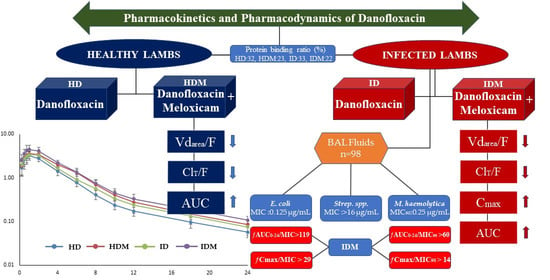
Pharmacokinetic Behavior and Pharmacokinetic/Pharmacodynamic Integration of Danofloxacin Following Single or Co-Administration with Meloxicam in Healthy Lambs and Lambs with Respiratory Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Clinical Examination and Creating Groups
2.3. Pharmacokinetic Study
2.3.1. Danofloxacin Analysis
2.3.2. Pharmacokinetic Analysis
2.3.3. In Vitro Protein Binding Assay of Danofloxacin
2.3.4. Statistical Analysis
2.4. Microbiological Analysis
2.4.1. Collection of Bronchoalveolar Lavage Fluid Samples
2.4.2. Bacterial Isolation and Identification
2.4.3. Minimum Inhibitory Concentration
2.4.4. Pharmacokinetic/Pharmacodynamic Integration
3. Results
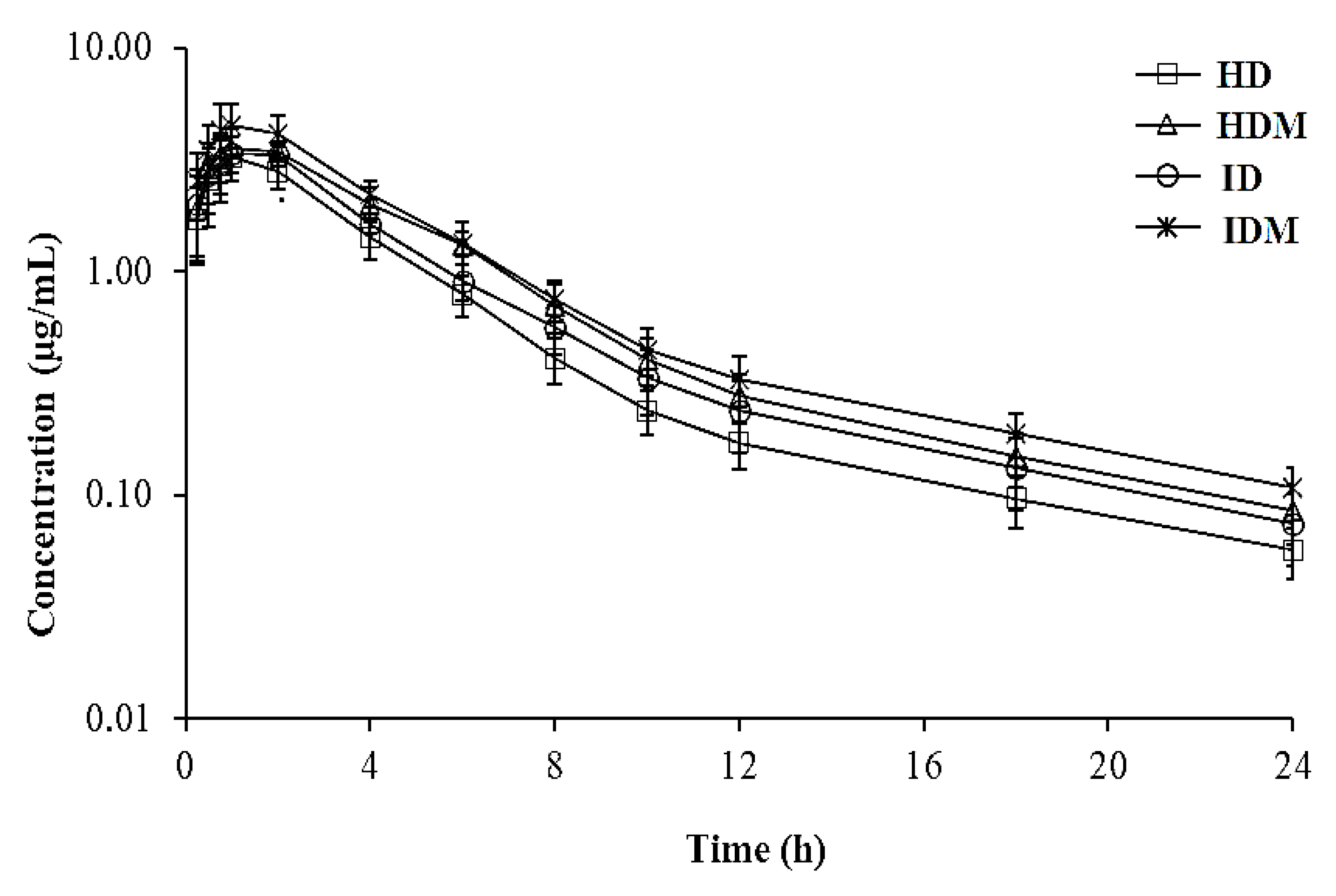
3.1. Pharmacokinetics
3.2. In Vitro Protein Binding of Danofloxacin
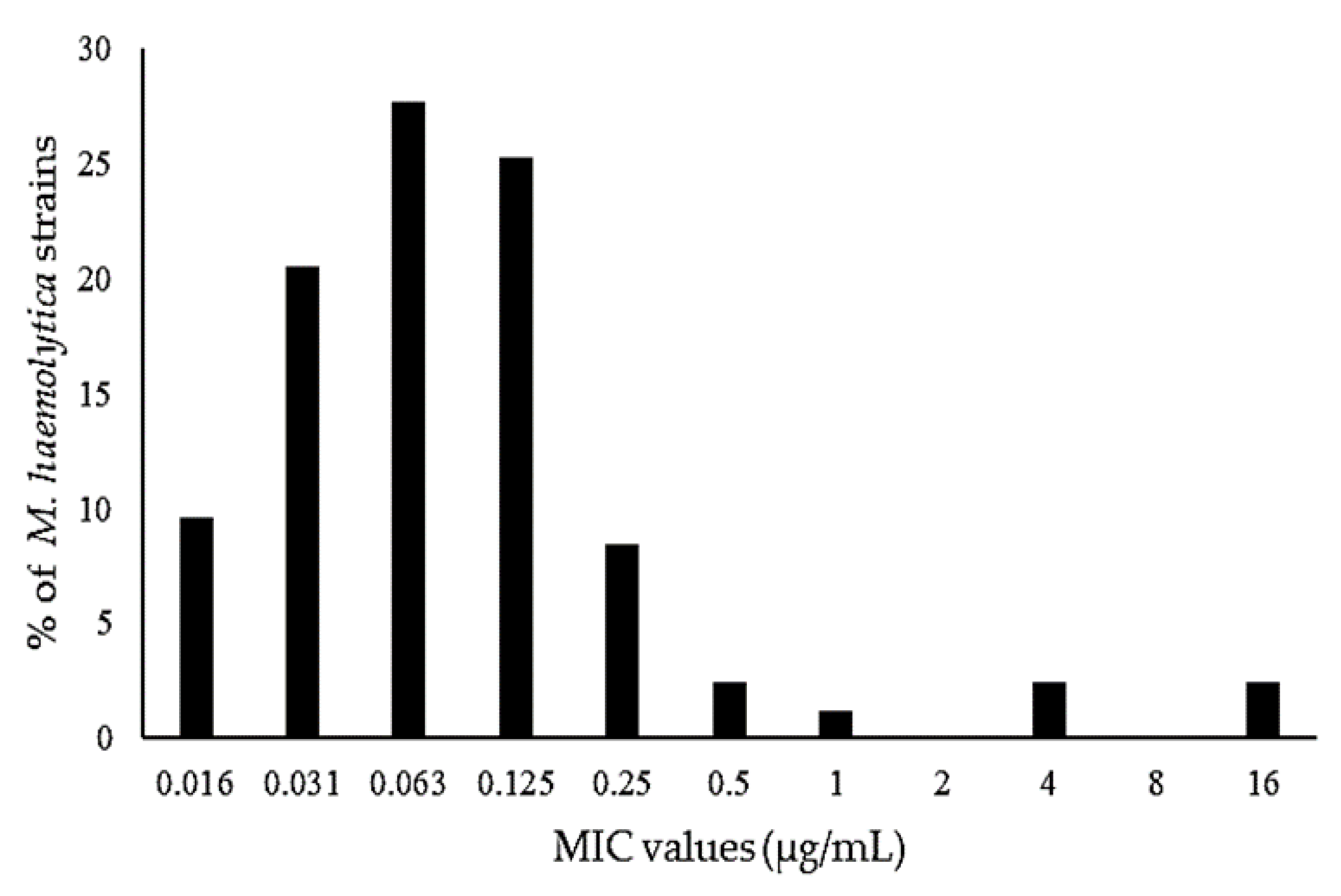
3.3. Pharmacodynamics
3.4. Pharmacokinetic/Pharmacodynamic Integration
4. Discussion
4.1. Pharmacokinetics of Danofloxacin
4.2. Pharmacokinetic/Pharmacodynamic Integration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodwin, K.; Jackson, R.; Brown, C.; Davies, P.; Morris, R.; Perkins, N. Pneumonic lesions in lambs in New Zealand: Patterns of prevalence and effects on production. N. Z. Vet. J. 2004, 52, 175–179. [Google Scholar] [CrossRef]
- Lacasta, D.; Ferrer, L.; Ramos, J.; González, J.; De las Heras, M. Influence of climatic factors on the development of pneumonia in lambs. Small Rumin. Res. 2008, 80, 28–32. [Google Scholar] [CrossRef]
- Scott, P.R. Treatment and control of respiratory disease in sheep. Vet. Clin. N. Am. Small Anim. Pract. 2011, 27, 175–186. [Google Scholar] [CrossRef]
- Odugbo, M.; Odama, L.; Umoh, J.; Lamorde, A. Pasteurella multocida pneumonic infection in sheep: Prevalence, clinical and pathological studies. Small Rumin. Res. 2006, 66, 273–277. [Google Scholar] [CrossRef]
- Rawat, N.; Gilhare, V.R.; Kushwaha, K.K.; Hattimare, D.D.; Khan, F.F.; Shende, R.K.; Jolhe, D.K. Isolation and molecular characterization of Mannheimia haemolytica and Pasteurella multocida associated with pneumonia of goats in Chhattisgarh. Vet. World 2019, 12, 331. [Google Scholar] [CrossRef]
- Goodwin-Ray, K.A. Pneumonia and Pleurisy in Sheep: Studies of Prevalence, Risk Factors, Vaccine Efficacy and Economic Impact. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2006. [Google Scholar]
- Estes, L. Review of pharmacokinetics and pharmacodynamics of antimicrobial agents. Mayo Clin. Proc. 1998, 73, 1114–1122. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Lipman, J. Optimizing Antibiotic Use in the Intensive Care Unit. Clin. Pulm. Med. 2010, 17, 162–169. [Google Scholar] [CrossRef]
- EMA. Committee for Veterinary Medicinal Products. Danofloxacin Summary Report (2). 1997. Available online: https://www.ema.europa.eu/en/documents/mrl-report/danofloxacin-summary-report-2-committee-veterinary-medicinal-products_en.pdf (accessed on 27 April 2021).
- EMA. Committee for Veterinary Medicinal Products. Danofloxacin (Extension to All Food Producing Species) Summary Report (6). 2002. Available online: https://www.ema.europa.eu/en/documents/mrl-report/danofloxacin-extension-all-food-producing-species-summary-report-6-committee-veterinary-medicinal_en.pdf (accessed on 27 April 2021).
- Friis, C. Penetration of danofloxacin into the respiratory tract tissues and secretions in calves. Am. J. Vet. Res. 1993, 54, 1122–1127. [Google Scholar]
- Giles, C.J.; Magonigle, R.A.; Grimshaw, W.T.; Tanner, A.C.; Risk, J.E.; Lynch, M.J.; Rice, J.R. Clinical pharmacokinetics of parenterally administered danofloxacin in cattle. J. Vet. Pharmacol. Ther. 1991, 14, 400–410. [Google Scholar] [CrossRef]
- Riviere, J.E.; Papich, M.G. Fluoroquinolone Antimicrobial Drugs. In Veterinary Pharmacology and Therapeutics, 10th ed.; Papich, M.G., Ed.; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 953–987. [Google Scholar]
- Stetsko, T.; Kocjumbas, I.; Muzyka, V.; Lisova, N.; Pyatnichko, O.; Ostrovska, L. Antimicrobial activity of danofloxacin, effectiveness and safeness of a new antimicrobial preparation based on its basis for treatment of calves with respiratory bacterial infections. Sci. Tech. Bull. State Sci. Res. Control Inst. Vet. Med. Prod. Fodd. Addit. Inst. Animal Biol. 2019, 20, 126–137. [Google Scholar] [CrossRef]
- Atef, M.; El-Gendi, A.Y.; Amer, M.M.; Abd El-Aty, A.M. Some pharmacokinetic data for danofloxacin in healthy goats. Vet. Res. Commun. 2001, 25, 367–377. [Google Scholar] [CrossRef]
- Schrickx, J.; Fink-Gremmels, J. Danofloxacin-mesylate is a substrate for ATP-dependent efflux transporters. Br. J. Pharmacol. 2007, 150, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Godinho, K.; Benchaoui, H.; Tilt, N.; Ramage, C.; Quirie, M.; Donachie, W.; De La Puente-Redondo, V.; Rowan, T. Efficacy of danofloxacin in the treatment of pneumonic pasteurellosis in specific pathogen-free lambs. Vet. Rec. 2007, 160, 770–771. [Google Scholar] [CrossRef]
- Rowan, T.; Sunderland, S.; Smith, D.; Sarasola, P.; Giles, C. Efficacy of danofloxacin in the treatment of respiratory disease in European cattle. Vet. Rec. 2004, 154, 585–589. [Google Scholar] [CrossRef]
- EMA. Committee for Veterinary Medicinal Products. Meloxicam Summary Report (1). 1997. Available online: https://www.ema.europa.eu/en/documents/mrl-report/meloxicam-summary-report-1-committee-veterinary-medicinal-products_en.pdf (accessed on 27 April 2021).
- Sim, T. Meloxicam Product Approved for Sheep in Australia after World-First Tri-Lateral Review. sheepcentral.com. Available online: https://www.sheepcentral.com/meloxicam-product-approved-for-sheep-in-australia-after-world-first-tri-lateral-review/ (accessed on 29 July 2020).
- Bednarek, D.; Zdzisinska, B.; Kondracki, M.; Kandefer-Szerszen, M. Effect of steroidal and non-steroidal anti-inflammatory drugs in combination with long-acting oxytetracycline on non-specific immunity of calves suffering from enzootic bronchopneumonia. Vet. Microbiol. 2003, 96, 53–67. [Google Scholar] [CrossRef]
- Curry, S.L.; Cogar, S.M.; Cook, J.L. Nonsteroidal antiinflammatory drugs: A review. J. Am. Anim. Hosp. Assoc. 2005, 41, 298–309. [Google Scholar] [CrossRef]
- Ramírez-Romero, R.; Brogden, K.A.; Gallup, J.M.; Sonea, I.M.; Ackermann, M.R. Mast cell density and substance P-like immunoreactivity during the initiation and progression of lung lesions in ovineMannheimia (Pasteurella) haemolytica pneumonia. Microb. Pathog. 2001, 30, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Lekeux, P. A therapeutic strategy for treatment of the bovine respiratory disease complex: The rationale for the combination of a nonsteroidal anti-inflammatory drug with an antibiotic. Cattle Pract. 2007, 15, 115–119. [Google Scholar]
- Lockwood, P.; Johnson, J.; Katz, T. Clinical efficacy of flunixin, carprofen and ketoprofen as adjuncts to the antibacterial treatment of bovine respiratory disease. Vet. Rec. 2003, 152, 392–394. [Google Scholar] [CrossRef]
- Bednarek, D.; Kondracki, M.; Friton, G.; Trela, T.; Niemczuk, K. Effect of steroidal and non-steroidal anti-inflammatory drugs on inflammatory markers in calves with experimentally-induced bronchopneumonia. Berl. Munch. Tierarztl. Wochenschr. 2005, 118, 305–308. [Google Scholar]
- Friton, G.; Cajal, C.; Ramirez-Romero, R. Long-term effects of meloxicam in the treatment of respiratory disease in fattening cattle. Vet. Rec. 2005, 156, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Jager, N.G.; van Hest, R.M.; Lipman, J.; Taccone, F.S.; Roberts, J.A. Therapeutic drug monitoring of anti-infective agents in critically ill patients. Expert Rev. Clin. Pharmacol. 2016, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Norris, R.; Paterson, D.L.; Martin, J.H. Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 2012, 73, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abo-EL-Sooud, K.; Al-Anati, L. Pharmacokinetic study of flunixin and its interaction with enrofloxacin after intramuscular administration in calves. Vet. World 2011, 4. [Google Scholar] [CrossRef]
- Altan, F.; Corum, O.; Yildiz, R.; Eser Faki, H.; Ider, M.; Ok, M.; Uney, K. Intravenous pharmacokinetics of moxifloxacin following simultaneous administration with flunixin meglumine or diclofenac in sheep. J. Vet. Pharmacol. Ther. 2020, 43, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Baroni, E.; Rubio, S.; Rodríguez, C.; De Lucas, J.; Fernández, H.; Andrés, M. Pharmacokinetic interactions of marbofloxacin with anti-inflammatory drugs in buffalo calves. Vet. Rec. 2011. [Google Scholar] [CrossRef]
- Durna Corum, D.; Corum, O.; Yildiz, R.; Eser Faki, H.; Ider, M.; Cetin, G.; Uney, K. Influences of tolfenamic acid and flunixin meglumine on the disposition kinetics of levofloxacin in sheep. Acta Vet. Hung. 2020, 68, 65–70. [Google Scholar] [CrossRef]
- Ogino, T.; Arai, T. Pharmacokinetic interactions of flunixin meglumine and enrofloxacin in ICR mice. Exp. Anim. 2007, 56, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Ogino, T.; Mizuno, Y.; Ogata, T.; Takahashi, Y. Pharmacokinetic interactions of flunixin meglumine and enrofloxacin in dogs. Am. J. Vet. Res. 2005, 66, 1209–1213. [Google Scholar] [CrossRef]
- Tekeli, I.O.; Turk, E.; Durna Corum, D.; Corum, O.; Kirgiz, F.C.; Sakin, F.; Uney, K. Effect of ketoprofen co-administration on pharmacokinetics of cefquinome following repeated administration in goats. J. Vet. Pharmacol. Ther. 2020, 43, 440–447. [Google Scholar] [CrossRef]
- Elmas, M.; Yazar, E.; Uney, K.; Er, A.; Traş, B. Pharmacokinetics of enrofloxacin and flunixin meglumine and interactions between both drugs after intravenous co-administration in healthy and endotoxaemic rabbits. Vet. J. 2008, 177, 418–424. [Google Scholar] [CrossRef]
- Koc, F.; Atila, A.; Karakuş, E.; Üney, K. Influences of flunixin and tenoxicam on the pharmacokinetics of florfenicol in lipopolysaccharide-induced endotoxemia. Turk. J. Vet. Anim. Sci. 2015, 39, 168–173. [Google Scholar] [CrossRef]
- Sadariya, K.; Patel, J.; Bhavsar, S.; Thaker, A. Effect of Febrile Condition and Ketoprofen Co-administration on Pharmacokinetics of Moxifloxacin Following Intravenous Administration in Sheep. Isr. J. Vet. Med. 2014, 69, 68–73. [Google Scholar]
- Christodoulopoulos, G.; Warnick, L.; Papaioannou, N.; Fthenakis, G. Tilmicosin administration to young lambs with respiratory infection: Safety and efficacy considerations. J. Vet. Pharmacol. Ther. 2002, 25, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Corum, O.; Altan, F.; Yildiz, R.; Ider, M.; Ok, M.; Uney, K. Pharmacokinetics of enrofloxacin and danofloxacin in premature calves. J. Vet. Pharmacol. Ther. 2019, 42, 624–631. [Google Scholar] [CrossRef]
- Corum, O.; Durna Corum, D.; Atik, O.; Eser Faki, H.; Altan, F.; Uney, K. Pharmacokinetics and bioavailability of danofloxacin in chukar partridge (Alectoris chukar) following intravenous, intramuscular, subcutaneous, and oral administrations. J. Vet. Pharmacol. Ther. 2019, 42, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Real, R.; Egido, E.; Perez, M.; Gonzalez-Lobato, L.; Barrera, B.; Prieto, J.; Alvarez, A.; Merino, G. Involvement of breast cancer resistance protein (BCRP/ABCG2) in the secretion of danofloxacin into milk: Interaction with ivermectin. J. Vet. Pharmacol. Ther. 2011, 34, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Ruennarong, N.; Wongpanit, K.; Sakulthaew, C.; Giorgi, M.; Kumagai, S.; Poapolathep, A.; Poapolathep, S. Dispositions of enrofloxacin and its major metabolite ciprofloxacin in Thai swamp buffaloes. J. Vet. Med. Sci. 2015, 15-0464. [Google Scholar] [CrossRef] [PubMed]
- Gibaldi, M.; Perrier, D. Noncompartmental analysis based on statistical moment theory. Pharmacokinetics 1982, 2, 409–417. [Google Scholar]
- Greko, C.; Finn, M.; Franklin, A.; Bengtsson, B. Pharmacokinetic/pharmacodynamic relationship of danofloxacin against Mannheimia haemolytica in a tissue-cage model in calves. J. Antimicrob. Chemother. 2003, 52, 253–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, P.J.; Markey, B.K.; Leonard, F.C.; Hartigan, P.; Fanning, S.; Fitzpatrick, E. Veterinary Microbiology and Microbial Disease; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 300–308. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wyne, PA, USA, 2012. [Google Scholar]
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Assessing the antimicrobial susceptibility of bacteria obtained from animals. J. Antimicrob. Chemother. 2010, 65, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, F.S.; Ali, B.H.; Landoni, M.; Lees, P. Pharmacokinetics and PK-PD modelling of danofloxacin in camel serum and tissue cage fluids. Vet. J. 2003, 165, 104–118. [Google Scholar] [CrossRef]
- Aliabadi, F.S.; Landoni, M.F.; Lees, P. Pharmacokinetics (PK), pharmacodynamics (PD), and PK-PD integration of danofloxacin in sheep biological fluids. Antimicrob. Agents Chemother. 2003, 47, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Aliabadi, F.S.; Lees, P. Pharmacokinetics and pharmacodynamics of danofloxacin in serum and tissue fluids of goats following intravenous and intramuscular administration. Am. J. Vet. Res. 2001, 62, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Haritova, A.M.; Rusenova, N.V.; Parvanov, P.R.; Lashev, L.D.; Fink-Gremmels, J. Pharmacokinetic-pharmacodynamic modelling of danofloxacin in turkeys. Vet. Res. Commun. 2006, 30, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Li, J.; Cheng, P.; Xiao, T.; Muhammad, I.; Yu, H.; Liu, R.; Zhang, X. Susceptibility breakpoint for Danofloxacin against swine Escherichia coli. BMC Vet. Res. 2019, 15, 51. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests for Bacteria Isolated from Animals: CLSI Supplement VET01S, 3rd ed.; Clinical and Laboratory Standards Institute: Wyne, PA, USA, 2015. [Google Scholar]
- Sweeney, M.T.; Papich, M.G.; Watts, J.L. New interpretive criteria for danofloxacin antibacterial susceptibility testing against Mannheimia haemolytica and Pasteurella multocida associated with bovine respiratory disease. J. Vet. Diagn. Investig. 2017, 29, 224–227. [Google Scholar] [CrossRef]
- Escudero, E.; Cárceles, C.; Fernandez-Varon, E.; Marin, P.; Benchaoui, H. Pharmacokinetics of danofloxacin 18% in lactating sheep and goats. J. Vet. Pharmacol. Ther. 2007, 30, 572–577. [Google Scholar] [CrossRef]
- Toutain, P.-L.; Bousquet-Mélou, A. Plasma clearance. J. Vet. Pharmacol. Ther. 2004, 27, 415–425. [Google Scholar] [CrossRef]
- Elmas, M.; Yazar, E.; Uney, K.; Er, A. Influence of Escherichia coli endotoxin-induced endotoxaemia on the pharmacokinetics of enrofloxacin after intravenous administration in rabbits. J. Vet. Med. A 2006, 53, 410–414. [Google Scholar] [CrossRef]
- EMA. Committee for Veterinary Medicinal Products. Danofloxacin (Extension to Pig) Summary Report (1). 1998. Available online: https://www.ema.europa.eu/en/documents/mrl-report/danofloxacin-extension-pigs-summary-report-1-committee-veterinary-medicinal-products_en.pdf (accessed on 27 April 2021).
- EMA. Committee for Veterinary Medicinal Products. Meloxicam (Extrapolation to Rabbits and Goats) Summary Report (7). 2006. Available online: https://www.ema.europa.eu/en/documents/mrl-report/meloxicam-extrapolation-rabbits-goats-summary-report-7-committee-veterinary-medicinal-products_en.pdf (accessed on 27 April 2021).
- Türck, D.; Roth, W.; Busch, U. A review of the clinical pharmacokinetics of meloxicam. Rheumatology 1996, 35, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Ejaz, P.; Bhojani, K.; Joshi, V. NSAIDs and kidney. J. Assoc. Physicians India 2004, 52, 371. [Google Scholar]
- Kim, G.-H. Renal effects of prostaglandins and cyclooxygenase-2 inhibitors. Electrolyte Blood Press. 2008, 6, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambaro, G.; Perazella, M. Adverse renal effects of anti-inflammatory agents: Evaluation of selective and nonselective cyclooxygenase inhibitors. J. Intern. Med. 2003, 253, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Pehlivan, B.; Cuvaş, Ö.; Başar, H.; Bakir, F.; Üstün, H.; Dikmen, B. Comparison of the effects of repeated dose treatments of lornoxicam and meloxicam on renal functions in rats. Turk. J. Med. Sci. 2010, 40, 371–376. [Google Scholar] [CrossRef]
- Garcia-Lino, A.M.; Blanco-Paniagua, E.; Astorga-Simon, E.N.; Alvarez-Fernandez, L.; Garcia-Mateos, D.; Alvarez-Fernandez, I.; Alvarez, A.I.; Merino, G. Abcg2 transporter affects plasma, milk and tissue levels of meloxicam. Biochem. Pharmacol. 2020, 175, 113924. [Google Scholar] [CrossRef] [PubMed]
- Gates, B.J.; Nguyen, T.T.; Setter, S.M.; Davies, N.M. Meloxicam: A reappraisal of pharmacokinetics, efficacy and safety. Expert Opin. Pharmacother. 2005, 6, 2117–2140. [Google Scholar] [CrossRef]
- Ismail, M.; El-Kattan, Y. Comparative pharmacokinetics of marbofloxacin in healthy and Mannheimia haemolytica infected calves. Res. Vet. Sci. 2007, 82, 398–404. [Google Scholar] [CrossRef]
- Mzyk, D.A.; Bublitz, C.M.; Martinez, M.N.; Davis, J.L.; Baynes, R.E.; Smith, G.W. Impact of bovine respiratory disease on the pharmacokinetics of danofloxacin and tulathromycin in different ages of calves. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Soliman, A.M.; Ayad, A.R.A. Pharmacokinetics and efficacy of tilmicosin in the treatment of Pasteurella haemolytica bronchopneumonia in calves. Pharmacol. Pharm. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Lan, W.; Wang, Y.; Jiang, L.; Jiang, Y.; Wang, Z. Comparative pharmacokinetics of danofloxacin in healthy and Pasteurella multocida infected ducks. J. Vet. Pharmacol. Ther. 2018, 41, 912–918. [Google Scholar] [CrossRef]
- Altan, F.; Ipek, D.N.S.; Corum, O.; Alp, S.Y.; Ipek, P.; Uney, K. The effects of Mannheimia haemolytica and albendazole on marbofloxacin pharmacokinetics in lambs. Trop. Anim. Health Prod. 2019, 51, 2603–2610. [Google Scholar] [CrossRef]
- De Paepe, P.; Belpaire, F.M.; Buylaert, W.A. Pharmacokinetic and pharmacodynamic considerations when treating patients with sepsis and septic shock. Clin. Pharmacokinet. 2002, 41, 1135–1151. [Google Scholar] [CrossRef]
- Martinez, M.; Modric, S. Patient variation in veterinary medicine: Part I. Influence of altered physiological states. J. Vet. Pharmacol. Ther. 2010, 33, 213–226. [Google Scholar] [CrossRef]
- Schmith, V.D.; Foss, J.F. Effects of inflammation on pharmacokinetics/pharmacodynamics: Increasing recognition of its contribution to variability in response. Clin. Pharmacol. Ther. 2008, 83, 809–811. [Google Scholar] [CrossRef]
- Renton, K.W. Regulation of drug metabolism and disposition during inflammation and infection. Expert Opin. Drug Metab. Toxicol. 2005, 1, 629–640. [Google Scholar] [CrossRef]
- Aitken, A.E.; Richardson, T.A.; Morgan, E.T. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 123–149. [Google Scholar] [CrossRef]
- Petrovic, V.; Teng, S.; Piquette-Miller, M. Regulation of drug transporters during infection and inflammation. Mol. Interv. 2007, 7, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.T.; Goralski, K.B.; Piquette-Miller, M.; Renton, K.W.; Robertson, G.R.; Chaluvadi, M.R.; Charles, K.A.; Clarke, S.J.; Kacevska, M.; Liddle, C.; et al. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab. Dispos. 2008, 36, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redondo, E.; Gázquez, A.; García, A.; Vadillo, S.; Masot, A. Dominant expression of interleukin-8 vs interleukin-1 β and tumour necrosis factor alpha in lungs of lambs experimentally infected with Mannheimia haemolytica. N. Z. Vet. J. 2011, 59, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Buyse, M.; German-Fattal, M.; Gimenez, F. Influence of the pro-inflammatory cytokines on P-glycoprotein expression and functionality. J. Pharm. Pharm. Sci. 2004, 7, 359–371. [Google Scholar]
- Kalitsky-Szirtes, J.; Shayeganpour, A.; Brocks, D.; Piquette-Miller, M. Suppression of drug-metabolizing enzymes and efflux transporters in the intestine of endotoxin-treated rats. Drug Metab. Dispos. 2004, 32, 20–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimche, S.M.; Nyagode, B.A.; Merrell, M.D.; Lee, C.-M.; Prasanphanich, N.S.; Cummings, R.D.; Morgan, E.T. Hepatic cytochrome P450s, phase II enzymes and nuclear receptors are downregulated in a Th2 environment during Schistosoma mansoni infection. Drug Metab. Dispos. 2014, 42, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poller, B.; Drewe, J.; Krähenbühl, S.; Huwyler, J.; Gutmann, H. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood–brain barrier. Cell Mol. Neurobiol. 2010, 30, 63–70. [Google Scholar] [CrossRef]
- Lindecrona, R.; Friis, C.; Nielsen, J. Pharmacokinetics and penetration of danofloxacin into the gastrointestinal tract in healthy and in Salmonella typhimurium infected pigs. Res. Vet. Sci. 2000, 68, 211–216. [Google Scholar] [CrossRef]
- Marquez, B.; Van Bambeke, F. ABC multidrug transporters: Target for modulation of drug pharmacokinetics and drug-drug interactions. Curr. Drug Targets 2011, 12, 600–620. [Google Scholar] [CrossRef]
- Shem-Tov, M.; Ziv, G.; Glickman, A.; Saran, A. Pharmacokinetics and penetration of danofloxacin from the blood into the milk of ewes. Vet. Res. 1997, 28, 571–579. [Google Scholar]
- Charlton, M.; Thompson, J. Pharmacokinetics in sepsis. BJA Educ. 2018, 19, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Endrenyi, L.; Fritsch, S.; Yan, W. Cmax/AUC is a clearer measure than Cmax for absorption rates in investigations of bioequivalence. Int. J. Clin. Pharmacol. Ther. Toxicol. 1991, 29, 394–399. [Google Scholar] [PubMed]
- Duan, J.Z. Drug-drug interaction pattern recognition. Drugs R & D 2010, 10, 9–24. [Google Scholar] [CrossRef]
- Lea-Henry, T.N.; Carland, J.E.; Stocker, S.L.; Sevastos, J.; Roberts, D.M. Clinical pharmacokinetics in kidney disease: Fundamental principles. Clin. J. Am. Soc. Nephrol. 2018, 13, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- McKellar, Q.A.; Gibson, I.F.; McCormack, R.Z. Pharmacokinetics and tissue disposition of danofloxacin in sheep. Biopharm. Drug Dispos. 1998, 19, 123–129. [Google Scholar] [CrossRef]
- DailyMed, L. ADVOCIN-danofloxacin Mesylate Injection, Solution Zoetis Inc. Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=d22c22fc-d530-423c-8d78-ca95fe195ba1&type=display (accessed on 1 December 2020).
- Giguère, S.; Dowling, P.M. Fluoroquinolones. Antimicrob. Ther. Vet. Med. 2013, 295–314. [Google Scholar] [CrossRef]
- Lode, H.; Borner, K.; Koeppe, P. Pharmacodynamics of fluoroquinolones. Clin. Infect. Dis. 1998, 27, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidhu, P.; Landoni, M.; Aliabadi, F.; Lees, P. PK–PD integration and modeling of marbofloxacin in sheep. Res. Vet. Sci. 2010, 88, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.-L.; del Castillo, J.R.; Bousquet-Mélou, A. The pharmacokinetic–pharmacodynamic approach to a rational dosage regimen for antibiotics. Res. Vet. Sci. 2002, 73, 105–114. [Google Scholar] [CrossRef]
- Blaser, J.; Stone, B.B.; Groner, M.C.; Zinner, S.H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob. Agents Chemother. 1987, 31, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Development of In Vitro Susceptibility Testing Criteria and Quality Control Parameters for Veterinary Antimicrobial Agents; Approved Guideline, 3rd ed.; Clinical and Laboratory Standards Institute: Wyne, PA, USA, 2008. [Google Scholar]
- Jackson, J.; Davidson, J.; Ter Hune, T.; Magonigle, R. A dose response study of the fluoroquinolone, danofloxacin, against induced bovine pneumonic pasteurellosis. In Proceedings of the XVI World Buiatrics Congress, Salvador, Bahia, Brazil, 13–17 August 1990. [Google Scholar]
- TerHune, T.N.; Skogerboe, T.L.; Shostrom, V.K.; Weigel, D.J. Comparison of pharmacokinetics of danofloxacin and enrofloxacin in calves challenged with Mannheimia haemolytica. Am. J. Vet. Res. 2005, 66, 342–349. [Google Scholar] [CrossRef]
| Parameters | HD | HDM | ID | IDM |
|---|---|---|---|---|
| Age (month) | 3.20 ± 0.23 | 3.25 ± 0.36 | 3.50 ± 0.30 | 3.54 ± 0.51 |
| Body weight (kg) | 27.50 ± 3.03 | 28.17 ± 3.27 | 28.86 ± 3.08 | 27.86 ± 3.44 |
| Rectal temperature (°C) | 39.77 ± 0.15 | 39.93 ± 0.12 | 41.01 ± 0.22 | 41.04 ± 0.22 |
| Respiratory rate (breath/min) | 42.17 ± 3.63 | 42.83 ± 2.79 | 61.00 ± 3.27 | 62.43 ± 3.46 |
| Parameters | HD | HDM | ID | IDM |
|---|---|---|---|---|
| t1/2ʎz (h) | 6.98 (5.75–8.15) | 6.46 (4.82–8.24) | 6.07 (5.01–7.48) | 6.73 (5.14–8.12) |
| ClT/F (L/h·kg) | 0.45 (0.39–0.51) a | 0.32 (0.28–0.36) b,c | 0.38 (0.31–0.42) b | 0.28 (0.21–0.31) c |
| Vdarea/F (L/kg) | 4.55 (3.52–5.40) a | 3.02 (2.01–4.18) b | 3.35 (2.98–4.20) a,b | 2.73 (1.94–3.44) b |
| Vdss/F (L/kg) | 2.46 (1.94–3.23) a | 1.88 (1.39–2.24) b | 2.12 (1.61–2.51) a,b | 1.70 (1.23–2.21) b |
| Tmax (h) | 1.01 (0.75–2.00) | 1.25 (1.00–2.00) | 1.34 (1.00–2.00) | 1.23 (0.75–2.00) |
| Cmax (µg/mL) | 3.36 (2.46–4.46) b | 3.73 (2.89–4.30) a,b | 3.61 (2.97–4.46) a,b | 4.59 (3.29–6.44) a |
| AUC0–24 (h.µg/mL) | 12.68 (11.04–14.61) c | 17.69 (15.94–20.32) a,b | 14.99 (13.59–17.97) b,c | 20.23 (18.37–25.93) a |
| AUC0–∞ (h·µg/mL) | 13.26 (11.63–15.26) c | 18.52 (16.56–20.73) a,b | 15.65 (13.96–19.06) b,c | 21.30 (19.19–27.29) a |
| Cmax/AUC | 0.25 (0.20–30) | 0.20 (0.15–0.25) | 0.23 (0.17–0.29) | 0.22 (0.17–27) |
| MRT (h) | 5.43 (4.54–6.27) | 5.81 (4.79–6.95) | 5.54 (3.86–6.78) | 6.02 (4.72–7.51) |
| Danofloxacin Concentration (µg/mL) | PBHD | PBHDM | PBID | PBIDM |
|---|---|---|---|---|
| 0.05 | 36.3 ± 2.1 a | 24.3 ± 2.1 b | 36.0 ± 3.6 a | 22.0 ± 3.0 b |
| 0.5 | 32.0 ± 4.6 a | 21.0 ± 3.0 b | 33.0 ± 1.0 a | 24.3 ± 3.8 a,b |
| 5.0 | 27.7 ± 3.1 a,* | 22.6 ± 4.0 a,b | 29.3 ± 3.5 a | 18.3 ± 3.5 b |
| Mean ± SD | 32.0 ± 4.8 a | 22.7 ± 3.1 b | 32.8 ± 3.9 a | 21.6 ± 4.0 b |
| Animal No | ƒAUC0–24/MIC | ƒCmax/MIC | ||
|---|---|---|---|---|
| ID | IDM | ID | IDM | |
| Mannheimia haemolytica, MIC90 of 0.25 µg/mL | ||||
| 1 | 38.42 | 57.62 | 7.99 | 10.32 |
| 2 | 37.83 | 44.98 | 10.89 | 13.35 |
| 3 | 36.50 | 81.31 | 9.34 | 20.21 |
| 4 | 29.70 | 59.95 | 11.99 | 11.26 |
| 5 | 50.42 | 56.37 | 10.43 | 19.17 |
| 6 | 59.97 | 58.85 | 8.66 | 14.76 |
| 7 | 44.08 | 60.90 | 9.15 | 14.45 |
| GM (min–max) | 41.44 (29.70–59.97) | 59.21 (44.98–81.31) | 9.69 (7.99–11.99) | 14.40 (10.32–20.21) |
| Escherichia coli, MIC of 0.125 µg/mL | ||||
| 1 | 76.84 | 115.23 | 15.98 | 20.65 |
| 2 | 75.66 | 89.96 | 21.78 | 26.69 |
| 3 | 73.01 | 162.63 | 18.69 | 40.42 |
| 4 | 59.40 | 119.89 | 23.98 | 22.53 |
| 5 | 100.83 | 112.74 | 20.86 | 38.33 |
| 6 | 119.94 | 117.71 | 17.31 | 29.53 |
| 7 | 88.16 | 121.80 | 18.30 | 28.90 |
| GM (min–max) | 82.89 (59.40–119.94) | 118.43 (89.96–162.63) | 19.39 (15.98–23.98) | 28.80 (20.65–40.42) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ural, M.N.; Uney, K. Pharmacokinetic Behavior and Pharmacokinetic/Pharmacodynamic Integration of Danofloxacin Following Single or Co-Administration with Meloxicam in Healthy Lambs and Lambs with Respiratory Infections. Antibiotics 2021, 10, 1190. https://doi.org/10.3390/antibiotics10101190
Ural MN, Uney K. Pharmacokinetic Behavior and Pharmacokinetic/Pharmacodynamic Integration of Danofloxacin Following Single or Co-Administration with Meloxicam in Healthy Lambs and Lambs with Respiratory Infections. Antibiotics. 2021; 10(10):1190. https://doi.org/10.3390/antibiotics10101190
Chicago/Turabian StyleUral, Mehmet Nihat, and Kamil Uney. 2021. "Pharmacokinetic Behavior and Pharmacokinetic/Pharmacodynamic Integration of Danofloxacin Following Single or Co-Administration with Meloxicam in Healthy Lambs and Lambs with Respiratory Infections" Antibiotics 10, no. 10: 1190. https://doi.org/10.3390/antibiotics10101190
APA StyleUral, M. N., & Uney, K. (2021). Pharmacokinetic Behavior and Pharmacokinetic/Pharmacodynamic Integration of Danofloxacin Following Single or Co-Administration with Meloxicam in Healthy Lambs and Lambs with Respiratory Infections. Antibiotics, 10(10), 1190. https://doi.org/10.3390/antibiotics10101190