/antibiotics10111290








