Alternative and Complementary Therapies against Foodborne Salmonella Infections
Abstract
:1. Introduction
2. Results
2.1. The Prevalence Rate of Salmonella
2.2. Initial Evaluation of Different Solvent Extracts
2.3. Antimicrobial Susceptibility
2.4. Characterization of Cinnamon Oil Active Principal Compounds by Using TLC
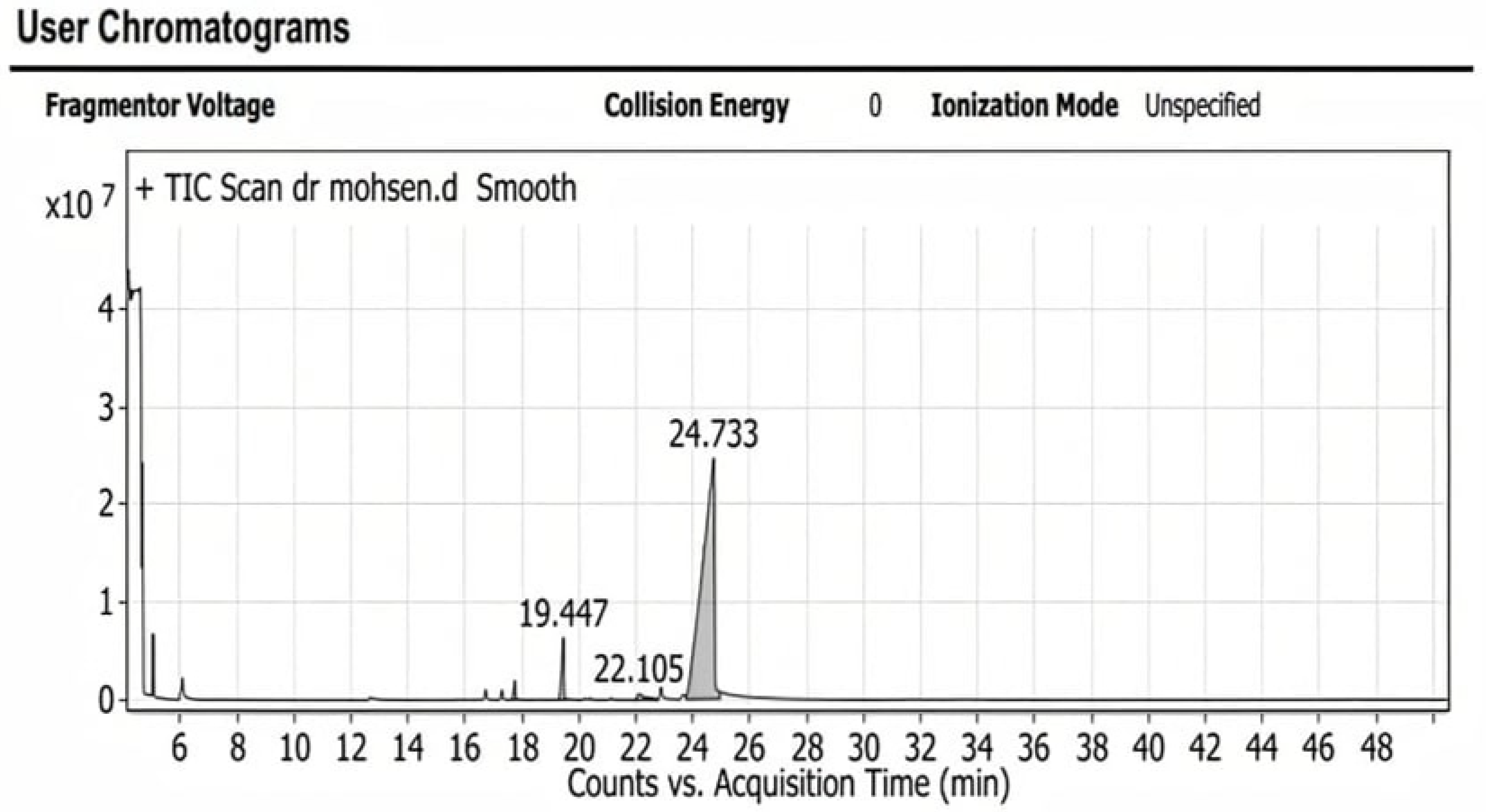
2.5. Characterization of Compounds Present in the Oily Extract (Cinnamon Oil) by Using GC-MS
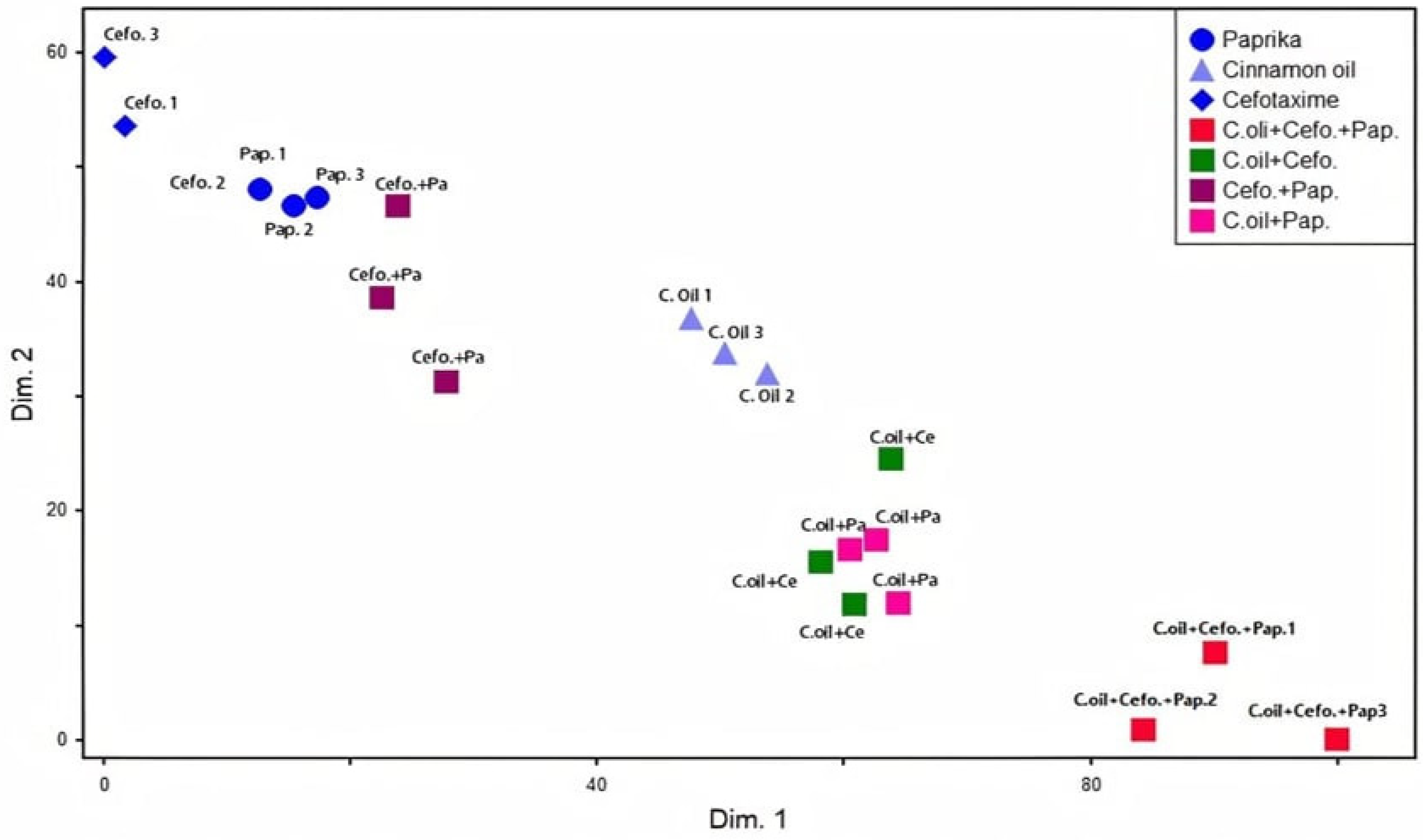
2.6. Anti-Virulence Activities of Cinnamon Oil
3. Discussion
4. Materials and Methods
4.1. Sampling
4.2. Isolation and Identification
4.3. Extraction of Herbals
4.3.1. Oily Extraction
4.3.2. Aqueous Extraction
4.3.3. Alcoholic Extraction
4.4. Agar Well Diffusion Assay for the evaluation of Antibacterial Activities
4.5. Antibiotic Sensitivity Testing
4.5.1. Disc Diffusion Method
4.5.2. Antibiotic Stock Solution Prepared for Effective Antibiotics
4.5.3. Determination of the Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (MBC)
4.6. Measuring the Synergetic Effect of a Herbal–Antibiotic Combination
4.7. Evaluation of Active Principle for Effective Extract
4.7.1. Thin Layer Chromatography and Bio-Autography
4.7.2. Characterization of the Isolated Compound Using GC-MS Analysis
4.8. Molecular Docking on Salmonella Virulence Factor
4.9. Bioinformatics and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tadesse, D.A.; Zhao, S.; Tong, E.; Ayers, S.; Singh, A.; Bartholomew, M.J.; McDermott, P.F. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950–2002. Emerg. Infect. Dis. 2012, 18, 741–749. [Google Scholar] [CrossRef]
- Sharma, S. Food preservatives and their harmful effects. Int. J. Sci. Res. Publ. 2015, 5, 1–2. [Google Scholar]
- Okeke, I.N.; Laxminarayan, R.; Bhutta, Z.A.; Duse, A.G.; Jenkins, P.; O’Brien, T.F.; Pablos-Mendez, A.; Klugman, K.P. Antimicrobial resistance in developing countries. Part I: Recent trends and current status. Lancet Infect. Dis. 2005, 5, 481–493. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshafie, H.S.; Viggiani, L.; Mostafa, M.S.; El-Hashash, M.A.; Camele, I.; Bufo, S.A. Biological Activity and Chemical Identification of Ornithine Lipid Produced by Burkholderia Gladioli Pv. Agaricicola ICMP 11096 Using LC-MS and NMR Analyses. J. Biol. Res. 2018, 90, 2. [Google Scholar] [CrossRef]
- Camele, I.; Elshafie, H.S.; Caputo, L.; Sakr, S.H.; De Feo, V. Bacillus Mojavensis: Biofilm Formation and Biochemical Investigation of Its Bioactive Metabolites. J. Biol. Res. 2019, 92, 1. [Google Scholar] [CrossRef]
- Camele, I.P.; Elshafie, H.S.; Caputo, L.; De-Feo, V. Anti-quorum Sensing and Antimicrobial Effect of Mediterranean Plant Essential Oils Against phytopathogenic bacteria. Front. Microbiol. 2019, 10, 2619. [Google Scholar] [CrossRef] [PubMed]
- Salamci, E.; Kordali, S.; Kotan, R.; Cakir, A.; Kaya, Y. Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum var. chiliophyllum. Biochem. Syst. Ecol. 2007, 35, 569–581. [Google Scholar] [CrossRef]
- Valero, M.; Frances, E. Synergistic bactericidal effect of carvacrol, cinnamaldehyde or thymol and refrigeration to inhibit Bacillus cereus in carrot broth. Food Microbiol. 2006, 23, 68–73. [Google Scholar] [CrossRef]
- Kalemba, D.K.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiol. 2001, 18, 463–470. [Google Scholar] [CrossRef]
- Walsh, S.E.; Maillard, J.Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Activity and mechanisms of action of selected biocidal agents on Gram-positive and-negative bacteria. J. Appl. Microbiol. 2003, 94, 240–247. [Google Scholar] [CrossRef]
- Cavalitto, C.J.; Bailey, J.H. The antibacterial principles of (Allium sativum L.): Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1994, 66, 195–200. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Street, D.; Delgado, S.I.; Klontz, K.C. Recalls of food and cosmetics due to microbial contamination reported to the U.S. Food and Drug Administration. J. Food Protect. 2000, 63, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Abdolpour, F.; Monsef-Esfahani, H.R.; Farsam, H. A TLC bioautographic assay for the detection of nitrofurantoin resistance reversal compound. J. Chromatogr. B 2007, 850, 528–530. [Google Scholar] [CrossRef]
- Bassolé, I.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomazelli, J.O.; Kuhn, F.; Padilha, P.M.; Vicente, L.M.; Costa, S.W.; Boligon, A.A.; Scapinellob, J.; Nesi, C.N.; Dal-Magrob, J.; Lamo-Castellví, S. Microencapsulation of essential thyme oil by spray drying and its antimicrobial evaluation against Vibrio alginolyticus and Vibrio parahaemolyticus. Braz. J. Biol. 2018, 78, 311–317. [Google Scholar] [CrossRef]
- Sojic, B.; Pavlic, B.; Ikonic, P.; Tomovic, V.; Ikonic, B.; Zekovic, Z.; Kocic-Tanackov, S.; Jokanovic, M.; Skaljac, S.; Ivic, M. Coriander essential oil as natural food additive improves quality and safety of cooked pork sausages with different nitrite levels. Meat Sci. 2019, 157, 107879. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Ghaith, D.M.; Zafer, M.M.; Said, H.M.; Elanwary, S.; Elsaban, S.; Al-Agamy, M.H.; Bohol, M.F.; Bendary, M.M.; Al-Qahtani, A.; Al-Ahdal, M.N. Genetic diversity of carbapenem-resistant Klebsiella pneumoniae causing neonatal sepsis in intensive care unit, Cairo, Egypt. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 39, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, N.K.; Abd El-Hamid, M.I.; Bendary, M.M.; El-Azazy, A.A.; Ammar, A.M. Existence of vancomycin resistance amongmethicillin resistant S. aureues recovered from animal and human sources in Egypt. Slov. Vet. Res. 2018, 55, 221–230. [Google Scholar]
- Abd El-Hamid, M.I.; Bendary, M.M.; Merwad, A.M.; Elsohaby, I.; Ghaith, D.M.; Alshareef, W. What is behind phylogenetic analysis of hospital, community and livestock associated methicillin-resistant Staphylococcus aureus? Transbound. Emerg. Dis. 2019, 66, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, M.; Shaheen, A.; Bouhy, A.; Bendary, M. Alternative therapy to manage otitis media caused by multidrug-resistant fungi. Arch. Microbiol. 2020, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Bendary, M.M.; Solyman, S.M.; Azab, M.M.; Mahmoud, N.F.; Hanora, A.M. Genetic diversity of multidrug resistant Staphylococcus aureus isolated from clinical and non clinical samples in Egypt. Cell. Mol. Biol. 2016, 62, 55. [Google Scholar] [PubMed]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Badawi, M.; Ismail, T.A.; Bendary, M.M.; Abdelaziz, A.M.; Mosbah, R.A.; Mohamed, D.I.; Arisha, A.H.; Abd El-Hamid, M.I. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella typhimurium. Sci. Rep. 2021, 11, 7742. [Google Scholar] [CrossRef]
- Bendary, M.M.; Solyman, S.M.; Azab, M.M.; Mahmoud, N.F.; Hanora, A.M. Characterization of Methicillin Resistant Staphylococcus aureus isolated from human and animal samples in Egypt. Cell. Mol. Biol. 2016, 62, 94–100. [Google Scholar]
- Abd El-Hamid, M.I.; Abd El-Aziz, N.K.; Samir, M.; El-Naenaeey, E.Y.; Abo Remela, E.M.; Mosbah, R.A.; Bendary, M.M. Genetic Diversity of Campylobacter jejuni Isolated from Avian and Human Sources in Egypt. Front. Microbiol. 2019, 10, 2353. [Google Scholar] [CrossRef]
- Kra´sniewska, K.; Kosakowska, O.; Pobiega, K.; Gniewosz, M. The Influence of Two-Component Mixtures from Spanish OriganumOil with Spanish Marjoram Oil or Coriander Oil on Antilisterial Activity and Sensory Quality of a Fresh Cut Vegetable Mixture. Foods 2020, 9, 1740. [Google Scholar] [CrossRef]
- Aranaz-Andrés, J.M.; Aibar-Remón, C.; Vitaller-Murillo, J.; Ruiz-López, P.; Limón-Ramírez, R.; Terol-García, E. Incidence of adverse events related to health care in Spain: Results of the Spanish National Study of Adverse Events. J. Epidemiol. Community Health 2008, 62, 1022–1029. [Google Scholar] [CrossRef]
- Mosallam, F.M.; Helmy, E.A.; Bendary, M.M.; El-Batal, I.A. Potency of a novel synthesized Ag- eugenol nanoemulsion for treating some bacterial and fungal pathogens. J. Mater. Res. 2021, 36, 1524–1537. [Google Scholar] [CrossRef]
- Bendary, M.M.; Ibrahim, D.; Mosbah, R.A.; Mosallam, F.; Hegazy, W.H.; Awad, N.S.; Alshareef, W.A.; Alomar, S.Y.; Zaitone, S.A.; Abd El-Hamid, M.I. Thymol Nanoemulsion: A New Therapeutic Option for Extensively Drug Resistant Foodborne Pathogens. Antibiotics 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.A.; Khayat, M.T.; Ibrahim, T.S.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Repurposing Anti-diabetic Drugs to Cripple Quorum Sensing in Pseudomonas aeruginosa. Microorganism 2020, 8, 1285. [Google Scholar] [CrossRef] [PubMed]
- Terentjeva, M.; Avsejenko, J.; Streikiša, M.; Utināne, A.; Kovaļenko, K.; Bērziņš, A. Prevalence and antimicrobial resistance of Salmonella in meat and meat products in Latvia. Ann. Agric. Environ. Med. 2017, 24, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Kramarenko, T.; Nurmoja, I.; Kärssin, A.; Meremäe, K.; Hörman, A.; Roasto, M. The prevalence and serovar diversity of Salmonella in various food products in Estonia. Food Control 2014, 42, 43–47. [Google Scholar] [CrossRef]
- Nazer, A.K.; Dadras, H.; Eskandari, S. Aerobic bacteria isolated from eggs and day-old chicks and their antibacterial resistance in Shiraz, Iran. Iran. J. Vet. Res. 2006, 7, 20–30. [Google Scholar]
- Karlowsky, J.A.; Jones, M.E.; Thornsberry, C.; Friedland, I.R.; Sahm, D.F. Trends in antimicrobial susceptibilities among Enterobacteriaceae isolated from hospitalized patients in the United States from 1998 to 2001. Antimicrob. Agents Chemother. 2003, 47, 1672–1680. [Google Scholar] [CrossRef] [Green Version]
- Miranda, C.; Silva, V.; Igrejas, G.; Poeta, P. Impact of European pet antibiotic use on enterococci and staphylococci antimicrobial resistance and human health. Future Microbiol. 2021, 16, 185–201. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Gruľová, D.; Zheljazkov, V.D.; De Feo, V.; Camele, I. Biological investigations of essential oils extracted from three Juniperus species and evaluation of their antimicrobial, antioxidant and cytotoxic activities. J. Appl. Microbiol. 2020, 129, 1261–1271. [Google Scholar] [CrossRef]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. Synergistic antibacterial, antifungal and antioxidant efficacy of cinnamon and clove essential oils in combination. Arch. Microbiol. 2020, 202, 1439–1448. [Google Scholar] [CrossRef]
- Dardona, Z.W.; Al-Hindi, A. Evaluating the effect of selected midicinal plant extracts and their synergistic effect with metronidazole against Entamoeba histolytica. Eur. J. Biomed. Pharm. Sci. 2014, 6, 5. [Google Scholar]
- Yap, P.X.; Krishnan, T.; Chan, K.G.; Lim, S.E. Antibacterial mode of action of Cinnamomum verum bark essential oil, alone and in combination with piperacillin, against a multi-drug-resistant Escherichia coli strain. J. Microbiol. Biotechnol. 2015, 25, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- TyaTyagi, A.K.; Malik, A. Morpho structural damage in food-spoiling bacteria due to the lemon grass oil and its vapour: SEM, TEM, and AFM investigations. Evid. Based Compl. Altern. Med. 2012, 2012, 692625. [Google Scholar]
- Bouhdid, S.; Abrini, J.; Zhiri, A.; Espuny, M.J.; Manresa, A. Investigation of functional and morphological changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Origanum compactum essential oil. J. Appl. Microbiol. 2009, 106, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.; Princy, V.; Christian, B.A.; SundaraBaalaji, N.; Syed, I.; Basheer, A. Investigations of binding mode insight in Salmonella typhi type-III secretion system tip protein (SipD): A molecular docking and MD simulation study. Inform. Med. Unlocked 2017, 9, 166–172. [Google Scholar] [CrossRef]
- Jakee, J.I.; Ata, N.S.; El-Moez, S.A.; Kandiel, M.M.; Radwan, N.M. Assessment of the prevalence of Salmonellae in food. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 30–42. Available online: https://www.researchgate.net/publication/270903903_Assessment_of_the_Prevalence_of_Salmonellae_in_Food (accessed on 29 October 2021).
- Christense, H.; Nordentoft, S.; Olsen, J.E. Phylogenetic relationships of Salmonella based on rRNA sequences. Int. J. Syst. Bacteriol. 1998, 48, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, J.H.; Turnidge, J.D. Susceptibility test methods: Dilution and disk diffusion methods. Man. Clin. Microbiol. 2015, 1253–1273. [Google Scholar]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied. Sci. 2020, 12, 1. [Google Scholar] [CrossRef]
- Betoni, J.C.; Mantovani, R.P.; Barbosa, L.N.; Di-Stasi, L.C.; Fernandes, J.A. Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Mem. Inst. Oswaldo Cruz 2006, 101, 387–390. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Ma, X.; Deng, L.; Zhao, X.; Wei, Y.; Gao, Z.; Jia, J.; Xu, J.; Sun, C. Fresh garlic extract enhances the antimicrobial activities of antibiotics on resistant strains in vitro. Jundishapur. J. Microbiol. 2015, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standard single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papich, M.G. Antimicrobials, susceptibility testing, and minimum inhibitory concentrations (MIC) in veterinary infection treatment. The Veterinary Clinics of North America. Small Anim. Pract. 2013, 43, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute M07. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed.; Approved Standard CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Moussaoui, F.; Alaoui, T. Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants. Asian Pac. J. Trop. Biomed. 2016, 6, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Saleem, R.; Meinwald, J. Synthesis of novel hypotensive aromatic thiocarbamate glycosides. J. Chem. Soc. Perkin. Trans. 2000, 1, 391–394. [Google Scholar] [CrossRef]
- Ogunlesi, M.; Okiei, W.; Osibote, E.A. Analysis of the essential oil from the leaves of Sesamum radiatum, a potential medication for male infertility factor, by gas chromatography-mass spectrometry. Afr. J. Biotechnol. 2010, 9, 1060–1067. [Google Scholar]
- Han, X.; Yang, K.; Gross, R.W. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass. Spectrom. Rev. 2012, 31, 134–178. [Google Scholar] [CrossRef] [Green Version]
- Mokhtarikhah, G.; Ebadi, M.T.; Ayyari, M. Qualitative changes of spearmint essential oil as affected by drying methods. Ind. Crops Prod. 2020, 153, 112492. [Google Scholar] [CrossRef]
- Abagyan, R.; Totrov, M.; Kuznetsov, D. ICM? A new method for protein modeling and design: Applications to docking and structure prediction from the distorted native conformation. J. Comput. Chem. 1994, 15, 488–506. [Google Scholar] [CrossRef]
- Neves, M.A.; Totrov, M.; Abagyan, R. Docking and scoring with ICM: The benchmarking results and strategies for improvement. J. Comput. Aided Mol. Des. 2012, 26, 675–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolde, R. Pheatmap: Pretty Heatmaps, R Package Version 1.0.12. 2019. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 29 October 2021).


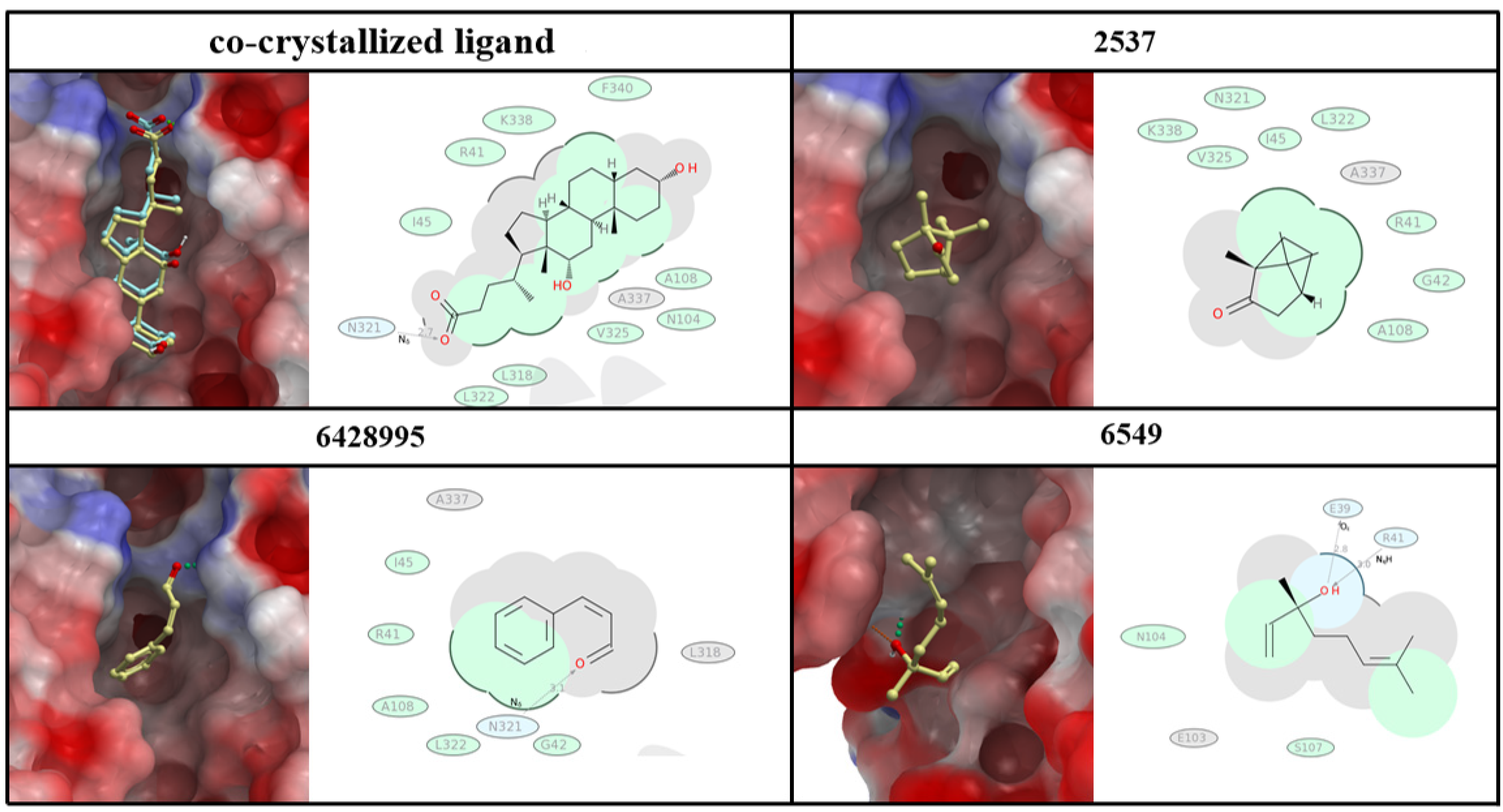
| PubChem CID | Score | Molecular Weight | logP | Heavy Atoms | HB Donor | HB Acceptor | Rotatable Bonds |
|---|---|---|---|---|---|---|---|
| Co-crystallized ligand (deoxycholate) | −17.02 | 392.571 | 4.51 | 28 | 3 | 4 | 4 |
| Z-3-Phenylacrylaldehyde and its stereoisomer, 2-Propenal,3-phenyl | −14.16 | 132.16 | 1.91 | 10 | 0 | 1 | 2 |
| Camphor | −11.15 | 152.23 | 2.4 | 11 | 0 | 1 | 0 |
| Linalool | −13.19 | 154.25 | 2.67 | 11 | 1 | 1 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaly, M.F.; Nasr, Z.M.; Abousaty, A.I.; Seadawy, H.G.; Shaheen, M.A.A.; Albogami, S.; Al-Sanea, M.M.; Bendary, M.M. Alternative and Complementary Therapies against Foodborne Salmonella Infections. Antibiotics 2021, 10, 1453. https://doi.org/10.3390/antibiotics10121453
Ghaly MF, Nasr ZM, Abousaty AI, Seadawy HG, Shaheen MAA, Albogami S, Al-Sanea MM, Bendary MM. Alternative and Complementary Therapies against Foodborne Salmonella Infections. Antibiotics. 2021; 10(12):1453. https://doi.org/10.3390/antibiotics10121453
Chicago/Turabian StyleGhaly, Mohamed F., Zahraa M. Nasr, Amira I. Abousaty, Hanan G. Seadawy, Mohamed A. A. Shaheen, Sarah Albogami, Mohammad M. Al-Sanea, and Mahmoud M. Bendary. 2021. "Alternative and Complementary Therapies against Foodborne Salmonella Infections" Antibiotics 10, no. 12: 1453. https://doi.org/10.3390/antibiotics10121453
APA StyleGhaly, M. F., Nasr, Z. M., Abousaty, A. I., Seadawy, H. G., Shaheen, M. A. A., Albogami, S., Al-Sanea, M. M., & Bendary, M. M. (2021). Alternative and Complementary Therapies against Foodborne Salmonella Infections. Antibiotics, 10(12), 1453. https://doi.org/10.3390/antibiotics10121453






