Metabolomic Profiling and Biological Activities of Pleurotus columbinus Quél. Cultivated on Different Agri-Food Byproducts
Abstract
:1. Introduction
2. Results and Discussion
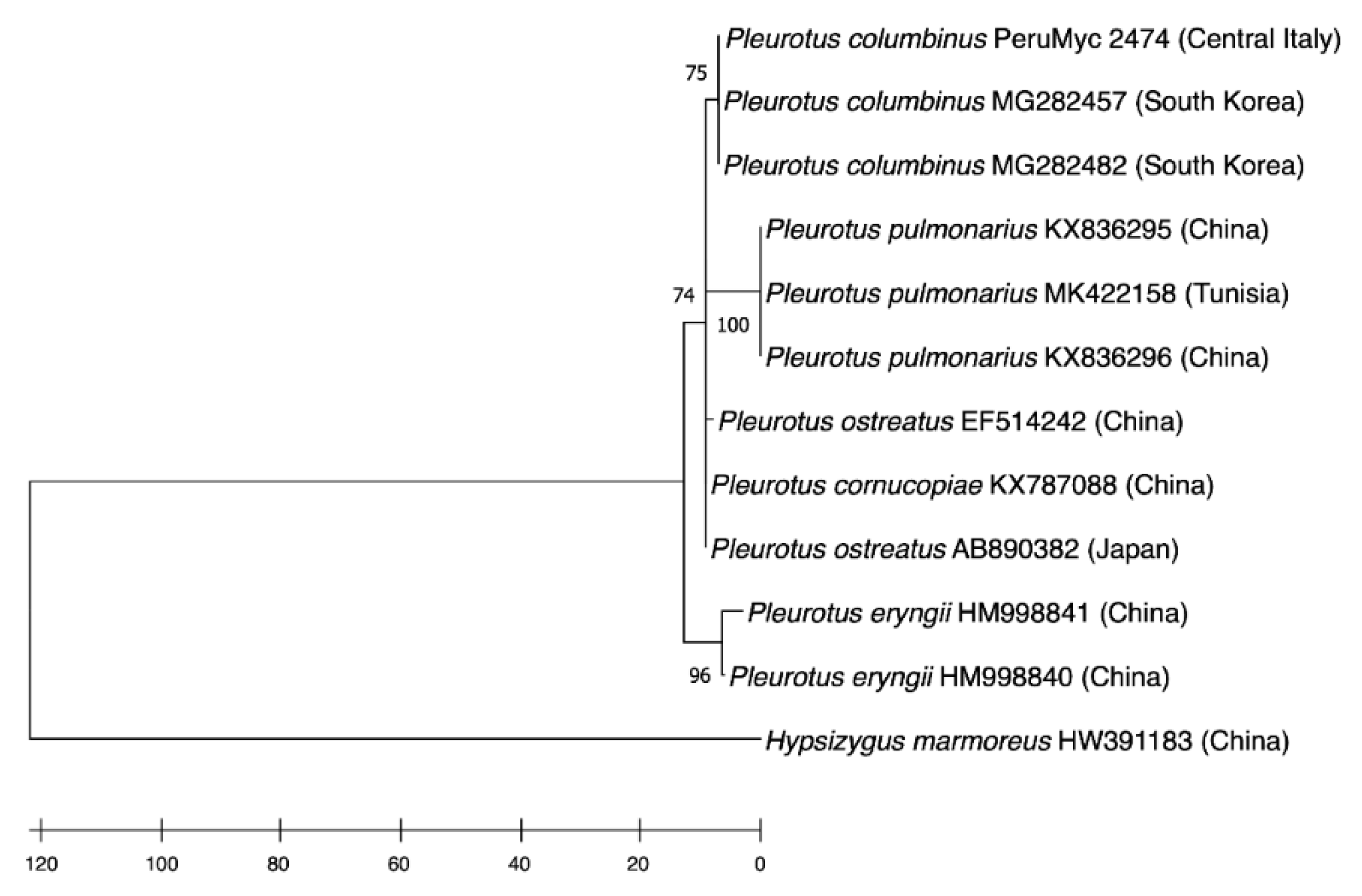
2.1. Mushroom Identification
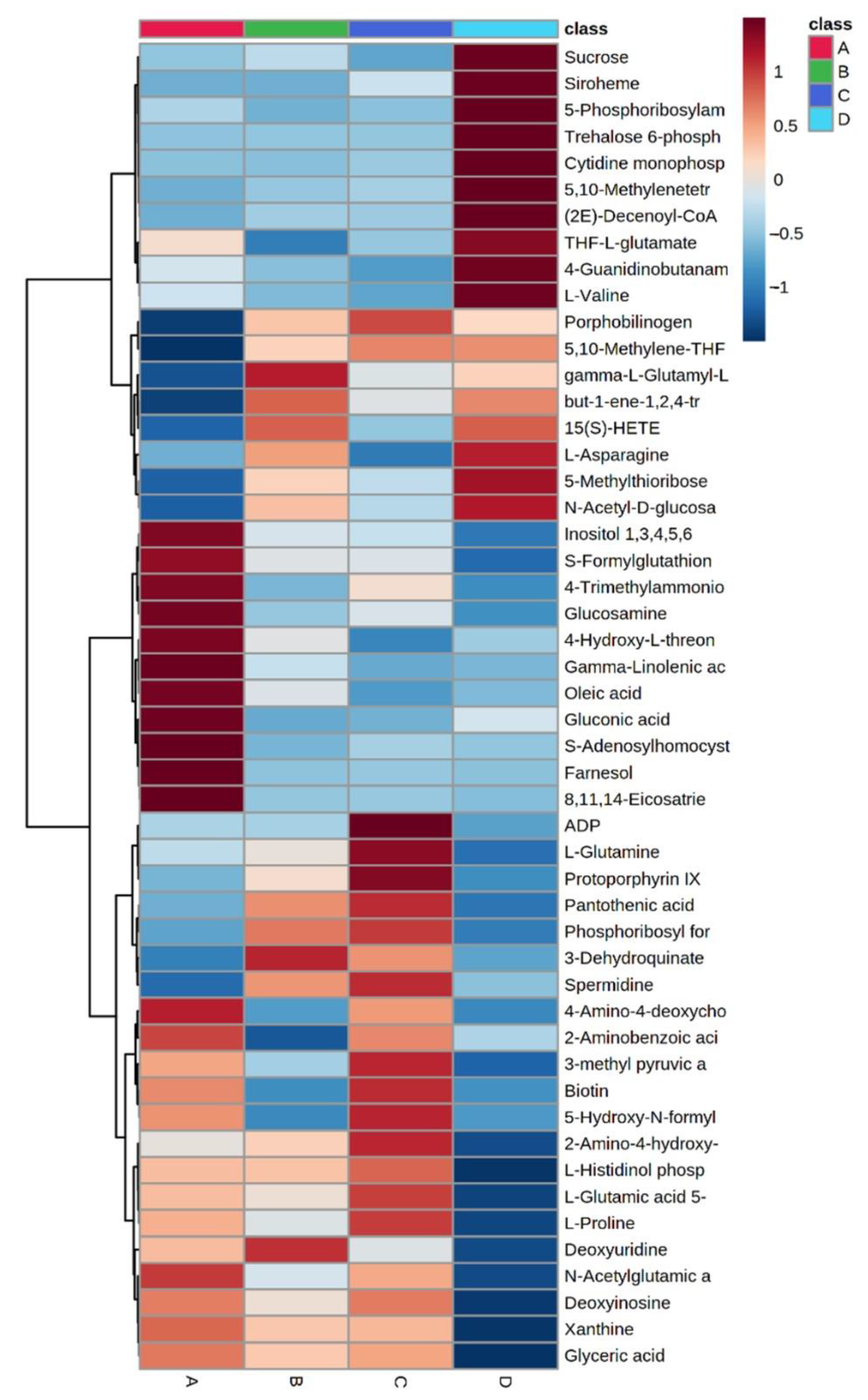
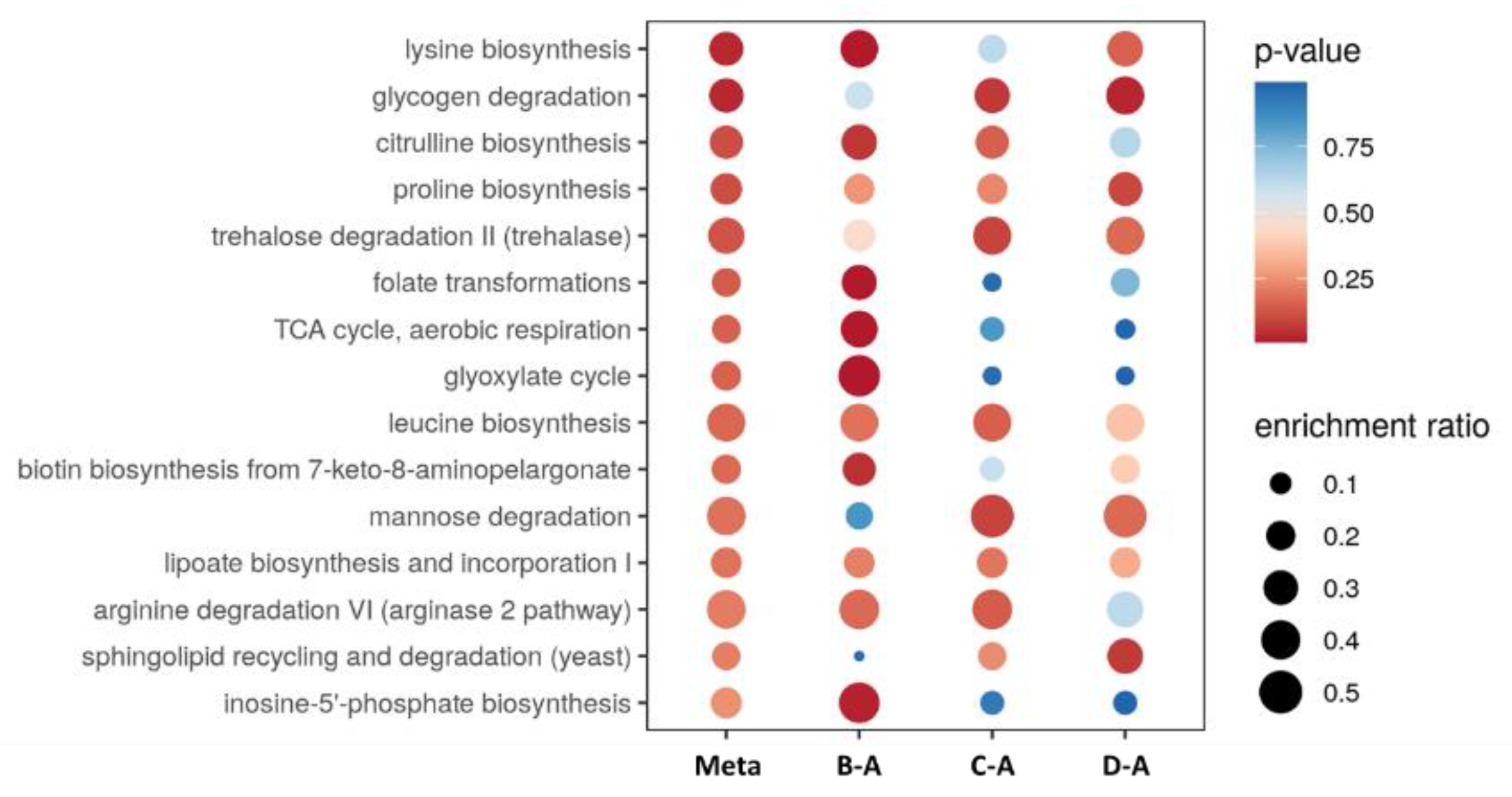
2.2. Untargeted LC-MS/MS-Based Metabolomics
2.3. Phenolic and Flavonoid Determination via HPLC-DAD-MS
2.4. Antimicrobial and Antioxidant Effects
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Mushroom Material
3.3. Molecular Identification
3.4. Spawn Production
3.5. Mushroom Cultivation Substrates
3.6. Preparation of Mushrooms Methanol Extracts
3.7. Untargeted LC-MS/MS-Based Metabolomics and Statistical Analysis
3.8. Phenolic and Flavonoid Determination: HPLC-DAD-MS Analyses
3.9. Free Radical-Scavenging Activity
3.9.1. DPPH Assay
3.9.2. ABTS Assay
3.9.3. β-Carotene-Linoleic Acid Assay
3.10. Antimicrobial Tests
3.10.1. Bacterial and Fungal Strains
3.10.2. Antibacterial Activity
3.10.3. Antifungal Activity
3.10.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gargano, M.L.; van Griensven, L.J.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Ianni, F.; Blasi, F.; Angelini, P.; Di Simone, S.C.; Angeles Flores, G.; Cossignani, L.; Venanzoni, R. Extraction Optimization by Experimental Design of Bioactives from Pleurotus ostreatus and Evaluation of Antioxidant and Antimicrobial Activities. Processes 2021, 9, 743. [Google Scholar] [CrossRef]
- Philippoussis, A.N. Production of mushrooms using agro-industrial residues as substrates. In Biotechnology for Agro-Industrial Residues Utilisation; Springer: Berlin/Heidelberg, Germany, 2009; pp. 163–196. [Google Scholar]
- Angelini, P.; Pagiotti, R.; Granetti, B. Effect of antimicrobial activity of Melaleuca alternifolia essential oil on antagonistic potential of Pleurotus species against Trichoderma harzianum in dual culture. World J. Microbiol. Biotechnol. 2008, 24, 197–202. [Google Scholar] [CrossRef]
- Lavelli, V.; Proserpio, C.; Gallotti, F.; Laureati, M.; Pagliarini, E. Circular reuse of bio-resources: The role of Pleurotus spp. in the development of functional foods. Food Funct. 2018, 9, 1353–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiantas, K.; Tsiaka, T.; Koutrotsios, G.; Siapi, E.; Zervakis, G.I.; Kalogeropoulos, N.; Zoumpoulakis, P. On the identification and quantification of ergothioneine and lovastatin in various mushroom species: Assets and challenges of different analytical approaches. Molecules 2021, 26, 1832. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C. Biotechnological, nutritional and therapeutic uses of Pleurotus spp.(Oyster mushroom) related with its chemical composition: A review on the past decade findings. Trends Food Sci. Technol. 2016, 50, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Jayasuriya, W.; Handunnetti, S.M.; Wanigatunge, C.A.; Fernando, G.H.; Abeytunga, D.T.U.; Suresh, T.S. Anti-inflammatory activity of Pleurotus ostreatus, a culinary medicinal mushroom, in wistar rats. Evid. Based Complementary Altern. Med. 2020, 2020, 6845383. [Google Scholar] [CrossRef] [Green Version]
- Jedinak, A.; Dudhgaonkar, S.; Wu, Q.-l.; Simon, J.; Sliva, D. Anti-inflammatory activity of edible oyster mushroom is mediated through the inhibition of NF-κB and AP-1 signaling. Nutr. J. 2011, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Ajmal, M.; Nickten, J.; Aslam, S.; Ali, M. Nutritional value of Pleurotus (Flabellatus) Djamor (R-22) cultivated on sawdusts of different woods. Pak. J. Bot. 2013, 45, 1105–1108. [Google Scholar]
- Llauradó, G.; Morris, H.J.; Lebeque, Y.; Gutiérrez, A.; Fontaine, R.; Bermúdez, R.C.; Perraud-Gaime, I. Phytochemical screening and effects on cell-mediated immune response of Pleurotus fruiting bodies powder. Food Agric. Immunol. 2013, 24, 295–304. [Google Scholar] [CrossRef]
- Maiti, S.; Mallick, S.K.; Bhutia, S.K.; Behera, B.; Mandal, M.; Maiti, T.K. Antitumor effect of culinary-medicinal oyster mushroom, Pleurotus ostreatus (Jacq.: Fr.) P. Kumm., derived protein fraction on tumor-bearing mice models. Int. J. Med. Mushrooms 2011, 13, 427–440. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhao, C.; Zheng, S.; Mei, X.; Huang, K.; Wang, G.; He, X. Anti-obesity and hypolipidemic effect of water extract from Pleurotus citrinopileatus in C57 BL/6J mice. Food Sci. Nutr. 2019, 7, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Venturella, G.; Gargano, M.; Compagno, R. The genus Pleurotus in Italy. Flora Mediterr. 2015, 25, 143–155. [Google Scholar]
- Wang, H.; Ng, T. Isolation of a novel ubiquitin-like protein from Pleurotus ostreatus mushroom with anti-human immunodeficiency virus, translation-inhibitory, and ribonuclease activities. Biochem. Biophys. Res. Commun. 2000, 276, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory effects of edible and medicinal mushrooms and their bioactive immunoregulatory products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.; Naraian, R.; Singh, V. Medicinal properties of Pleurotus species (oyster mushroom): A review. World J. Fungal Plant Biol. 2012, 3, 1–12. [Google Scholar]
- Adebayo, E.; Elkanah, F.; Afolabi, F.; Ogundun, O.; Alabi, T.; Oduoye, O. Molecular characterization of most cultivated Pleurotus species in sub-western region Nigeria with development of cost effective cultivation protocol on palm oil waste. Heliyon 2021, 7, e06215. [Google Scholar] [CrossRef] [PubMed]
- Bonatti, M.; Karnopp, P.; Soares, H.; Furlan, S. Evaluation of Pleurotus ostreatus and Pleurotus sajor-caju nutritional characteristics when cultivated in different lignocellulosic wastes. Food Chem. 2004, 88, 425–428. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.C.; Zervakis, G.I. Toward an increased functionality in oyster (Pleurotus) mushrooms produced on grape marc or olive mill wastes serving as sources of bioactive compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef]
- Yildiz, S.; Yildiz, Ü.C.; Gezer, E.D.; Temiz, A. Some lignocellulosic wastes used as raw material in cultivation of the Pleurotus ostreatus culture mushroom. Process. Biochem. 2002, 38, 301–306. [Google Scholar] [CrossRef]
- Owaid, M.N.; Abed, I.A.; Al-Saeedi, S.S.S. Applicable properties of the bio-fertilizer spent mushroom substrate in organic systems as a byproduct from the cultivation of Pleurotus spp. Inf. Process. Agric. 2017, 4, 78–82. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2020, 49, 1–14. [Google Scholar] [CrossRef]
- Angelini, P.; Pagiotti, R.; Venanzoni, R.; Granetti, B. Antifungal and allelopathic effects of Asafoetida against Trichoderma harzianum and Pleurotus spp. Allelopath. J. 2009, 23, 357–368. [Google Scholar]
- Carrasco-González, J.A.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Nutritional composition and nutraceutical properties of the Pleurotus fruiting bodies: Potential use as food ingredient. J. Food Compos. Anal. 2017, 58, 69–81. [Google Scholar] [CrossRef]
- Jeznabadi, E.K.; Jafarpour, M.; Eghbalsaied, S. King oyster mushroom production using various sources of agricultural wastes in Iran. Int. J. Recycl. Org. Waste Agric. 2016, 5, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Kinge, T.; Adi, E.; Mih, A.; Ache, N.; Nji, T. Effect of substrate on the growth, nutritional and bioactive components of Pleurotus ostreatus and Pleurotus florida. Afr. J. Biotechnol. 2016, 15, 1476–1486. [Google Scholar] [CrossRef]
- Ritota, M.; Manzi, P. Pleurotus spp. cultivation on different agri-food by-products: Example of biotechnological application. Sustainability 2019, 11, 5049. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, G. Genus Pleurotus (Jacq.: Fr.) P. Kumm.(Agaricomycetideae): Diversity, taxonomic problems, and cultural and traditional medicinal uses. Int. J. Med. Mushrooms 2000, 2, 29. [Google Scholar] [CrossRef]
- Hiber, O. Die Gattung Pleurotus (FR.) Kummer unter besonderer Berucksichtigung des Pleurotus eryngii-Formenkomplexes. Bibl. Mycol. 1982, 87, 137–172. [Google Scholar]
- Zervakis, G.; Balis, C. A pluralistic approach in the study of Pleurotus species with emphasis on compatibility and physiology of the European morphotaxa. Mycol. Res. 1996, 100, 717–731. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, V.P.; Singh, N.K. A Review on Phytochemistry and Pharmacology of Medicinal as well as Poisonous Mushrooms. Mini Rev. Med. Chem. 2018, 18, 1095–1109. [Google Scholar] [CrossRef]
- Rodrigues Barbosa, J.; Dos Santos Freitas, M.M.; da Silva Martins, L.H.; de Carvalho Junior, R.N. Polysaccharides of mushroom Pleurotus spp.: New extraction techniques, biological activities and development of new technologies. Carbohydr. Polym. 2020, 229, 115550. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, A.; Tripathi, A. Biological activities of Pleurotus spp. polysaccharides: A review. J. Food Biochem. 2021, 45, e13748. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, S.M.; El-Mahdy, T.S.; Awad, M.F.; Elleboudy, N.S.; Farag, M.M.S.; Aboshanab, K.M.; Yassien, M.A. Antiviral, Cytotoxic, and Antioxidant Activities of Three Edible Agaricomycetes Mushrooms: Pleurotus columbinus, Pleurotus sajor-caju, and Agaricus bisporus. J. Fungi 2021, 7, 645. [Google Scholar] [CrossRef] [PubMed]
- Irshad, A.; Shahid, M.; Asghar, M.; Khan, J.A. Antioxidant potential analysis of P. Ostreatus, P. Sajor-Caju, P. Sapidus and P. Columbinus. J. Biol. Regul. Homeost. Agents 2017, 31, 705–709. [Google Scholar] [PubMed]
- Luo, F.; Zhong, Z.; Liu, L.; Igarashi, Y.; Xie, D.; Li, N. Metabolomic differential analysis of interspecific interactions among white rot fungi Trametes versicolor, Dichomitus squalens and Pleurotus ostreatus. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bas, C.; Kuyper, T.W.; Noordeloos, M.; Vellinga, E. Flora Agaricina Neerlandica—Critical Monographs on the Families of Agarics and Boleti Occurring in the Netherlands; AA Balkema: Rotterdam, The Netherlands, 1990; Volume 2. [Google Scholar]
- Mišković, J.; Rašeta, M.; Čapelja, E.; Krsmanović, N.; Novaković, A.; Karaman, M. Mushroom Species Stereum hirsutum as Natural Source of Phenolics and Fatty Acids as Antioxidants and Acetylcholinesterase Inhibitors. Chem. Biodivers. 2021. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.R.; El-Sayed, H. Molecular identification and antimicrobial activities of some wild Egyptian mushrooms: Bjerkandera adusta as a promising source of bioactive antimicrobial phenolic compounds. J. Genet. Eng. Biotechnol. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S. Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef]
- Singer, R. The Agaricales in Modern Taxonomy; Koeltz Sc. Books: Koenigstein, Germany, 1986. [Google Scholar]
- Gams, W.; Hoekstra, E.; Aptroot, A. CBS Course of Mycology; Centraalbureau voor Schimmelcultures: Baarn, The Netherlands, 1998. [Google Scholar]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms; Ten Speed Press: Berkeley, CA, USA, 2011. [Google Scholar]
- Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Tirillini, B.; Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S.C. Evaluation of antioxidant, antimicrobial and tyrosinase inhibitory activities of extracts from Tricholosporum goniospermum, an edible wild mushroom. Antibiotics 2020, 9, 513. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Li, S.; Park, Y.; Duraisingham, S.; Strobel, F.H.; Khan, N.; Soltow, Q.A.; Jones, D.P.; Pulendran, B. Predicting network activity from high throughput metabolomics. PLoS Comput. Biol. 2013, 9, e1003123. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- di Giacomo, V.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Politi, M.; Antolini, M.D.; Acquaviva, A. Metabolomic profile and antioxidant/anti-inflammatory effects of industrial hemp water extract in fibroblasts, keratinocytes and isolated mouse skin specimens. Antioxidants 2021, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.; Browse, D.; Reading, S.; Benjamin, I. A simple and accurate mathematical method for calculation of the EC50. J. Pharmacol. Toxicol. Methods 1999, 41, 55–58. [Google Scholar] [CrossRef]
- Öztürk, M.; Duru, M.E.; Kivrak, Ş.; Mercan-Doğan, N.; Türkoglu, A.; Özler, M.A. In vitro antioxidant, anticholinesterase and antimicrobial activity studies on three Agaricus species with fatty acid compositions and iron contents: A comparative study on the three most edible mushrooms. Food Chem. Toxicol. 2011, 49, 1353–1360. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Shon, M.-Y.; Kim, T.-H.; Sung, N.-J. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 2003, 82, 593–597. [Google Scholar] [CrossRef]
- Prieto, M.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M. β-Carotene assay revisited. Application to characterize and quantify antioxidant and prooxidant activities in a microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef] [Green Version]
- Vaz, J.A.; Barros, L.; Martins, A.; Santos-Buelga, C.; Vasconcelos, M.H.; Ferreira, I.C. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011, 126, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Angelini, P.; Matei, F.; Flores, G.A.; Pellegrino, R.M.; Vuguziga, L.; Venanzoni, R.; Tirillini, B.; Emiliani, C.; Orlando, G.; Menghini, L. Metabolomic Profiling, Antioxidant and Antimicrobial Activity of Bidens pilosa. Processes 2021, 9, 903. [Google Scholar] [CrossRef]
| Quantity (µg/mL ± S.D.) | ||||
|---|---|---|---|---|
| Extracts | A | B | C | D |
| Gallic acid | 2.76 ± 0.12 | 1.29 ± 0.04 | 2.74 ± 0.05 | 1.97 ± 0.38 |
| Hydroxytyrosol | not detected | not detected | 4.43 ± 0.39 | 2.91 ± 0.77 |
| Catechin | 7.60 ± 0.21 | 12.25 ± 0.42 | 26.90 ± 1.07 | 14.54 ± 0.13 |
| Chlorogenic acid | not detected | not detected | 1.62 ± 0.13 | 1.10 ± 0.01 |
| Epicatechin | not detected | 5.69 ± 0.18 | 7.97 ± 0.19 | 11.98 ± 0.01 |
| Benzoic acid | 0.13 ± 0.02 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.015 ± 0.01 |
| Extracts | MIC (µg mL−1) * | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | Fluconazole (µg/mL) | ||
| Yeasts | Strain (ID) C. tropicalis (YEPGA 6184) | 125.99 (100–200) | 79.37 (50–100) | 200- > 200 | 200- > 200 | 2 |
| C. albicans (YEPGA 6379) | 125.99 (100–200) | 200- > 200 | 200- > 200 | 200- > 200 | 1 | |
| C. parapsilosis (YEPGA 6551) | 31.49 (25–50) | 39.68 (25–59) | 200- > 200 | 200- > 200 | 4 | |
| C. albicans (YEPGA 6183) | 62.99 (50–100) | 158.74 (100–200) | 200- > 200 | 200- > 200 | 2 | |
| Gram - | Ciprofloxacin (µg/mL) | |||||
| Bacteria | E. coli (ATCC 10536) | 19.84 (12.5–25) | 9.92 (6.25–12.5) | 31.49 (25–50) | 15.74 (12.5–25) | <0.12 |
| E. coli (PeryMycA 2) | 158.74 (100–200) | 79.27 (50–100) | 9.92 (6.25–12.5) | 19.84 (12.5–25) | 1.23 (0.98–1.95) | |
| E. coli (PeruMycA 3) | 200- > 200 | 200- > 200 | 79.37 (50–100) | 125.99 (100–200) | 0.62 (0.49–0.98) | |
| P. aeruginosa (PeruMycA 5) | 62.99 (50–100) | 62.99 (50–100) | 125.99 (100–200) | 125.99 (100–200) | 1.23 (0.98–1.95) | |
| S. typhy (PeruMycA 7) | 125.99 (100–200) | 79.37 (50–100) | 79.37 (50–100) | 125.99 (100–200) | 0.38 (0.24–0.49) | |
| Gram + | ||||||
| B. cereus (PeruMycA 4) | 31.49 (25–50) | 200- > 200 | 125.99 (100–200) | 125.99 (100–200) | <0.12 | |
| B. subtilis (PeruMycA 6) | 79.37 (50–100) | 79.37 (50–100) | 125.99 (100–200) | 158.74 (100–200) | <0.12 | |
| S. aureus (ATCC 6538) | 158.74 (100–200) | 125.99 (100–200) | 200- > 200 | 125.99 (100–200) | 0.62 (0.98–0.49) | |
| Griseofulvin (µg/mL) | ||||||
| Dermatophytes | T. mentagrophytes (CCF 4823) | 28.24 (12.5–25) | 20.37 (6.25–12.5) | 129.37 (50–100) | 81.50 (25–50) | 2.52 (2–4) |
| T. tonsurans (CCF 4834) | 129.37 (50–100) | 112.99 (50–100) | 89.68 (25–50) | 112.99 (50–100) | 0.198 (0.125–0.25) | |
| T.rubrum (CCF 4933) | 158.74 (100–200) | 200- > 200 | 16.17 (6.25–12.5) | 158.74 (100–200) | 1.26 (1–2) | |
| A. quadrifidum (CCF5792) | 89.68 (25–50) | 81.50 (25–50) | 62.99 (50–100) | 15.74 (12.5–25) | >8 | |
| T. erinacei (CCF5930) | 39.68 (25–50) | 158.74 (100–200) | 79.37 (50–100) | 31.49 (25–50) | 3.174 (2–4) | |
| N. gypseum (CCF6261) | 31.49 (25–50) | 125.99 (100–200) | 79.37 (50–100) | 62.99 (50–100) | 1.587 (1–2) | |
| A. currei (CCF5207) | 62.99 (50–100) | 39.68 (25–50) | 31.49 (25–50) | 19.84 (12.5–25) | >8 | |
| A. insingulare (CCF5417) | 19.84 (12.5–25) | 79.37 (50–100) | 62.99 (50–100) | 31.49 (25–50) | >8 | |
| Extracts | DPPH Test EC50 (µg/mL ± SD) | ABTS Test EC50 (µg/mL ± SD) | Linoleic Assay EC50 (µg/mL ± SD) |
|---|---|---|---|
| A | 4.98 ± 0.53 c | 6.16 ± 0.53 c | 11.29 ± 1.11 c |
| B | 2.25 ± 0.19 a | 4.34 ± 0.45 a | 8.47 ± 0.62 ab |
| C | 3.81 ± 0.32 c | 5.52 ± 0.51 c | 11.65 ± 0.99 c |
| D | 2.58 ± 0.21 ab | 4.61 ± 0.37 ab | 8.74 ± 0.86 ab |
| Trolox (µg TE) | 0.28 ± 0.03 | 0.66 ± 0.07 | 0.56 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelini, P.; Pellegrino, R.M.; Tirillini, B.; Flores, G.A.; Alabed, H.B.R.; Ianni, F.; Blasi, F.; Cossignani, L.; Venanzoni, R.; Orlando, G.; et al. Metabolomic Profiling and Biological Activities of Pleurotus columbinus Quél. Cultivated on Different Agri-Food Byproducts. Antibiotics 2021, 10, 1245. https://doi.org/10.3390/antibiotics10101245
Angelini P, Pellegrino RM, Tirillini B, Flores GA, Alabed HBR, Ianni F, Blasi F, Cossignani L, Venanzoni R, Orlando G, et al. Metabolomic Profiling and Biological Activities of Pleurotus columbinus Quél. Cultivated on Different Agri-Food Byproducts. Antibiotics. 2021; 10(10):1245. https://doi.org/10.3390/antibiotics10101245
Chicago/Turabian StyleAngelini, Paola, Roberto Maria Pellegrino, Bruno Tirillini, Giancarlo Angeles Flores, Husam B. R. Alabed, Federica Ianni, Francesca Blasi, Lina Cossignani, Roberto Venanzoni, Giustino Orlando, and et al. 2021. "Metabolomic Profiling and Biological Activities of Pleurotus columbinus Quél. Cultivated on Different Agri-Food Byproducts" Antibiotics 10, no. 10: 1245. https://doi.org/10.3390/antibiotics10101245
APA StyleAngelini, P., Pellegrino, R. M., Tirillini, B., Flores, G. A., Alabed, H. B. R., Ianni, F., Blasi, F., Cossignani, L., Venanzoni, R., Orlando, G., Menghini, L., & Ferrante, C. (2021). Metabolomic Profiling and Biological Activities of Pleurotus columbinus Quél. Cultivated on Different Agri-Food Byproducts. Antibiotics, 10(10), 1245. https://doi.org/10.3390/antibiotics10101245















