In Vivo Pharmacodynamics of β-Lactams/Nacubactam against Carbapenem-Resistant and/or Carbapenemase-Producing Enterobacter cloacae and Klebsiella pneumoniae in Murine Pneumonia Model
Abstract
:1. Introduction
2. Results
2.1. The Minimum Inhibitory Concentrations (MICs) of ATM, FEP, MEM, and NAC
2.2. ATM, FEP, MEM, and NAC Pharmacokinetics (PK) in the Epithelial Lining Fluid (ELF) and Plasma
2.3. Pharmacodynamic (PD) Study with Murine Pneumonia Model
2.3.1. Antimicrobial Efficacies of NAC Monotherapy
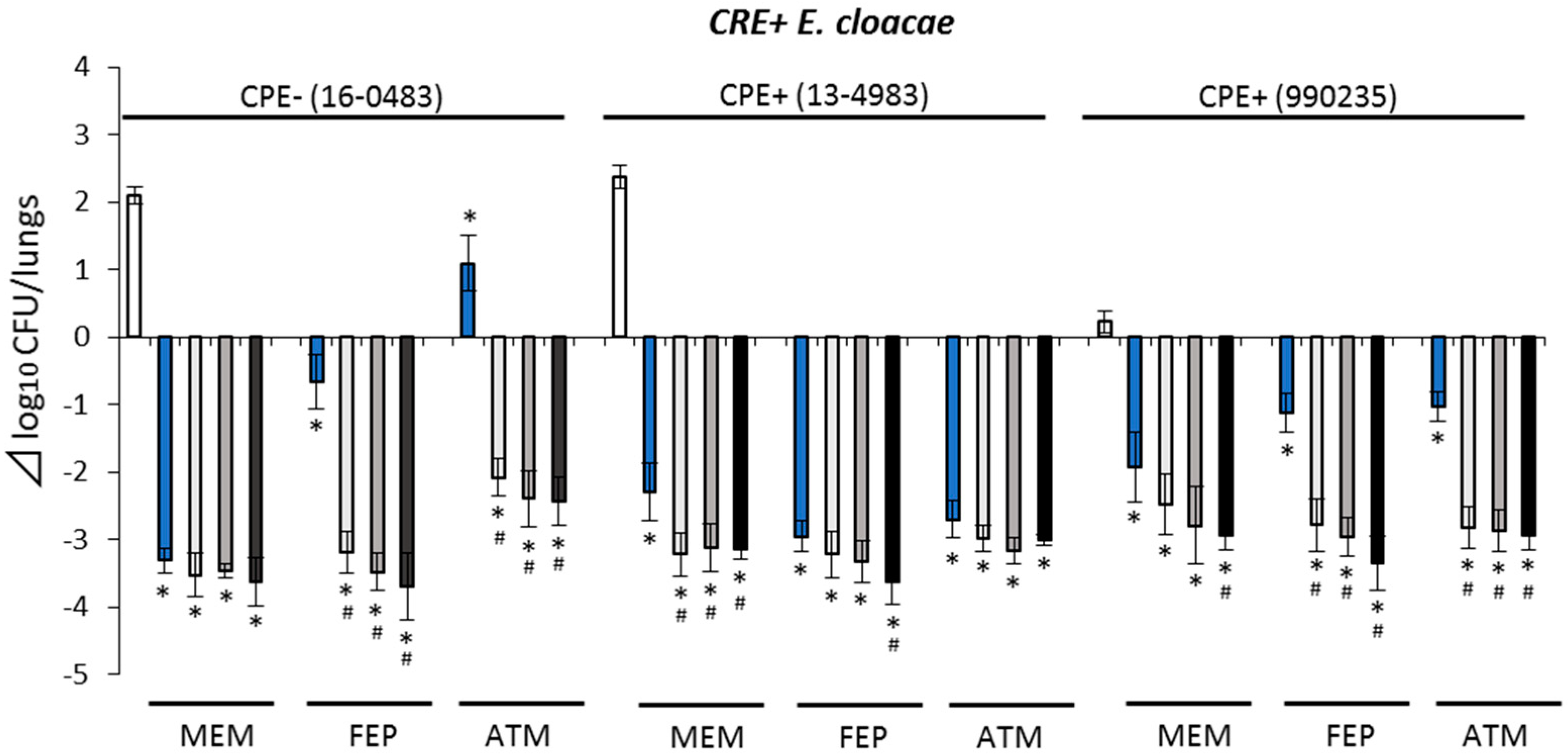
2.3.2. Antimicrobial Efficacies against CRE+ Isolates
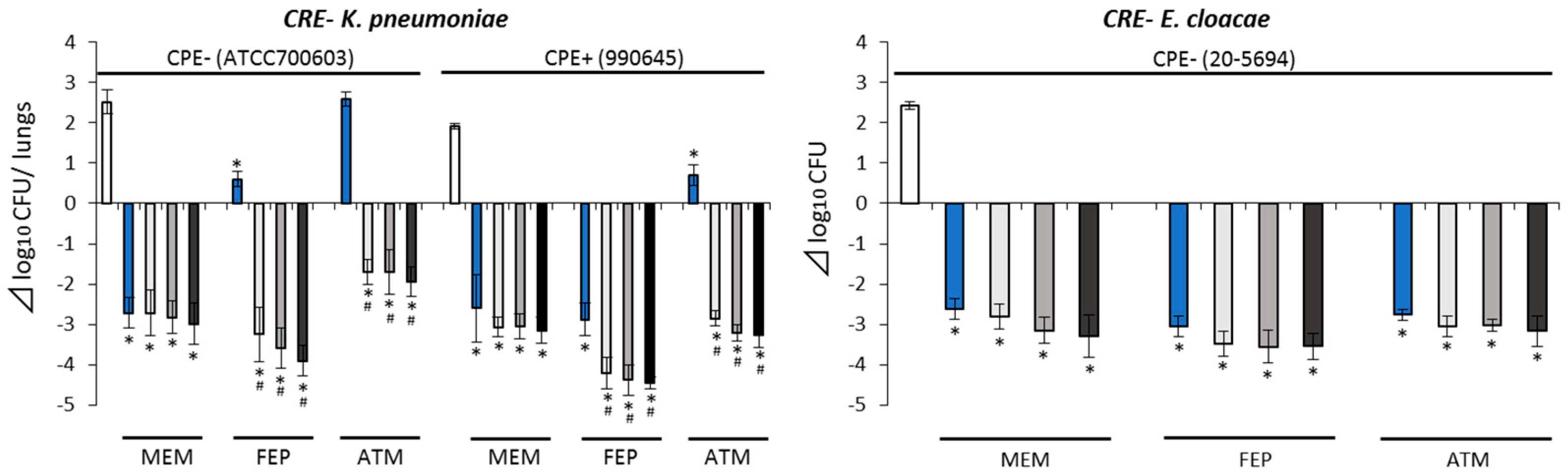
2.3.3. Antimicrobial Efficacies against CRE− Isolates
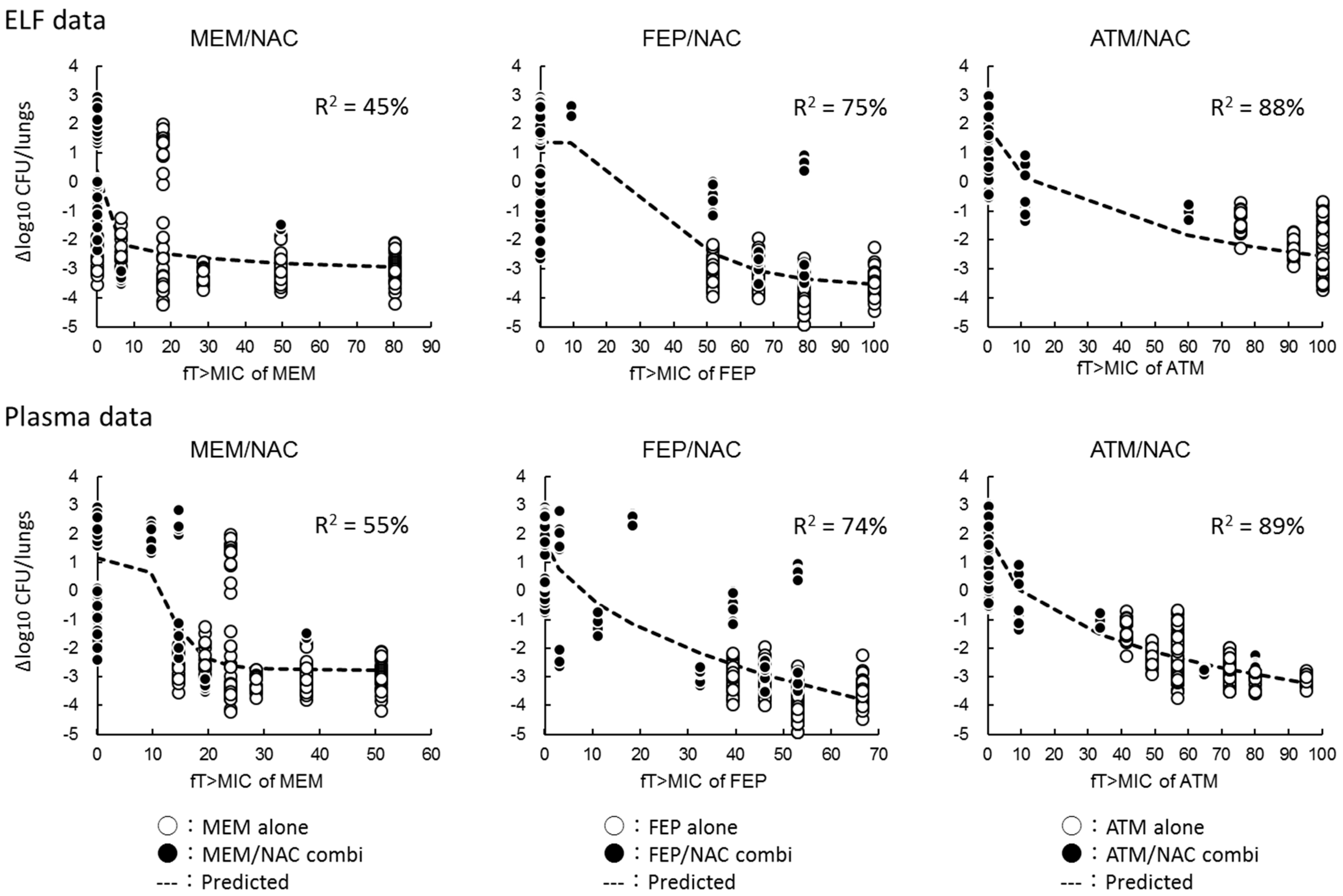
2.4. Relationships between Antimicrobial Activities and the Percentage of Free Drug Time above MIC (%fT > MIC)
3. Discussion
4. Materials and Methods
4.1. Antimicrobials
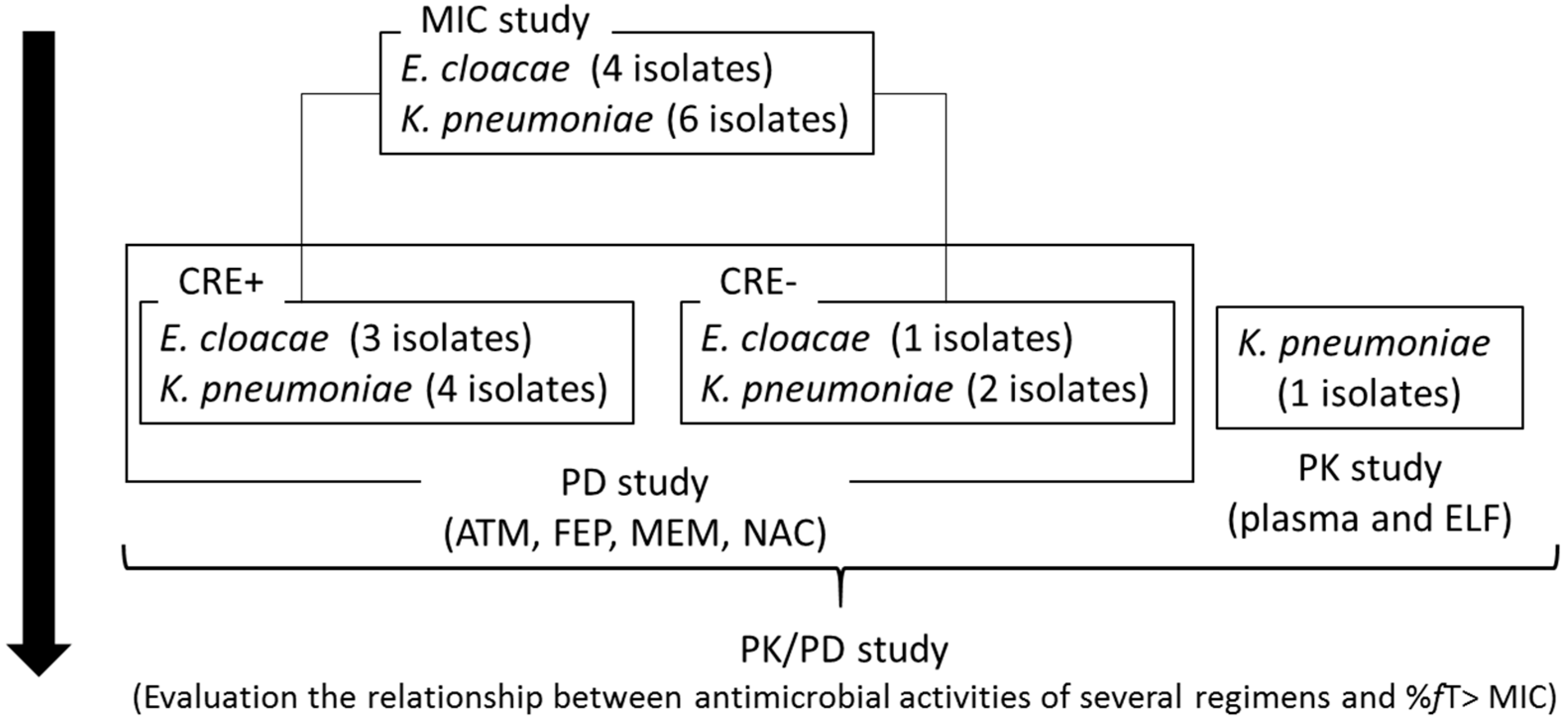
4.2. Microorganisms
4.3. Susceptibility Test
4.4. Animals
4.5. Pneumonia Model
4.6. PK Studies
4.7. Instrumentation and Chromatographic Conditions
4.8. PD Study
4.9. PK/PD Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qureshi, Z.A.; Paterson, D.L.; Potoski, B.A.; Kilayko, M.C.; Sandovsky, G.; Sordillo, E.; Polsky, B.; Adams-Haduch, J.M.; Doi, Y. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: Superiority of combination antimicrobial regimens. Antimicrob. Agents Chemother. 2012, 56, 2108–2113. [Google Scholar] [CrossRef] [Green Version]
- Tumbarello, M.; Viale, P.; Viscoli, C.; Trecarichi, E.M.; Tumietto, F.; Marchese, A.; Spanu, T.; Ambretti, S.; Ginocchio, F.; Cristini, F.; et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: Importance of combination therapy. Clin. Infect. Dis. 2012, 55, 943–950. [Google Scholar] [CrossRef] [Green Version]
- van Duin, D.; Arias, C.A.; Komarow, L.; Chen, L.; Hanson, B.M.; Weston, G.; Cober, E.; Garner, O.B.; Jacob, J.T.; Satlin, M.J.; et al. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): A prospective cohort study. Lancet Infect. Dis. 2020, 20, 731–741. [Google Scholar] [CrossRef]
- Logan, L.K.; Weinstein, R.A. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 2017, 215, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef]
- Carattoli, A.; Villa, L.; Poirel, L.; Bonnin, R.A.; Nordmann, P. Evolution of IncA/C blaCMY-2-carrying plasmids by acquisition of the blaNDM-1 carbapenemase gene. Antimicrob. Agents Chemother. 2012, 56, 783–786. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, N.; Asada, R.; Kawahara, R.; Hagiya, H.; Akeda, Y.; Shanmugakani, R.K.; Yoshida, H.; Yukawa, S.; Yamamoto, K.; Takayama, Y.; et al. Prevalence of, and risk factors for, carriage of carbapenem-resistant Enterobacteriaceae among hospitalized patients in Japan. J. Hosp. Infect. 2017, 97, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Asai, N.; Sakanashi, D.; Suematsu, H.; Kato, H.; Hagihara, M.; Nishiyama, N.; Koizumi, Y.; Yamagishi, Y.; Mikamo, H. The epidemiology and risk factor of carbapenem-resistant enterobacteriaceae colonization and infections: Case control study in a single institute in Japan. J. Infect. Chemother. 2018, 24, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gu, B.; Huang, M.; Liu, H.; Xu, T.; Xia, W.; Wang, T. Epidemiology of carbapenem resistant Enterobacteriaceae (CRE) during 2000-2012 in Asia. J. Thorac. Dis. 2015, 7, 376–385. [Google Scholar] [PubMed]
- Cai, Z.; Tao, J.; Jia, T.; Fu, H.; Zhang, X.; Zhao, M.; Du, H.; Yu, H.; Shan, B.; Huang, B.; et al. Multicenter Evaluation of the Xpert Carba-R Assay for Detection and Identification of Carbapenemase Genes in Sputum Specimens. J. Clin. Microbiol. 2020, 58, e00644-20. [Google Scholar] [CrossRef] [PubMed]
- Morinaka, A.; Tsutsumi, Y.; Yamada, M.; Suzuki, K.; Watanabe, T.; Abe, T.; Furuuchi, T.; Inamura, S.; Sakamaki, Y.; Mitsuhashi, N.; et al. OP0595, a new diazabicyclooctane: Mode of action as a serine β-lactamase inhibitor, antibiotic and β-lactam ‘enhancer’. J. Antimicrob. Chemother. 2015, 70, 2779–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livermore, D.M.; Warner, M.; Mushtaq, S.; Woodford, N. Interactions of OP0595, a novel triple-action diazabicyclooctane, with β-lactams against OP0595-resistant Enterobacteriaceae mutants. Antimicrob. Agents Chemother. 2016, 60, 554–560. [Google Scholar] [CrossRef] [Green Version]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; Woodford, N. Activity of OP0595/β425 lactam combinations against Gram-negative bacteria with extended-spectrum, AmpC and carbapenem-hydrolysing β-lactamases. J. Antimicrob. Chemother. 2015, 70, 3032–3041. [Google Scholar] [CrossRef] [Green Version]
- Hagihara, M.; Kato, H.; Sugano, T.; Okade, H.; Sato, N.; Shibata, Y.; Sakanashi, D.; Asai, N.; Koizumi, Y.; Suematsu, H.; et al. Pharmacodynamic evaluation of meropenem, cefepime, and aztreonam combined with a novel β-lactamase inhibitor, nacubactam, against carbapenem-resistant and/or carbapenemase-producing Klebsiella pneumoniae and Escherichia coli using a murine thigh infection model. Int. J. Antimicrob. Agents 2021, 57, 106330. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; M100-ED28; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Docquier, J.D.; Mangani, S. An update on β-lactamase inhibitor discovery and development. Drug Resist. Updat. 2018, 36, 13–29. [Google Scholar] [CrossRef]
- Mushtaq, S.; Vickers, A.; Woodford, N.; Haldimann, A.; Livermore, D.M. Activity of nacubactam (RG6080/OP0595) combinations against MBL-producing Enterobacteriaceae. J. Antimicrob. Chemother 2019, 74, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Du, X.; Kuti, J.L.; Nicolau, D.P. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob. Agents Chemother. 2007, 51, 1725–1730. [Google Scholar] [CrossRef] [Green Version]
- Craig, W.A.; Redington, J.; Ebert, S.C. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J. Antimicrob. Chemother 1991, 27 (Suppl. C), 29–40. [Google Scholar] [CrossRef]
- Sumita, Y.; Nouda, H.; Tada, E.; Kohzuki, T.; Kato, M.; Okuda, T.; Fukasawa, M. Pharmacokinetics of meropenem, a new carbapenem antibiotic, parenterally administered to laboratory animals. Chemotherapy 1992, 40, 123–131. [Google Scholar]
- Hirano, M.; Masuyoshi, S.; Kondo, S.; Asai, Y.; Oki, T. Distribution, metabolism and excretion of cefepime in rats. Chemotherapy 1991, 39, 97–103. [Google Scholar]
- Kita, Y.; Fugono, T.; Imada, A. Comparative pharmacokinetics of carumonam and aztreonam in mice, rats, rabbits, dogs, and cynomolgus monkeys. Antimicrob. Agents Chemother. 1986, 29, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, M.; Uematsu, T.; Kanamaru, M.; Ueno, K. Phase I Study of Meropenem. Chemotherapy 1992, 40, 258–275. [Google Scholar]
- Nakajima, M.; Uematsu, T.; Kanamaru, M.; Chuna, I.; Kidono, M.; Shimizu, K. Phase I Study of Cefepime (BMY-28242): Single Dose Study in Healthy Male Volunteers. Chemotherapy 1991, 39, 104–116. [Google Scholar]
- Nakashima, M.; Uematsu, T.; Takiguchi, Y.; Maeda, Y. Pharmacokinetics and Safety of Azthreonam in Healthy Japanese Volunteers. Jpn. Pharmacol. Ther. 1985, 16, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Mallalieu, N.L.; Winter, E.; Fettner, S.; Patel, K.; Zwanziger, E.; Attley, G.; Rodriguez, I.; Kano, A.; Salama, S.M.; Bentley, D.; et al. Safety and Pharmacokinetic Characterization of Nacubactam, a Novel β-Lactamase Inhibitor, Alone and in Combination with Meropenem, in Healthy Volunteers. Antimicrob. Agents Chemother. 2020, 64, e02229-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakawa, Y. Global spread of multidrug-resistant microbes including CRE and clinical alerts. Jpn. J. Chemother. 2015, 63, 187–197. [Google Scholar]
- Lasko, M.J.; Abdelraouf, K.; Nicolau, D.P. In Vivo Activity of WCK 4282 (High-Dose Cefepime/Tazobactam) against Serine-β-lactamase-Producing Enterobacterales and Pseudomonas aeruginosa in the Neutropenic Murine Lung Infection Model. Antimicrob. Agents Chemother. 2021, 76, 993–1000. [Google Scholar]
- Maglio, D.; Ong, C.; Banevicius, M.A.; Geng, Q.; Nightingale, C.H.; Nicolau, D.P. Determination of the in vivo pharmacodynamic profile of cefepime against extended-spectrum-beta-lactamase-producing Escherichia coli at various inocula. Antimicrob. Agents Chemother. 2004, 48, 1941–1947. [Google Scholar] [CrossRef] [Green Version]
- Tessier, P.R.; Keel, R.A.; Hagihara, M.; Crandon, J.L.; Nicolau, D.P. Comparative in vivo efficacies of epithelial lining fluid exposures of tedizolid, linezolid, and vancomycin for methicillin-resistant Staphylococcus aureus in a mouse pneumonia model. Antimicrob. Agents Chemother. 2012, 56, 2342–2346. [Google Scholar] [CrossRef] [Green Version]

| Types | Species | Strains | Genotypes | >MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MEM | MEM/NAC * | FEP | FEP/NAC * | ATM | ATM/NAC * | NAC | ||||
| CRE+/CPE+ | E. cloacae | 13-4983 990235 | IMP-1, IMP-1 | 4 64 | 1 * 8 * | 8 64 | 2 * 4 * | 0.12 64 | 0.03 * 1 * | 2 8 |
| K. pneumoniae | ATCC BAA-1705 | KPC | 8 | 0.25 * | 32 | 1 * | >128 | 1 * | 2 | |
| 13-3445 | IMP-1 | 64 | 4 * | 128 | 2 * | 0.5 | 0.25 * | 2 | ||
| 594 | IMP-6 | 32 | 2 * | 4 | 2 * | 8 | 0.25 * | >64 | ||
| CRE+/CPE− | E. cloacae | 16-0483 | 4 | 2 * | 4 | 1 * | 128 | 2 * | 4 | |
| K. pneumoniae | 16-2183 | CTX-M-9 | 8 | 2 * | >128 | 4 * | >128 | 4 * | >128 | |
| CRE−/CPE+ | K. pneumoniae | 990645 | DHA-1, IMP-1 | 0.25 | 0.25 | 2 | 1 | 64 | 1 | 2 |
| CRE−/CPE− | K. pneumoniae | ATCC700603 | SHV-18 | 0.03 | 0.03 | 1 | 0.25 * | >128 | 1 * | 4 |
| E. cloacae | 20-5694 | 0.03 | 0.03 | 0.03 | 0.03 | 0.12 | 0.12 | 4 | ||
| Antimicrobials | Dose (mg/kg) | Tmax (h) | Cmax (µg/mL) | AUC0–∞ (µg h/mL) | T1/2 (h) | Vd/F (L/kg) | CL/F (L/h/kg) |
|---|---|---|---|---|---|---|---|
| In ELF | |||||||
| Nacubactam | 100 | 0.5 | 31 | 39 | 1.63 | NA | NA |
| Meropenem | 100 | 0.5 | 3 | 6 | 0.69 | NA | NA |
| Cefepime | 100 | 1 | 18 | 32 | 0.84 | NA | NA |
| Aztreonam | 100 | 0.5 | 16 | 27 | 0.94 | NA | NA |
| In plasma | |||||||
| Nacubactam | 100 | 0.25 | 116 | 131 | 0.61 | 0.67 | 0.76 |
| Meropenem | 100 | 0.25 | 53 | 36 | 0.4 | 1.6 | 2.79 |
| Cefepime | 100 | 0.25 | 80 | 84 | 0.41 | 0.71 | 1.19 |
| Aztreonam | 100 | 0.5 | 96 | 120 | 0.36 | 0.43 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagihara, M.; Kato, H.; Sugano, T.; Okade, H.; Sato, N.; Shibata, Y.; Sakanashi, D.; Hirai, J.; Asai, N.; Suematsu, H.; et al. In Vivo Pharmacodynamics of β-Lactams/Nacubactam against Carbapenem-Resistant and/or Carbapenemase-Producing Enterobacter cloacae and Klebsiella pneumoniae in Murine Pneumonia Model. Antibiotics 2021, 10, 1179. https://doi.org/10.3390/antibiotics10101179
Hagihara M, Kato H, Sugano T, Okade H, Sato N, Shibata Y, Sakanashi D, Hirai J, Asai N, Suematsu H, et al. In Vivo Pharmacodynamics of β-Lactams/Nacubactam against Carbapenem-Resistant and/or Carbapenemase-Producing Enterobacter cloacae and Klebsiella pneumoniae in Murine Pneumonia Model. Antibiotics. 2021; 10(10):1179. https://doi.org/10.3390/antibiotics10101179
Chicago/Turabian StyleHagihara, Mao, Hideo Kato, Toshie Sugano, Hayato Okade, Nobuo Sato, Yuichi Shibata, Daisuke Sakanashi, Jun Hirai, Nobuhiro Asai, Hiroyuki Suematsu, and et al. 2021. "In Vivo Pharmacodynamics of β-Lactams/Nacubactam against Carbapenem-Resistant and/or Carbapenemase-Producing Enterobacter cloacae and Klebsiella pneumoniae in Murine Pneumonia Model" Antibiotics 10, no. 10: 1179. https://doi.org/10.3390/antibiotics10101179
APA StyleHagihara, M., Kato, H., Sugano, T., Okade, H., Sato, N., Shibata, Y., Sakanashi, D., Hirai, J., Asai, N., Suematsu, H., Yamagishi, Y., & Mikamo, H. (2021). In Vivo Pharmacodynamics of β-Lactams/Nacubactam against Carbapenem-Resistant and/or Carbapenemase-Producing Enterobacter cloacae and Klebsiella pneumoniae in Murine Pneumonia Model. Antibiotics, 10(10), 1179. https://doi.org/10.3390/antibiotics10101179







