Genetic Profile of Linezolid-Resistant M. tuberculosis Clinical Strains from Moscow
Abstract
:1. Introduction
2. Results
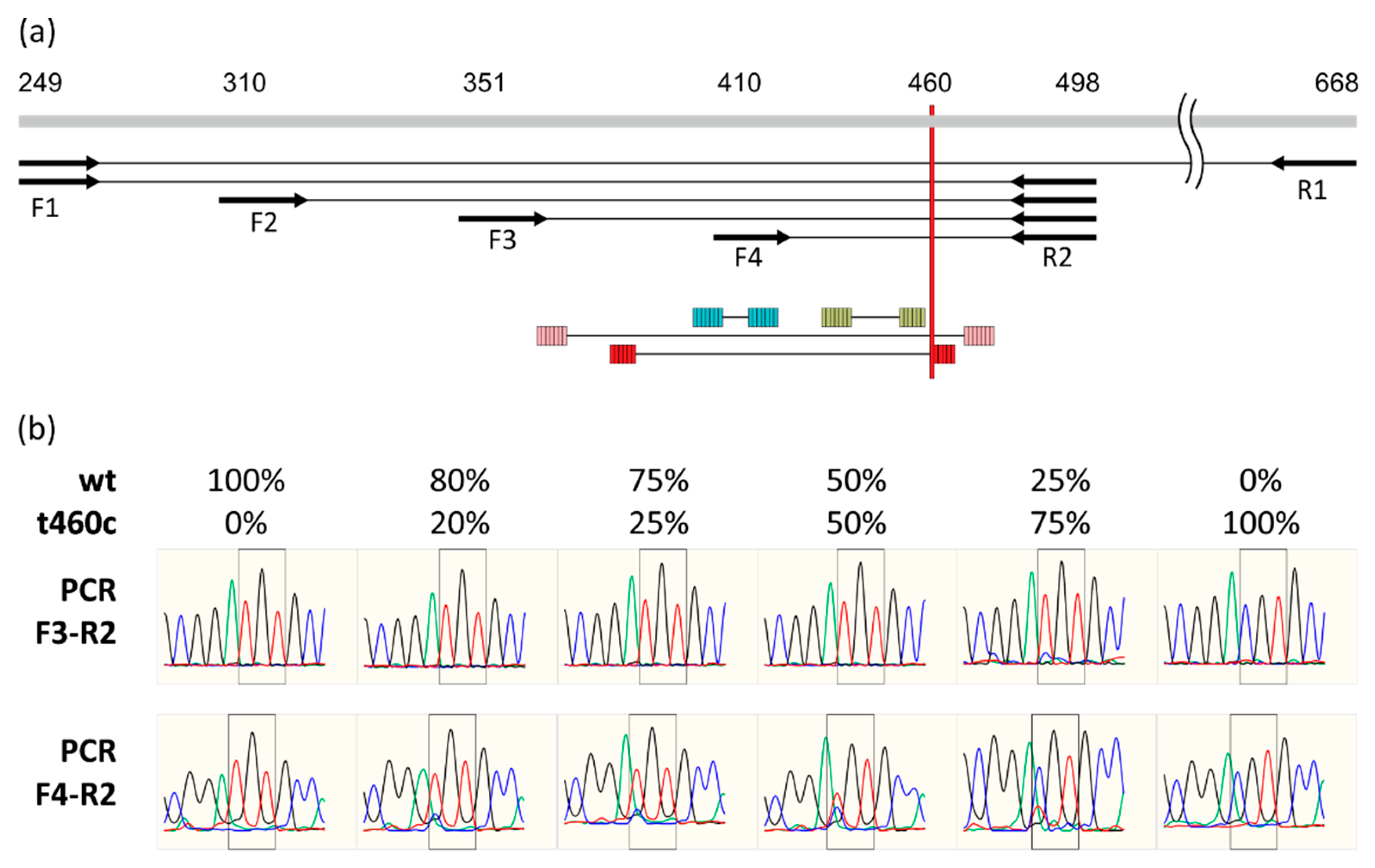
2.1. Detection of rplC t460c Mutation in Heteroresistant State
2.2. Genetic and Phenotypic Properties of Clinical Isolates
2.3. Clinical Aspects of Linezolid Acquisition
3. Discussion
4. Materials and Methods
4.1. Mycobacterium Tuberculosis Strains
4.2. DNA Isolation and Sequencing
4.3. MIRU-VNTR Typing
4.4. Whole-Genome Sequencing and Bionformatic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glaziou, P.; Floyd, K.; Raviglione, M.C. Global Epidemiology of Tuberculosis. Semin. Respir. Crit. Care Med. 2018, 39, 271–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gygli, S.M.; Borrell, S.; Trauner, A.; Gagneux, S. Antimicrobial Resistance in Mycobacterium Tuberculosis: Mechanistic and Evolutionary Perspectives. FEMS Microbiol. Rev. 2017, 41, 354–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliori, G.B.; Sotgiu, G.; Gandhi, N.R.; Falzon, D.; DeRiemer, K.; Centis, R.; Hollm-Delgado, M.G.; Palmero, D.; Pérez-Guzmán, C.; Vargas, M.H.; et al. Drug Resistance beyond Extensively Drug-Resistant Tuberculosis: Individual Patient Data Meta-Analysis. Eur. Respir. J. 2013, 42, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiberi, S.; Scardigli, A.; Centis, R.; D’Ambrosio, L.; Muñoz-Torrico, M.; Salazar-Lezama, M.Á.; Spanevello, A.; Visca, D.; Zumla, A.; Migliori, G.B.; et al. Classifying New Anti-Tuberculosis Drugs: Rationale and Future Perspectives. Int. J. Infect. Dis. 2017, 56, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Tiberi, S.; du Plessis, N.; Walzl, G.; Vjecha, M.J.; Rao, M.; Ntoumi, F.; Mfinanga, S.; Kapata, N.; Mwaba, P.; McHugh, T.D.; et al. Tuberculosis: Progress and Advances in Development of New Drugs, Treatment Regimens, and Host-Directed Therapies. Lancet Infect. Dis. 2018, 18, e183–e198. [Google Scholar] [CrossRef]
- The World Health Organization. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-155052-9. [Google Scholar]
- The World Health Organization (WHO). Meeting Report of the WHO Expert Consultation on the Definition of Extensively Drug-Resistant Tuberculosis; WHO: Geneva, Switzerland, 27–29 October 2020. [Google Scholar]
- Borisov, S.E.; Dheda, K.; Enwerem, M.; Romero Leyet, R.; D’Ambrosio, L.; Centis, R.; Sotgiu, G.; Tiberi, S.; Alffenaar, J.-W.; Maryandyshev, A.; et al. Effectiveness and Safety of Bedaquiline-Containing Regimens in the Treatment of MDR- and XDR-TB: A Multicentre Study. Eur. Respir. J. 2017, 49, 1700387. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Gao, J.; Yu, Y.; Li, Q.; Bai, G.; Shu, W.; Gao, M.; Liu, Y.; Wang, L.; Wang, Y.; et al. Low Rate of Acquired Linezolid Resistance in Multidrug-Resistant Tuberculosis Treated with Bedaquiline-Linezolid Combination. Front. Microbiol. 2021, 12, 655653. [Google Scholar] [CrossRef]
- Beckert, P.; Hillemann, D.; Kohl, T.A.; Kalinowski, J.; Richter, E.; Niemann, S.; Feuerriegel, S. RplC T460C Identified as a Dominant Mutation in Linezolid-Resistant Mycobacterium Tuberculosis Strains. Antimicrob. Agents Chemother. 2012, 56, 2743–2745. [Google Scholar] [CrossRef] [Green Version]
- Long, K.S.; Vester, B. Resistance to Linezolid Caused by Modifications at Its Binding Site on the Ribosome. Antimicrob. Agents Chemother. 2012, 56, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.-G.; Yuan, X.-L.; He, D.-D.; Hu, G.-Z.; Miao, M.-S.; Xu, E.-P. Research Progress on the Oxazolidinone Drug Linezolid Resistance. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9274–9281. [Google Scholar] [CrossRef]
- Srivastava, S.; Magombedze, G.; Koeuth, T.; Sherman, C.; Pasipanodya, J.G.; Raj, P.; Wakeland, E.; Deshpande, D.; Gumbo, T. Linezolid Dose That Maximizes Sterilizing Effect While Minimizing Toxicity and Resistance Emergence for Tuberculosis. Antimicrob. Agents Chemother. 2017, 61, e00751-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allix-Béguec, C.; Harmsen, D.; Weniger, T.; Supply, P.; Niemann, S. Evaluation and Strategy for Use of MIRU-VNTRplus, a Multifunctional Database for Online Analysis of Genotyping Data and Phylogenetic Identification of Mycobacterium Tuberculosis Complex Isolates. J. Clin. Microbiol. 2008, 46, 2692–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merker, M.; Blin, C.; Mona, S.; Duforet-Frebourg, N.; Lecher, S.; Willery, E.; Blum, M.G.B.; Rüsch-Gerdes, S.; Mokrousov, I.; Aleksic, E.; et al. Evolutionary History and Global Spread of the Mycobacterium Tuberculosis Beijing Lineage. Nat. Genet. 2015, 47, 242–249. [Google Scholar] [CrossRef]
- Mokrousov, I.; Narvskaya, O.; Vyazovaya, A.; Otten, T.; Jiao, W.-W.; Gomes, L.L.; Suffys, P.N.; Shen, A.-D.; Vishnevsky, B. Russian “Successful” Clone B0/W148 of Mycobacterium Tuberculosis Beijing Genotype: A Multiplex PCR Assay for Rapid Detection and Global Screening. J. Clin. Microbiol. 2012, 50, 3757–3759. [Google Scholar] [CrossRef] [Green Version]
- Mokrousov, I. Insights into the Origin, Emergence, and Current Spread of a Successful Russian Clone of Mycobacterium Tuberculosis. Clin. Microbiol. Rev. 2013, 26, 342–360. [Google Scholar] [CrossRef] [Green Version]
- Crudu, V.; Merker, M.; Lange, C.; Noroc, E.; Romancenco, E.; Chesov, D.; Günther, G.; Niemann, S. Nosocomial Transmission of Multidrug-Resistant Tuberculosis. Int. J. Tuberc. Lung Dis. 2015, 19, 1520–1523. [Google Scholar] [CrossRef]
- Vyazovaya, A.; Mokrousov, I.; Solovieva, N.; Mushkin, A.; Manicheva, O.; Vishnevsky, B.; Zhuravlev, V.; Narvskaya, O. Tuberculous Spondylitis in Russia and Prominent Role of Multidrug-Resistant Clone Mycobacterium Tuberculosis Beijing B0/W148. Antimicrob. Agents Chemother. 2015, 59, 2349–2357. [Google Scholar] [CrossRef] [Green Version]
- Engström, A.; Antonenka, U.; Kadyrov, A.; Kalmambetova, G.; Kranzer, K.; Merker, M.; Kabirov, O.; Parpieva, N.; Rajabov, A.; Sahalchyk, E.; et al. Population Structure of Drug-Resistant Mycobacterium Tuberculosis in Central Asia. BMC Infect. Dis. 2019, 19, 908. [Google Scholar] [CrossRef]
- Perdigão, J.; Silva, C.; Maltez, F.; Machado, D.; Miranda, A.; Couto, I.; Rabna, P.; Florez de Sessions, P.; Phelan, J.; Pain, A.; et al. Emergence of Multidrug-Resistant Mycobacterium Tuberculosis of the Beijing Lineage in Portugal and Guinea-Bissau: A Snapshot of Moving Clones by Whole-Genome Sequencing. Emerg. Microbes Infect. 2020, 9, 1342–1353. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; He, W.; Jiao, W.; Xia, H.; Sun, L.; Wang, S.; Xiao, J.; Ou, X.; Zhao, Y.; Shen, A. Molecular Characterization of Multidrug-Resistant Tuberculosis against Levofloxacin, Moxifloxacin, Bedaquiline, Linezolid, Clofazimine, and Delamanid in Southwest of China. BMC Infect. Dis. 2021, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Zong, Z.; Jing, W.; Shi, J.; Wen, S.; Zhang, T.; Huo, F.; Shang, Y.; Liang, Q.; Huang, H.; Pang, Y. Comparison of In Vitro Activity and MIC Distributions between the Novel Oxazolidinone Delpazolid and Linezolid against Multidrug-Resistant and Extensively Drug-Resistant Mycobacterium Tuberculosis in China. Antimicrob. Agents Chemother. 2018, 62, e00165-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasserman, S.; Louw, G.; Ramangoaela, L.; Barber, G.; Hayes, C.; Omar, S.V.; Maartens, G.; Barry, C.; Song, T.; Meintjes, G. Linezolid Resistance in Patients with Drug-Resistant TB and Treatment Failure in South Africa. J. Antimicrob. Chemother. 2019, 74, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, J.; Cui, P.; Shi, W.; Shi, X.; Niu, H.; Chan, D.; Yew, W.W.; Zhang, W.; Zhang, Y. Mycobacterium Tuberculosis Mutations Associated with Reduced Susceptibility to Linezolid. Antimicrob. Agents Chemother. 2016, 60, 2542–2544. [Google Scholar] [CrossRef] [Green Version]
- Theisen, A.; Reichel, C.; Rüsch-Gerdes, S.; Haas, W.H.; Rockstroh, J.K.; Spengler, U.; Sauerbruch, T. Mixed-Strain Infection with a Drug-Sensitive and Multidrug-Resistant Strain of Mycobacterium Tuberculosis. Lancet 1995, 345, 1512. [Google Scholar] [CrossRef]
- Hanekom, M.; Streicher, E.M.; Van de Berg, D.; Cox, H.; McDermid, C.; Bosman, M.; Gey van Pittius, N.C.; Victor, T.C.; Kidd, M.; van Soolingen, D.; et al. Population Structure of Mixed Mycobacterium Tuberculosis Infection Is Strain Genotype and Culture Medium Dependent. PLoS ONE 2013, 8, e70178. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.S.; Modongo, C.; Ncube, R.; Sepako, E.; Klausner, J.D.; Zetola, N.M. Advanced Immune Suppression Is Associated with Increased Prevalence of Mixed-Strain Mycobacterium Tuberculosis Infections among Persons at High Risk for Drug-Resistant Tuberculosis in Botswana. J. Infect. Dis. 2015, 211, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Eldholm, V.; Norheim, G.; von der Lippe, B.; Kinander, W.; Dahle, U.R.; Caugant, D.A.; Mannsåker, T.; Mengshoel, A.T.; Dyrhol-Riise, A.M.; Balloux, F. Evolution of Extensively Drug-Resistant Mycobacterium Tuberculosis from a Susceptible Ancestor in a Single Patient. Genome Biol. 2014, 15, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Thibert, L.; Chedore, P.; Shandro, C.; Jamieson, F.; Tyrrell, G.; Christianson, S.; Soualhine, H.; Wolfe, J. Canadian Multicenter Laboratory Study for Standardized Second-Line Antimicrobial Susceptibility Testing of Mycobacterium Tuberculosis. J. Clin. Microbiol. 2011, 49, 4112–4116. [Google Scholar] [CrossRef] [Green Version]
- Barrera, L.; Cooreman, E.; de Dieu Iragena, J.; Drobniewski, F.; Duda, P.; Havelkova, M.; Hoffner, S.; Kam, K.M.; Kim, S.J.; Labelle, S.; et al. Policy Guidance on Drug-Susceptibility Testing (DST) of Second-Line Antituberculosis Drugs; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Rüsch-Gerdes, S.; Pfyffer, G.E.; Casal, M.; Chadwick, M.; Siddiqi, S. Multicenter Laboratory Validation of the BACTEC MGIT 960 Technique for Testing Susceptibilities of Mycobacterium Tuberculosis to Classical Second-Line Drugs and Newer Antimicrobials. J. Clin. Microbiol. 2006, 44, 688–692. [Google Scholar] [CrossRef] [Green Version]
- Zimenkov, D.V.; Nosova, E.Y.; Kulagina, E.V.; Antonova, O.V.; Arslanbaeva, L.R.; Isakova, A.I.; Krylova, L.Y.; Peretokina, I.V.; Makarova, M.V.; Safonova, S.G.; et al. Examination of Bedaquiline- and Linezolid-Resistant Mycobacterium Tuberculosis Isolates from the Moscow Region. J. Antimicrob. Chemother. 2017, 72, 1901–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supply, P.; Allix, C.; Lesjean, S.; Cardoso-Oelemann, M.; Rüsch-Gerdes, S.; Willery, E.; Savine, E.; de Haas, P.; van Deutekom, H.; Roring, S.; et al. Proposal for Standardization of Optimized Mycobacterial Interspersed Repetitive Unit-Variable-Number Tandem Repeat Typing of Mycobacterium Tuberculosis. J. Clin. Microbiol. 2006, 44, 4498–4510. [Google Scholar] [CrossRef] [Green Version]
- Afgan, E.; Baker, D.; van den Beek, M.; Blankenberg, D.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Eberhard, C.; et al. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2016 Update. Nucleic Acids Res. 2016, 44, W3–W10. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the Biology of Mycobacterium Tuberculosis from the Complete Genome Sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Long-Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Garrison, E.; Marth, G. Haplotype-Based Variant Detection from Short-Read Sequencing. arXiv 2012, arXiv:1207.3907. [Google Scholar]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A Program for Annotating and Predicting the Effects of Single Nucleotide Polymorphisms, SnpEff: SNPs in the Genome of Drosophila Melanogaster Strain W1118; Iso-2; Iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuker, M. Mfold Web Server for Nucleic Acid Folding and Hybridization Prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
| ID | MIRU-VNTR 1 | Genotype | Beijing Clonal Complex | LZR Phenotype | |||
|---|---|---|---|---|---|---|---|
| MGIT | MIC | rrl | rplC2 | ||||
| #1 | 255432322122236152213423 | LAM 121-52 | - | R | 8 | wt | t460c |
| #2 | 252422322122236162213423 | LAM 121-51 | - | R | 8 | wt | t460c |
| #3 | 253335444432658253213423 | Beijing 94-32 | CC1 | R | 1 | g2270t | wt |
| #4 | 253336444442658253213423 | Beijing 94-32 | CC1 | R | 4 | wt | t460c |
| #5 | 273334444432656253213423 | Beijing 98-32 | CC3 | R | 4 | a2810c; g2814t | wt |
| #6 | 274335344432657253213423 | Beijing 100-32 | CC2 | R | 4 | wt | t460c |
| #7 | 213722354433236252213423 | URAL 163-15 | - | R | 4 | wt | t460c |
| #8 | 273335444432657253213423 | Beijing 100-32 | CC2 | R | 4 | wt | t460c |
| #9 | 253335444432658253213423 | Beijing 94-32 | CC1 | R | 4 | wt | t460c |
| #10 | 274334244423647252213423 | Beijing 9515-32 | BL7 | R | 4 | wt | t460c |
| #11 | 253335443432658253213423 | Beijing 94-32 | CC1 | R | 16 | wt | t460c |
| #12 | 273335444432657253213423 | Beijing 100-32 | CC2 | R | 2 | wt | (t460c) |
| #13 | 253334444232658253213423 | Beijing 94-32 | CC1 | R | 2 | wt | t460c |
| #14 | 273345444432657253213423 | Beijing 100-32 | CC2 | R | 4 | g2814t | wt |
| #15 | 2(7/6)3(3/1)4544(3/4)432 (5/6)(4/5)8253213423 | mixed | - | R | 2 | wt | (t460c) |
| #16 | 253346444442658253213423 | Beijing 94-32 | CC1 | R | 4 | wt | t460c |
| ID | Genotype | Drug resistance Profile 1 | Case | TB Form 2 | HIV | Days Form Treatment Start | Outcome | |
|---|---|---|---|---|---|---|---|---|
| H.R.Z.E.S | Fq.Sl.Ps.Et.Cs | |||||||
| #1 | LAM 121-52 | R.R.R.R.R | R.R.R.R.S | Previous ineffective course | Cirrhotic | - | 769 | Failure |
| #2 | LAM 121-51 | R.R.S.R.S | R.R.R.R.S | Chronic | Fibro-cavernous | - | 392 | Lost to follow up |
| #3 | Beijing 94-32 | R.R.R.R.S | R.S.S.S.S | Chronic | Fibro-cavernous | - | 267 | Failure |
| #4 | Beijing 94-32 | R.R.R.R.R | R.R.R.R.S | Primary | Infiltrative | - | 398 | Death |
| #5 | Beijing 98-32 | R.R.R.R.R | R.R.R.R.S | Chronic | Fibro-cavernous | - | 832 | Death |
| #6 | Beijing 100-32 | R.R.R.R.R | R.R.R.R.S | Relapse | Caseous pneumonia | - | 0 | Death |
| #7 | URAL 163-15 | R.R.R.R.R | R.R.R.R.S | Chronic | Fibro-cavernous | - | 206 | Lost to follow up |
| #8 | Beijing 100-32 | R.R.R.R.R | R.R.R.R.S | Relapse | Disseminated | - | 1052 | Death |
| #9 | Beijing 94-32 | R.R.S.R.R | R.S.R.S.S | Chronic | Fibro-cavernous | - | 386 | Failure |
| #10 | Beijing 100-32 | ?.?.?.?.? | ?.?.?.?.? | ND | ND | ND | ND | Lost to follow up |
| #11 | Beijing 94-32 | R.R.R.S.R | R.S.S.R.S | Primary | TB meningitis | - | 0 | Death |
| #12 | Beijing 100-32 | R.R.R.S.R | R.R.R.R.S | Chronic | Fibro-cavernous | + | 1072 | Failure |
| #13 | Beijing 94-32 | R.R.S.S.R | R.R.R.R.R | Chronic | Fibro-cavernous | - | 984 | Failure |
| #14 | Beijing 100-32 | R.R.S.R.R | R.R.R.R.S | Relapse | Infiltrative | - | 876 | Lost to follow up |
| #15 | mixed | R.R.R.R.R | R.R.S.R.S | Primary | Disseminated | + | 0 | Death |
| #16 | Beijing 94-32 | R.R.R.S.R | R.R.R.R.S | Relapse | Disseminated | + | 330 | Death |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ushtanit, A.; Mikhailova, Y.; Lyubimova, A.; Makarova, M.; Safonova, S.; Filippov, A.; Borisov, S.; Zimenkov, D. Genetic Profile of Linezolid-Resistant M. tuberculosis Clinical Strains from Moscow. Antibiotics 2021, 10, 1243. https://doi.org/10.3390/antibiotics10101243
Ushtanit A, Mikhailova Y, Lyubimova A, Makarova M, Safonova S, Filippov A, Borisov S, Zimenkov D. Genetic Profile of Linezolid-Resistant M. tuberculosis Clinical Strains from Moscow. Antibiotics. 2021; 10(10):1243. https://doi.org/10.3390/antibiotics10101243
Chicago/Turabian StyleUshtanit, Anastasia, Yulia Mikhailova, Alexandra Lyubimova, Marina Makarova, Svetlana Safonova, Alexey Filippov, Sergey Borisov, and Danila Zimenkov. 2021. "Genetic Profile of Linezolid-Resistant M. tuberculosis Clinical Strains from Moscow" Antibiotics 10, no. 10: 1243. https://doi.org/10.3390/antibiotics10101243
APA StyleUshtanit, A., Mikhailova, Y., Lyubimova, A., Makarova, M., Safonova, S., Filippov, A., Borisov, S., & Zimenkov, D. (2021). Genetic Profile of Linezolid-Resistant M. tuberculosis Clinical Strains from Moscow. Antibiotics, 10(10), 1243. https://doi.org/10.3390/antibiotics10101243






