Infection Control in the Era of COVID-19: A Narrative Review
Abstract
1. Introduction
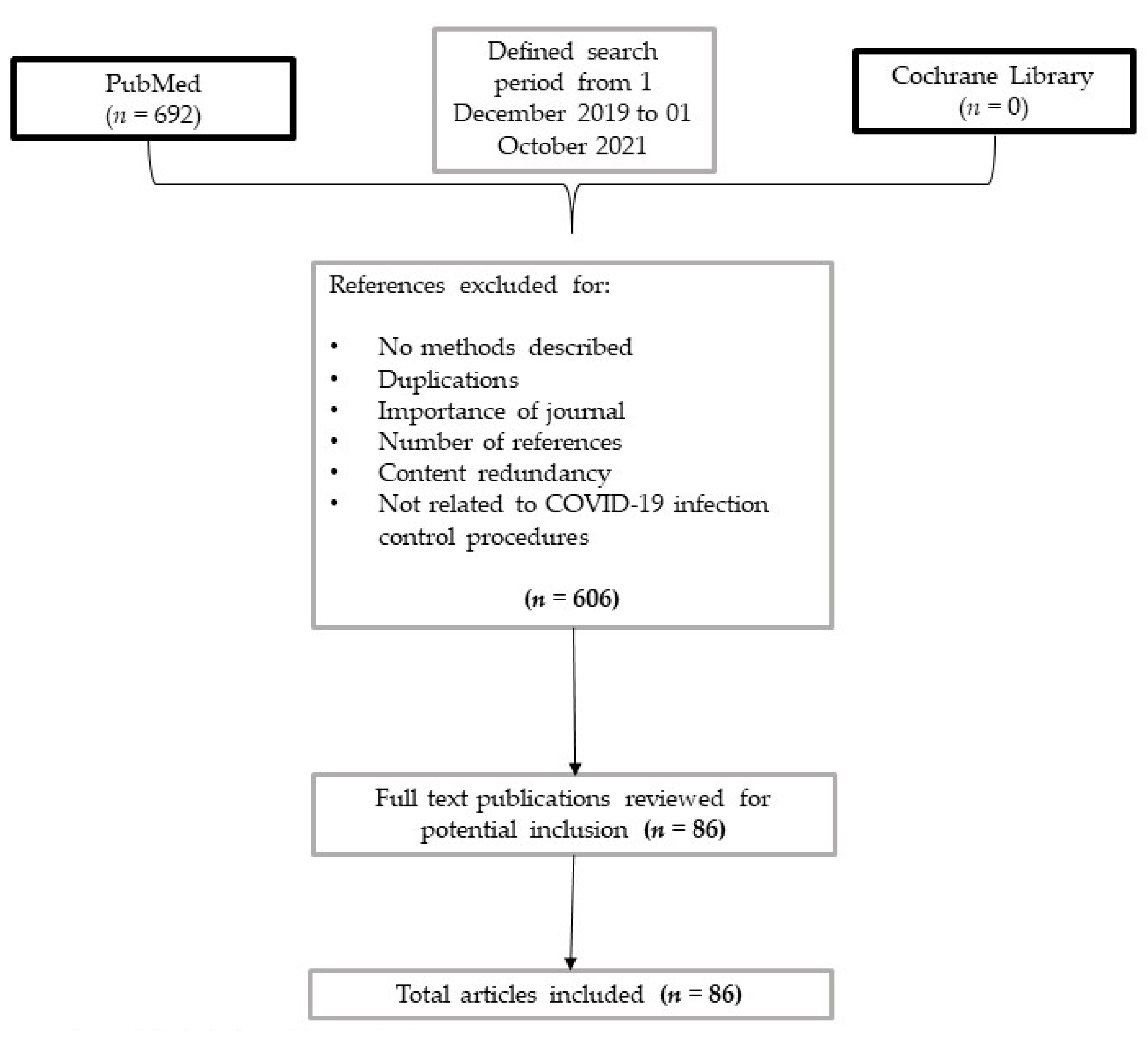
2. Methods
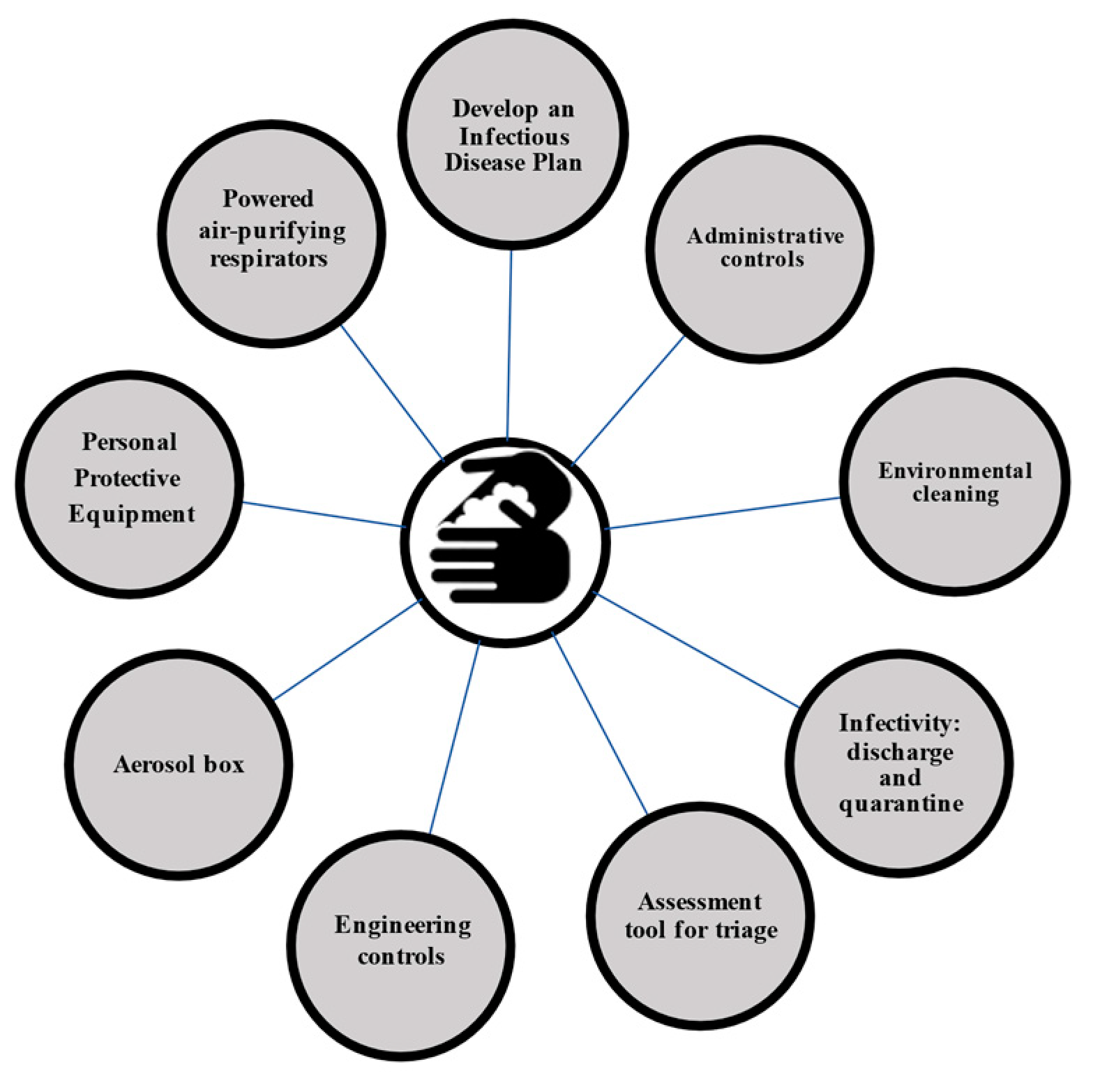
3. Results
3.1. Develop an Infectious Disease Plan
3.2. Administrative Controls
3.3. Assessment Tool for Triage
3.4. Engineering Controls
- a.
- Viral Clearance Periods
- b.
- Theatre bundling
- c.
- Physical barriers
3.5. Aerosol Box
3.6. Personal Protective Equipment (PPE)
3.7. PPE Reuse
3.8. Types of PPE Masques
3.9. Powered Air-Purifying Respirators (PAPRs)
3.10. Environmental Cleaning
3.11. Infectivity: Discharge and Quarantine
- a.
- Semi-quantitative Polymerase Chain Reaction (PCR)
- b.
- Discharge and Quarantine
- c.
- Antigen Testing for Nosocomial Infections
- d.
- Decision-making for pursuing work for HCW
3.12. Corpse Handling and Management
4. Discussion
4.1. Discrpancy and Limitations among International Guidelines
4.2. Changes in Guidelines
4.3. Unanswered Questions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Pneumonia of Unknown Cause—China. Available online: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ (accessed on 12 October 2021).
- De Rosa, F.G.; Palazzo, A.; Rosso, T.; Shbaklo, N.; Mussa, M.; Boglione, L.; Borgogno, E.; Rossati, A.; Mornese Pinna, S.; Scabini, S.; et al. Risk Factors for Mortality in COVID-19 Hospitalized Patients in Piedmont, Italy: Results from the Multicenter, Regional, CORACLE Registry. J. Clin. Med. 2021, 10, 1951. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Lupia, T.; Corcione, S.; de Rosa, F.G. COVID-19: In the uncertainty, do not try this at home. Intern. Emerg. Med. 2020, 15, 1599–1600. [Google Scholar] [CrossRef]
- Yan, Y.; Shin, W.I.; Pang, Y.X.; Meng, Y.; Lai, J.; You, C.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. The First 75 Days of Novel Coronavirus (SARS-CoV-2) Outbreak: Recent Advances, Prevention, and Treatment. Int. J. Environ. Res. Public Health 2020, 17, 2323. [Google Scholar] [CrossRef]
- Lupia, T.; Scabini, S.; Pinna, S.M.; di Perri, G.; de Rosa, F.G.; Corcione, S. 2019 novel coronavirus (2019-nCoV) outbreak: A new challenge. J. Glob. Antimicrob. Resist. 2020, 21, 22–27. [Google Scholar] [CrossRef]
- John Hopkins Corona Virus Resource Center. COVID-19 Dashboard by the Center for Systems Science and Engi-neering (CSSE) at Johns Hopkins University (JHU). Available online: https://coronavirus.jhu.edu/map.html (accessed on 6 October 2021).
- European Centre for Disease Prevention and Control. Novel Coronavirus Disease 2019 (COVID-19) Pandemic: Increased Transmission in the EU/EEA and the UK—Sixth Update—12 March 2020; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- Seto, W.H.; Tsang, D.; Yung, R.W.H.; Ching, T.Y.; Ng, T.K.; Ho, M.; Ho, L.M.; Peiris, J.S.M. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet 2003, 361, 1519–1520. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Lau, S.K.P.; Woo, P.C.Y.; Kwok, Y.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007, 20, 660–694. [Google Scholar] [CrossRef]
- Chan, W.M.; Yuen, K.S.C.; Fan, D.S.P.; Lam, D.S.C.; Chan, P.K.S.; Sung, J.J.Y. Tears and conjunctival scrapings for coronavirus in patients with SARS. Br. J. Ophthalmol. 2004, 88, 968–969. [Google Scholar] [CrossRef]
- Zumla, A.; Hui, D.S.; Perlman, S. Middle East respiratory syndrome. Lancet 2015, 386, 995–1007. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Zhao, G.; Chu, H.; Wang, D.; Yan, H.; Poon, V.; Wen, L.; Wong, B.; Zhao, X.; et al. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci. Adv. 2017, 3, 4966–4981. [Google Scholar] [CrossRef]
- Chou, R.; Dana, T.; Buckley, D.I.; Selph, S.; Fu, R.; Totten, A.M. Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers: A Living Rapid Review. Ann. Intern. Med. 2020, 173, 120–136. [Google Scholar] [CrossRef]
- Cawcutt, K.A.; Starlin, R.; Rupp, M.E. Fighting fear in healthcare workers during the COVID-19 pandemic. Infect. Control. Hosp. Epidemiol. 2020, 41, 1192–1193. [Google Scholar] [CrossRef]
- Lai, X.; Wang, X.; Yang, Q.; Xu, X.; Tang, Y.; Liu, C.; Tan, L.; Lai, R.; Wang, H.; Zhang, X.; et al. Will healthcare workers improve infection prevention and control behaviors as COVID-19 risk emerges and increases, in China? Antimicrob. Resist. Infect. Control. 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Fix, G.M.; Reisinger, H.S.; Etchin, A.; McDannold, S.; Eagan, A.; Findley, K.; Gifford, A.; Gupta, K.; McInnes, D.K. Health care workers’ perceptions and reported use of respiratory protective equipment: A qualitative analysis. Am. J. Infect. Control 2019, 47, 1162–1166. [Google Scholar] [CrossRef]
- Yen, M.-Y.; Lu, Y.-C.; Huang, P.-H.; Chen, C.-M.; Chen, Y.-C.; Lin, Y.E. Quantitative evaluation of infection control models in the prevention of nosocomial transmission of SARS virus to healthcare workers: Implication to nosocomial viral infection control for healthcare workers. Scand. J. Infect. Dis. 2010, 42, 510–515. [Google Scholar] [CrossRef]
- World Health Organization. Infection Prevention and Control during Health Care when Novel Coronavirus (nCoV) Infection is Suspected. Available online: https://www.who.int/publications/i/item/10665-331495 (accessed on 6 October 2021).
- Bureau of Disease Prevention and Control of the National Health Commission of the People’s Republic of China. Novel Coronavirus Pneumonia and Prevention Control. Program, 5th ed.; Bureau of Disease Prevention and Control of the National Health Commission of the People’s Republic of China: Beijing, China, 2020. (In Chinese) [Google Scholar]
- Belfroid, E.; van Steenbergen, J.; Timen, A.; Ellerbroek, P.; Huis, A.; Hulscher, M. Preparedness and the importance of meeting the needs of healthcare workers: A qualitative study on Ebola. J. Hosp. Infect. 2018, 98, 212–218. [Google Scholar] [CrossRef]
- Storr, J.; Twyman, A.; Zingg, W.; Damani, N.; Kilpatrick, C.; Reilly, J.; Price, L.; Egger, M.; Grayson, M.L.; Kelley, E.; et al. Core components for effective infection prevention and control programmes: New WHO evidence-based recommendations. Antimicrob. Resist. Infect. Control. 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Ilesanmi, O.S.; Afolabi, A.A.; Akande, A.; Raji, T. Mohammed, “Infection prevention and control during COVID-19 pandemic: Realities from health care workers in a north central state in Nigeria. Epidemiol. Infect. 2021, 149, 1–34. [Google Scholar] [CrossRef]
- Ramazzini, T.F.C. 24th Collegium Ramazzini Statement: Prevention of Work-Related Infection in the COVID-19 Pandemic. Ann. Glob. Health 2020, 86, 1–3. [Google Scholar] [CrossRef]
- MKhalefa, A.; Khadabadi, N.A.; Moores, T.S.; Hossain, F.S. Evidence-based review of safe theatre practice during the COVID-19 pandemic beyond personal protective equipment. Ann. R. Coll. Surg. Engl. 2021, 103, 88–95. [Google Scholar] [CrossRef]
- Solstad, E.; Pettersen, J.; Robbins, I. Hospitals as professional organizations and the perception of distances. Financ. Account. Manag. 2021, 37, 20–36. [Google Scholar] [CrossRef]
- Yetmar, Z.A.; Issa, M.; Munawar, S.; Burton, M.C.; Pureza, V.; Sohail, M.R.; Mehmood, T. Inpatient Care of Patients with COVID-19: A Guide for Hospitalists. Am. J. Med. 2020, 133, 1019–1024. [Google Scholar] [CrossRef]
- Ağalar, C.; Engin, D.Ö. Protective measures for COVID-19 for healthcare providers and laboratory personnel. Turk. J. Med. Sci. 2020, 50, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Wake, R.M.; Morgan, M.; Choi, J.; Winn, S. Reducing nosocomial transmission of COVID-19: Implementation of a COVID-19 triage system. Clin. Med. J. R. Coll. Physicians Lond. 2020, 20, E141–E145. [Google Scholar] [CrossRef] [PubMed]
- Coia, J.E.; Ritchie, L.; Adisesh, A.; Booth, C.M.; Bradley, C.; Bunyan, D.; Carson, G.; Fry, C.; Hoffman, P.; Jenkins, D.; et al. Guidance on the use of respiratory and facial protection equipment. J. Hosp. Infect. 2013, 85, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.M. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic–a narrative review. Anaesthesia 2020, 75, 920–927. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahman, K.; Sun, Y.; Qureshi, M.; Abdi, I.; Chughtai, A.; Seale, H. Current knowledge of COVID-19 and infection prevention and control strategies in healthcare settings: A global analysis. Infect. Control. Hosp. Epidemiol. 2020, 41, 1196–1206. [Google Scholar] [CrossRef]
- Sorbello, M.; Rosenblatt, W.; Hofmeyr, R.; Greif, R.; Urdaneta, F. Aerosol boxes and barrier enclosures for airway management in COVID-19 patients: A scoping review and narrative synthesis. Br. J. Anaesth. 2020, 125, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Chahar, P.; Dugar, S.; Marciniak, D. Airway management considerations in patients with COVID-19. Clevel. Clin. J. Med. 2020, 87, 1–4. [Google Scholar] [CrossRef]
- Bearden, D.M.; Aiken, P.B.; Cheng, Y.H.; Mai, E.; Peters, T.M. COVID-19, “COVID-19: A primer for healthcare providers. Wiener Klinische Wochenschrift 2020, 132, 390–395. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, S.; Xu, Y. Donning and doffing of personal protective equipment protocol and key points of nursing care for patients with COVID-19 in ICU. Stroke Vasc. Neurol. 2020, 5, 302–307. [Google Scholar] [CrossRef]
- Respiratory Care Committee of Chinese Thoracic Society. Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia. Chin. J. Tuberc. Respir. Dis. 2020, 43, 288–296. [Google Scholar] [CrossRef]
- Toomey, E.C.; Conway, Y.; Burton, C.; Smith, S.; Smalle, M.; Chan, X.-H.S.; Adisesh, A.; Tanveer, S.; Ross, L.; Thomson, I.; et al. Extended use or reuse of single-use surgical masks and filtering face-piece respirators during the coronavirus disease 2019 (COVID-19) pandemic: A rapid systematic review. Infect. Control. Hosp. Epidemiol. 2021, 42, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, J.; Li, P.; Zhang, C. Ultraviolet germicidal irradiation for filtering facepiece respirators disinfection to facilitate reuse during COVID-19 pandemic: A review. Photodiagnosis Photodyn. Ther. 2020, 31, 101943. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Hu, T.; Liu, L.; Chen, R.; Guo, Q.; Yang, L.; Cheng, Y.; Huang, J.; Du, L. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J. Evid.-Based Med. 2020, 13, 93–101. [Google Scholar] [CrossRef]
- Ha, J.F. The COVID-19 pandemic, personal protective equipment and respirator: A narrative review. Int. J. Clin. Pract. 2020, 74, e13578. [Google Scholar] [CrossRef]
- Center of Disease and Control Hospital Respiratory Protection Program Toolkit. NIOSH. 2015. Available online: https://www.cdc.gov/niosh/docs/2015-117/pdfs/2015-117.pdf?id=10.26616/NIOSHPUB2015117 (accessed on 6 October 2021).
- Wu, Y.C.; Chen, C.S.; Chan, Y.J. The outbreak of COVID-19: An overview. J. Chin. Med Assoc. 2020, 83, 217–220. [Google Scholar] [CrossRef]
- Roberge, R.J. Evaluation of the rationale for concurrent use of N95 filtering facepiece respirators with loose-fitting powered air-purifying respirators during aerosol-generating medical procedures. Am. J. Infect. Control. 2008, 36, 135–141. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Infection Prevention and Control for the Care of Patients with 2019-nCoV in Healthcare Settings. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/nove-coronavirus-infection-prevention-control-patients-healthcaresettings.pdf (accessed on 13 October 2021).
- Shimabukuro, P.; Duarte, M.; Imoto, A.; Atallah, A.; Franco, E.; Peccin, M.; Taminato, M. Environmental cleaning to prevent COVID-19 infection. A rapid systematic review. Sao Paulo Med. J. 2020, 138, 505–514. [Google Scholar] [CrossRef]
- Walker, C.M.; Ko, G. Effect of Ultraviolet Germicidal Irradiation on Viral Aerosols. Environ. Sci. Technol. 2007, 41, 5460–5465. [Google Scholar] [CrossRef]
- Wang, J.; Feng, H.; Zhang, S.; Ni, Z.; Lingmei, N.; Chen, Y.; Zhuo, L.; Zhong, Z.; Qu, T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020, 94, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.; Tan, Y.; Chia, P.; Lee, T.; Ng, O.; Su, M.; Wong, M.; Marimuthu, K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA J. Am. Med Assoc. 2020, 323, 1610–1612. [Google Scholar] [CrossRef]
- Center of Disease and Control. Infection Control: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html (accessed on 13 October 2021).
- Homza, M.; Zelena, H.; Janosek, J.; Tomaskova, H.; Jezo, E.; Kloudova, A.; Mrazek, J.; Svagera, Z.; Prymula, R. Covid-19 antigen testing: Better than we know? A test accuracy study. Infect. Dis. 2021, 53, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Porte, L.; Legarraga, P.; Vollrath, V.; Aguilera, X.; Munita, J.M.; Araos, R.; Pizarro, G.; Vial, P.; Iruretagoyena, M.; Dittrich, S.; et al. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int. J. Infect. Dis. 2020, 99, 328. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 3. [Google Scholar] [CrossRef]
- Nagura-Ikeda, M.; Imai, K.; Tabata, S.; Miyoshi, K.; Murahara, N.; Mizuno, T.; Horiuchi, M.; Kato, K.; Imoto, Y.; Iwata, M.; et al. Clinical Evaluation of Self-Collected Saliva by Quantitative Reverse Transcription-PCR (RT-QPCR), Direct RT-QPCR, Reverse Transcription-Loop-Mediated Isothermal Amplification, and a Rapid Antigen Test To Diagnose COVID-19. J. Clin. Microbiol. 2020, 58, e01438-20. [Google Scholar] [CrossRef]
- World Health Organization. Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays. Available online: https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays (accessed on 30 July 2021).
- European Center of Disease and Control Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK. Available online: https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-and-uk#no-link (accessed on 30 July 2021).
- Bielicki, J.; Duval, X.; Gobat, N.; Goossens, H.; Koopmans, M.; Tacconelli, E.; Werf, S. Monitoring approaches for health-care workers during the COVID-19 pandemic. Lancet Infect. Dis. 2020, 20, e261–e267. [Google Scholar] [CrossRef]
- Robert Koch Institut. Coronavirus SARS-CoV-2—Kontaktpersonen-Nachverfolgung bei SARS-CoV-2-Infektionen. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Kontaktperson/Management.html (accessed on 13 June 2021).
- Nationales Zentrum Fur Infektionpravention Aktuelle Ereignisse—Swissnoso. Available online: https://www.swissnoso.ch/forschung-entwicklung/aktuelle-ereignisse/ (accessed on 13 June 2021).
- Rhee, C.; Baker, M.A.; Klompas, M. The COVID-19 infection control arms race. Infect. Control. Hosp. Epidemiol. 2020, 41, 1. [Google Scholar] [CrossRef]
- UK Health Security Agency. UKHSA Review into IPC Guidance—GOV.UK. Available online: https://www.gov.uk/government/publications/ukhsa-review-into-ipc-guidance (accessed on 6 October 2021).
- Madrid Bans Wearing FFP2 and FFP3 Masks, Deemed ‘Selfish’—Lisbob. Available online: https://www.lisbob.net/en/blog-expats-spain/madrid-bans-wearing-ffp2-and-ffp3-masks-deemed-selfish (accessed on 29 July 2021).
- Nicolle, L. La science et les mesures de sécurité contre le SRAS. Can. J. Anesth. 2003, 50, 983–988. [Google Scholar] [CrossRef]
- Casanova, L.M.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Effect of single- versus double-gloving on virus transfer to health care workers’ skin and clothing during removal of personal protective equipment. Am. J. Infect. Control. 2012, 40, 369–374. [Google Scholar] [CrossRef]
- Yao, W.; Wang, T.; Jiang, B.; Gao, F.; Wang, L.; Zheng, H.; Xiao, W.; Yao, S.; Mei, W.; Chen, X. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: Lessons learnt and international expert recommendations. Br. J. Anaesth. 2020, 125, e28–e37. [Google Scholar] [CrossRef]
- Zamora, J.E.; Murdoch, J.; Simchison, B.; Day, A.G. Contamination: A comparison of 2 personal protective systems. CMAJ 2006, 175, 249–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, S.D.; Boles, C.L.; Perencevich, E.N.; Diekema, D.J.; Nonnenmann, M.W. Bioaerosol concentrations generated from toilet flushing in a hospital-based patient care setting. Antimicrob. Resist. Infect. Control. 2018, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, H.; Kobayashi, T.; Yang, Y.; Hayashi, K.; Miyama, T.; Kinoshita, R.; Linton, N.M.; Jung, S.; Yuan, B.; Suzuki, A.; et al. The Rate of Underascertainment of Novel Coronavirus (2019-nCoV) Infection: Estimation Using Japanese Passengers Data on Evacuation Flights. J. Clin. Med. 2020, 9, 419. [Google Scholar] [CrossRef]
- Luo, F.; Darwiche, K.; Singh, S.; Torrego, A.; Steinfort, D.P.; Gasparini, S.; Liu, D.; Zhang, W.; Fernandez-Bussy, S.; Herth, F.J.F.; et al. Performing Bronchoscopy in Times of the COVID-19 Pandemic: Practice Statement from an International Expert Panel. Respiration 2020, 99, 417–422. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Z.; Zhang, S.; Li, X.; Lin, L.; Li, C.; Cui, Y.; Fu, R.; Dong, Y.; Chi, X.; et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020, 26, 1586–1591. [Google Scholar] [CrossRef]
- Lu, J.; Gu, J.; Li, K.; Xu, C.; Su, W.; Lai, Z.; Deqian, Z.; Yu, C.; Xu, B.; Yang, Z.; et al. COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 2020, 26, 1628–1631. [Google Scholar] [CrossRef]
| Topic | Number of Assessed Studies |
|---|---|
| IPC bundles | 36 |
| PPE | 21 |
| Disinfection and filtration techniques (UV, negative pressure, heat) | 16 |
| IPC for special procedures (tracheostomy, broncoscopy, endoscopy, CT scan) | 8 |
| ICU & LTCF | 5 |
| Recommendation | Description |
|---|---|
| Infectious Disease Plan [17,18] |
|
| Administrative Controls [17,29] |
|
| Triage [21] |
|
| Engineering controls [18,22] |
|
| Personal Protective Equipment [30,31,36] |
|
| Environmental cleaning [15] |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shbaklo, N.; Lupia, T.; De Rosa, F.G.; Corcione, S. Infection Control in the Era of COVID-19: A Narrative Review. Antibiotics 2021, 10, 1244. https://doi.org/10.3390/antibiotics10101244
Shbaklo N, Lupia T, De Rosa FG, Corcione S. Infection Control in the Era of COVID-19: A Narrative Review. Antibiotics. 2021; 10(10):1244. https://doi.org/10.3390/antibiotics10101244
Chicago/Turabian StyleShbaklo, Nour, Tommaso Lupia, Francesco G. De Rosa, and Silvia Corcione. 2021. "Infection Control in the Era of COVID-19: A Narrative Review" Antibiotics 10, no. 10: 1244. https://doi.org/10.3390/antibiotics10101244
APA StyleShbaklo, N., Lupia, T., De Rosa, F. G., & Corcione, S. (2021). Infection Control in the Era of COVID-19: A Narrative Review. Antibiotics, 10(10), 1244. https://doi.org/10.3390/antibiotics10101244








