Consumption of Antibacterials for Systemic Use in Slovakia: A National Study and the Quality Indicators for Outpatient Antibiotic Use
Abstract
:1. Introduction
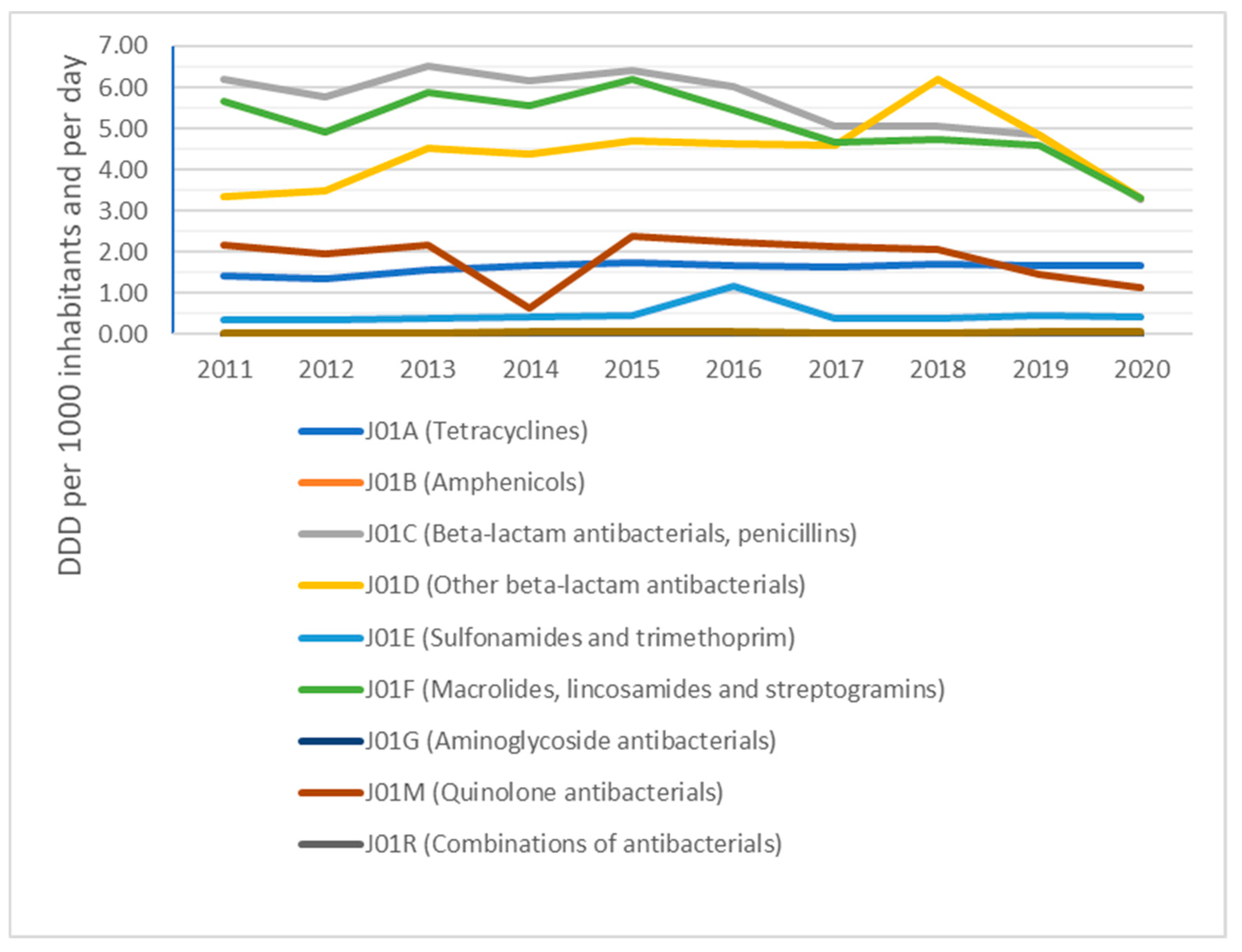
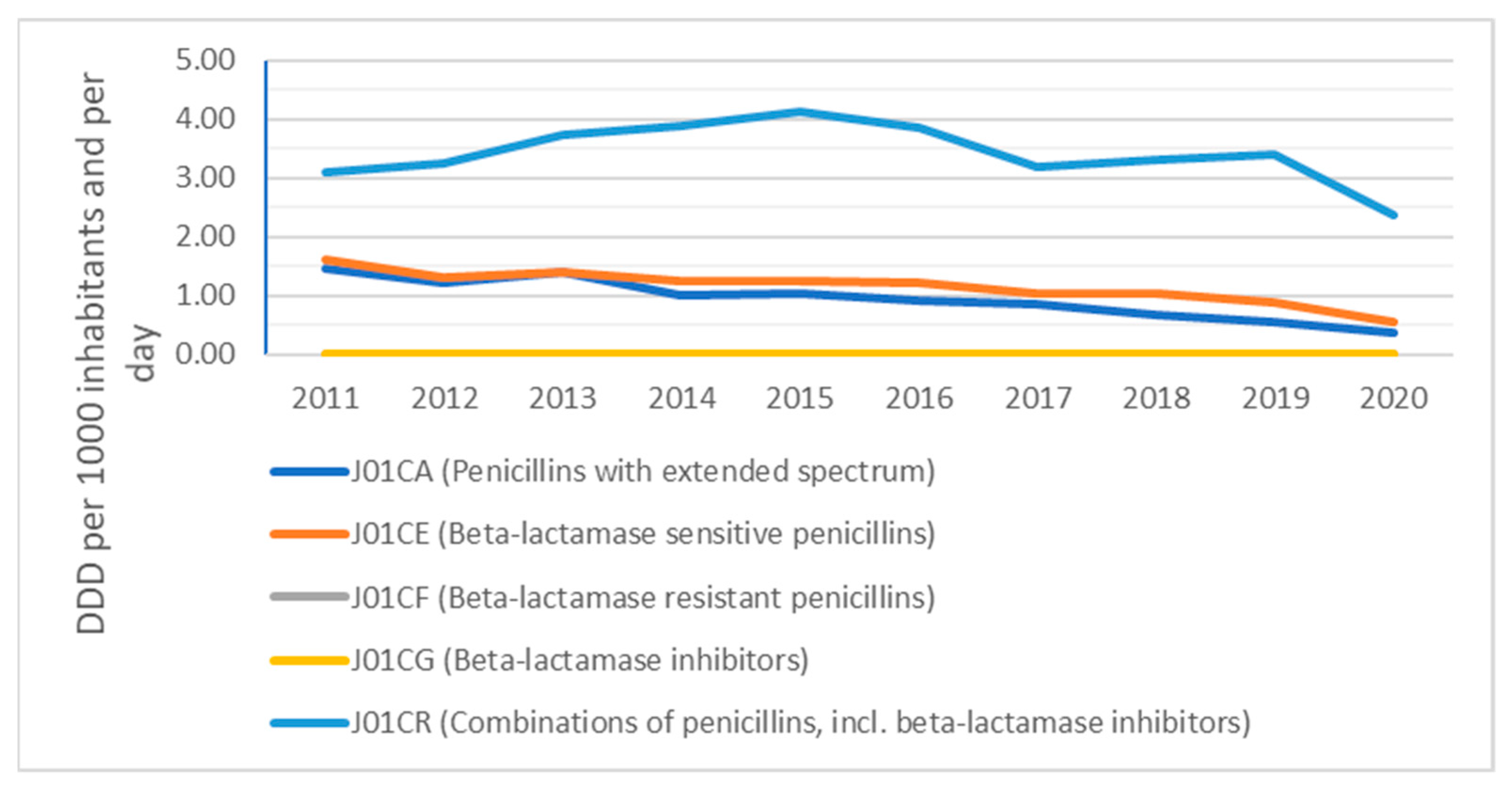
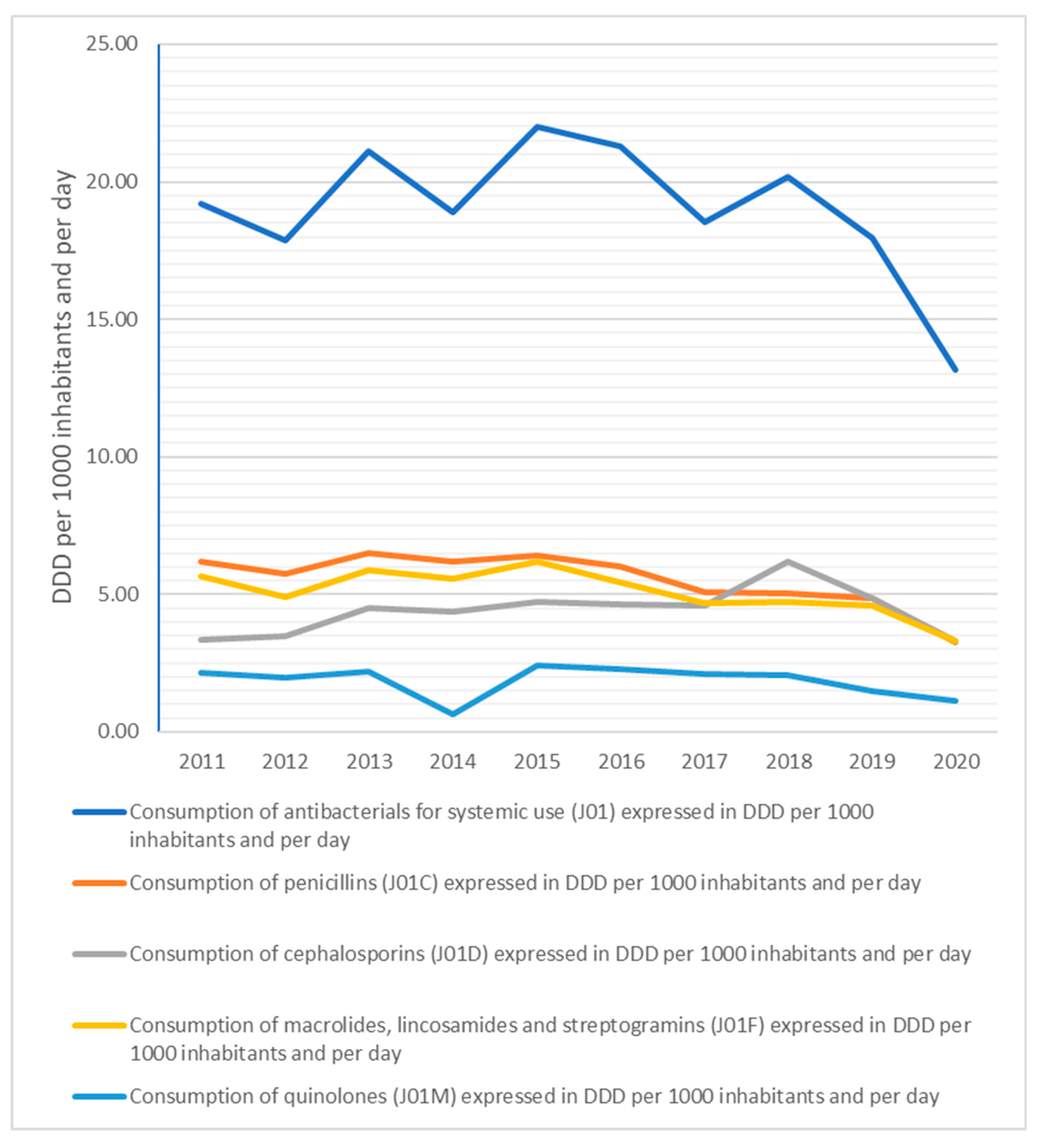
2. Results
3. Discussion
- Patient pressure. It is the patients who often demand that doctors prescribe antibiotics, even when there is no reason to do so. A significant number of patients who request a prescription for antibiotics will also receive them.
- Emergency rooms. Patients often go to emergency rooms for a prescription for antibiotics, even if their GP refuses to prescribe them.
- Effectiveness of prescriptions and availability of doctors in the region.
- Economic power of the region. The number of doctor visits is also related to the strength of the economy or the unemployment rate in the region. In the case of economically more developed regions, patients with influenza or other common diseases seek out doctors less often (holidays, housework, “sick days”) than in regions with higher unemployment [25].
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Smatana, M.; Pažitný, P.; Kandilaki, D.; Laktišová, M.; Sedláková, D.; Palušková, M.; Ginneken, E.V.; Spranger, A. Slovakia: Health system review. Health Syst. Transit. 2016, 18, 210. [Google Scholar]
- Ministry of Health. Act No. 363/2011 Coll. on the Scope and Conditions of Payments for Medicines, Medical Devices and Dietetic Foods from Public Health Insurance and Amending Certain Acts, as Amended. Available online: http://www.zakonypreludi.sk/zz/2011-363 (accessed on 31 May 2021).
- Tesar, T.; Obsitnik, B.; Kaló, Z.; Kristensen, F.B. How Changes in Reimbursement Practices Influence the Financial Sustainability of Medicine Policy: Lessons Learned from Slovakia. Front. Pharmacol. 2019, 10, 664. [Google Scholar] [CrossRef] [Green Version]
- Van de Sande-Bruinsma, N.; Grundmann, H.; Verloo, D.; Tiemersma, E.; Monen, J.; Goossens, H.; Ferech, M.; European Antimicrobial Resistance Surveillance System; European Surveillance of Antimicrobial Project Groups. Antimicrobial drug use and resistance in Europe. Emerg. Infect. Dis. 2008, 14, 1722–1730. [Google Scholar] [CrossRef]
- Robertson, J.; Vlahović-Palčevski, V.; Iwamoto, K.; Högberg, L.D.; Godman, B.; Monnet, D.L.; Garner, S.; Weist, K.; ESAC-Net Study Group; WHO Europe AMC Network Study Group. Variations in the consumption of antimicrobial medicines in the European region, 2014–2018: Findings and implications from ESAC-Net and WHO Europe. Front. Pharmacol. 2021, 12, 639207. [Google Scholar] [CrossRef]
- Versporten, A.; Bruyndonckx, R.; Adriaenssens, N.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S.; ESAC-Net Study Group. Consumption of cephalosporins in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76 (Suppl. 2), ii22–ii29. [Google Scholar] [CrossRef]
- Adriaenssens, N.; Bruyndonckx, R.; Versporten, A.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S.; ESAC-Net Study Group. Consumption of quinolones in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76 (Suppl. 2), ii37–ii44. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, S.G.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 9, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Hofer, U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Iwamoto, K.; Hoxha, I.; Ghazaryan, L.; Abilova, V.; Cvijanovic, A.; Pyshnik, H.; Darakhvelidze, M.; Makalkina, L.; Jakupi, A.; et al. Antimicrobial Medicines Consumption in Eastern Europe and Central Asia—An Updated Cross-National Study and Assessment of Quantitative Metrics for Policy Action. Front. Pharmacol. 2019, 9, 1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy Hara, G.; Kanj, S.S.; Pagani, L.; Abbo, L.; Endimiani, A.; Wertheim, H.F.L.; Amabile-Cuevas, C.; Tattevin, P.; Mehtar, S.; Cardoso, F.L.; et al. Ten key points for the appropriate use of antibiotics in hospitalised patients: A consensus from the Antimicrobial Stewardship and Resistance Working Groups of the International Society of Chemotherapy. Int. J. Antimicrob. Agents 2016, 48, 239–246. [Google Scholar] [CrossRef]
- Kelkar, D.; Galwankar, S. State of the globe: Antimicrobial stewardship guides rationale use of antimicrobials to enable faster cure and prevent drug resistance. J. Glob. Infect. Dis. 2013, 5, 43–44. [Google Scholar] [PubMed]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Pharmaceutical Strategy for Europe {SWD (2020) 286 final}. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=COM:2020:0761:FIN (accessed on 31 May 2021).
- French, G.L. Clinical impact and relevance of antibiotic resistance. Adv. Drug Deliv. Rev. 2005, 57, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- Wojkowska-Mach, J.; Godman, B.; Glassman, A.; Kurdi, A.; Pilc, A.; Rozanska, A.; Skoczynski, S.; Walaszek, M.; Bochenek, T. Antibiotic consumption and antimicrobial resistance in Poland; findings and implications. Antimicrob. Resist. Infect. Control 2018, 7, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruyndonckx, R.; Adriaenssens, N.; Versporten, A.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Weist, K.; Coenen, S.; ESAC-Net Study Group. Consumption of antibiotics in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76 (Suppl. 2), ii7–ii13. [Google Scholar] [CrossRef] [PubMed]
- Bruyndonckx, R.; Adriaenssens, N.; Hens, N.; Versporten, A.; Monnet, D.L.; Molenberghs, G. Consumption of penicillins in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76 (Suppl. 2), ii14–ii21. [Google Scholar] [CrossRef]
- Adriaenssens, N.; Bruyndonckx, R.; Versporten, A.; Hens, N.; Monnet, D.L.; Molenberghs, G. Consumption of macrolides, lincosamides and streptogramins in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76 (Suppl. 2), ii30–ii36. [Google Scholar] [CrossRef]
- European Medicines Agency. Disabling and Potentially Permanent Side Effects Lead to Suspension or Restrictions of Quinolone and Fluoroquinolone Antibiotics. Available online: https://www.ema.europa.eu/en/documents/referral/quinolone-fluoroquinolone-article-31-referral-disabling-potentially-permanent-side-effects-lead_en.pdf (accessed on 10 September 2021).
- Versporten, A.; Bruyndonckx, R.; Adriaenssens, N.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S.; ESAC-Net Study Group. Consumption of tetracyclines, sulphonamides and trimethoprim, and other antibacterials in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76 (Suppl. 2), ii45–ii59. [Google Scholar] [CrossRef]
- Del Mar, C.B.; Scott, A.M.; Glasziou, P.P.; Hoffmann, T.; Van Driel, M.L.; Beller, E.; Phillips, S.M.; Dartnell, J. Reducing antibiotic prescribing in Australian general practice: Time for a national strategy. Med. J. Aust. 2017, 207, 401–406. [Google Scholar] [CrossRef]
- Hupkova, H.; Kochan, J.; Gezo, M.; Hrmova, D. S-meddial project of cooperation with pediatricians on providing individual feedback on antibiotics prescription in order to improve prescription habits and to rationalize drug spending. Value Health 2007, 6, A309. [Google Scholar] [CrossRef]
- Hupkova, H.; Foltán, V.; Hroncova, D.; Gezo, M. Integrated educational project with individual feedback from claims data leads to improved antibiotic prescription and resistance decrease. Value Health 2010, 3, A204. [Google Scholar] [CrossRef] [Green Version]
- Tesař, T.; Rapoš, D.; Egnerová, A.; Babeľa, R. Antibiotic prescription as a public health care issue. Slovenský lekár 2012, 7–8, 123–129. [Google Scholar]
- Health Insurance Fund Trust. Where Do We Pay the Most for Antibiotics? Available online: https://www.dovera.sk/aktuality/3707-kde-platime-za-antibiotika-najviac-pozrite-sa-na-mape (accessed on 31 May 2021).
- Pavelka, M.; Van-Zandvoort, K.; Abbott, S.; Sherratt, K.; Majdan, M.; CMMID COVID-19 working group; Jarcuska, P.; Krajci, M.; Funk, S. The impact of population-wide rapid antigen testing on SARS-CoV-2 prevalence in Slovakia. medRxiv 2020, 372, 635–641. [Google Scholar] [CrossRef]
- Holt, E. Slovakia to test all adults for SARS-CoV-2. Lancet 2020, 396, 1386–1387. [Google Scholar] [CrossRef]
- Frnda, J.; Durica, M. On Pilot Massive COVID-19 Testing by Antigen Tests in Europe. Case Study: Slovakia. Infect. Dis. Rep. 2021, 13, 45–57. [Google Scholar] [CrossRef]
- Mirzaei, R.; Goodarzi, P.; Asadi, M.; Soltani, A.; Aljanabi, H.A.A.; Jeda, A.S.; Dashtbin, S.; Jalalifar, S.; Mohammdzadeh, R.; Teimori, A.; et al. Bacterial co-infections with SARS-CoV-2. IUBMB Life 2020, 72, 2097–2111. [Google Scholar] [CrossRef]
- Coenen, S.; Ferech, M.; Haaijer-Ruskamp, F.M.; Butler, C.C.; Stichele, R.H.V.; Verheij, T.J.M.; Monnet, L.D.; Little, P.; Goossens, H. European Surveillance of Antimicrobial Consumption (ESAC): Quality indicators for outpatient antibiotic use in Europe. Qual. Saf. Health Care 2007, 16, 440–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriaenssens, N.; Bruyndonckx, R.; Versporten, A.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S.; ESAC-Net Study Group. Quality appraisal of antibiotic consumption in the community, European Union/European Economic Area, 2009 and 2017. J. Antimicrob. Chemother. 2021, 76 (Suppl. 2), ii60–ii67. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–2015: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2020, 21, 107–115. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf (accessed on 31 May 2021).
- World Health Organization. AWaRe Classification Database. Available online: https://www.who.int/medicines/news/2019/WHO_releases2019AWaRe_classification_antibiotics/en/ (accessed on 31 May 2021).
- European Centre for Disease Prevention and Control European Food Safety Authority and European Medicines Agency. ECDC/EFSA/EMA Second Joint Report on the Integrated Analysis of the Consumption of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Humans and Food-Producing Animals—Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report 2017. Available online: https://www.ecdc.europa.eu/en/publications-data/ecdcefsaema-second-joint-report-integrated-analysis-consumption-antimicrobial (accessed on 31 May 2021).
- Fletcher-Lartey, S.; Yee, M.; Gaarslev, C.; Khan, R. Why do general practitioners prescribe antibiotics for upper respiratory tract infections to meet patient expectations: A mixed methods study. BMJ Open 2016, 6, e012244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirota, M.; Round, T.; Samaranayaka, S.; Kostopoulou, O. Expectations for antibiotics increase their prescribing: Causal evidence about localized impact. Health Psychol. 2017, 36, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, S.R.; Rakhmanova, L.; Out-O’Reilly, E.; Reay, S.; Thomas, M.G.; Sajtos, L. The use of a poster to reduce expectations to receive antibiotics for a common cold. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- State Institute for Drug Control. About SIDC. Available online: https://www.sukl.sk/hlavna-stranka/english-version/about-sidc?page_id=259 (accessed on 31 May 2021).
- MCR. Consumption of Medicinal Products in Slovakia. Available online: https://www.mcr.sk/spotreba-liekov-na-slovensku/ (accessed on 31 May 2021).
- National Health Information Centre. About NHIC. Available online: http://www.nczisk.sk/en/Pages/default.aspx (accessed on 31 May 2021).
- European Centre for Disease Prevention and Control European Food Safety Authority and European Medicines Agency. ECDC, EFSA and EMA Joint Scientific Opinion on a List of Outcome Indicators as Regards Surveillance of Antimicrobial Resistance and Antimicrobial Consumption in Humans and Food-Producing Animals. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/5017 (accessed on 31 May 2021).
- WHO Collaborating Centre for Drug Statistics Methodology. ATC Classification Index with DDDs 2021. Oslo 2021. Available online: https://www.whocc.no/ (accessed on 31 May 2021).
- European Surveillance of Antimicrobial Consumption Network (ESAC-Net). Antimicrobial Consumption (AMC) Reporting Protocol. Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/surveillance-and-disease-data/report-protocol (accessed on 31 May 2021).
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Consumption Database (ESAC-Net). Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/surveillance-and-disease-data/database (accessed on 31 May 2021).
- European Centre for Disease Prevention and Control (ECDC). The European Surveillance System (TESSy). Available online: https://www.ecdc.europa.eu/en/publications-data/european-surveillance-system-tessy (accessed on 31 May 2021).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesar, T.; Masarykova, L.; Lehocka, L.; Porubcova, S.; Cicova, M.; Wawruch, M. Consumption of Antibacterials for Systemic Use in Slovakia: A National Study and the Quality Indicators for Outpatient Antibiotic Use. Antibiotics 2021, 10, 1180. https://doi.org/10.3390/antibiotics10101180
Tesar T, Masarykova L, Lehocka L, Porubcova S, Cicova M, Wawruch M. Consumption of Antibacterials for Systemic Use in Slovakia: A National Study and the Quality Indicators for Outpatient Antibiotic Use. Antibiotics. 2021; 10(10):1180. https://doi.org/10.3390/antibiotics10101180
Chicago/Turabian StyleTesar, Tomas, Lucia Masarykova, Lubica Lehocka, Slavka Porubcova, Monika Cicova, and Martin Wawruch. 2021. "Consumption of Antibacterials for Systemic Use in Slovakia: A National Study and the Quality Indicators for Outpatient Antibiotic Use" Antibiotics 10, no. 10: 1180. https://doi.org/10.3390/antibiotics10101180
APA StyleTesar, T., Masarykova, L., Lehocka, L., Porubcova, S., Cicova, M., & Wawruch, M. (2021). Consumption of Antibacterials for Systemic Use in Slovakia: A National Study and the Quality Indicators for Outpatient Antibiotic Use. Antibiotics, 10(10), 1180. https://doi.org/10.3390/antibiotics10101180





