Looking Back to Amycolatopsis: History of the Antibiotic Discovery and Future Prospects
Abstract
:1. Introduction
2. The History and Genomic Analysis of Amycolatopsis
3. Amycolatopsis Genomic Potential for Antibiotic Production
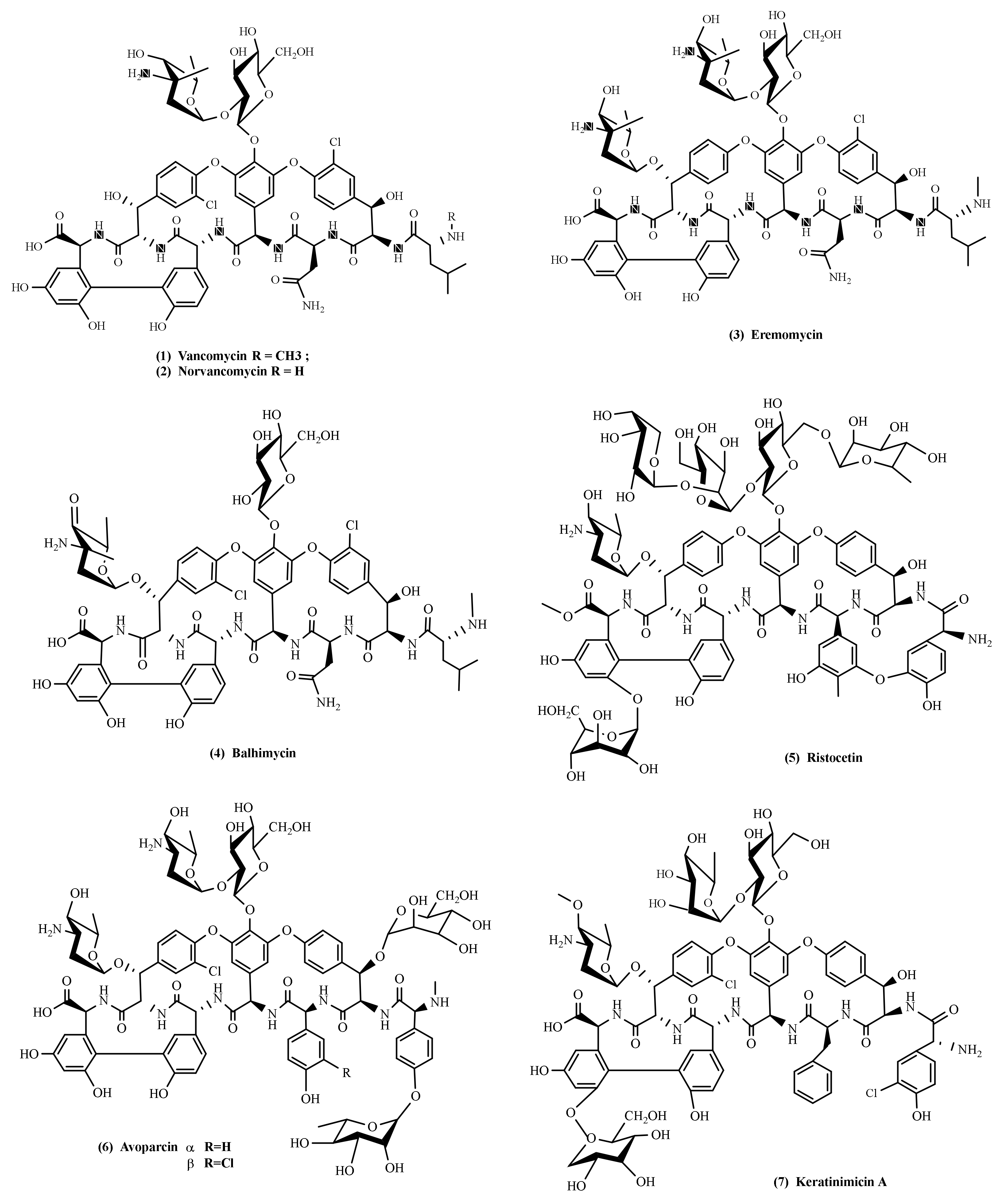
4. Glycopeptide Antibiotics
4.1. Vancomycin
4.2. Eremomycin
4.3. Norvancomycin
4.4. Balhimycin
4.5. Ristocetin (Ristomycin)
4.6. Avoparcin and Emergence of Vancomycin Resistance
5. Polyketide Antibiotics
5.1. Rifamycin’s Discovery and Structure
5.2. Mechanism of Rifampicin Action and Occurrence of Resistance
5.3. Polyketide Backbone Rearrangement
6. Old New Polyenes
6.1. Kanglemycin A
6.2. Chelocardin (Otherwise Known as Cetocycline or Cetotetrine)
6.3. Vancoresmycin
6.4. Rifamycin O
7. Antibiotics Produced by Amycolatopsis Isolated from Poorly Studied Ecological Habitats
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ventura, M.; Canchaya, C.; Tauch, A.; Chandra, G.; Fitzgerald, G.F.; Chater, K.F.; van Sinderen, D. Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 2007, 71, 495–548. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Xu, T.; Wang, J.; Hou, Y.; Liu, C.; Liu, S.; Wu, S. Secondary Metabolites of the Genus Amycolatopsis: Structures, Bioactivities and Biosynthesis. Molecules 2021, 26, 1884. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, Q.; Shen, Q.; Wang, H. Progress in understanding the genetic information and biosynthetic pathways behind Amycolatopsis antibiotics, with implications for the continued discovery of novel drugs. ChemBioChem 2016, 17, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Singh, P.; Lal, R. Genetics and genomics of the genus Amycolatopsis. Indian J. Microbiol. 2016, 56, 233–246. [Google Scholar] [CrossRef] [Green Version]
- Dávila Costa, J.S.; Amoroso, M.J. Current biotechnological applications of the genus Amycolatopsis. World J. Microbiol. Biotechnol. 2014, 30, 1919–1926. [Google Scholar] [CrossRef]
- Penkhrue, W.; Sujarit, K.; Kudo, T.; Ohkuma, M.; Masaki, K.; Aizawa, T.; Pathom-Aree, W.; Khanongnuch, C.; Lumyong, S. Amycolatopsis oliviviridis sp. nov., a novel polylactic acid-bioplastic-degrading actinomycete isolated from paddy soil. Int. J. Syst. Evol. Microbiol. 2018, 68, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Lechevalier, M.P.; Prauser, H.; Labeda, D.P.; Ruan, J.S. Two new genera of nocardioform actinomycetes: Amycolata gen. nov. and Amycolatopsis gen. nov. Int. J. Syst. Bacteriol. 1986, 36, 29–37. [Google Scholar] [CrossRef]
- Tang, S.K.; Wang, Y.; Guan, T.W.; Lee, J.C.; Kim, C.J.; Li, W.J. Amycolatopsis halophila sp. nov., a halophilic actinomycete isolated from a salt lake. Int. J. Syst. Evol. Microbiol. 2010, 60 Pt 5, 1073–1078. [Google Scholar] [CrossRef] [Green Version]
- Carlsohn, M.R.; Growth, I.; Tan, G.Y.A.; Schütze, B.; Saluz, H.P.; Munder, T.; Yang, J.; Wink, J.; Goodfellow, M. Amycolatopsis saalfeldensis sp. nov., a novel actinomycete isolated from a medieval alum slate mine. Int. J. Syst. Evol. Microbiol. 2007, 57 Pt 7, 1640–1646. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, M.; González, I.; Díaz-Muñoz, C.; Martínez, G.; Genilloud, O. Comparative genomics and biosynthetic potential analysis of two lichen-isolated Amycolatopsis strains. Front. Microbiol. 2018, 9, 369. [Google Scholar] [CrossRef]
- Bian, J.; Li, Y.; Wang, J.; Song, F.H.; Liu, M.; Dai, H.Q.; Ren, B.; Gao, H.; Hu, X.; Liu, Z.H.; et al. Amycolatopsis marina sp. nov., an actinomycete isolated from an ocean sediment. Int. J. Syst. Evol. Microbiol. 2009, 59 Pt 3, 477–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodfellow, M.; Kim, S.B.; Minnikin, D.E.; Whitehead, D.; Zhou, Z.-H.; Mattinson-Rose, A.D. Amycolatopsis sacchari sp. nov., a moderately thermophilic actinomycete isolated from vegetable matter. Int. J. Syst. Evol. Microbiol. 2001, 51, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.S.; Zhang, B.; Zhang, M.; Guo, Z.K.; Deng, X.Z.; Shi, J.; Li, W.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Rifamorpholines A–E, potential antibiotics from locust-associated actinobacteria Amycolatopsis sp. Hca4. Org. Biomol. Chem. 2017, 15, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Beemelmanns, C.; Ramadhar, T.R.; Kim, K.H.; Klassen, J.L.; Cao, S.; Wyche, T.P.; Hou, Y.; Poulsen, M.; Bugni, T.S.; Currie, C.R.; et al. Macrotermycins A-D, glycosylated macrolactams from a termite-associated Amycolatopsis sp. M39. Org. Lett. 2017, 19, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Paściak, M.; Liu, Z.; Xie, Q.; Gamian, A. Amycolatopsis palatopharyngis sp. nov., a potentially pathogenic actinomycete isolated from a human clinical source. Int. J. Syst. Evol. Microbiol. 2004, 54 Pt 2, 359–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, G.Y.A.; Goodfellow, M. Amycolatopsis. In Bergey’s Manual of Systematic Bacteriology; Whitman, W.B., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–40. [Google Scholar]
- Labeda, D.P.; Donahue, J.M.; Williams, N.M.; Sells, S.F.; Henton, M.M. Amycolatopsis kentuckyensis sp. nov., Amycolatopsis lexingtonensis sp. nov. and Amycolatopsis pretoriensis sp. nov., isolated from equine placentas. Int. J. Syst. Evol. Microbiol 2003, 53 Pt 5, 1601–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LPSN—List of Prokaryotic Names with Standing in Nomenclature. Available online: http://www.bacterio.net/amycolatopsis.html (accessed on 22 June 2021).
- The National Center for Biotechnology Information (Assembly). Available online: www.ncbi.nlm.nih.gov/assembly (accessed on 22 June 2021).
- Adamek, M.; Alanjary, M.; Sales-Ortells, H.; Goodfellow, M.; Bull, A.T.; Winkler, A.; Wibberg, D.; Kalinowski, J.; Ziemert, N. Comparative genomics reveals phylogenetic distribution patterns of secondary metabolites in Amycolatopsis species. BMC Genomics 2018, 19, 426. [Google Scholar] [CrossRef] [Green Version]
- Sangal, V.; Goodfellow, M.; Blom, J.; Tan, G.Y.A.; Klenk, H.P.; Sutcliffe, I.C. Revisiting the taxonomic status of the biomedically and industrially important genus Amycolatopsis, using a phylogenomic approach. Front. Microbiol. 2018, 9, 2281. [Google Scholar] [CrossRef]
- Tan, G.Y.; Ward, A.C.; Goodfellow, M. Exploration of Amycolatopsis diversity in soil using genus-specific primers and novel selective media. Syst. Appl. Microbiol. 2006, 29, 557–569. [Google Scholar] [CrossRef]
- Doroghazi, J.R.; Metcalf, W.W. Comparative genomics of actinomycetes with a focus on natural product biosynthetic genes. BMC Genomics 2013, 14, 611. [Google Scholar] [CrossRef] [Green Version]
- Everest, G.J.; le Roes-Hill, M.; Rohland, J.; Enslin, S.; Meyers, P.R. Amycolatopsis roodepoortensis sp. nov. and Amycolatopsis speibonae sp. nov.: Antibiotic-producing actinobacteria isolated from South African soils. J. Antibiot. (Tokyo) 2014, 67, 813–818. [Google Scholar] [CrossRef]
- Minimum Information about a Biosynthetic Gene cluster. Available online: http://mibig.secondarymetabolites.org (accessed on 22 June 2021).
- Medema, M.H.; Kottmann, R.; Yilmaz, P.; Cummings, M.; Biggins, J.B.; Blin, K.; de Bruijn, I.; Chooi, Y.H.; Claesen, J.; Coates, R.C.; et al. Minimum information about a biosynthetic gene cluster. Nat. Chem. Biol. 2015, 11, 625–631. [Google Scholar] [CrossRef]
- Everest, G.J.; Meyers, P.R. Evaluation of the antibiotic biosynthetic potential of the genus Amycolatopsis and description of Amycolatopsis circi sp. nov., Amycolatopsis equina sp. nov. and Amycolatopsis hippodromi sp. nov. J. Appl. Microbiol. 2011, 111, 300–311. [Google Scholar] [CrossRef]
- Banskota, A.H.; Mcalpine, J.B.; Sørensen, D.; Ibrahim, A.; Aouidate, M.; Piraee, M.; Alarco, A.M.; Farnet, C.M.; Zazopoulos, E. Genomic analyses lead to novel secondary metabolites. Part 3. ECO-0501, a novel antibacterial of a new class. J. Antibiot. (Tokyo) 2006, 59, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wu, Y.; Zhang, C.; Davis, K.M.; Moon, K.; Bushin, L.B.; Seyedsayamdost, M.R. A genetics-free method for high-throughput discovery of cryptic microbial metabolites. Nat. Chem. Biol. 2019, 15, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lu, W.; Ahmadi, M.K.; Montiel, D.; Ternei, M.A.; Brady, S.F. Atolypenes, Tricyclic Bacterial Sesterterpenes Discovered Using a Multiplexed In Vitro Cas9-TAR Gene Cluster Refactoring Approach. ACS Synth Biol. 2019, 8, 109–118. [Google Scholar] [CrossRef]
- Hopp, D.C.; Rabenstein, J.; Rhea, J.; Smith, C.; Romari, K.; Clarke, M.; Francis, L.; Irigoyen, M.; Milanowski, D.; Luche, M.; et al. Mutactimycin E, a new anthracycline antibiotic with Gram-positive activity. J. Antibiot. (Tokyo) 2008, 61, 675–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.; Kim, S.H.; Shin, Y.; Bae, M.; Kim, B.Y.; Lee, S.K.; Oh, K.B.; Shin, J.; Oh, D.C. A new benzofuran glycoside and indole alkaloids from a sponge-associated rare actinomycete, Amycolatopsis sp. Mar. Drugs. 2014, 12, 2326–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauermeister, A.; Calil, F.A.; Pinto, F.d.C.L.; Medeiros, T.C.T.; Almeida, L.C.; Silva, L.J.; de Melo, I.S.; Zucchi, T.D.; Costa-Lotufo, L.V.; Moraes, L.A.B. Pradimicin-IRD from Amycolatopsis sp. IRD-009 and its antimicrobial and cytotoxic activities. Nat. Prod. Res. 2019, 33, 1713–1720. [Google Scholar] [CrossRef]
- Izuta, S.; Kosaka, S.; Kawai, M.; Miyano, R.; Matsuo, H.; Matsumoto, A.; Nonaka, K.; Takahashi, Y.; Ōmura, S.; Nakashima, T. Dipyrimicin A and B, microbial compounds isolated from Amycolatopsis sp. K16-0194. J. Antibiot. (Tokyo) 2018, 71, 535–537. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Högberg, T.; Drake, S.D.; Burgstahler, A.W.; Jackson, M.; Lee, B.; Sheldon, R.I.; Gracey, H.E.; Kohl, W.; Theriault, R.J. Isolation and structural determination of siderochelin C, a fermentation product of an unusual Actinomycetes sp. J. Antibiot. (Tokyo) 1984, 37, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Ye, F.W.; Shen, Y.M. Siderochelins with anti-mycobacterial activity from Amycolatopsis sp. LZ149. Chin. J. Nat. Med. 2015, 13, 69–72. [Google Scholar] [CrossRef]
- Shimanaka, K.; Kinoshita, N.; Iinuma, H.; Hamada, M.; Takeuchi, T. Novel antibiotics, amythiamicins. I. Taxonomy, fermentation, isolation, physico-chemical properties, and antimicrobial activity. J. Antibiot. (Tokyo) 1994, 47, 668–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimanaka, K.; Takahashi, Y.; Iinuma, H.; Naganawa, H.; Takeuchi, T. Novel antibiotics, amythiamicins. III. Structure elucidations of amythiamicins A, B and C. J. Antibiot. (Tokyo) 1994, 47, 1153–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwun, M.J.; Cheng, J.; Yang, S.H.; Lee, D.R.; Suh, J.W.; Hong, H.J. Draft genome sequence of ristocetin-producing strain Amycolatopsis sp. strain MJM2582 isolated in South Korea. Genome Announc. 2014, 2, e01091-14. [Google Scholar] [CrossRef] [Green Version]
- Truman, A.W.; Kwun, M.J.; Cheng, J.; Yang, S.H.; Suh, J.W.; Hong, H.J. Antibiotic resistance mechanisms inform discovery: Identification and characterization of a novel amycolatopsis strain producing ristocetin. Antimicrob. Agents Chemother. 2014, 58, 5687–5895. [Google Scholar] [CrossRef] [Green Version]
- Grundy, W.E.; Sinclair, A.C.; Theriault, R.J.; Goldstein, A.W.; Rickher, C.J.; Warren, H.B., Jr.; Oliver, T.J.; Sylvester, J.C. Ristocetin, microbiologic properties. Antibiot. Annu. 1956, 687–692. [Google Scholar]
- Igarashi, M.; Sawa, R.; Kinoshita, N.; Hashizume, H.; Nakagawa, N.; Homma, Y.; Nishimura, Y.; Akamatsu, Y. Pargamicin A, a novel cyclic peptide antibiotic from Amycolatopsis sp. J. Antibiot. (Tokyo) 2008, 61, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Hashizume, H.; Iijima, K.; Yamashita, K.; Kimura, T.; Wada, S.I.; Sawa, R.; Igarashi, M. Valgamicin C, a novel cyclic depsipeptide containing the unusual amino acid cleonine, and related valgamicins A, T and V produced by Amycolatopsis sp. ML1-hF4. J. Antibiot. (Tokyo) 2017, 71, 129–134. [Google Scholar] [CrossRef]
- Zheng, K.X.; Jiang, Y.; Jiang, J.X.; Huang, R.; He, J.; Wu, S.H. A new phthalazinone derivative and a new isoflavonoid glycoside from lichen-associated Amycolatopsis sp. Fitoterapia 2019, 135, 85–89. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, Y.; Huang, R.; Jiang, B.; Zheng, K.; Wu, S. Diverse secondary metabolites from a lichen-derived Amycolatopsis strain. Curr. Microbiol. 2020, 77, 2104–2110. [Google Scholar] [CrossRef]
- Pishchany, G.; Mevers, E.; Ndousse-Fetter, S.; Horvath, D.J., Jr.; Paludo, C.R.; Silva-Junior, E.A.; Koren, S.; Skaar, E.P.; Clardy, J.; Kolter, R. Amycomicin is a potent and specific antibiotic discovered with a targeted interaction screen. Proc. Natl. Acad. Sci. USA. 2018, 115, 10124–10129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasari, V.R.; Muthyala, M.K.; Nikku, M.Y.; Donthireddy, S.R. Novel pyridinium compound from marine actinomycete, Amycolatopsis alba var. nov. DVR D4 showing antimicrobial and cytotoxic activities in vitro. Microbiol. Res. 2012, 167, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, S.; Lu, J.; Esumi, H.; Yamazaki, Y.; Kinoshita, N.; Honma, Y.; Hamada, M.; Ohsono, M.; Ishizuka, M.; Takeuchi, T. Kigamicins, novel antitumor antibiotics. I. Taxonomy, isolation, physico-chemical properties and biological activities. J. Antibiot. (Tokyo) 2003, 56, 1004–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, X.; Zhu, J.; Wang, H.; Lu, C. Carbamothioic S-acid derivative and kigamicins, the activated production of silent metabolites in Amycolatopsis alba DSM 44262Δabm9 elicited by N-acetyl-D-glucosamine. Nat. Prod. Res. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Shen, Y. Identification of the Bacterial Maytansinoid Gene Cluster asc Provides Insights into the Post-PKS Modifications of Ansacarbamitocin Biosynthesis. Org. Lett. 2019, 21, 5823–5826. [Google Scholar] [CrossRef]
- Omura, S.; Tanaka, H.; Tanaka, Y.; Spiri-Nakagawa, P.; Oiwa, R.; Takahashi, Y.; Matsuyama, K.; Iwai, Y. Studies on bacterial cell wall inhibitors. VII. Azureomycins A and B, new antibiotics produced by Pseudonocardia azurea nov. sp. Taxonomy of the producing organism, isolation, characterization and biological properties. J. Antibiot. (Tokyo) 1979, 32, 985–994. [Google Scholar] [CrossRef] [Green Version]
- Khatri, I.; Subramanian, S.; Mayilraj, S. Genome sequencing and annotation of Amycolatopsis azurea DSM 43854(T). Genom. Data. 2014, 12, 44–45. [Google Scholar] [CrossRef] [Green Version]
- Dobashi, K.; Matsuda, N.; Hamada, M.; Naganawa, H.; Takita, T.; Takeuchi, T. Novel antifungal antibiotics octacosamicins A and B. I. Taxonomy, fermentation and isolation, physico-chemical properties and biological activities. J. Antibiot. (Tokyo) 1988, 41, 1525–1532. [Google Scholar] [CrossRef] [Green Version]
- Dobashi, K.; Naganawa, H.; Takahashi, Y.; Takita, T.; Takeuchi, T. Novel antifungal antibiotics octacosamicins A and B. II. The structure elucidation using various NMR spectroscopic methods. J. Antibiot. (Tokyo) 1988, 41, 1533–1541. [Google Scholar] [CrossRef]
- Nadkarni, S.R.; Patel, M.V.; Chatterjee, S.; Vijayakumar, E.K.; Desikan, K.R.; Blumbach, J.; Ganguli, B.N.; Limbert, M. Balhimycin, a new glycopeptide antibiotic produced by Amycolatopsis sp. Y-86,21022. Taxonomy, production, isolation and biological activity. J. Antibiot. (Tokyo) 1994, 47, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Kunstmann, M.P.; Mitscher, L.A.; Porter, J.N.; Shay, A.J.; Darken, M.A. LL-AV290, a new antibiotic. I. Fermentation, isolation, and characterization. Antimicrob. Agents Chemother. 1968, 8, 242–245. [Google Scholar]
- Ellestad, G.A.; Swenson, W.; McGahren, W.J. Epimerization and stereochemistry of avoparcin. J. Antibiot. (Tokyo) 1983, 36, 1683–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acar, J.; Casewell, M.; Freeman, J.; Friis, C.; Goossens, H. Avoparcin and virginiamycin as animal growth promoters: A plea for science in decision-making. Clin. Microbiol. Infect. 2000, 6, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Neu, H.C.; Chin, N.X.; Niu, W.W. In vitro activity of the new glycopeptide decaplanin. Eur. J. Clin. Microbiol. Infect. Dis. 1992, 11, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Wink, J.; Gandhi, J.; Kroppenstedt, R.M.; Seibert, G.; Sträubler, B.; Schumann, P.; Stackebrandt, E. Amycolatopsis decaplanina sp. nov., a novel member of the genus with unusual morphology. Int. J. Syst. Evol. Microbiol. 2004, 54 Pt 1, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Aobulikasimu, N.; Zhang, Z.; Liu, C.; Cao, B.; Lin, B.; Guan, P.; Mu, Y.; Jiang, Y.; Han, L.; et al. Amycolasporins and dibenzoyls from lichen-associated Amycolatopsis hippodrome and their antibacterial and anti-inflammatory activities. J. Nat. Prod. 2020, 83, 3545–3553. [Google Scholar] [CrossRef]
- Spohn, M.; Kirchner, N.; Kulik, A.; Jochim, A.; Wolf, F.; Muenzer, P.; Borst, O.; Gross, H.; Wohlleben, W.; Stegmann, E. Overproduction of Ristomycin A by activation of a silent gene cluster in Amycolatopsis japonicum MG417-CF17. Antimicrob. Agents Chemother. 2014, 58, 6185–6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Muñoz, J.C.; Selem-Mojica, N.; Mullowney, M.W.; Kautsar, S.A.; Tryon, J.H.; Parkinson, E.I.; De Los Santos, E.L.C.; Yeong, M.; Cruz-Morales, P.; Abubucker, S.; et al. A computational framework to explore large-scale biosynthetic diversity. Nat. Chem. Biol. 2020, 16, 60–68. [Google Scholar] [CrossRef]
- Shorin, V.A.; Yudinstev, S.D.; Kunrat, I.A.; Goldberg, L.E.; Pevzner, N.S.; Brazhnikova, M.G.; Lomakina, N.N.; Oparysheva, E.F. New antibiotics, actinoidin. Antibiotiki 1957, 2, 44–49. (In Russian) [Google Scholar]
- Wink, J.M.; Kroppenstedt, R.M.; Ganguli, B.N.; Nadkarni, S.R.; Schumann, P.; Seibert, G.; Stackebrandt, E. Three new antibiotics producing species of the genus Amycolatopsis, Amycolatopsis balhimycina sp. nov., A. tolypomycina sp. nov., A. vancoresmycina sp. nov., and description of Amycolatopsis keratiniphila subsp. keratiniphila subsp. nov. and A. keratiniphila subsp. nogabecina subsp. nov. Syst. Appl. Microbiol. 2003, 26, 38–46. [Google Scholar] [CrossRef]
- Miller, A.K.; Celozzi, E.; Kong, Y.; Pelak, B.A.; Kropp, H.; Stapley, E.O.; Hendlin, D. Cephamycins, a new family of beta-lactam antibiotics. IV. In vivo studies. Antimicrob. Agents Chemother. 1972, 2, 287–290. [Google Scholar] [CrossRef] [Green Version]
- Stapley, E.O.; Jackson, M.; Hernandez, S.; Zimmerman, S.B.; Currie, S.A.; Mochales, S.; Mata, J.M.; Woodruff, H.B.; Hendlin, D. Cephamycins, a new family of beta-lactam antibiotics. I. Production by actinomycetes, including Streptomyces lactamdurans sp. n. Antimicrob. Agents Chemother. 1972, 2, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreiro, C.; Pisabarro, A.; Martín, J.F. Characterization of the ribosomal rrnD operon of the cephamycin-producer ’Nocardia lactamdurans’ shows that this actinomycete belongs to the genus Amycolatopsis. Syst. Appl. Microbiol. 2000, 23, 15–24. [Google Scholar] [CrossRef]
- Liras, P.; Demain, A.L. Enzymology of beta-lactam compounds with cephem structure produced by actinomycete. Methods Enzymol. 2009, 458, 401–429. [Google Scholar] [CrossRef]
- Wax, R.; Maises, W.; Weston, R.; Birnbaum, J. Efrotomycin, a new antibiotic from Streptomyces lactamdurans. J. Antibiot. (Tokyo) 1976, 29, 670–673. [Google Scholar] [CrossRef] [Green Version]
- Theriault, R.J.; Rasmussen, R.R.; Kohl, W.L.; Prokop, J.F.; Hutch, T.B.; Barlow, G.J. Benzanthrins A and B, a new class of quinone antibiotics. I. Discovery, fermentation and antibacterial activity. J. Antibiot. (Tokyo) 1986, 39, 1509–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, R.R.; Nuss, M.E.; Scherr, M.H.; Mueller, S.L.; McAlpine, J.B.; Mitscher, L.A. Benzanthrins A and B, a new class of quinone antibiotics. II. Isolation, elucidation of structure and potential antitumor activity. J. Antibiot. (Tokyo) 1986, 39, 1515–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philip, J.E.; Schenck, J.R.; Hargie, M.P. Ristocetins A and B, two new antibiotics; isolation and properties. Antibiot. Annu. 1957, 699–705. [Google Scholar]
- Roberts, G.D.; Carr, S.A.; Rottschaefer, S.; Jeffs, P.W. Structural characterization of glycopeptide antibiotics related to vancomycin by fast atom bombardment mass spectrometry. J. Antibiot. (Tokyo) 1985, 38, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wu, X.; Zhu, J.; Shen, Y. Amexanthomycins A-J, pentangular polyphenols produced by Amycolatopsis mediterranei S699∆rifA. Appl. Microbiol. Biotechnol. 2018, 102, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Iijima, M.; Masuda, T.; Kinoshita, N.; Iinuma, H.; Naganawa, H.; Hamada, M.; Ishizuka, M.; Takeuchi, T. Dethymicin, a novel immunosuppressant isolated from an Amycolatopsis. Fermentation, isolation, physico-chemical properties and biological activities. J. Antibiot. (Tokyo) 1992, 45, 1819–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.J.; Fu, Y.; Yan, G.H.; Bao, G.H.; Xu, C.F.; He, C.H. Isolation and structure of a new ansamycin antibiotic kanglemycin A from a Nocardia. J. Antibiot. (Tokyo) 1988, 41, 264–267. [Google Scholar] [CrossRef]
- Mosaei, H.; Molodtsov, V.; Kepplinger, B.; Harbottle, J.; Moon, C.W.; Jeeves, R.E.; Ceccaroni, L.; Shin, Y.; Morton-Laing, S.; Marrs, E.C.L.; et al. Mode of action of Kanglemycin A, an ansamycin natural product that is active against rifampicin-resistant Mycobacterium tuberculosis. Mol. Cell. 2018, 72, 263–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sensi, P.; Margalith, P.; Timbal, M.T. Rifomycin, a new antibiotic; preliminary report. Farmaco Sci. 1959, 14, 146–147. [Google Scholar]
- Birner, J.; Hodgson, P.R.; Lane, W.R.; Baxter, E.H. An Australian isolate of Nocardia mediterranea producing rifamycin SV. J. Antibiot. (Tokyo) 1972, 25, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.J.; Han, B.L.; Yameshita, N.; Sato, M. 31-Homorifamycin W, a novel metabolite from Amycolatopsis mediterranei. J. Antibiot. (Tokyo) 1994, 47, 613–615. [Google Scholar] [CrossRef]
- Tang, B.; Zhao, W.; Zheng, H.; Zhuo, Y.; Zhang, L.; Zhao, G.P. Complete genome sequence of Amycolatopsis mediterranei S699 based on de novo assembly via a combinatorial sequencing strategy. J. Bacteriol. 2012, 194, 5699–5700. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.R.; Zhang, J.L.; Tian, X.Y.; Wu, X.K.; Li, T.H.; Lu, C.H.; Shen, Y.M. Isolation of 11,12-seco-Rifamycin W derivatives reveals a cleavage pattern of the rifamycin ansa chain. Org. Lett. 2019, 21, 900–903. [Google Scholar] [CrossRef]
- Anderson, M.G.; Khoo, C.L.; Rickards, R.W. Oxidation processes in the biosynthesis of the tetracenomycin and elloramycin antibiotics. J. Antibiot. (Tokyo) 1989, 42, 640–643. [Google Scholar] [CrossRef]
- Qiao, X.; Gan, M.; Wang, C.; Liu, B.; Shang, Y.; Li, Y.; Chen, S. Tetracenomycin X exerts antitumour activity in lung cancer cells through the downregulation of cyclin D1. Mar. Drug. 2019, 17, 63. [Google Scholar] [CrossRef] [Green Version]
- Brigham, R.B.; Pittenger, R.C. Streptomyces orientalis, n. sp., the source of vancomycin. Antibiot. Chemother. (Northfield) 1956, 6, 642–647. [Google Scholar]
- Boeck, L.D.; Mertz, F.P.; Wolter, R.K.; Higgens, C.E. N-demethylvancomycin, a novel antibiotic produced by a strain of Nocardia orientalis. Taxonomy and fermentation. J. Antibiot. (Tokyo) 1984, 37, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Hunt, A.H.; Marconi, G.G.; Elzey, T.K.; Hoehn, M.M. A51568A: N-demethylvancomycin. J. Antibiot. (Tokyo) 1984, 37, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Qi, D.; Cheng, X.; Song, Z.; Li, W.; He, B. Antibiotic activities and affinities for bacterial cell wall analogue of N-demethylvancomycin and its derivatives. J. Antibiot. (Tokyo) 1998, 51, 750–756. [Google Scholar] [CrossRef] [Green Version]
- Lapchinskaia, O.A.; Katrukha, G.S.; Pogozheva, V.V.; Ponomarenko, V.I.; Filicheva, V.A.; Kharitonova, L.A.; Lapchinskaia, M.Y.; Yakovenko, A.N.; Nifantiev, N.E.; Shashkov, A.S.; et al. Amycolatopsis orientalis Strain—Producer of the Antibiotic Dimethylvancomycin and Method of the Antibiotic Preparation. Patent RU 2633511, 12 October 2017. (In Russian). [Google Scholar]
- Shashkov, A.S.; Tsvetkov, D.E.; Grachev, A.A.; Nifantiev, N.E.; Lapchinskaia, O.A.; Lavrova-Balashova, M.F.; Ponomarenko, V.I.; Katrukha, G.S. Structural analysis of antibiotic INA 9301 from Amycolatopsis orientalis. NPC 2008, 3, 1631–1638. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Lei, X.; Chen, M.; Jiang, B.; Wu, L.; Zhang, X.; Zheng, Z.; Hu, X.; You, X.; Si, S.; et al. Three structurally-related impurities in norvancomycin drug substance. J. Antibiot. (Tokyo) 2017, 70, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Zhang, C.; Jiang, Z.; Li, X.; Shi, Y.; Liu, M.; Xie, Y.; Wang, L.; Hong, B. Complete genome sequence of Amycolatopsis orientalis CPCC200066, the producer of norvancomycin. J. Biotechnol. 2017, 10, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Tsunakawa, M.; Tenmyo, O.; Tomita, K.; Naruse, N.; Kotake, C.; Miyaki, T.; Konishi, M.; Oki, T. Quartromicin, a complex of novel antiviral antibiotics. I. Production, isolation, physico-chemical properties and antiviral activity. J. Antibiot. (Tokyo) 1992, 45, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacey, M.S.; Jefson, M.R.; Huang, L.H.; Cullen, W.P.; Maeda, H.; Tone, J.; Nishiyama, S.; Kaneda, K.; Ishiguro, M. UK-69,753, a novel member of the efrotomycin family of antibiotics. I. Taxonomy of the producing organism, fermentation and isolation. J. Antibiot. (Tokyo) 1989, 42, 1453–1459. [Google Scholar] [CrossRef] [Green Version]
- Jefson, M.R.; Bordner, J.; Reese, C.P.; Whipple, E.B. UK-69,753, a novel member of the efrotomycin family of antibiotics. II. Structure determination and biological activity. J. Antibiot. (Tokyo) 1989, 42, 1610–1618. [Google Scholar] [CrossRef]
- Box, S.J.; Elson, A.L.; Gilpin, M.L.; Winstanley, D.J. MM 47761 and MM 49721, glycopeptide antibiotics produced by a new strain of Amycolatopsis orientalis. Isolation, purification and structure determination. J. Antibiot. (Tokyo) 1990, 43, 931–937. [Google Scholar] [CrossRef] [Green Version]
- Box, S.J.; Coates, N.J.; Davis, C.J.; Gilpin, M.L.; Houge-Frydrych, C.S.; Milner, P.H. MM 55266 and MM 55268, glycopeptide antibiotics produced by a new strain of Amycolatopsis. Isolation, purification and structure determination. J. Antibiot. (Tokyo) 1991, 44, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Berdnikova, T.F.; Shashkov, A.S.; Katrukha, G.S.; Lapchinskaia, O.A.; Iurkevich, N.V.; Grachev, A.A.; Nifant’ev, N.E. The structure of antibiotic eremomycin B. Russ. J. Bioorg. Chem. 2009, 35, 497–503. [Google Scholar] [CrossRef]
- Gause, G.F.; Brazhnikova, M.G.; Lomakina, N.N.; Berdnikova, T.F.; Fedorova, G.B.; Tokareva, N.L.; Borisova, V.N.; Batta, G. Eremomycin—New glycopeptide antibiotics. Chemical properties and structure. J. Antibiot. (Tokyo) 1989, 42, 1790–1799. [Google Scholar] [CrossRef] [Green Version]
- Gauze, G.F.; Brazhnikova, M.G.; Laĭko, A.V.; Sveshnikova, M.A.; Preobrazhenskaia, T.P. Eremomycin—A new antibiotic from the cyclic glycopeptide group. Antibiot. Med. Biotekhnol. 1987, 32, 571–576. (In Russian) [Google Scholar] [PubMed]
- Tsuji, N.; Kobayashi, M.; Kamigauchi, T.; Yoshimura, Y.; Terui, Y. New glycopeptide antibiotics. I. The structures of orienticins. J. Antibiot. (Tokyo) 1988, 41, 819–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, N.; Kamigauchi, T.; Kobayashi, M.; Terui, Y. New glycopeptide antibiotics: II. The isolation and structures of chloroorienticins. J. Antibiot. (Tokyo) 1988, 41, 1506–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolston, K.V.; Nguyen, H.; Messer, M. In vitro activity of LY264826, a new glycopeptide antibiotic, against Gram-positive bacteria isolated from patients with cancer. Antimicrob. Agents Chemother. 1990, 34, 2137–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunimoto, S.; Someno, T.; Yamazaki, Y.; Lu, J.; Esumi, H.; Naganawa, H. Kigamicins, novel antitumor antibiotics. II. Structure determination. J. Antibiot. (Tokyo) 2003, 56, 1012–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, T.; Ohba, S.; Kawada, M.; Osono, M.; Ikeda, D.; Esumi, H.; Kunimoto, S. Antitumor effect of kigamicin D on mouse tumor models. J. Antibiot. (Tokyo) 2006, 59, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, G.Y.A.; Robinson, S.; Lacey, E.; Brown, R.; Kim, W.; Goodfellow, M. Amycolatopsis regifaucium sp. nov., a novel actinomycete that produces kigamicins. Int. J. Syst. Evol. Microbiol. 2007, 57 Pt 11, 2562–2567. [Google Scholar] [CrossRef] [Green Version]
- Biryukov, M.V.; Zakalyukina, S.E.; Osterman, I.A. Strain of Amycolatopsis rifamycinica—Producer of the Antibiotic Tetracenomycin X. Patent RU 2724537, 23 June 2020. (In Russian). [Google Scholar]
- Osterman, I.A.; Wieland, M.; Maviza, T.P.; Lashkevich, K.A.; Lukianov, D.A.; Komarova, E.S.; Zakalyukina, Y.V.; Buschauer, R.; Shiriaev, D.I.; Leyn, S.A.; et al. Tetracenomycin X inhibits translation by binding within the ribosomal exit tunnel. Nat. Chem. Biol. 2020, 16, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Schwalen, C.J.; Hudson, G.A.; Kille, B.; Mitchell, D.A. Bioinformatic expansion and discovery of thiopeptide antibiotics. J. Am. Chem. Soc. 2018, 140, 9494–9501. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, L.; Zhao, L.; Chen, X.; Miao, C.; Hu, L.; Huang, X.; Chen, Y.; Li, Y. Echinosporin antibiotics isolated from Amycolatopsis strain and their antifungal activity against root-rot pathogens of the Panax notoginseng. Folia Microbiol. 2019, 64, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Tsuchida, T.; Umekita, M.; Kinoshita, N.; Iinuma, H.; Sawa, T.; Hamada, M.; Takeuchi, T. Epoxyquinomicins A, B, C and D, new antibiotics from Amycolatopsis. I. Taxonomy, fermentation, isolation and antimicrobial activities. J. Antibiot. (Tokyo) 1997, 50, 900–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, N.; Tsuchida, T.; Sawa, R.; Iinuma, H.; Nakamura, H.; Naganawa, H.; Sawa, T.; Takeuchi, T. Epoxyquinomicins A, B, C and D, new antibiotics from Amycolatopsis. III. Physico-chemical properties and structure determination. J. Antibiot. (Tokyo) 1997, 50, 912–915. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, T.; Inuma, H.; Kinoshita, N.; Ikeda, T.; Sawa, T.; Hamada, M.; Takeuchi, T. Azicemicins A and B, a new antimicrobial agent produced by Amycolatopsis. I. Taxonomy, fermentation, isolation, characterization and biological activities. J. Antibiot. (Tokyo) 1995, 48, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, T.; Sawa, R.; Takahashi, Y.; Iinuma, H.; Sawa, T.; Naganawa, H.; Takeuchi, T. Azicemicins A and B, new antimicrobial agents produced by Amycolatopsis. II. Structure determination. J. Antibiot. (Tokyo) 1995, 48, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Proctor, R.; Craig, W.; Kunin, C. Cetocycline, tetracycline analog: In vitro studies of antimicrobial activity, serum binding, lipid solubility, and uptake by bacteria. Antimicrob. Agents Chemother. 1978, 13, 598–604. [Google Scholar] [CrossRef] [Green Version]
- Lukežič, T.; Pikl, Š.; Zaburannyi, N.; Remškar, M.; Petković, H.; Müller, R. Heterologous expression of the atypical tetracycline chelocardin reveals the full set of genes required for its biosynthesis. Microb. Cell Fact. 2020, 19, 230. [Google Scholar] [CrossRef]
- Lukežič, T.; Fayad, A.A.; Bader, C.; Harmrolfs, K.; Bartuli, J.; Groß, S.; Lešnik, U.; Hennessen, F.; Herrmann, J.; Pikl, Š.; et al. Engineering atypical tetracycline formation in Amycolatopsis sulphurea for the production of modified chelocardin antibiotics. ACS Chem. Biol. 2019, 14, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Mongolla, P.; Chandrasekhar, C.; Poornachandra, Y.; Siva, B.; Babu, K.S.; Ramakrishna, K.V.S. Anti-proliferative and antioxidant activities of 1-methoxy-3-methyl-8-hydroxy-anthraquinone, a hydroxyanthraquinoid extrolite produced by Amycolatopsis thermoflava strain SFMA-103. Microbiol. Biotechnol. Lett. 2017, 45, 200–208. [Google Scholar] [CrossRef]
- Kishi, T.; Yamana, H.; Muroi, M.; Harada, S.; Asai, M. Tolypomycin, a new antibiotic. 3. Isolation and characterization of tolypomycin Y. J. Antibiot. (Tokyo) 1972, 25, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Lapchinskaia, O.A.; Katrukha, G.S.; Terekhova, L.P.; Pogozheva, V.V.; Filicheva, V.A.; Kharitonova, L.A.; Lapchinskaia, M.Y.; Yakovenko, A.N.; Ponomarenko, V.I.; Orlova, G.I. The Amycolatopsis umgeniensis strain is a producer of the antibiotic eremomycin. Patent RU 2689699 C1, 28 May 2019. (In Russian). [Google Scholar]
- Hopmann, C.; Kurz, M.; Brönstrup, M.; Wink, J.; LeBeller, D. Isolation and structure elucidation of vancoresmycin—A new antibiotic from Amycolatopsis sp. ST 101170. Tetrahedron Lett. 2002, 43, 435–438. [Google Scholar] [CrossRef]
- Kepplinger, B.; Morton-Laing, S.; Seistrup, K.H.; Marrs, E.C.L.; Hopkins, A.P.; Perry, J.D.; Strahl, H.; Hall, M.J.; Errington, J.; Allenby, N.E.E. Mode of action and heterologous expression of the natural product antibiotic vancoresmycin. ACS Chem. Biol. 2018, 13, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Binda, E.; Marinelli, F.; Marcone, G.L. Old and new glycopeptide antibiotics: Action and resistance. Antibiotics 2014, 3, 572–594. [Google Scholar] [CrossRef] [Green Version]
- McCormick, M.H.; Stark, W.M.; Pittenger, G.E.; Pittenger, R.C.; McGuire, J.M. Vancomycin, a new antibiotic. І. Chemical and biological properties. Antibiot. Annu. 1956, 3, 606–611. [Google Scholar]
- Rubinstein, E.; Keynan, Y. Vancomycin revisited—60 years later. Front. Public Health 2014, 2, 217. [Google Scholar] [CrossRef] [Green Version]
- Levine, D.P. Vancomycin: A history. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S5–S12. [Google Scholar] [CrossRef]
- Wang, W.Y.; Yang, S.B.; Wu, Y.J.; Shen, X.F.; Chen, S.X. Enhancement of A82846B yield and proportion by overexpressing the halogenase gene in Amycolatopsis orientalis SIPI18099. Appl. Microbiol. Biotechnol. 2018, 102, 5635–5643. [Google Scholar] [CrossRef]
- Patel, R. Enterococcal-type glycopeptide resistance genes in non-enterococcal organisms. FEMS Microbiol. Lett. 2000, 185, 1–7. [Google Scholar] [CrossRef]
- Sivagnanam, S.; Deleu, D. Red man syndrome. Crit. Care. 2003, 7, 119–120. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.C.; Boger, D.L. Maxamycins: Durable antibiotics derived by rational redesign of vancomycin. Acc. Chem. Res. 2020, 53, 2587–2599. [Google Scholar] [CrossRef]
- Xu, L.; Huang, H.; Wei, W.; Zhong, Y.; Tang, B.; Yuan, H.; Zhu, L.; Huang, W.; Ge, M.; Yang, S.; et al. Complete genome sequence and comparative genomic analyses of the vancomycin-producing Amycolatopsis orientalis. BMC Genom. 2014, 15, 363. [Google Scholar] [CrossRef] [Green Version]
- Yim, G.; Thaker, M.N.; Koteva, K.; Wright, G. Glycopeptide antibiotic biosynthesis. J. Antibiot. (Tokyo) 2014, 67, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, B.K.; Walsh, C.T. Vancomycin assembly: Nature’s way. Angew. Chem. Int. Ed Engl. 2003, 42, 730–765. [Google Scholar] [CrossRef]
- Nagarajan, R. Structure-activity relationships of vancomycin-type glycopeptide antibiotics. J. Antibiot. (Tokyo) 1993, 46, 1181–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gause, G.F.; Preobrazhenskaya, T.P.; Laiko, A.V.; Selezneva, T.I.; Sveshnikova, M.A.; Brazhnikova, M.G.; Fedorova, G.B.; Borisova, V.N.; Tolstykh, I.V.; Proshlyakova, V.V.; et al. The antibiotic “eremomycin” and the method of its preparation. Patent SU 1475150 A1, 27 May 1997. (In Russian). [Google Scholar]
- Gauze, G.F.; Brazhnikova, M.G.; Lomakina, N.N.; Gol’dberg, L.E.; Laiko, A.V. Eremomycin—A new antibiotic of the polycyclic glycopeptide group. Antibiot. Khimioter. 1989, 34, 348–352. (In Russian) [Google Scholar] [PubMed]
- Filippos’iants, S.T.; Malkova, I.V.; Gol’dberg, L.E. Glycopeptide antibiotics: Eremomycin, vancomycin, and teicoplanin. Comparison of several parameters of pharmacokinetics and antimicrobial activity. Antibiot. Khimioter. 1989, 34, 523–526. (In Russian) [Google Scholar] [PubMed]
- Alduina, R.; Gallo, G.; Renzone, G.; Weber, T.; Scaloni, A.; Puglia, A.M. Novel Amycolatopsis balhimycina biochemical abilities unveiled by proteomics. FEMS Microbiol. Lett. 2014, 351, 209–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohlleben, W.; Mast, Y.; Muth, G.; Röttgen, M.; Stegmann, E.; Weber, T. Synthetic Biology of secondary metabolite biosynthesis in actinomycetes: Engineering precursor supply as a way to optimize antibiotic production. FEBS Lett. 2012, 586, 2171–2176. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.C. Ristocetin. In Antibiotics. Mechanism of Action; Gottlieb, D., Shaw, P., Eds.; Springer: New York, NY, USA, 1967; Volume 1, pp. 84–89. [Google Scholar]
- Keesler, D.A.; Flood, V.H. Current issues in diagnosis and treatment of von Willebrand disease. Res. Pract. Thromb. Haemost. 2017, 2, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGahren, W.J.; Martin, J.H.; Morton, G.O.; Hargreaves, R.T.; Leese, R.A.; Lovell, F.M.; Ellestad, G.A.; O’Brien, E.; Holker, J.S.E. Structure of avoparcin components. J. Am. Chem. Soc. 1980, 102, 1671–1684. [Google Scholar] [CrossRef]
- Van de Kerk-van Hoof, A.; Heck, A.J. Interactions of α- and β-avoparcin with bacterial cell-wall receptor-mimicking peptides studied by electrospray ionization mass spectrometry. J. Antimicrob. Chemother. 1999, 44, 593–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouliouris, T.; Raven, K.E.; Ludden, C.; Blane, B.; Corander, J.; Horner, C.S.; Hernandez-Garcia, J.; Wood, P.; Hadjirin, N.F.; Radakovic, M.; et al. Genomic surveillance of Enterococcus faecium reveals limited sharing of strains and resistance genes between livestock and humans in the United Kingdom. mBio 2018, 9, e01780-18. [Google Scholar] [CrossRef] [Green Version]
- Birkegård, A.C.; Græsbøll, K.; Clasen, J.; Halasa, T.; Toft, N.; Folkesson, A. Continuing occurrence of vancomycin resistance determinants in Danish pig farms 20 years after removing exposure to avoparcin. Vet. Microbiol. 2019, 232, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Stogios, P.J.; Savchenko, A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020, 29, 654–669. [Google Scholar] [CrossRef]
- Kahne, D.; Leimkuhler, C.; Lu, W.; Walsh, C. Glycopeptide and lipoglycopeptide antibiotics. Chem. Rev. 2005, 105, 425–448. [Google Scholar] [CrossRef]
- Boneca, I.G.; Chiosis, G. Vancomycin resistance: Occurrence, mechanisms and strategies to combat it. Expert. Opin. Ther. Targets. 2003, 7, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R.; Derlot, E.; Duval, J.; Courvalin, P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N. Engl. J. Med. 1988, 319, 157–161. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, T.; Crank, C.W. Vancomycin-resistant enterococcal infections: Epidemiology, clinical manifestations, and optimal management. Infect. Drug. Resist. 2015, 8, 217–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arredondo-Alonso, S.; Top, J.; McNally, A.; Puranen, S.; Pesonen, M.; Pensar, J.; Marttinen, P.; Braat, J.C.; Rogers, M.R.C.; van Schaik, W.; et al. Plasmids shaped the recent emergence of the major nosocomial pathogen Enterococcus faecium. mBio 2020, 11, e03284-19. [Google Scholar] [CrossRef] [Green Version]
- Shariati, A.; Dadashi, M.; Moghadam, M.T.; van Belkum, A.; Yaslianifard, S.; Darban-Sarokhalil, D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12689. [Google Scholar] [CrossRef]
- Bartley, J. First case of VRSA identified in Michigan. Infect. Control Hosp. Epidemiol. 2002, 23, 480. [Google Scholar] [CrossRef]
- Stegmann, E.; Frasch, H.J.; Kilian, R.; Pozzi, R. Self-resistance mechanisms of actinomycetes producing lipid II-targeting antibiotics. Int. J. Med. Microbiol. 2015, 305, 190–195. [Google Scholar] [CrossRef]
- Marshall, C.G.; Lessard, I.A.; Park, I.; Wright, G.D. Glycopeptide antibiotic resistance genes in glycopeptide-producing organisms. Antimicrob. Agents Chemother. 1998, 42, 2215–2220. [Google Scholar] [CrossRef] [Green Version]
- Schäberle, T.F.; Vollmer, W.; Frasch, H.J.; Hüttel, S.; Kulik, A.; Röttgen, M.; von Thaler, A.K.; Wohlleben, W.; Stegmann, E. Self-resistance and cell wall composition in the glycopeptide producer Amycolatopsis balhimycina. Antimicrob. Agents Chemother. 2011, 55, 4283–4289. [Google Scholar] [CrossRef] [Green Version]
- Wehrli, W. Ansamycins. Chemistry, biosynthesis and biological activity. Top. Curr. Chem. 1977, 72, 21–49. [Google Scholar] [CrossRef]
- Farr, B.; Mandell, G.L. Rifampin. Med. Clin. North Am. 1982, 66, 157–168. [Google Scholar] [CrossRef]
- Lal, R.; Khanna, M.; Kaur, H.; Srivastava, N.; Tripathi, K.K.; Lal, S. Rifamycins: Strain improvement program. Crit. Rev. Microbiol. 1995, 21, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Peano, C.; Damiano, F.; Forcato, M.; Pietrelli, A.; Palumbo, C.; Corti, G.; Siculella, L.; Fuligni, F.; Tagliazucchi, G.M.; De Benedetto, G.E.; et al. Comparative genomics revealed key molecular targets to rapidly convert a reference rifamycin-producing bacterial strain into an overproducer by genetic engineering. Metab. Eng. 2014, 26, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Floss, H.G. Antibiotic biosynthesis: From natural to unnatural compounds. J. Ind. Microbiol. Biotechnol. 2001, 27, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Lei, C.; Li, F.; Zhang, X.; Wang, J.; Zhang, W.; Fan, Z.; Li, W.; Tang, G.L.; Xiao, Y.; et al. Deciphering the late steps of rifamycin biosynthesis. Nat. Commun. 2018, 9, 2342. [Google Scholar] [CrossRef]
- Robertsen, H.L.; Musiol-Kroll, E.M. Actinomycete-derived polyketides as a source of antibiotics and lead structures for the development of new antimicrobial drugs. Antibiotics 2019, 8, 157. [Google Scholar] [CrossRef] [Green Version]
- Bergamini, N.; Fowst, G. Rifamycin SV. A review. Arzneimittelforschung 1965, 15, 951–1002. [Google Scholar]
- Aristoff, P.A.; Garcia, G.A.; Kirchhoff, P.D.; Showalter, H.D. Rifamycins-obstacles and opportunities. Tuberculosis 2010, 90, 94–118. [Google Scholar] [CrossRef]
- Sensi, P. History of the development of rifampin. Rev. Infect. Dis. 1983, 5, S402–S406. [Google Scholar] [CrossRef]
- Xu, J.; Wan, E.; Kim, C.J.; Floss, H.G.; Mahmud, T. Identification of tailoring genes involved in the modification of the polyketide backbone of rifamycin B by Amycolatopsis mediterranei S699. Microbiology (Reading) 2005, 151 Pt 8, 2515–2528. [Google Scholar] [CrossRef] [Green Version]
- Ghisalba, O.; Traxler, P.; Nüesch, J. Early intermediates in the biosynthesis of ansamycins. I. Isolation and identification of protorifamycin I. J. Antibiot. (Tokyo) 1978, 31, 1124–1131. [Google Scholar] [CrossRef]
- Ghisalba, O.; Traxler, P.; Fuhrer, H.; Richter, W.J. Early intermediates in the biosynthesis of ansamycins. II. Isolation and identification of proansamycin B-M1 and protorifamycin i-M1. J. Antibiot. (Tokyo) 1979, 32, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Ghisalba, O.; Traxler, P.; Fuhrer, H.; Richter, W.J. Early intermediates in the biosynthesis of ansamycins. III. Isolation and identification of further 8-deoxyansamycins of the rifamycin-type. J. Antibiot. (Tokyo) 1980, 33, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, E.; Gallo, G.G.; Antonini, P.; White, R.J. Structure of rifamycin W, a novel ansamycin from a mutant of Nocardia mediterranea. Tetrahedron 1974, 30, 3087–3091. [Google Scholar] [CrossRef]
- Cricchio, R.; Antonini, P.; Ferrari, P.; Ripamonti, A.; Tuan, G.; Martinelli, E. Rifamycin Z, a novel ansamycin from a mutant of Nocardia mediterranea. J. Antibiot. (Tokyo) 1981, 34, 1257–1260. [Google Scholar] [CrossRef] [Green Version]
- Sensi, P.; Timbal, M.T.; Maffii, G. Rifomycin IX. Two new antibiotics of rifomycin family: Rifomycin S and rifomycin SV. Preliminary report. Experientia 1960, 16, 412. [Google Scholar] [CrossRef]
- Lancini, G.; Hengeller, C. Isolation of rifamycin SV from a mutant Streptomyces mediterranei strain. J. Antibiot. (Tokyo) 1969, 22, 637–638. [Google Scholar] [CrossRef]
- Martinelli, E.; Antonini, P.; Cricchio, R.; Lancini, G.; White, R.J. Rifamycin R, a novel metabolite from a mutant of Nocardia mediterranea. J. Antibiot. (Tokyo) 1978, 31, 949–951. [Google Scholar] [CrossRef] [Green Version]
- Lancini, G.; Sartori, G. Rifamycin G, a further product of Nocardia mediterranei metabolism. J. Antibiot. (Tokyo) 1976, 29, 466–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitich, J.; Prelog, V.; Sensi, P. Rifomycin Y and its transformation products. Experientia 1967, 23, 505–507. [Google Scholar] [CrossRef]
- Lancini, G.C.; Thiemann, J.E.; Sartori, G.; Sensi, P. Biogenesis of rifamycins. The conversion of rifamycin B into rifamycin Y. Experientia 1967, 23, 899–900. [Google Scholar] [CrossRef]
- Stratmann, A.; Schupp, T.; Toupet, C.; Schilling, W.; Oberer, L.; Traber, R. New insights into rifamycin B biosynthesis: Isolation of proansamycin B and 34a-deoxy-rifamycin W as early macrocyclic intermediates indicating two separated biosynthetic pathways. J. Antibiot. (Tokyo) 2002, 55, 396–406. [Google Scholar] [CrossRef]
- Sensi, P.; Ballotta, R.; Greco, M. Rifomycin. V. Rifomycin O, a new antibiotic of the rifomycin family. Farmaco Sci. 1960, 15, 228–234. [Google Scholar] [PubMed]
- Hanh, B.T.B.; Park, J.W.; Kim, T.H.; Kim, J.S.; Yang, C.S.; Jang, K.; Cui, J.; Oh, D.C.; Jang, J. Rifamycin O, an alternative anti-Mycobacterium abscessus agent. Molecules 2020, 25, 1597. [Google Scholar] [CrossRef] [Green Version]
- Cricchio, R.; Antonini, P.; Sartori, G. Thiazorifamycins. III. Biosynthesis of rifamycins P, Q and verde, novel metabolites from a mutant of Nocardia mediterranea. J. Antibiot. (Tokyo) 1980, 33, 842–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancini, G.C.; Gallo, G.G.; Sartori, G.; Sensi, P. Isolation and structure of rifamycin L and its biogenetic relationship with other rifamycins. J. Antibiot. (Tokyo) 1969, 22, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Hengeller, C.; Lancini, G.; Sensi, P. 27-Demethoxy-27-Hydroxyrifamycin Derivatives. US Patent 3743635A, 3 July 1973. [Google Scholar]
- Traxler, P.; Schupp, T.; Fuhrer, H.; Richter, W.J. 3-Hydroxyrifamycin S and further novel ansamycins from a recombinant strain R-21 of Nocardia mediterranei. J. Antibiot. (Tokyo) 1981, 34, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Portero, J.-L.; Rubio, M. New anti-tuberculosis therapies. Expert Opin. Ther. Patents 2007, 17, 617–637. [Google Scholar] [CrossRef]
- Tomioka, H.; Namba, K. Development of antituberculous drugs: Current status and future prospects. Kekkaku 2006, 81, 753–774. (In Japanese) [Google Scholar]
- Chakraborty, S.; Rhee, K.Y. Tuberculosis drug development: History and evolution of the mechanism-based paradigm. Cold Spring Harb. Perspect. Med. 2015, 5, a021147. [Google Scholar] [CrossRef] [Green Version]
- Udwadia, Z.F. MDR, XDR, TDR tuberculosis: Ominous progression. Thorax 2012, 67, 286–288. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.-F.; Liu, X.-Q.; Peng, S.-Y.; Zhou, Q.; Xu, B.; Yuan, H.; Tang, G.-L. Characterization of the rifamycin-degrading monooxygenase from rifamycin producers implicating its involvement in saliniketal biosynthesis. Front. Microbiol. 2020, 11, 971. [Google Scholar] [CrossRef]
- Rothstein, D.M. Rifamycins, alone and in combination. Cold Spring Harb. Perspect. Med. 2016, 6, a027011. [Google Scholar] [CrossRef]
- Nigam, A.; Almabruk, K.H.; Saxena, A.; Yang, J.; Mukherjee, U.; Kaur, H.; Kohli, P.; Kumari, R.; Singh, P.; Zakharov, L.N.; et al. Modification of rifamycin polyketide backbone leads to improved drug activity against rifampicin-resistant Mycobacterium tuberculosis. J. Biol. Chem. 2014, 289, 21142–21152. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Ye, F.; Song, Y.; Zhang, X.; Lu, C.; Shen, Y. Rifamycin W analogues from Amycolatopsis mediterranei S699 Δrif-orf5 strain. Biomolecules 2021, 11, 920. [Google Scholar] [CrossRef]
- Ye, F.; Shi, Y.; Zhao, S.; Li, Z.; Wang, H.; Lu, C.; Shen, Y. 8-Deoxy-rifamycin derivatives from Amycolatopsis mediterranei S699 ΔrifT strain. Biomolecules 2020, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Peek, J.; Xu, J.; Wang, H.; Suryavanshi, S.; Zimmerman, M.; Russo, R.; Park, S.; Perlin, D.S.; Brady, S.F. A Semisynthetic Kanglemycin shows in vivo efficacy against high-burden rifampicin resistant pathogens. ACS Infect. Dis. 2020, 6, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I. Tetracycline analogs whose primary target is not the bacterial ribosome. Antimicrob. Agents Chemother. 1994, 38, 637–640. [Google Scholar] [CrossRef] [Green Version]
- Stepanek, J.J.; Lukežič, T.; Teichert, I.; Petković, H.; Bandow, J.E. Dual mechanism of action of the atypical tetracycline chelocardin. Biochim. Biophys. Acta 2016, 1864, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Lešnik, U.; Lukežič, T.; Podgoršek, A.; Horvat, J.; Polak, T.; Šala, M.; Jenko, B.; Harmrolfs, K.; Ocampo-Sosa, A.; Martínez-Martínez, L.; et al. Construction of a new class of tetracycline lead structures with potent antibacterial activity through biosynthetic engineering. Angew. Chem. Int. Ed. Engl. 2015, 54, 3937–3940. [Google Scholar] [CrossRef] [PubMed]
- Grandclaudon, C.; Birudukota, N.V.S.; Elgaher, W.A.M.; Jumde, R.P.; Yahiaoui, S.; Arisetti, N.; Hennessen, F.; Hüttel, S.; Stadler, M.; Herrmann, J.; et al. Semisynthesis and biological evaluation of amidochelocardin derivatives as broad-spectrum antibiotics. Eur. J. Med. Chem. 2020, 188, 112005. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, S.; Mayilraj, S. Genome sequencing and annotation of Amycolatopsis vancoresmycina strain DSM 44592T. Genom. Data 2014, 2, 16–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bergeijk, D.A.; Terlouw, B.R.; Medema, M.H.; van Wezel, G.P. Ecology and genomics of Actinobacteria: New concepts for natural product discovery. Nat. Rev. Microbiol. 2020, 18, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Y.; Xiao, Y.S.; Zhang, B.; Shao, F.L.; Guo, Z.K.; Zhang, J.J.; Jiao, R.H.; Sun, Y.; Xu, Q.; Tan, R.X.; et al. Amycolamycins A and B, two enediyne-derived compounds from a locust-associated Actinomycete. Org. Lett. 2017, 19, 6208–6211. [Google Scholar] [CrossRef] [PubMed]
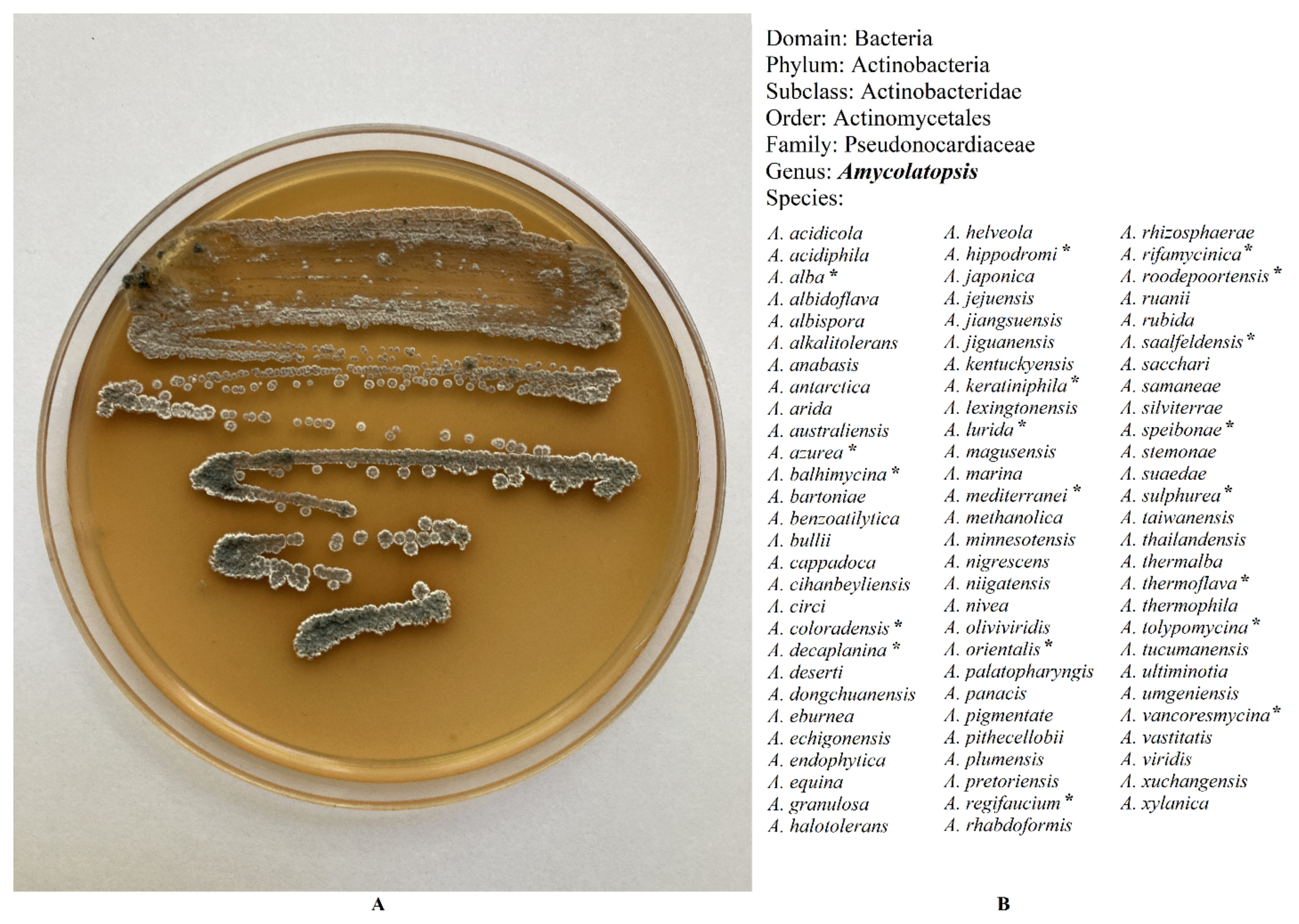

| Genus | Average Total Genome Length (Mb) [19] | Number of Biosynthetic Gene Clusters [25] |
|---|---|---|
| Actinoplanes | 9 | 11 |
| Actinomadura | 9 | 10 |
| Amycolatopsis | 9 | 25 |
| Micromonospora | 7 | 26 |
| Nocardia | 8 | 6 |
| Streptomyces | 9 | 637 |
| Streptosporangium | 10 | 3 |
| Species, Strains | Antibiotics | Properties | References |
|---|---|---|---|
| Amycolatopsis sp. 17128 | Mutactimycin A, D, E | Antimicrobial activity against Gram-positive bacteria (including MRSA 1) | [31] |
| Amycolatopsis sp. Cra33g | Amycolactam | Significant cytotoxicity | [32] |
| Amycolatopsis sp. Hca4 | Rifamorpholines A–E | Antimicrobial activity against Gram-positive bacteria (including MRSA) | [13] |
| Amycolatopsis sp. IRD-009 | Pradimicin-IRD | Antimicrobial activity against Gram-positive and Gram-negative bacteria; Cytotoxic activity against cancer cell lines | [33] |
| Amycolatopsis sp. К16-0194 | Dipyrimicins A and B | Dipyrimicin A exhibits strong antimicrobial and cytotoxic activities; Dipyrimicin B exhibits antimicrobial activity against Escherichia coli | [34] |
| Amycolatopsis sp. LZ149 | Siderochelins A, D, E, and F | Siderochelin A exhibits antimicrobial activity against Gram-positive bacteria and Escherichia coli; Siderochelins A, D and E exhibit antimicrobial activity against Mycobacterium smegmatis | [35,36] |
| Amycolatopsis sp. M39 | Macrotermycins A–D | Macrotermycins A and C had antimicrobial activity against Gram-positive bacteria (particularly staphylococcal infections); Selective antifungal activity (against a fungal parasite of the termite fungal garden) | [14] |
| Amycolatopsis sp. MI481-42F4 | Аmythiamicins A, B, C and D | Antimicrobial activity against Gram-positive bacteria (including MDR 2 strains) | [37,38] |
| Amycolatopsis sp. MJM2582 | Ristocetin (Ristomycin) | Antimicrobial activity against Gram-positive pathogenic infections (particularly staphylococcal infections); Applied to the in vitro diagnosis of conditions such as von Willebrand disease and Bernard–Soulier syndrome | [39,40,41] |
| Amycolatopsis sp. ML1-hF4 | Рargamicins A | Antimicrobial activity against Staphylococcus aureus strains (including MRSA) and Enterococcus faecalis, E. faecium strains (including VRE 3) | [42] |
| Valgamicins A, C, T and V | Weak activity against Gram-positive and Gram-negative bacteria; Valgamicins A, C and T exhibit moderate cytotoxicity against human tumor cell lines | [43] | |
| Amycolatopsis sp. YIM 130642 | Amycophthalazinone A | Weak antimicrobial and antifungal activities | [44] |
| Amycolatopsis sp. YIM 130687 | 2-carbamoyl-3-hydroxy-1,4-naphthoquinone | Strong antimicrobial (including MRSA) and antifungal activities | [45] |
| Amycolatopsis sp. AA4 | Amycomicin | Strong antimicrobial activity against Staphylococcus aureus | [46] |
| A. alba | 1(10-aminodecyl) pyridinium | Antimicrobial activity against Gram-positive and Gram-negative bacteria; Cytotoxic activity against cancer cell lines | [47] |
| Kigamicins A-E | Antimicrobial activity against Gram-positive bacteria (including MRSA); Kigamicin D is an anticancer agent | [48,49] | |
| Maytansinoids 1–14 | Maytansinoids 7 and 13 showed antitumor activities against four human cancer cell lines | [50] | |
| A. australiensis | Antibiotic biosynthetic genes were identified | [27] | |
| A. azurea | Azureomycins A and B | Strong antimicrobial activity against Gram-positive bacteria | [51,52] |
| Оctacosamicins A and B | Very weak or no activity against Gram-positive and Gram-negative bacteria; Moderate activity against fungi and yeast | [53,54] | |
| A. balhimycina | Balhimycin | Antimicrobial activity against Gram-positive bacteria (including MRSA) | [55] |
| A. coloradensis | Avoparcin (avotan) | Antimicrobial activity against Gram-positive bacteria; Animal growth promoter | [56,57,58] |
| A. decaplanina | Decaplanin | Antimicrobial activity against Gram-positive bacteria (including antibiotic-resistant enterococci and clinical isolates) | [59,60] |
| A. hippodromi | Amycolasporins A−C | Antimicrobial activity against Gram-positive and Gram-negative bacteria | [61] |
| A. japonica | Ristocetin (Ristomycin) | Antimicrobial activity against Gram-positive bacteria, (particularly staphylococcal infections); Applied to the in vitro diagnosis of conditions such as von Willebrand disease and Bernard–Soulier syndrome | [62] |
| A. jejuensis | Antibiotic biosynthetic genes were identified | [63] | |
| A. keratiniphila | Keratinimicins A–D; Keratinicyclin A–C | Keratinimicins A and C exibit strong antimicrobial activity against Gram-positive bacteria (particularly staphylococcal infections); keratinicyclin В exibit moderate antimicrobial activity against Streptococcus spp. and Clostridium difficile | [29] |
| A. keratiniphila subsp. keratiniphila | Antibiotic biosynthetic genes were identified | [27] | |
| A. keratiniphila subsp. nogabecina | Nogabecin (Actinoidin B) | Antimicrobial activity against Gram-positive bacteria | [64,65] |
| A. lactamdurans * | Cephamycin C | Antimicrobial activity against Gram-positive and Gram-negative bacteria (including resistant strains); very efficient antibiotic against anaerobic microbes | [66,67,68,69] |
| Efrotomycin | Antimicrobial activity against Gram-positive bacteria | [68,70] | |
| A. lurida | Benzanthrins A and B | Antimicrobial activity against Gram-positive bacteria; Inhibit the growth tumor cells in tissue culture | [71,72] |
| A. lurida | Ristocetin (Ristomycin) | Antimicrobial activity against Gram-positive bacteria, (particularly staphylococcal infections); Applied to the in vitro diagnosis of conditions, such as von Willebrand disease and Bernard–Soulier syndrome | [41,73,74] |
| A. mediterranei | Amexanthomycins A–J | Inhibitory activity against human DNA topoisomerases | [75] |
| Dethymicin | Antimicrobial activity against Gram-positive bacteria (including MRSA); Immunosuppressant | [76] | |
| Kanglemycin A | Antimicrobial activity against Gram-positive bacteria (including rifampicin-resistant ones and M. tuberculosis with MDR) | [77,78] | |
| Rifamycines | Strong antimicrobial activity against Gram-positive bacteria (particularly mycobacteria) | [79,80,81,82,83] | |
| Tetracenomycin Х | Antimicrobial activity against Gram-positive bacteria; Showed antitumour activity in vivo | [84,85] | |
| A. minnesotensis | Antibiotic biosynthetic genes were identified | [27] | |
| A. nigrescens | Antibiotic biosynthetic genes were identified | [27] | |
| A. niigatensis | Antibiotic biosynthetic genes were identified | [27] | |
| A. orientalis | Vancomycin | A last-line drug for the treatment of infections caused by almost all clinically significant Gram-positive bacteria (including MRSA) | [3,86] |
| N–Demethylvancomycin | Antimicrobial activity against Gram-positive bacteria (including MRSA) | [87,88,89,90] | |
| N,N–Demethylvancomycin | Antimicrobial activity against Gram-positive bacteria | [91] | |
| Norvancomycin | Antimicrobial activity against Gram-positive bacteria (particularly MRSA and MRSE 4) | [92,93] | |
| Quartromicin (the complex of at least six antibiotics components A1, A2, A3, D1, D2, and D3) | Antiviral activity against herpes simplex virus type 1, influenza virus type A and human immunodeficiency virus | [94] | |
| UK-69753 | Strong antimicrobial activity in vitro and in vivo against the swine Gram-negative anaerobic pathogen Treponema hyodysenteriae | [95,96] | |
| MM 47761 and MM 4972; MM 55266, and MM 55268 | Antimicrobial activity against Gram-positive bacteria | [97,98] | |
| Eremomycin В | Antimicrobial activity against Gram-positive bacteria | [99,100,101] | |
| Orienticins A-D | Antimicrobial activity against S. aureus (including MRSA) | [102] | |
| Сhloroorienticins A-E | Antimicrobial activity against S. aureus (including MRSA) | [103] | |
| LY264826 | Antimicrobial activity against Gram-positive bacteria (including MRSA) | [104] | |
| ECO-0501 | Strong antimicrobial activity against Gram-positive bacteria (including MRSA and VRE) | [28] | |
| A. palatopharyngis | Antibiotic biosynthetic genes were identified | [27] | |
| A. regifaucium | Kigamicins A-E | Antimicrobial activity against Gram-positive bacteria (including MRSA); Kigamicin D is an anticancer agent | [48,105,106,107] |
| A. rifamycinica | Tetracenomycin Х | Moderate antimicrobial activity against Gram-positive organisms (including resistant strains); Activity against certain tumor cell lines | [108,109] |
| A. roodepoortensis | Antibiotic biosynthetic genes were identified; Antimicrobial activity against Gram-positive (particularly mycobacteria) and Gram-negative bacteria | [24] | |
| A. rubida | Antibiotic biosynthetic genes were identified | [27] | |
| A. saalfeldensis | Saalfelduracin | Strong antimicrobial activity against drug-resistant Gram-positive bacteria | [110] |
| A. speibonae | Antibiotic biosynthetic genes were identified; Antimicrobial activity against Gram-positive bacteria (particularly mycobacteria) | [24] | |
| A. speibonae | Echinosporin 7-deoxyechinosporin | Antifungal activity against root-rot pathogens of the Panax notoginseng | [111] |
| A. sulphurea | Epoxyquinomicins A-D | Epoxyquinomicins A and B exhibit antimicrobial activity against Gram-positive bacteria; Epoxyquinomicins C and D exhibit almost no antimicrobial activity and no cytotoxicity; All these antibiotics showed improvement of collagen induced arthritis in vivo | [112,113] |
| Azicemicins A and B | Moderate antimicrobial activity against Gram-positive bacteria (particularly mycobacteria) | [114,115] | |
| Chelocardin (Cetocycline) | Antimicrobial activity against Gram-positive and Gram-negative (including tetracycline-resistant pathogens and MDR pathogens) | [116,117,118] | |
| A. taiwanensis | Antibiotic biosynthetic genes were identified | [27] | |
| A. thermoflava | Antibiotic biosynthetic genes were identified | [27] | |
| 1-methoxy-3-methyl-8-hydroxy-anthraquinone | Antibiotic biosynthetic genes were identified Anticancer activity against lung cancer and lymphoblastic leukemia cells | [119] | |
| A. tolypomycina | Tolypomycin | Strong antimicrobial activities against Gram-positive bacteria | [65,120] |
| A. tucumanensis | Antibiotic biosynthetic genes were identified | [27] | |
| A. umgeniensis | Eremomycin В | Antimicrobial activity against Gram-positive bacteria | [121] |
| A. vancoresmycina | Vancoresmycin | Antimicrobial activity against Gram-positive bacteria (including resistant strains) | [122,123] |
| А. xylanica | Antibiotic biosynthetic genes were identified | [27] | |
| Rifamycin Metabolites | Possible Precursor | Properties | References |
|---|---|---|---|
| Proansamycin X | The first hypothetical macrocyclic intermediate of rifamycin biosynthesis has never been isolated and identified | [83,169] | |
| Protorifamycin I (8-deoxyansamycins W) | Proansamycin X | No activity against Gram-positive bacteria or Gram-negative bacteria | [170] |
| modified protorifamycins (derived from protorifamycin I) and defective rifamycins (8-deoxyrifamycins) | Protorifamycin I | No antibiotic activity | [171,172] |
| Rifamycin W | Proansamycin X | No activity against Gram-positive bacteria or Gram-negative bacteria | [173] |
| Rifamycin Z | Rifamycin W | No activity against Gram-positive bacteria or Gram-negative bacteria | [174] |
| 31-Homorifamycin W | Rifamycin W | No significant antibacterial, antifungal, or antiviral activity | [81] |
| Rifamycin SV | Rifamycin W | Strong activity against Gram-positive bacteria (particularly mycobacteria) | [175,176] |
| Rifamycin S | Rifamycin SV | Strong activity against Gram-positive bacteria (particularly mycobacteria) | [175] |
| Rifamycin R | Rifamycin S | Strong activity against Gram-positive bacteria (particularly mycobacteria) | [177] |
| Rifamycin G | Rifamycin S | Activity against M. tuberculosis | [178] |
| Rifamycin Y | Rifamycin B | Antibiotically inactive | [179,180] |
| Rifamycin YO, YS, Isorifamycin Y | Rifamycin Y | Antibiotically inactive | [179] |
| Protorifamycin B, 34a-deoxy-rifamycin W, Rifamycin W-28-desmethyl-28-carboxy, Rifamycin W-hemiacetal | Rifamycin W | No data | [181] |
| Rifamycin O | Rifamycin L | Activity against M.abscessus | [164,182,183] |
| Thiazorifamycins: Rifamycin Q, Rifamycin P, Rifamycin Verde | Rifamycin S | No data | [184] |
| Rifamycin L | Rifamycin S | Good antimicrobial activity against Gram-positive and Gram-negative bacteria | [164,185] |
| Rifamycin В | Rifamycin S | Activity against Gram-positive bacteria (particularly mycobacteria) | [79,164] |
| 27-Demethoxy-27-hydroxyrifamycin derivatives | Rifamycin SV | Activity against several Gram-negative bacteria | [186] |
| 3-Hydroxyrifamycin S and further novel ansamycins S, G and W type | Rifamycin S and W, respectively | Ansamycins W type are devoid of any biological activity. Other ansamacins exhibit activity against Gram-positive and Gram-negative bacteria | [187] |
| Rifamorpholines А-Е | Rifamycin S | Rifamorpholines B and D exhibit antimicrobial activity against methicillin-resistant S. aureus (MRSA) | [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisil, O.V.; Efimenko, T.A.; Efremenkova, O.V. Looking Back to Amycolatopsis: History of the Antibiotic Discovery and Future Prospects. Antibiotics 2021, 10, 1254. https://doi.org/10.3390/antibiotics10101254
Kisil OV, Efimenko TA, Efremenkova OV. Looking Back to Amycolatopsis: History of the Antibiotic Discovery and Future Prospects. Antibiotics. 2021; 10(10):1254. https://doi.org/10.3390/antibiotics10101254
Chicago/Turabian StyleKisil, Olga V., Tatiana A. Efimenko, and Olga V. Efremenkova. 2021. "Looking Back to Amycolatopsis: History of the Antibiotic Discovery and Future Prospects" Antibiotics 10, no. 10: 1254. https://doi.org/10.3390/antibiotics10101254
APA StyleKisil, O. V., Efimenko, T. A., & Efremenkova, O. V. (2021). Looking Back to Amycolatopsis: History of the Antibiotic Discovery and Future Prospects. Antibiotics, 10(10), 1254. https://doi.org/10.3390/antibiotics10101254






