Daily Administered Dual-Light Photodynamic Therapy Provides a Sustained Antibacterial Effect on Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Outcome Endpoints
2.2. Statistical Analysis
3. Results
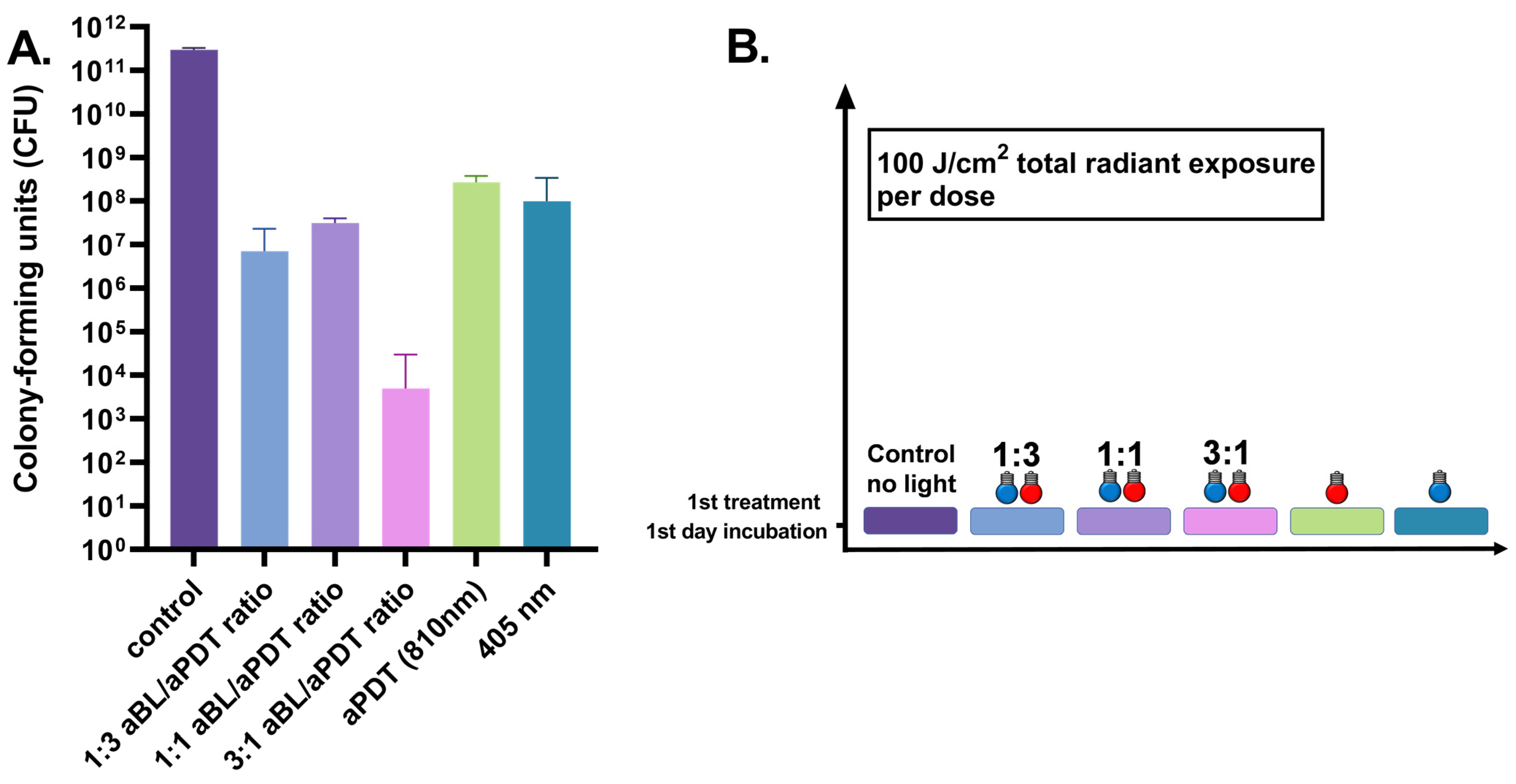
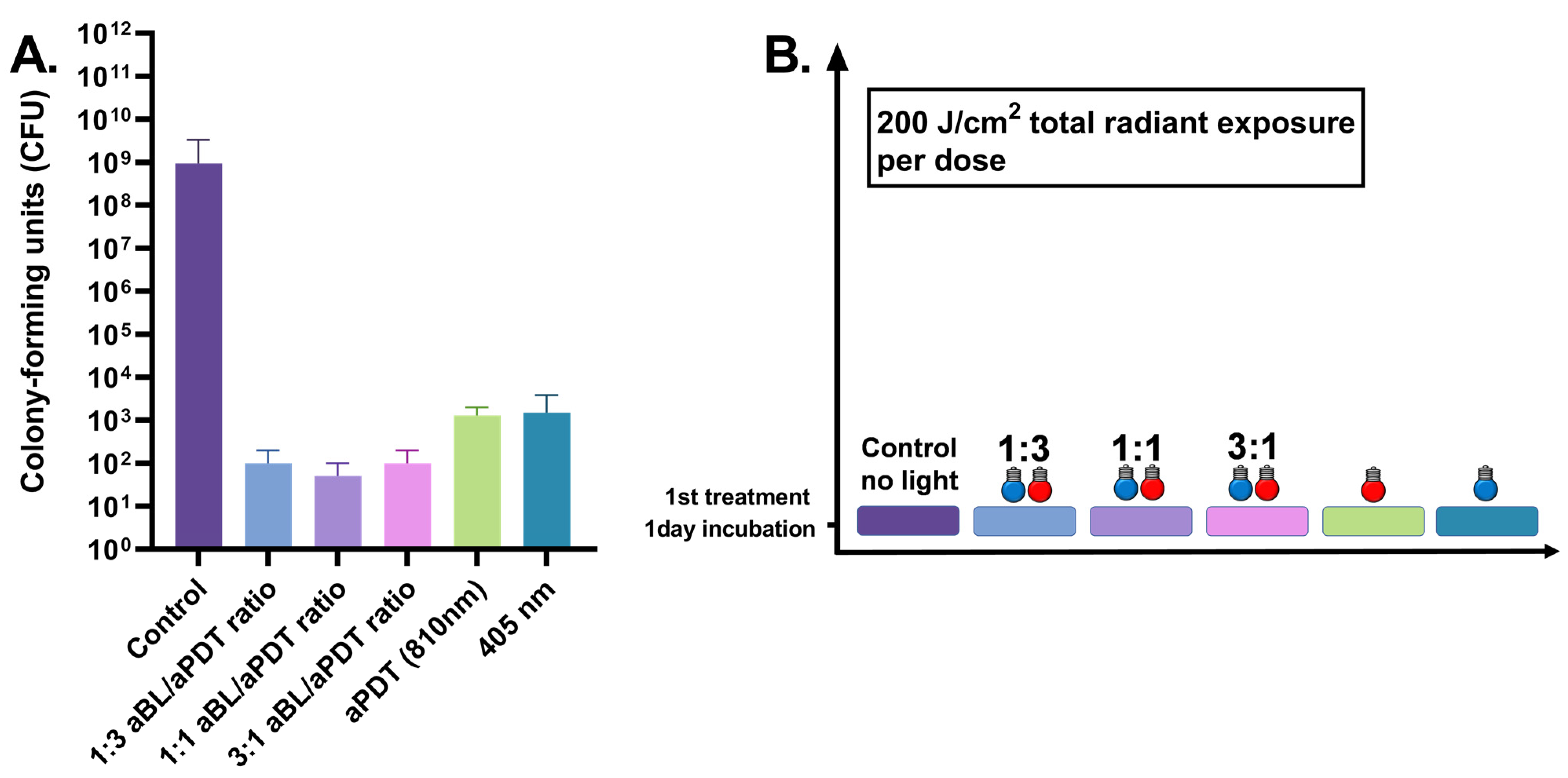
3.1. One-Day S. aureus Biofilm Treated Once by 100 J/cm2
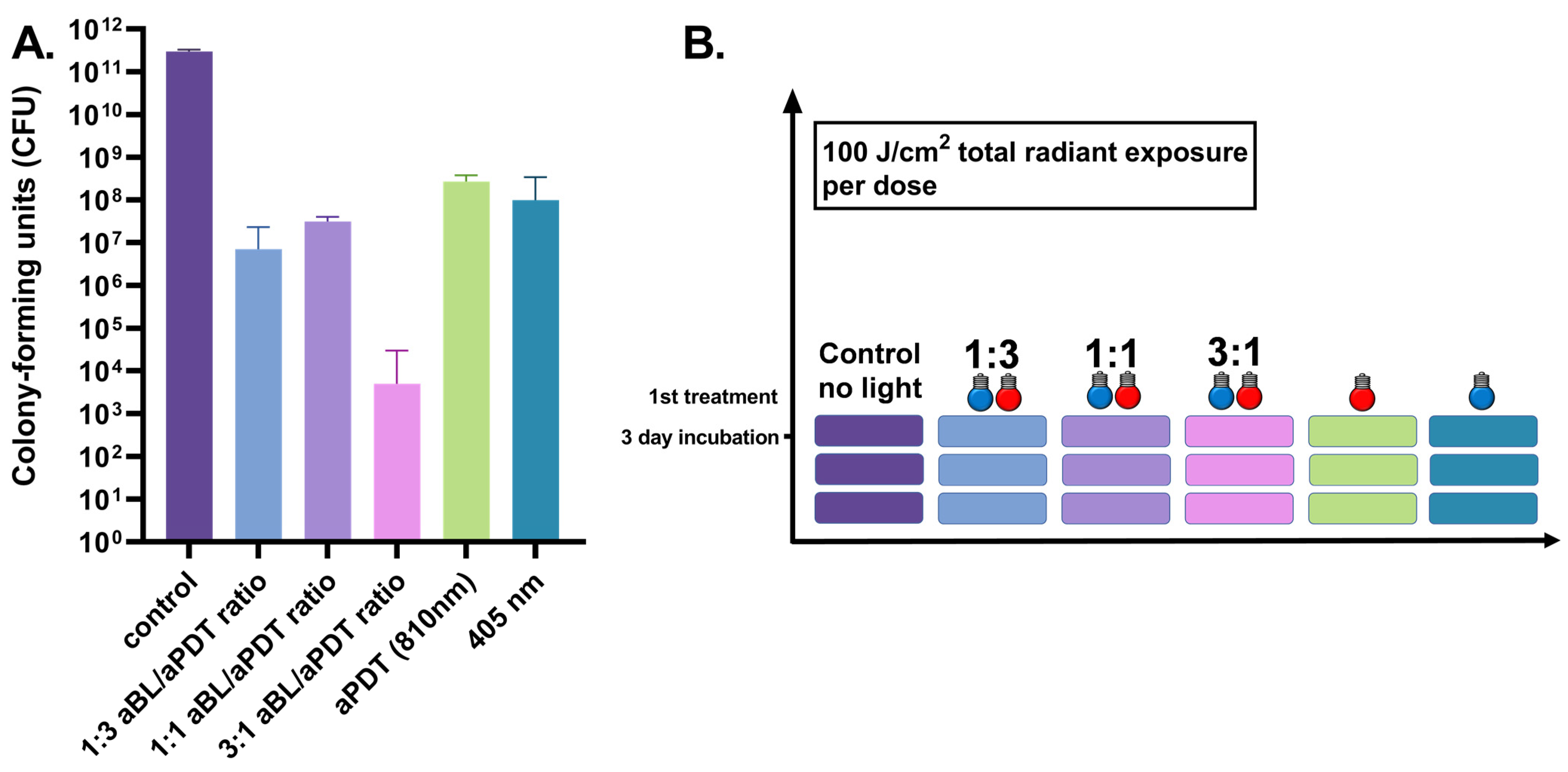
3.2. Three-Day S. aureus Biofilm Treated Once by 100 J/cm2
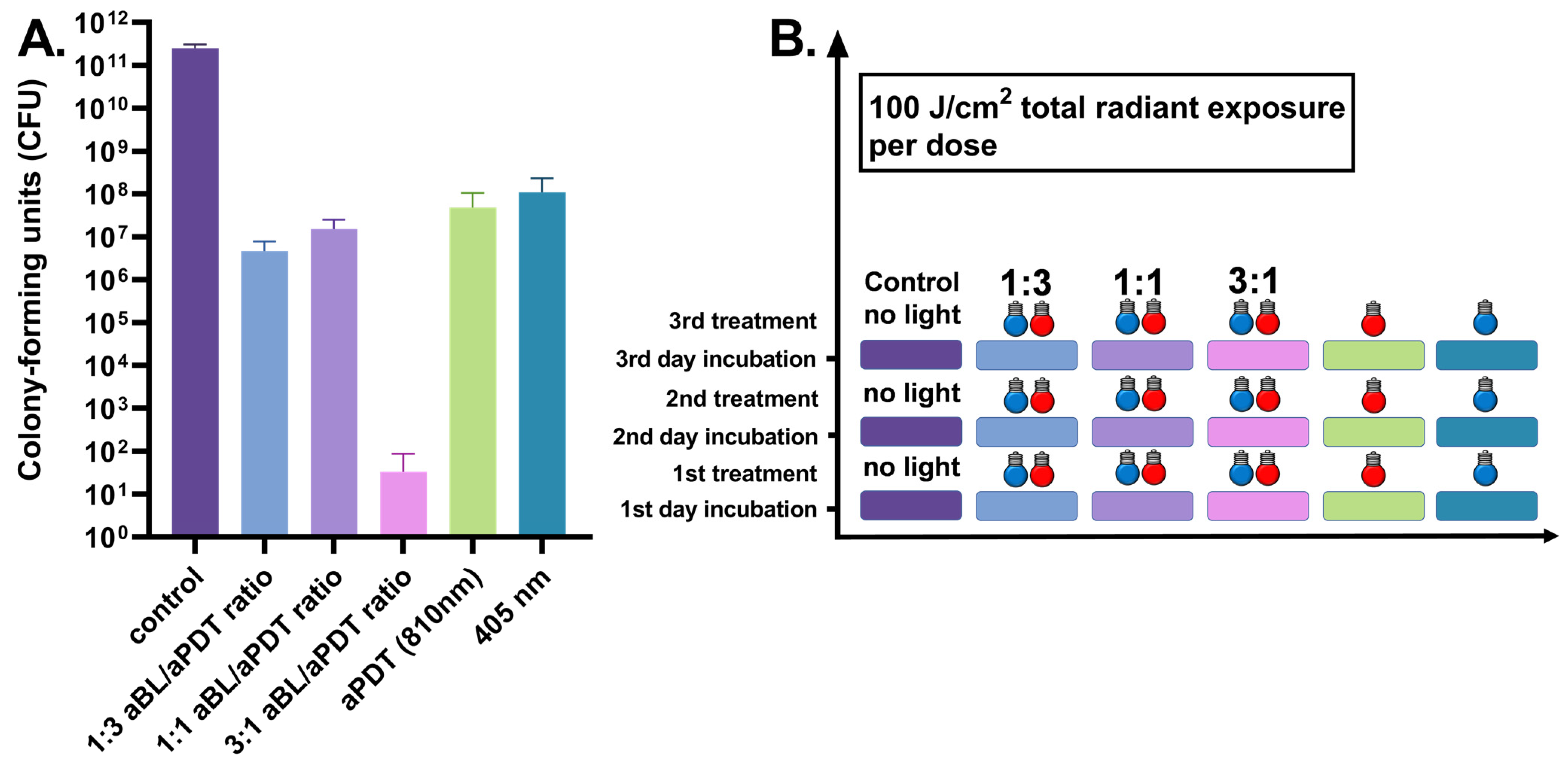
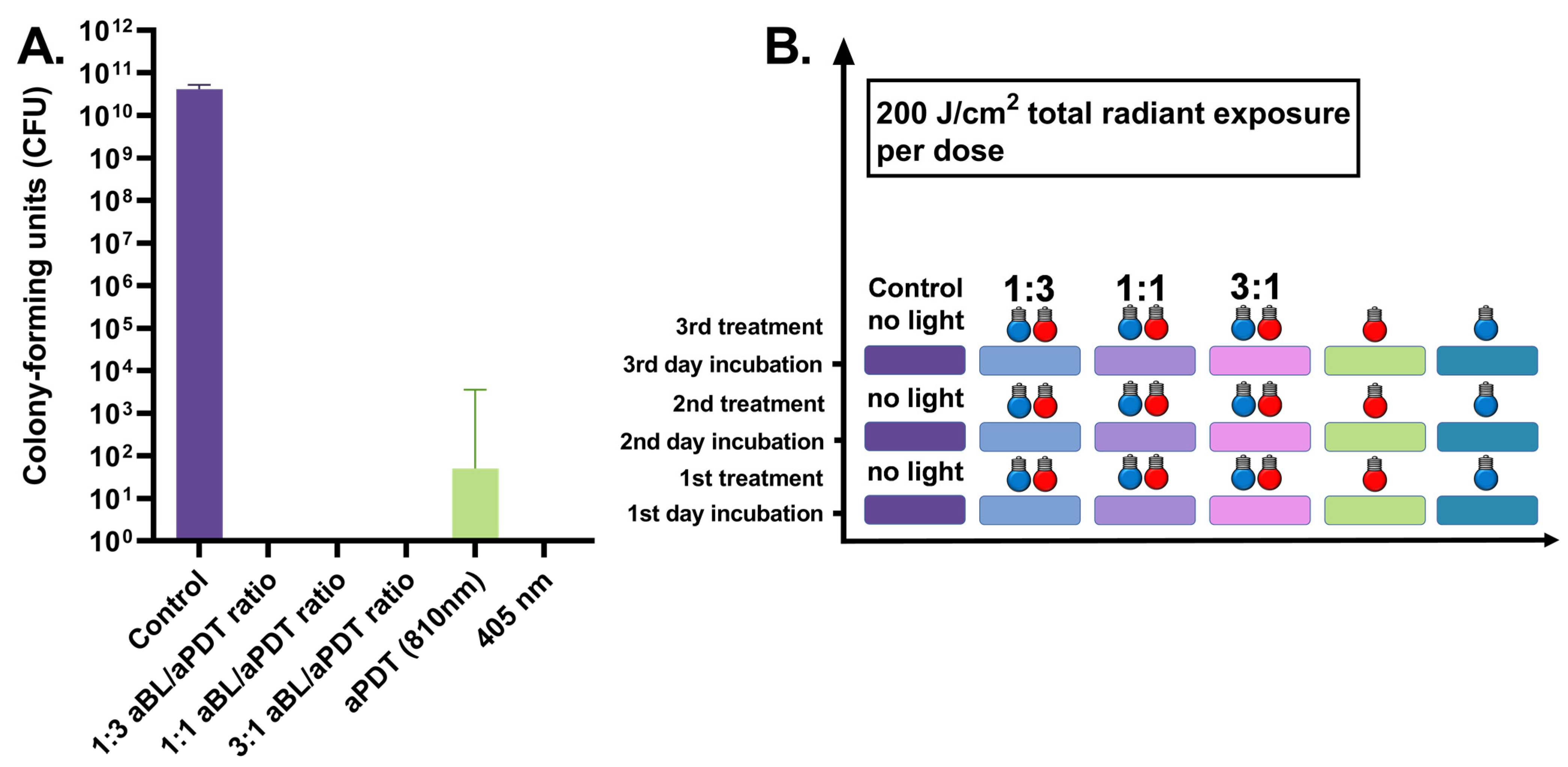
3.3. Three-Day S. aureus Biofilm Treated Daily by 100 J/cm2
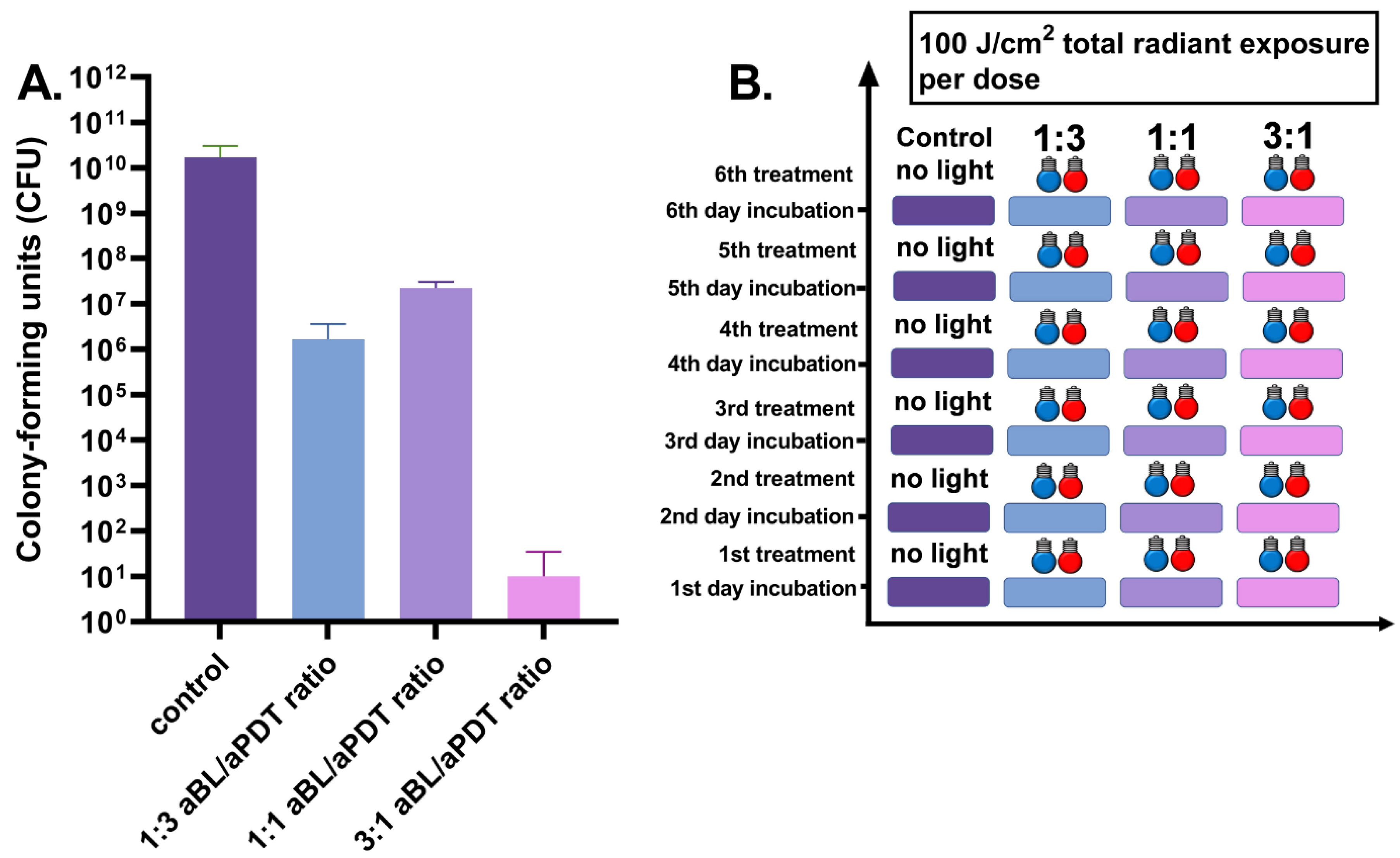
3.4. Six-Day S. aureus Biofilm Treated Daily by 100 J/cm2
3.5. One-Day S. aureus Biofilm Treated Once by 200 J/cm2
3.6. Three-Day S. aureus Biofilm Treated Daily by 200 J/cm2
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ondusko, D.; Nolt, D. Staphylococcus aureus. Pediatr. Rev. 2018, 39, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federspiel, J.; Stearns, S.; Peppercorn, A.; Chu, V.; Fowler, V., Jr. Increasing U.S. rates of endocarditis with Staphylococcus aureus: 1999–2008. Arch. Intern. Med. 2012, 4, 363–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdoch, D.R.; Corey, R.G.; Hoen, B.; Miró, M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The international collaboration on endocarditis-prospective cohort study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Kern, W. Management of Staphylococcus aureus bacteremia and endocarditis: Progresses and challenges. Curr. Opin. Infect. Dis. 2010, 23, 346–358. [Google Scholar] [CrossRef]
- Skinner, D.; Keefer, C. Significance of bacteremia caused by Staphylococcus aureus A study of one hundred and twenty-two cases and a review of the literature concerned with experimental infection in animals. Arch. Intern. Med. 1941, 5, 851–875. [Google Scholar] [CrossRef]
- den Heijer, C.D.J.; van Bijnen, E.M.E.; Paget, W.J.; Pringle, M.; Goossens, H.; Bruggeman, C.A.; Schellevis, F.G.; Stobberingh, E.E. Prevalence and resistance of commensal Staphylococcus aureus, including methicillin-resistant S. aureus, in nine European countries: A cross-sectional study. Lancet Infect. Dis. 2013, 13, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Elie-Turenne, M.-C.; Fernandes, H.; Mediavilla, J.R.; Rosenthal, M.; Mathema, B.; Singh, A.; Cohen, T.R.; Pawar, K.A.; Shahidi, H.; Kreiswirth, B.N.; et al. Prevalence and characteristics of Staphylococcus aureus colonization among healthcare professionals in an urban teaching hospital. Infect. Control Hosp. Epidemiol. 2010, 31, 574–580. [Google Scholar] [CrossRef]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Mediavilla, J.; Chen, L.; Mathema, B.; Kreiswirth, B. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr. Opin. Microbiol. 2012, 15, 588–595. [Google Scholar] [CrossRef]
- Ogawara, H. Comparison of antibiotic resistance mechanisms in antibiotic-producing and pathogenic bacteria. Molecules 2019, 24, 3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plackett, B. Why big pharma has abandoned antibiotics. Nature 2020, 586, 50–52. [Google Scholar] [CrossRef]
- Mohr, K. History of antibiotics research. Curr. Top Microbiol. Immunol. 2016, 398, 237–272. [Google Scholar] [CrossRef] [PubMed]
- Årdal, C.; Balasegaram, M.; Laxminarayan, R.; McAdams, D.; Outterson, K.; Rex, J.H.; Sumpradit, N. Antibiotic development—Economic, regulatory and societal challenges. Nat. Rev. Microbiol. 2020, 18, 267–274. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resist. Updat. 2017, 33–35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-H.; Lee, J.-K.; Um, H.-S.; Chang, B.-S.; Lee, S.-Y.; Lee, M.-K. Phototoxic effect of blue light on the planktonic and biofilm state of anaerobic periodontal pathogens. J. Periodontal Implant Sci. 2013, 43, 72–78. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 5, 571–589. [Google Scholar]
- Yin, R.; Dai, T.; Avci, P.; Jorge, A.E.S.; de Melo, W.; Vecchio, D.; Huang, Y.; Gupta, A.; Hamblin, M.R. Light based anti-infectives: Ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr. Opin. Pharmacol. 2013, 5, 2109–2121. [Google Scholar] [CrossRef] [Green Version]
- Nikinmaa, S.; Alapulli, H.; Auvinen, P.; Vaara, M.; Rantala, J.; Kankuri, E.; Sorsa, T.; Meurman, J.; Pätilä, T. Dual-light photodynamic therapy administered daily provides a sustained antibacterial effect on biofilm and prevents streptococcus mutans adaptation. PLoS ONE 2020, 15, e0232775. [Google Scholar] [CrossRef]
- Zmantar, T.; Kouidhi, B.; Miladi, H.; Mahdouani, K.; Bakhrouf, A. A microtiter plate assay for Staphylococcus aureus biofilm quantification at various pH levels and hydrogen peroxide supplementation. New Microbiol. 2010, 2, 137–145. [Google Scholar]
- Nikinmaa, S.; Patila, T. Dual-Light Photodynamic Therapy Administered Daily Provides a Sustained Antibacterial Effect on Biofilm and Prevents Streptococcus mutans Adaptation. 2021. Available online: https://www.protocols.io/view/dual-light-photodynamic-therapy-administered-daily-bfbcjiiw (accessed on 25 April 2021).
- Tavares, A.; Carvalho, C.M.B.; Faustino, M.A.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Alves, E.; et al. Antimicrobial photodynamic therapy: Study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Onyango, L.; Alreshidi, M. Adaptive metabolism in staphylococci: Survival and persistence in environmental and clinical settings. J. Pathog. 2018, 2018, 1092632. [Google Scholar] [CrossRef] [Green Version]
- Guffrey, J.; Payne, W.; Jones, T.; Martin, K. Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 nm light from a supraluminous diode array. Photomed. Laser Surg. 2013, 31, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Burchard, T.; Karygianni, L.; Hellwig, E.; Follo, M.; Wrbas, T.; Wittmer, A.; Al-Ahmad, A. Inactivation of oral biofilms using visible light and water filtered infrared A radiation and indocyanine green. Future Med. Chem. 2019, 11, 1721–1740. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.-W.; Wu, E.-C.; Ko, W.-C.; Lee, C.-C.; Hor, L.-I.; Huang, I.-H. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus by indocyanine green and near infrared light. Dermatol. Sin. 2018, 36, 8–15. [Google Scholar] [CrossRef]
- Tomb, R.; White, T.; Coia, J.; Anderson, J.; MacGregor, S.; Maclean, M. Review of the comparative susceptibility of microbial species to photoinactivation using 380–480 nm violet-blue light. Photochem. Photobiol. 2018, 3, 445–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoenes, K.; Hess, M.; Vatter, P.; Spellerberg, B.; Hessling, M. 405 nm and 450 nm photoinactivation of saccharomyces cerevisiae. Eur. J. Microbiol. Immunol. 2018, 8, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Masson-Mayers, D.; Bumah, V.; Biener, G.; Raicu, V.; Enwemeka, C. The relative antimicrobial effect of blue 405 nm LED and blue 405 nm laser on methicillin-resistant Staphylococcus aureus in vitro. Lasers Med. Sci. 2015, 30, 2265–2271. [Google Scholar] [CrossRef] [PubMed]
- Leanse, L.; Goh, X.; Cheng, J.-X.; Hooper, D.; Dai, T. Dual-wavelenght photo-killing of methicillin-resistant Staphylococcus aureus. JCI Insight 2020, 5, e134343. [Google Scholar] [CrossRef] [PubMed]
- Guffrey, J.; Wilborn, J. Effects of combined 405 nm and 880-nm light on Staphylococcus aureus and pseudomonas aeruginosa in vitro. Photomed. Laser Surg. 2006, 6, 680–683. [Google Scholar] [CrossRef] [Green Version]
- Esposito, S.; Noviello, S.; Leone, S. Epidemiology and microbiology of skin and soft tissue infections. Curr. Opin. Infect. Dis. 2016, 2, 109–115. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, R.; Campos, C.; de Castro Franco, L.; Rocha, A.; Ercole, F. Incidence and risk factors for surgical site infection in general surgeries. Rev. Lat.-Am. Enfermagem. 2017, 25, e2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, C.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef]
- McGarry, S.; Engemann, J.; Schmader, K.; Sexton, D.; Kaye, K. Surgical site infection due to Staphylococcus aureus among elderly patients: Mortality, duration of hospitalization and cost. Infect. Control Hosp. Epidemiol. 2004, 6, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, A.; Murata, T.; Imai, K.; Yamamoto, Y.; Fujimoto, Y. Treatment procedures and associated medical costs of methicillin-resistant Staphylococcus aureus infection in Japan: A retrospective analysis using a database of Japanese employment-based health insurance. SAGE Open Med. 2019, 7, 2050312119871181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Experiment | Figure | Repeats | Number of Treaments | Radiant Exposure (J/cm2) | Wavelenghts (nm) | Irradiance of 405 nm (mW/cm2) | Irradiance of 810 nm (mW/cm2) | ICG (+/−) | Biofilm Age at the End of Experiment (Days) |
|---|---|---|---|---|---|---|---|---|---|
| 1-day aBL | 1 | 6 | 1 | 100 | 405 | 80 | 0 | − | 1 |
| 1-day aPDT | 1 | 6 | 1 | 100 | 810 | 0 | 100 | + | 1 |
| 1-day single dose 1:3 | 1 | 6 | 1 | 100 | 405 + 810 | 42 | 135 | + | 1 |
| 1-day single dose 1:1 | 1 | 6 | 1 | 100 | 405 + 810 | 73 | 79 | + | 1 |
| 1-day single dose 3:1 | 1 | 6 | 1 | 100 | 405 + 810 | 130 | 38 | + | 1 |
| 1-day control | 1 | 3 | N/A | N/A | N/A | N/A | N/A | − | 1 |
| 1-day aBL | 5 | 6 | 1 | 200 | 405 | 80 | 0 | − | 1 |
| 1-day aPDT | 5 | 6 | 1 | 200 | 810 | 0 | 100 | + | 1 |
| 1-day single dose 1:3 | 5 | 6 | 1 | 200 | 405 + 810 | 42 | 135 | + | 1 |
| 1-day single dose 1:1 | 5 | 6 | 1 | 200 | 405 + 810 | 73 | 79 | + | 1 |
| 1-day single dose 3:1 | 5 | 6 | 1 | 200 | 405 + 810 | 130 | 38 | + | 1 |
| 1-day control | 5 | 6 | N/A | N/A | N/A | N/A | N/A | − | 1 |
| 3-day aBL | 2 | 6 | 1 | 100 | 405 | 80 | 0 | − | 3 |
| 3-day aPDT | 2 | 6 | 1 | 100 | 810 | 0 | 100 | + | 3 |
| 3-day single dose 1:3 | 2 | 6 | 1 | 100 | 405 + 810 | 42 | 135 | + | 3 |
| 3-day single dose 1:1 | 2 | 6 | 1 | 100 | 405 + 810 | 73 | 79 | + | 3 |
| 3-day single dose 3:1 | 2 | 6 | 1 | 100 | 405 + 810 | 130 | 38 | + | 3 |
| 3-day control | 2 | 3 | N/A | N/A | N/A | N/A | N/A | − | 3 |
| 3-day daily dose aBL | 3 | 6 | 3 | 100 | 405 | 80 | 0 | − | 3 |
| 3-day daily dose aPDT | 3 | 6 | 3 | 100 | 810 | 0 | 100 | + | 3 |
| 3-day daily dose 1:3 | 3 | 6 | 3 | 100 | 405 + 810 | 42 | 135 | + | 3 |
| 3-day daily dose 1:1 | 3 | 6 | 3 | 100 | 405 + 810 | 73 | 79 | + | 3 |
| 3-day daily dose 3:1 | 3 | 6 | 3 | 100 | 405 + 810 | 130 | 38 | + | 3 |
| 3-day control | 3 | 3 | N/A | N/A | N/A | N/A | N/A | − | 3 |
| 3-day daily dose aBL | 6 | 6 | 3 | 200 | 405 | 80 | 0 | − | 3 |
| 3-day daily dose aPDT | 6 | 6 | 3 | 200 | 810 | 0 | 100 | + | 3 |
| 3-day daily dose 1:3 | 6 | 6 | 3 | 200 | 405 + 810 | 42 | 135 | + | 3 |
| 3-day daily dose 1:1 | 6 | 6 | 3 | 200 | 405 + 810 | 73 | 79 | + | 3 |
| 3-day daily dose 3:1 | 6 | 6 | 3 | 200 | 405 + 810 | 130 | 38 | + | 3 |
| 3-day control | 6 | 6 | N/A | N/A | N/A | N/A | N/A | − | 3 |
| 6-day aBL | 4 | 6 | 6 | 100 | 405 | 80 | 0 | − | 6 |
| 6-day aPDT | 4 | 6 | 6 | 100 | 810 | 0 | 100 | + | 6 |
| 6-day single dose 1:3 | 4 | 6 | 6 | 100 | 405 + 810 | 42 | 135 | + | 6 |
| 6-day single dose 1:1 | 4 | 6 | 6 | 100 | 405 + 810 | 73 | 79 | + | 6 |
| 6-day single dose 3:1 | 4 | 6 | 6 | 100 | 405 + 810 | 130 | 38 | + | 6 |
| 6-day control | 4 | 3 | N/A | N/A | N/A | N/A | N/A | − | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikinmaa, S.; Podonyi, A.; Raivio, P.; Meurman, J.; Sorsa, T.; Rantala, J.; Kankuri, E.; Tauriainen, T.; Pätilä, T. Daily Administered Dual-Light Photodynamic Therapy Provides a Sustained Antibacterial Effect on Staphylococcus aureus. Antibiotics 2021, 10, 1240. https://doi.org/10.3390/antibiotics10101240
Nikinmaa S, Podonyi A, Raivio P, Meurman J, Sorsa T, Rantala J, Kankuri E, Tauriainen T, Pätilä T. Daily Administered Dual-Light Photodynamic Therapy Provides a Sustained Antibacterial Effect on Staphylococcus aureus. Antibiotics. 2021; 10(10):1240. https://doi.org/10.3390/antibiotics10101240
Chicago/Turabian StyleNikinmaa, Sakari, Anna Podonyi, Peter Raivio, Jukka Meurman, Timo Sorsa, Juha Rantala, Esko Kankuri, Tuomas Tauriainen, and Tommi Pätilä. 2021. "Daily Administered Dual-Light Photodynamic Therapy Provides a Sustained Antibacterial Effect on Staphylococcus aureus" Antibiotics 10, no. 10: 1240. https://doi.org/10.3390/antibiotics10101240






