Modeling the Structure of Crystalline Alamethicin and Its NMR Chemical Shift Tensors
Abstract
:1. Introduction
2. Results and Discussion
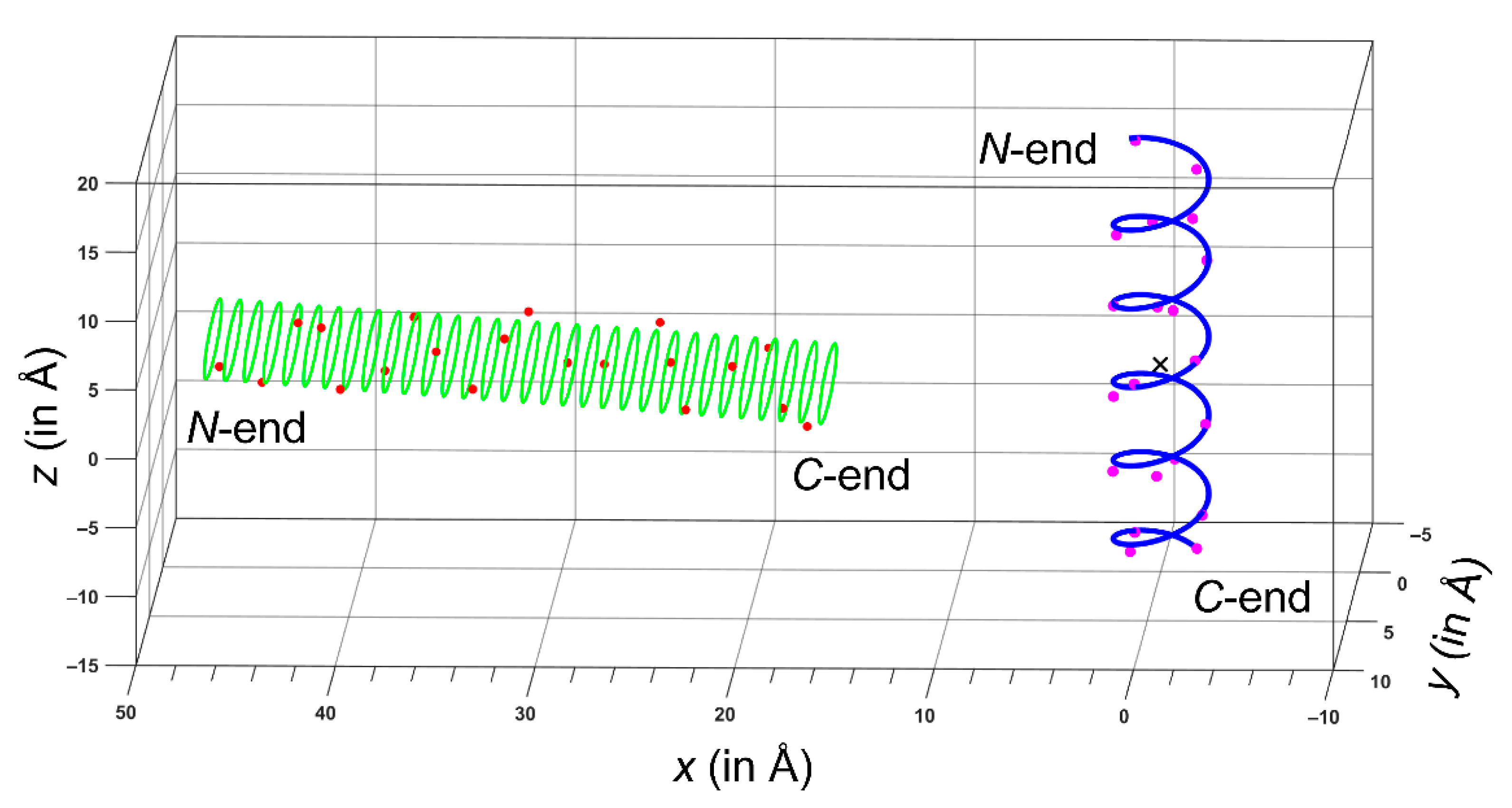
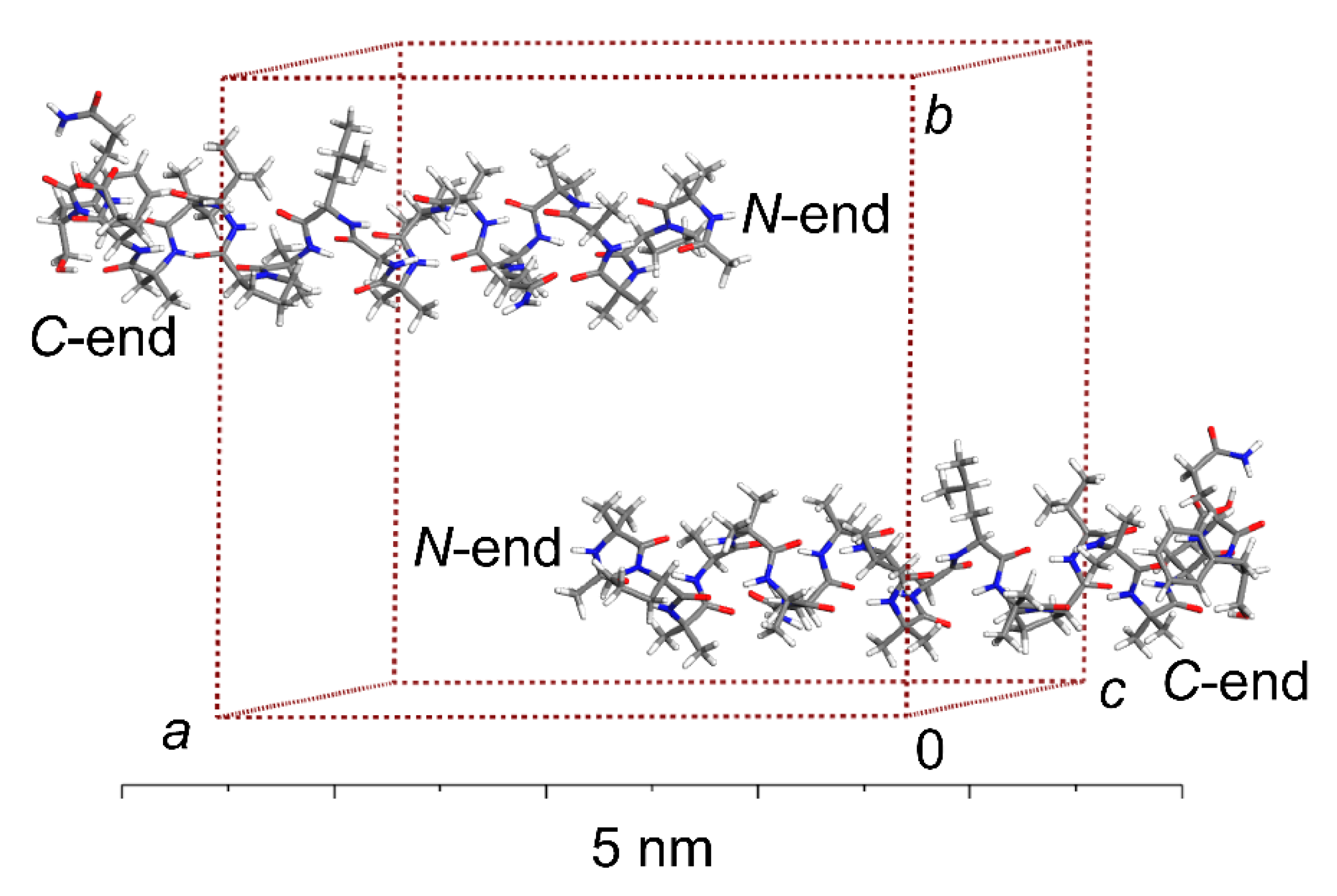
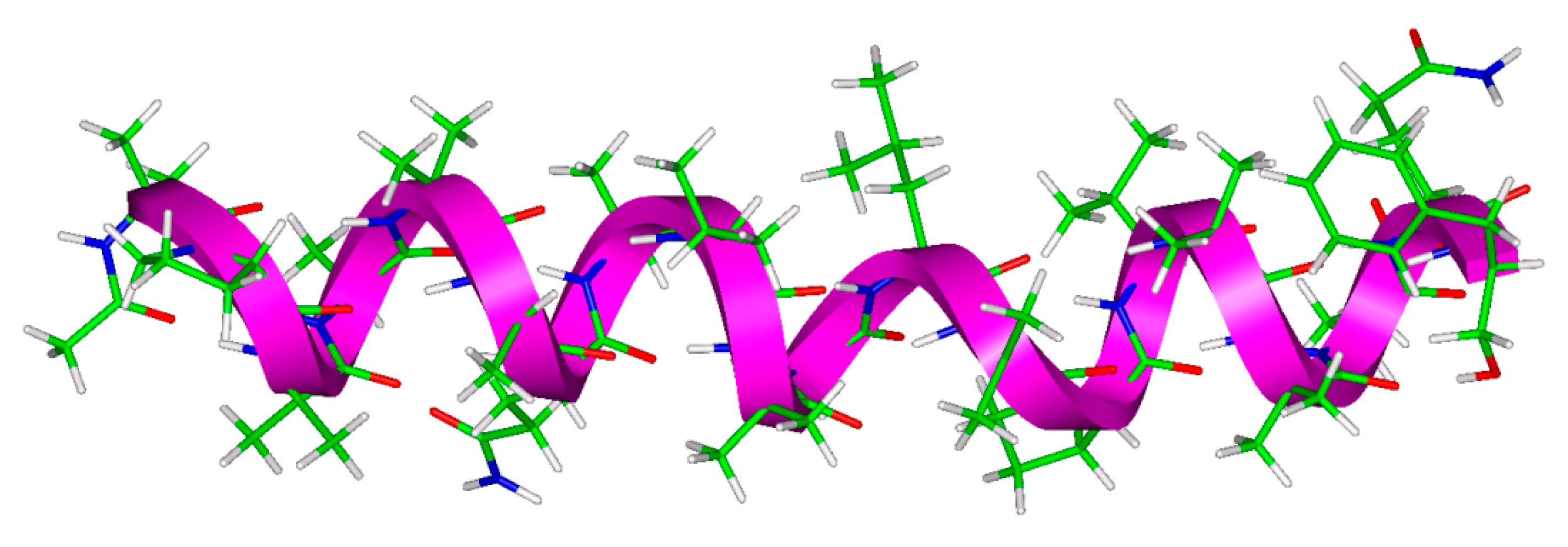
2.1. The ALM Structure
2.2. The 15N SSNMR Parameters of Amidic Nitrogens
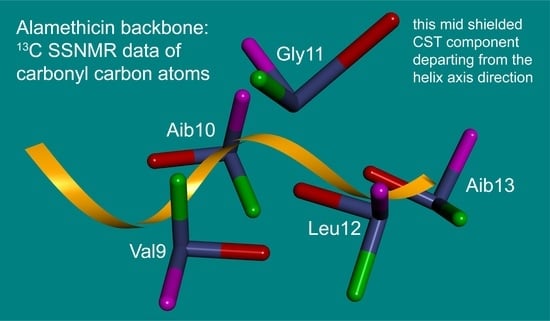
2.3. The 13C SSNMR Parameters and the Chemical Shift Oscillations of Carbonyl Carbons
3. Materials and Methods
3.1. The Periodic DFT Calculations
3.2. Simulations of the SSNMR Spectral Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hwon, J.H.; Powderly, W.G. The post anti-biotic era is here. Nature 2021, 373, 471. [Google Scholar] [CrossRef]
- Antimicrobial Resistance. Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 13 September 2021).
- Hanna, C.C.; Hermant, Y.O.; Harris, P.W.R.; Brimble, M.A. Discovery, Synthesis, and Optimization of Peptide-Based Antibiotics. Acc. Chem. Res. 2021, 54, 1878–1890. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Z.; Wang, X.; Niu, Y.; Zhang, S.; Xu, W.; Ren, C. Advances of peptides for antibacterial applications. Colloids Surf. B 2021, 202, 11682. [Google Scholar] [CrossRef]
- Kabelka, I.; Vácha, R. Advances in Molecular Understanding of α-Helical Membrane-Active Peptides. Acc. Chem. Res. 2021, 54, 2196–2204. [Google Scholar] [CrossRef]
- Marquette, A.; Bechinger, B. Biophysical Investigations Elucidating the Mechanisms of Action of Antimicrobial Peptides and Their Synergism. Biomolecules 2018, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Malanovic, N.; Marx, L.; Blondelle, S.E.; Pabst, G.; Semeraro, E.F. Experimental concepts for linking the biological activities of antimicrobial peptides to their molecular modes of action. BBA Biomembr. 2020, 1862, 183275. [Google Scholar] [CrossRef]
- Bechinger, B. The SMART model: Soft Membranes Adapt and Respond, also Transiently, in the presence of antimicrobial peptides. J. Pept. Sci. 2015, 21, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Simcock, P.W.; Bublitz, M.; Cipcigan, F.; Ryadnov, M.G.; Crain, J.; Stansfeld, P.J.; Sansom, M.S.P. Membrane Binding of Antimicrobial Peptides Is Modulated by Lipid Charge Modification. J. Chem. Theory Comput. 2021, 17, 1218–1228. [Google Scholar] [CrossRef]
- Aronica, P.G.A.; Reid, L.M.; Desai, N.; Li, J.; Fox, S.J.; Yadahalli, S.; Essex, J.W.; Verma, C.S. Computational Methods and Tools in Antimicrobial Peptide Research. J. Chem. Inf. Model. 2021, 61, 3172–3196. [Google Scholar] [CrossRef]
- Kirschbaum, J.; Krause, C.; Winzheimer, R.K.; Brückner, H. Sequences of alamethicins F30 and F50 reconsidered and reconciled. J. Pept. Sci. 2003, 9, 799–809. [Google Scholar] [CrossRef]
- Pieta, P.; Mirza, J.; Lipkowski, J. Direct visualization of the alamethicin pore formed in a planar phospholipid matrix. Proc. Natl. Acad. Sci. USA 2012, 109, 21223–21227. [Google Scholar] [CrossRef] [Green Version]
- McClintic, W.T.; Taylor, G.J.; Simpson, M.L.; Collier, C.P. Macromolecular Crowding Affects Voltage-Dependent Alamethicin Pore Formation in Lipid Bilayer Membranes. J. Phys. Chem. B 2020, 124, 5095–5102. [Google Scholar] [CrossRef]
- Molugu, T.R.; Lee, S.; Brown, M.F. Concepts and Methods of Solid-State NMR Spectroscopy Applied to Biomembranes. Chem. Rev. 2017, 117, 12087–12132. [Google Scholar] [CrossRef]
- Yeh, V.; Bonev, B.B. Solid state NMR of membrane proteins: Methods and applications. Biochem. Soc. Trans. 2021, 49, BST20200070. [Google Scholar] [CrossRef]
- Salnikov, E.S.; Friedrich, H.; Li, X.; Bertani, P.; Reissmann, S.; Hertweck, C.; O’Neil, J.D.J.; Raap, J.; Bechinger, B. Structure and Alignment of the Membrane-Associated Peptaibols Ampullosporin A and Alamethicin by Oriented 15N and 31P Solid-State NMR Spectroscopy. Biophys. J. 2009, 96, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Bertelsen, K.; Paaske, B.; Thøgersen, L.; Tajkhorshid, E.; Schiøtt, B.; Skrydstrup, T.; Nielsen, N.C.; Vosegaard, T. Residue-Specific Information about the Dynamics of Antimicrobial Peptides from 1H–15N Solid-State NMR Spectroscopy. J. Am. Chem. Soc. 2009, 131, 18335–18342. [Google Scholar] [CrossRef]
- Toraya, S.; Nishimura, K.; Naito, A. Dynamic Structure of Vesicle-Bound Melittin in a Variety of Lipid Chain Lenghts by Solid-State NMR. Biophys. J. 2004, 87, 3323–3335. [Google Scholar] [CrossRef] [Green Version]
- Nagao, T.; Mishima, D.; Jakhlantugs, N.; Wang, J.; Ishioka, D.; Yokota, K.; Norisada, K.; Kawamura, I.; Ueda, K.; Naito, A. Structure and orientation of antibiotic peptide alamethicin in phospholipid bilayers as revealed by chemical shift oscillation analysis of solid state nuclear magnetic resonance and molecular dynamics simulation. BBA Biomembr. 2015, 1848, 2789–2798. [Google Scholar] [CrossRef] [Green Version]
- Salnikov, E.S.; Aisebrey, C.; Raya, J.; Bechinger, B. Investigations of the Structure, Topology and Dynamics of Membrane-Associated Polypeptides by Solid-State NMR Spectroscopy. In Advances in Biological Solid-State NMR: Proteins and Membrane-Active Peptides, 1st ed.; Separovic, F., Naito, A., Eds.; Royal Society of Chemistry: London, UK, 2014; pp. 214–234. [Google Scholar] [CrossRef]
- Hansen, S.K.; Bertelsen, K.; Paaske, B.; Nielsen, N.C.; Vosegaard, T. Solid-state NMR methods for oriented membrane proteins. Prog. Nucl. Mag. Res. Sp. 2015, 88, 48–85. [Google Scholar] [CrossRef]
- Naito, A.; Matsumori, N.; Ramamoorthy, A. Dynamic membrane interactions of antibacterial and antifungal biomolecules, and amyloid peptides, revealed by solid-state NMR spectroscopy. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 307–323. [Google Scholar] [CrossRef]
- Hodgkinson, P. NMR Crystallography of Molecular Organics. Prog. Nucl. Mag. Res. Sp. 2020, 118, 10–53. [Google Scholar] [CrossRef]
- Czernek, J.; Brus, J. Monitoring the Site-Specific Solid-State NMR Data in Oligopeptides. Int. J. Mol. Sci. 2020, 21, 2700. [Google Scholar] [CrossRef]
- Czernek, J.; Brus, J. Polymorphic Forms of Valinomycin Investigated by NMR Crystallography. Int. J. Mol. Sci. 2020, 21, 4907. [Google Scholar] [CrossRef]
- Fox, R.O.; Richard, F.M. A voltage-gated ion-channel model inferred from the crystal structure of alamethicin at 1.5-Å resolution. Nature 1982, 300, 325–330. [Google Scholar] [CrossRef]
- Chugh, J.K.; Wallace, B.A. Peptaibols: Models for ion channels. Biochem. Soc. Trans. 2001, 29, 565–570. [Google Scholar] [CrossRef]
- Miura, Y. NMR studies of the conformation, stability, and dynamics of alamethicin in methanol. Eur. Biophys. J. 2020, 49, 113–124. [Google Scholar] [CrossRef]
- Lee, T.-H.; Hall, K.N.; Aguilar, M.-I. Antimicrobial Peptide Structure and Mechanism of Action: A Focus on the Role of Membrane Structure. Curr. Top. Med. Chem. 2016, 16, 25–39. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action, and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Birdsall, E.R.; Petti, M.K.; Saraswat, V.; Ostrander, J.S.; Arnold, M.S.; Zanni, M.T. Structure Changes of a Membrane Polypeptide under an Applied Voltage Observed with Surface-Enhanced 2D IR Spectroscopy. J. Phys. Chem. Lett. 2021, 12, 1786–1792. [Google Scholar] [CrossRef]
- Esteban-Martín, S.; Strandberg, E.; Fuertes, G.; Ulrich, A.S.; Salgado, J. Influence of Whole-Body Dynamics on 15N PISEMA NMR Spectra of Membrane Proteins: A Theoretical Analysis. Biophys. J. 2009, 96, 3233–3241. [Google Scholar] [CrossRef] [Green Version]
- Salnikov, E.; Bertani, P.; Raap, J.; Bechinger, B. Analysis of the amide 15N chemical shift tensor of the Ca tetrasubstituted constituent of membrane-active peptaibols, the a-aminoisobutyric acid residue, compared to those of di- and tri-substituted proteinogenic amino acid residues. J. Biomol. NMR 2009, 45, 373–387. [Google Scholar] [CrossRef]
- Czernek, J.; Brus, J. Theoretical predictions of the two-dimensional solid-state NMR spectra: A case study of the 13C—1H correlations in metergoline. Chem. Phys. Lett. 2013, 586, 56–60. [Google Scholar] [CrossRef]
- Czernek, J.; Brus, J. The covariance of the differences between experimental and theoretical chemical shifts as an aid for assigning two-dimensional heteronuclear correlation solid-state NMR spectra. Chem. Phys. Lett. 2014, 608, 334–339. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; De Menezes, S.M.C.; Granger, P.; Hoffman, R.E.; Zilm, K.W. Further conventions for NMR shielding and chemical shifts (IUPAC Recommendations 2008). Pure Appl. Chem. 2008, 82, 59–84. [Google Scholar] [CrossRef]
- Czernek, J.; Brus, J. Theoretical Investigations into the Variability of the N-15 Solid-State NMR Parameters Within an Antimicrobial Peptide Ampullosporin A. Phys. Res. 2018, 67, S349–S356. [Google Scholar] [CrossRef]
- Quine, J.R.; Achuthan, S.; Asbury, T.; Bertram, R.; Chapman, M.S.; Hu, J.; Cross, T.A. Intensity and mosaic spread analysis from PISEMA tensors in solid-state NMR. J. Magn. Reson. 2006, 179, 190–198. [Google Scholar] [CrossRef]
- Opella, S.J. Structure Determination of Membrane Proteins in Their Native Phospholipid Bilayer Environment by Rotationally Aligned Solid-State NMR Spectroscopy. Acc. Chem. Res. 2013, 49, 2145–2153. [Google Scholar] [CrossRef] [Green Version]
- Takeda, N.; Kuroki, S.; Kurosu, H.; Ando, S. 13C-NMR Chemical Shift Tensor and Hydrogen-Bonded Structure of Glycine-Containing Peptides in a Single Crystal. Biopolymers 1999, 50, 61–69. [Google Scholar] [CrossRef]
- Saito, H.; Ando, I.; Ramamoorthy, A. Chemical shift tensor—The heart of NMR: Insights into biological aspects of proteins. Prog. Nucl. Mag. Res. Sp. 2010, 57, 181–228. [Google Scholar] [CrossRef] [Green Version]
- Asakawa, N.; Kuroki, S.; Kurosu, H.; Ando, I.; Shoji, A.; Ozaki, T. Hydrogen-bonding effect on 13C NMR chemical shifts of L-alanine residue carbonyl carbons of peptides in the solid state. J. Am. Chem. Soc. 1992, 114, 3261–3265. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First principles simulation: Ideas, illustrations, and the CASTEP code. J. Phys. Condens. Matter. 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- BIOVIA Materials Studio. Dassault Systèmes; Vélizy-Villacoublay: Paris, France; Available online: https://www.3ds.com/products-services/biovia/products/molecular-modeling-simulation/biovia-materials-studio/ (accessed on 13 September 2021).
- Biswas, A.B.; Hughes, E.W.; Sharma, B.D.; Wilson, J.N. The crystal structure of α-glycylglycine. Acta Cryst. B 1968, 24, 40–50. [Google Scholar] [CrossRef]
- Rao, S.N.; Parthasarathy, R. Structure and conformational aspects of the nitrates of amino acids and peptides. I. Crystal structure of glycylglycine nitrate. Acta Cryst. B 1973, 29, 2379–2388. [Google Scholar] [CrossRef]
- Koetzle, T.F.; Hamilton, W.C. Precision neutron diffraction structure determination of protein and nucleic acid components. II. The crystal and molecular structure of the dipeptide glycylglycine monohydrochloride monohydrate. Acta Cryst. B 1972, 28, 2083–2090. [Google Scholar] [CrossRef]
- Gao, S.-P.; Pickard, C.J.; Perlov, A.; Milman, V. Core-Level Spectroscopy Calculation and the Plane Wave Pseudopotential Method. J. Phys. Condens. Matter. 2009, 21, 104203. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef] [Green Version]
- Pickard, C.J.; Mauri, F. All-electron magnetic response with pseudopotentials: NMR chemical shifts. Phys. Rev. B 2001, 63, 245101. [Google Scholar] [CrossRef] [Green Version]
- Yates, J.R.; Pickard, C.J.; Mauri, F. Calculations of NMR chemical shifts for extended systems using ultrasoft pseudopotentials. Phys. Rev. B 2007, 76, 024401. [Google Scholar] [CrossRef]
- Czernek, J. On the solid-state NMR spectra of naproxen. Chem. Phys. Lett. 2015, 619, 230–235. [Google Scholar] [CrossRef]
- Bechinger, B.; Sizun, C. Alignment and Structural Analysis of Membrane Polypeptides by 15N and 31P Solid-State NMR Spectroscopy. Concepts Magn. Reson. 2003, 18A, 130–145. [Google Scholar] [CrossRef]
- Paulino, J.; Yi, M.; Hung, I.; Gan, Z.; Wang, X.L.; Chekmenev, E.Y.; Zhou, H.X.; Cross, T.A. Functional stability of water wire–carbonyl interactions in an ion channel. Proc. Natl. Acad. Sci. USA 2020, 117, 11908–11915. [Google Scholar] [CrossRef]
- Hung, I.; Gan, Z.; Wu, G. Two- and Three-Dimensional 13C–17O Heteronuclear Correlation NMR Spectroscopy for Studying Organic and Biological Solid. J. Phys. Chem. Lett. 2021, 12, 8897–8902. [Google Scholar] [CrossRef]
- Hauser, K.; He, Y.; Garcia-Diaz, M.; Simmerling, C.; Coutsias, E. Characterization of Biomolecular Helices and Their Complementarity Using Geometric Analysis. J. Chem. Inf. Model 2017, 57, 864–874. [Google Scholar] [CrossRef] [Green Version]
- Czernek, J.; Brus, J. On the predictions of the 11B solid state NMR parameters. Chem. Phys. Lett. 2016, 655, 66–70. [Google Scholar] [CrossRef]
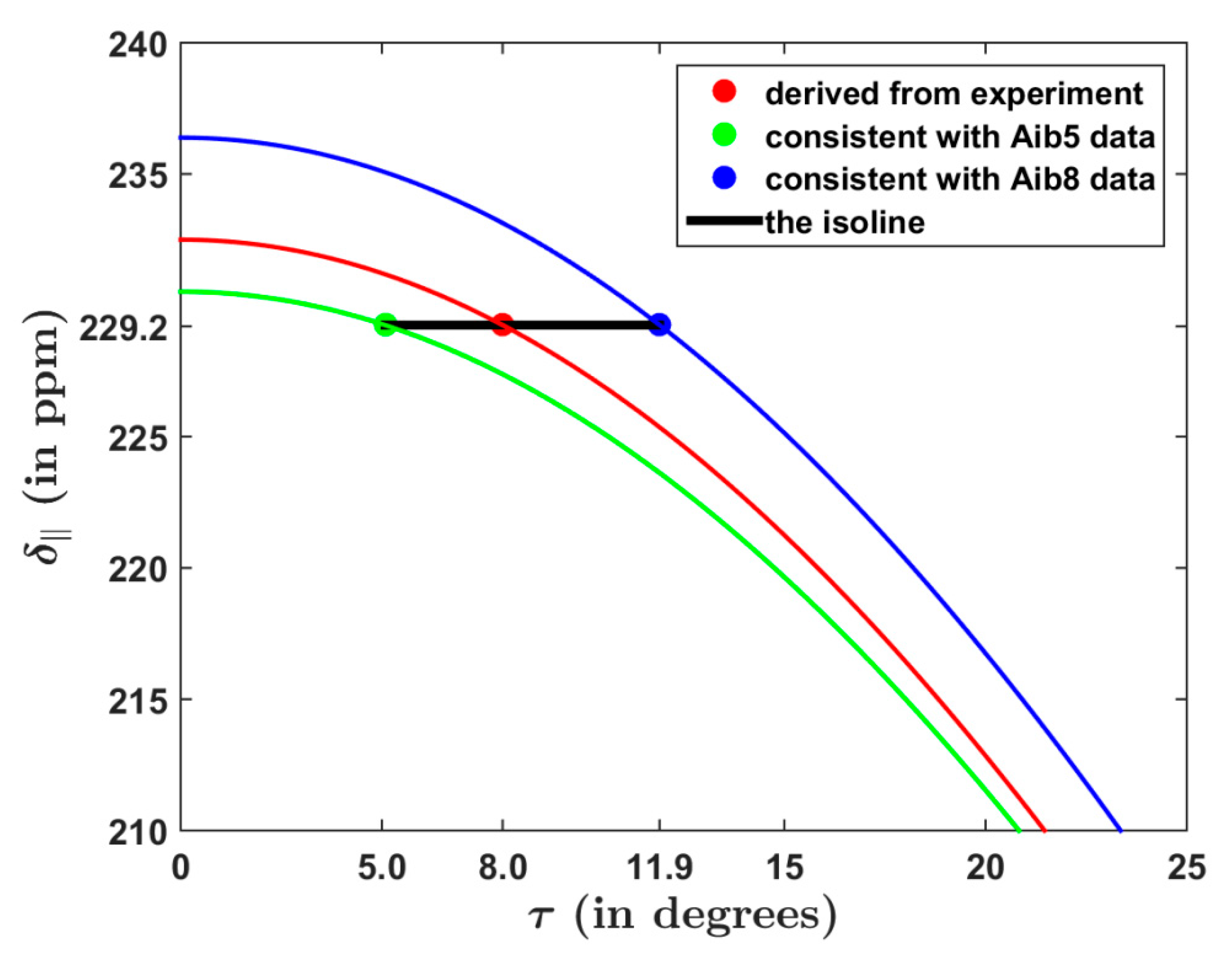
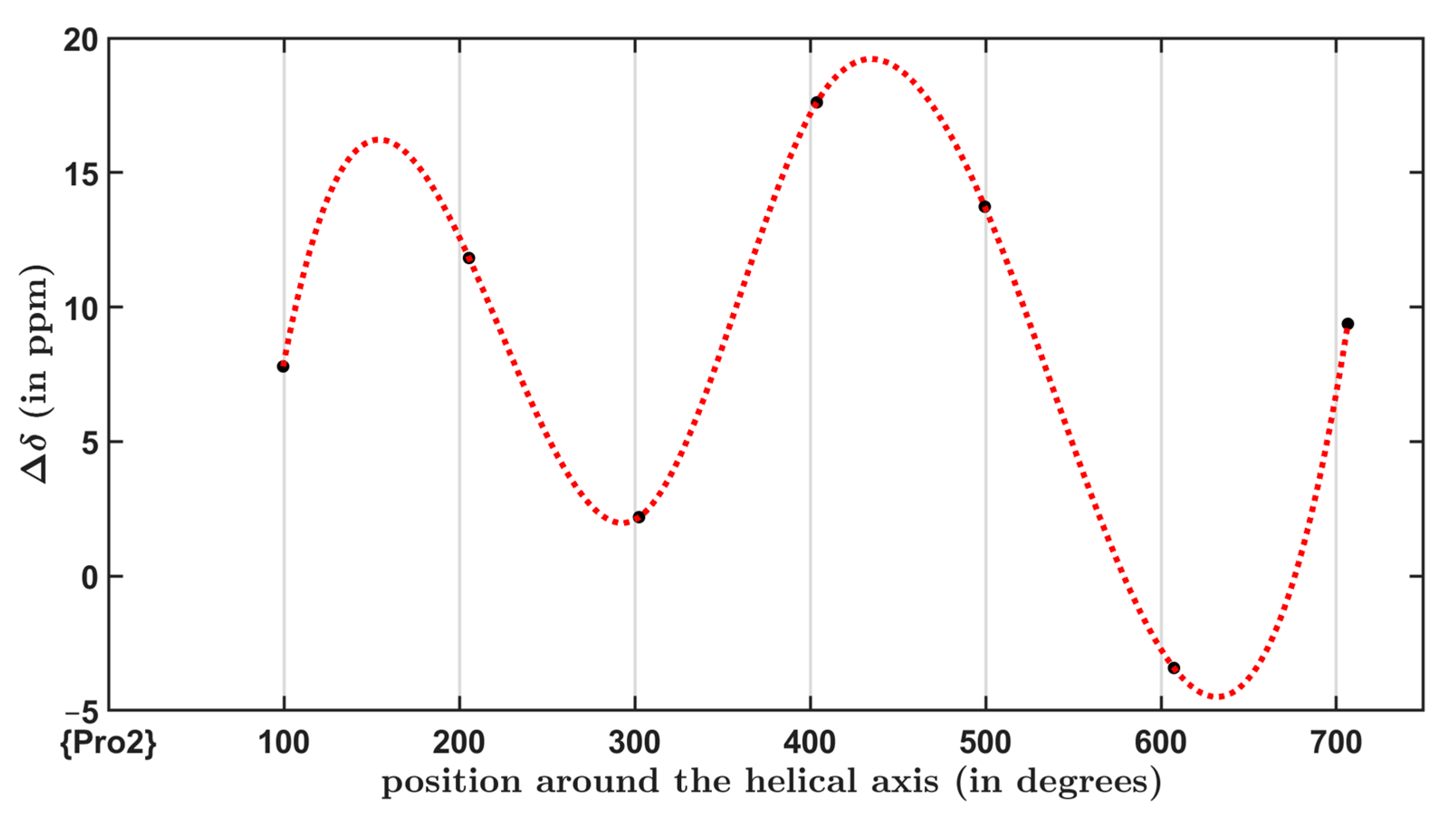
| Residue | (Degrees) | (Degrees) | (Degrees) | H-Bonding Involvement |
|---|---|---|---|---|
| Aib1 | −49 | −44 | −93 | →Aib5 |
| Pro2 | −65 | −34 | −99 | →Aib6 |
| Aib3 | −57 | −49 | −106 | →Gln7 |
| Ala4 | −66 | −43 | −109 | →Aib8 |
| Aib5 | −54 | −51 | −105 | →Val9, Aib1← |
| Ala6 | −68 | −37 | −105 | →Aib10, Pro2← |
| Gln7 | −61 | −45 | −106 | →Gly11, Aib3← |
| Aib8 | −56 | −46 | −102 | Ala4← |
| Val9 | −64 | −50 | −114 | →Leu12, Aib5← |
| Aib10 | −53 | −43 | −96 | →Aib13, Ala6← |
| Gly11 | −66 | −18 | −84 | Gln7← |
| Leu12 | −94 | −13 | −107 | →Aib16, Val9← |
| Aib13 | −51 | −42 | −93 | →Aib17, Aib10← |
| Pro14 | −68 | −23 | −91 | →Glu18 |
| Val15 | −67 | −48 | −115 | →Gln19 |
| Aib16 | −54 | −50 | −104 | →Phl20, Leu12← |
| Aib17 | −57 | −44 | −101 | →Phl20 hydroxyl, Aib13← |
| Glu18 | −61 | −37 | −98 | Pro14← |
| Gln19 | −78 | −35 | −113 | Val15← |
| Phl20 | −141 | ― 1 | ― 1 | Aib16←, Aib17← |
| Residue | (ppm) | (ppm) | (ppm) | (ppm) | (Degrees) | (Degrees) | (Degrees) |
|---|---|---|---|---|---|---|---|
| Aib1 | 136.7 | 77.1 | 91.5 | 241.7 | 2.5 | 14.1 | 12.9 |
| Pro2 | 133.6 | 47.7 | 125.4 | 227.6 | ― 1 | ― 1 | ― 1 |
| Aib3 | 120.3 | 63.5 | 75.0 | 222.3 | 7.0 | 14.8 | 28.7 |
| Ala4 | 117.7 | 48.3 | 81.7 | 223.2 | 1.1 | 16.2 | 17.2 |
| Aib5 | 125.7 | 68.2 | 78.4 | 230.5 | 4.3 | 13.1 | 31.3 |
| Ala6 | 116.6 | 53.7 | 75.5 | 220.7 | 2.9 | 18.2 | 16.1 |
| Gln7 | 119.7 | 56.8 | 76.0 | 226.3 | 4.6 | 18.2 | 30.1 |
| Aib8 | 127.8 | 68.5 | 78.6 | 236.4 | 4.2 | 12.9 | 51.5 |
| Val9 | 114.3 | 51.6 | 78.0 | 213.2 | 1.6 | 17.6 | 12.0 |
| Aib10 | 127.8 | 69.0 | 81.4 | 233.1 | 3.1 | 14.6 | 48.0 |
| Gly11 | 101.2 | 45.0 | 55.7 | 202.9 | 4.0 | 23.8 | 45.2 |
| Leu12 | 116.5 | 48.7 | 77.1 | 223.8 | 4.1 | 18.8 | 36.7 |
| Aib13 | 131.5 | 71.5 | 86.0 | 237.1 | 2.1 | 14.6 | 70.0 |
| Pro14 | 131.0 | 50.8 | 118.5 | 223.7 | ― 1 | ― 1 | ― 1 |
| Val15 | 116.4 | 57.6 | 71.6 | 220.0 | 5.3 | 20.1 | 48.5 |
| Aib16 | 125.8 | 62.9 | 83.4 | 231.1 | 1.6 | 10.9 | 17.8 |
| Aib17 | 122.6 | 65.5 | 79.3 | 223.0 | 4.6 | 14.4 | 10.1 |
| Glu18 | 115.8 | 56.7 | 73.1 | 217.5 | 4.9 | 20.8 | 13.9 |
| Gln19 | 117.8 | 52.7 | 75.2 | 225.4 | 2.0 | 19.1 | 24.3 |
| Phl20 | 112.0 | 56.9 | 65.8 | 213.3 | 5.4 | 19.9 | 15.8 |
| Residue | (ppm) | (ppm) | (ppm) | (ppm) | (Degrees) | (Degrees) | (Degrees) | (ppm) |
|---|---|---|---|---|---|---|---|---|
| Aib1 | 178.0 | 102 | 181.9 | 250.0 | 1.2 | 4.8 | 1.4 | 5.9 |
| Pro2 | 180.5 | 97.2 | 189.0 | 255.2 | 1.7 | 4.0 | 4.2 | 12.8 |
| Aib3 | 180.2 | 100.3 | 187.0 | 253.4 | 1.2 | 2.6 | 1.4 | 10.2 |
| Ala4 | 179.4 | 98.5 | 188.8 | 250.9 | 0.5 | 2.3 | 1.5 | 14.1 |
| Aib5 | 180.0 | 101.0 | 183.0 | 256.0 | 1.2 | 1.8 | 2.2 | 4.5 |
| Ala6 | 179.7 | 95.9 | 193.2 | 249.9 | 3.1 | 3.1 | 3.4 | 20.3 |
| Gln7 | 178.1 | 97.0 | 189.0 | 248.2 | 0.3 | 2.0 | 1.7 | 16.4 |
| Aib8 | 179.5 | 101.4 | 178.4 | 258.6 | 0.2 | 1.9 | 1.9 | −1.6 |
| Val9 | 179.1 | 96.8 | 187.0 | 253.5 | 0.6 | 0.8 | 0.9 | 11.9 |
| Aib10 | 182.1 | 99.6 | 189.3 | 257.3 | 1.4 | 1.4 | 2.6 | 10.9 |
| Gly11 | 171.7 | 94.4 | 166.8 | 253.8 | 2.2 | 3.0 | 2.3 | −7.3 |
| Leu12 | 180.9 | 97.3 | 198.7 | 246.8 | 0.8 | 4.2 | 0.8 | 26.6 |
| Aib13 | 178.2 | 102.1 | 182.7 | 249.8 | 0.5 | 5.3 | 1.5 | 6.8 |
| Pro14 | 179.1 | 94.4 | 190.0 | 252.8 | 0.7 | 1.3 | 3.0 | 16.4 |
| Val15 | 177.9 | 97.9 | 182.9 | 253.0 | 0.3 | 2.2 | 0.7 | 7.4 |
| Aib16 | 178.9 | 100.5 | 182.5 | 253.7 | 0.1 | 2.1 | 2.1 | 5.3 |
| Aib17 | 179.2 | 98.7 | 188.6 | 250.5 | 1.5 | 3.8 | 2.9 | 14.0 |
| Glu18 | 177.6 | 92.8 | 196.9 | 243.1 | 3.3 | 5.6 | 4.0 | 28.9 |
| Gln19 | 179.2 | 95.6 | 186.8 | 255.2 | 1.8 | 3.8 | 3.1 | 11.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czernek, J.; Brus, J. Modeling the Structure of Crystalline Alamethicin and Its NMR Chemical Shift Tensors. Antibiotics 2021, 10, 1265. https://doi.org/10.3390/antibiotics10101265
Czernek J, Brus J. Modeling the Structure of Crystalline Alamethicin and Its NMR Chemical Shift Tensors. Antibiotics. 2021; 10(10):1265. https://doi.org/10.3390/antibiotics10101265
Chicago/Turabian StyleCzernek, Jiří, and Jiří Brus. 2021. "Modeling the Structure of Crystalline Alamethicin and Its NMR Chemical Shift Tensors" Antibiotics 10, no. 10: 1265. https://doi.org/10.3390/antibiotics10101265
APA StyleCzernek, J., & Brus, J. (2021). Modeling the Structure of Crystalline Alamethicin and Its NMR Chemical Shift Tensors. Antibiotics, 10(10), 1265. https://doi.org/10.3390/antibiotics10101265