OXA-48 Carbapenemase-Producing Enterobacterales in Spanish Hospitals: An Updated Comprehensive Review on a Rising Antimicrobial Resistance
Abstract
1. Introduction
2. OXA-48-Like Enzymes: Mechanism of Resistance
Detection of OXA-48 CRE
3. Worldwide Spread
4. OXA-48 CRE Outbreaks in Spain
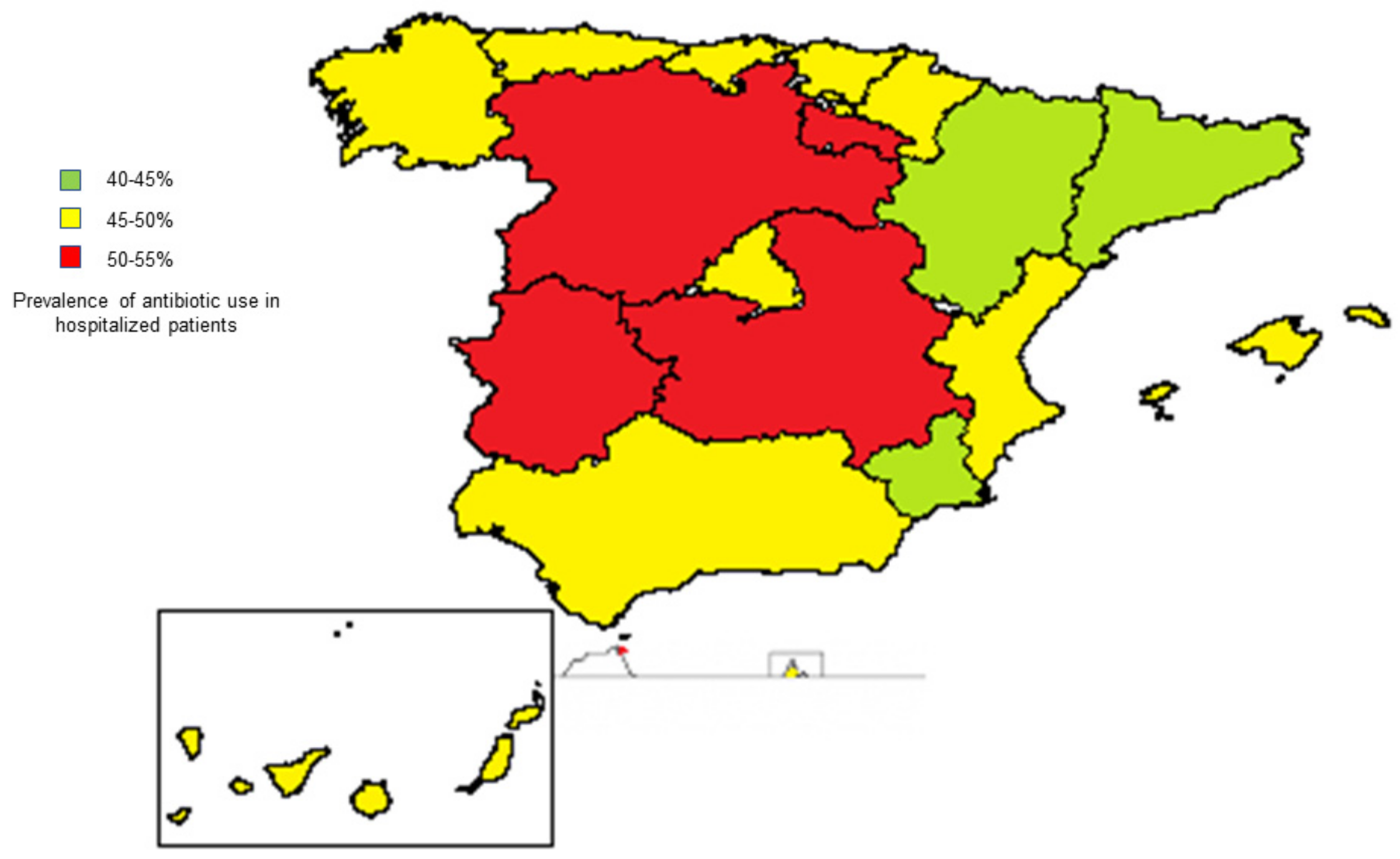
4.1. Frequency
4.2. Surgical Site Infections
- Adequate antibiotic prophylaxis before surgery.
- Skin antisepsis with 2% alcoholic chlorhexidine.
- Correct hair removal when necessary, without causing injuries or irritation.
- Adequate control of temperature and glycemia during the procedure.
- Rapid identification of infection signs after the surgery.
4.3. Colonization in the Intensive Care Unit (ICU)
4.4. Plasmid Transfer
4.5. Risk Factors for Acquiring OXA-48 CREs
5. Treatment
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pana, Z.D.; Zaoutis, T. Treatment of extended-spectrum ß-lactamase-producing Enterobacteriaceae (ESBLs) infections: What have we learned until now? F1000Res 2018, 7, 1347. [Google Scholar] [CrossRef] [PubMed]
- Adeolu, M.; Alnajar, S.; Naushad, S.S.; Gupta, R. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar]
- Hawkey, P.M. The growing burden of antimicrobial resistance. J. Antimicrob. Chemother. 2008, 62, i1–i9. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, R.G.; Johnson, J.K.; Bork, J.T.; Heil, E.L. Treatment options for extended-spectrum beta-lactamase (ESBL) and AmpC-producing bacteria. Expert Opin. Pharmacother. 2016, 17, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.C.; Lin, S.Y.; Chang, Y.T.; Lee, C.Y.; Chen, Y.H.; Hsueh, P.R. Management of infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae: Current evidence and future prospects. Expert Rev. Anti. Infect. Ther. 2018, 16, 205–218. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Facility Guidance for Control of Carbapenem-Resistant Enterobacteriaceae (CRE)—November 2015 Update CRE Toolkit. Available online: https://www.cdc.gov/hai/organisms/cre/Cre-toolkit/index.html (accessed on 1 December 2020).
- Nordmann, P.; Mariotte, S.; Naas, T.; Labia, R.; Nicolas, M.H. Biochemical properties of a carbapenem-hydrolyzing betalactamase from Enterobacter cloacae and cloning of the gene into Escherichia coli. Antimicrob. Agents Chemother. 1993, 37, 939–946. [Google Scholar] [CrossRef]
- Naas, T.; Nordmann, P. Analysis of a carbapenem hydrolyzing class A beta-lactamase from Enterobacter cloacae and of its LysR-type regulatory protein. Proc. Natl. Acad. Sci. USA 1994, 91, 7693–7697. [Google Scholar] [CrossRef]
- Haidar, G.; Clancy, C.J.; Chen, L.; Samanta, P.; Shields, R.K.; Kreiswirth, B.N.; Nguyen, M.H. Identifying spectra of activity and therapeutic niches for ceftazidime-avibactam and imipenem-relebactam against carbapenem-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, e00642-17. [Google Scholar] [CrossRef]
- Suay-García, B.; Pérez-Gracia, M.T. Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics 2019, 8, 122. [Google Scholar] [CrossRef]
- Ambler, R.P. The structure of β-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1980, 289, 321–331. [Google Scholar]
- Bush, K.; Jacoby, G.A. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Naas, T.; Poirel, L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Mairi, A.; Pantel, A.; Sotto, A.; Lavigne, J.P.; Touati, A. OXA-48-like carbapenemases producing Enterobacteriaceae in different niches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 587–604. [Google Scholar] [CrossRef]
- Evans, B.A.; Amyes, S.G.B. OXA β-lactamases. Clin. Microbiol. Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef]
- Poirel, L.; Potron, A.; Nordmann, P. OXA-48-like carbapenemases: The phantom menace. J. Antimicrob. Chemother. 2012, 67, 1597–1606. [Google Scholar] [CrossRef]
- Poirel, L.; Héritier, C.; Tolün, V.; Nordmann, P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2004, 48, 15–22. [Google Scholar] [CrossRef]
- Poirel, L.; Héritier, C.; Nordmann, P. Chromosome-encoded ambler class D beta-lactamase of Shewanella oneidensis as a progenitor of carbapenem-hydrolyzing oxacillinase. Antimicrob. Agents Chemother. 2004, 48, 348–351. [Google Scholar] [CrossRef]
- Kasap, M.; Torol, S.; Kolayli, F.; Dundar, D.; Vahaboglu, H. OXA-162, a novel variant of OXA-48 displays extended hydrolytic activity towards imipenem, meropenem and doripenem. J. Enzyme Inhib. Med. Chem. 2013, 28, 990–996. [Google Scholar] [CrossRef]
- Poirel, L.; Castanheira, M.; Carrër, A.; Rodriguez, C.P.; Jones, R.N.; Smayevsky, J.; Nordmann, P. OXA-163, an OXA-48-related class D β-lactamase with extended activity toward expanded-spectrum cephalosporins. Antimicrob. Agents Chemother. 2011, 55, 2546–2551. [Google Scholar] [CrossRef]
- Potron, A.; Nordmann, P.; Lafeuille, E.; Al Maskari, Z.; Al Rashdi, F.; Poirel, L. Characterization of OXA-181, a carbapenemhydrolyzing class D beta-lactamase from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2011, 55, 4896–4899. [Google Scholar] [CrossRef] [PubMed]
- Zong, Z. Discovery of bla(OXA-199), a chromosome-based bla(OXA-48)-like variant, in Shewanella xiamenensis. PLoS ONE 2012, 7, e48280. [Google Scholar] [CrossRef] [PubMed]
- Potron, A.; Nordmann, P.; Poirel, L. Characterization of OXA204, a carbapenem-hydrolyzing class D β-lactamase from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2013, 57, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Potron, A.; Rondinaud, E.; Poirel, L.; Belmonte, O.; Boyer, S.; Camiade, S.; Nordmann, P. Genetic and biochemical characterisation of OXA232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int. J. Antimicrob. Agents 2013, 41, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Oteo, J.; Hernández, J.M.; Espasa, M.; Fleites, A.; Sáez, D.; Bautista, V.; Pérez-Vázquez, M.; Fernández-García, M.D.; Delgado-Iribarren, A.; García-Picazo, L.; et al. Emergence of OXA-48-producing Klebsiella pneumoniae and the novel carbapenemases OXA-244 and OXA-245 in Spain. J. Antimicrob. Chemother. 2013, 68, 317–321. [Google Scholar] [CrossRef]
- Gomez, S.; Pasteran, F.; Faccone, D.; Bettiol, M.; Veliz, O.; De Belder, D.; Rapaport, M.; Gatti, B.; Petroni, A.; Corso, A. Intrapatient emergence of OXA-247: A novel carbapenemase found in a patient previously infected with OXA-163-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2013, 19, E233–E235. [Google Scholar] [CrossRef]
- Mataseje, L.F.; Abdesselam, K.; Vachon, J.; Mitchel, R.; Bryce, E.; Roscoe, D.; Boyd, D.A.; Embree, J.; Katz, K.; Kibsey, P.; et al. Results from the Canadian Nosocomial Infection Surveillance Program on Carbapenemase-Producing Enterobacteriaceae, 2010 to 2014. Antimicrob. Agents Chemother. 2016, 60, 6787–6794. [Google Scholar] [CrossRef]
- Sampaio, J.L.M.; Ribeiro, V.B.; Campos, J.C.; Rozales, F.P.; Magagnin, C.M.; Falci, D.R.; da Silva, R.C.F.; Falarosa, M.G.; Luz, D.I.; Vieira, F.J.; et al. Detection of OXA-370, an OXA-48- related class D β-lactamase, in Enterobacter hormaechei from Brazil. Antimicrob. Agents Chemother. 2014, 58, 3566–3567. [Google Scholar] [CrossRef]
- Dortet, L.; Oueslati, S.; Jeannot, K.; Tandé, D.; Naas, T.; Nordmann, P. Genetic and biochemical characterization of OXA-405, an OXA-48-type extended-spectrum β-lactamase without significant carbapenemase activity. Antimicrob. Agents Chemother. 2015, 59, 3823–3828. [Google Scholar] [CrossRef]
- Antonelli, A.; Di Palo, D.M.; Galano, A.; Becciani, S.; Montagnani, C.; Pecile, P.; Galli, L.; Rossolini, G.M. Intestinal carriage of Shewanella xiamenensis simulating carriage of OXA-48-producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2015, 82, 1–3. [Google Scholar] [CrossRef]
- Samuelsen, O.; Hansen, F.; Aasnaes, B.; Hasman, H.; Lund, B.A.; Leiros, H.S.; Lilje, B.; Janice, J.; Jakobsen, L.; Littauer, P.; et al. Dissemination and characteristics of a novel plasmid-encoded carbapenem-hydrolyzing class D beta-lactamase, OXA-436, found in isolates from four patients at six different hospitals in Denmark. Antimicrob. Agents Chemother. 2018, 62, e01260-17. [Google Scholar] [CrossRef] [PubMed]
- De Belder, D.; Ghiglione, B.; Pasteran, F.; de Mendieta, J.M.; Corso, A.; Curto, L.; Di Bella, A.; Gutkind, G.; Gomez, S.A.; Power, P. Comparative Kinetic Analysis of OXA-438 with Related OXA-48-Type Carbapenem-Hydrolyzing Class D β-Lactamases. ACS Infect. Dis. 2020, 6, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.; Hopkins, K.L.; Loy, R.; Doumith, M.; Meunier, D.; Hill, R.; Pike, R.; Mustafa, N.; Livermore, D.M.; Woodford, N. OXA-48-like carbapenemases in the UK: An analysis of isolates and cases from 2007 to 2014. J. Antimicrob. Chemother. 2017, 72, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Lutgring, J.D.; Zhu, W.; de Man, T.J.B.; Avillan, J.J.; Anderson, K.F.; Lonsway, D.R.; Rowe, L.A.; Batra, D.; Rasheed, J.K.; Limbago, B.M. Phenotypic and Genotypic Characterization of Enterobacteriaceae Producing Oxacillinase-48-Like Carbapenemases, United States. Emerg. Infect. Dis. 2018, 24, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Dabos, L.; Bogaerts, P.; Bonnin, R.A.; Zavala, A.; Sacre, P.; Iorga, B.I.; Huang, D.T.; Glupczynski, Y.; Naas, T. Genetic and biochemical characterization of OXA-519, a novel OXA-48-like beta-lactamase. Antimicrob. Agents Chemother. 2018, 62, e00469-18. [Google Scholar] [CrossRef]
- Dabos, L.; Jousset, A.B.; Bonnin, R.A.; Fortineau, N.; Zavala, A.; Retailleau, P.; Iorga, B.I.; Naas, T. Genetic and Biochemical Characterization of OXA-535, a Distantly Related OXA-48-Like β-Lactamase. Antimicrob. Agents Chemother. 2018, 62, e01198-18. [Google Scholar] [CrossRef]
- Howard, J.C.; Anderson, T.; Creighton, J.; Freeman, J.T. Geographical and temporal clustering of OXA-48-producing Escherichia coli ST410 causing community-onset urinary tract infection in Christchurch, New Zealand. J. Antimicrob. Chemother. 2018, 73, 2900–2901. [Google Scholar] [CrossRef]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-Lactamase DataBase (BLDB)—Structure and Function. J. Enzyme Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef]
- Aubert, D.; Naas, T.; Héritier, C.; Poirel, L.; Nordmann, P. Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of β-lactam resistance genes. J. Bacteriol. 2006, 188, 6506–6514. [Google Scholar] [CrossRef]
- Beyrouthy, R.; Robin, F.; Dabboussi, F.; Mallat, H.; Hamzé, M.; Bonnet, R. Carbapenemase and virulence factors of Enterobacteriaceae in North Lebanon between 2008 and 2012: Evolution via endemic spread of OXA-48. J. Antimicrob. Chemother. 2014, 69, 2699–2705. [Google Scholar] [CrossRef]
- Argente, M.; Miró, E.; Martí, C.; Vilamala, A.; Alonso-Tarrés, C.; Ballester, F.; Calderón, A.; Gallés, C.; Gasós, A.; Mirelis, B.; et al. Molecular characterization of OXA-48 carbapenemase-producing Klebsiella pneumoniae strains after a carbapenem resistance increase in Catalonia. Enferm. Infecc. Microbiol. Clin. 2019, 37, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Skalova, A.; Chudejova, K.; Rotova, V.; Medvecky, M.; Studentova, V.; Chudackova, E.; Lavicka, P.; Bergerova, T.; Jakubu, V.; Zemlickova, H.; et al. Molecular Characterization of OXA-48-Like-Producing Enterobacteriaceae in the Czech Republic and Evidence for Horizontal Transfer of pOXA-48-Like Plasmids. Antimicrob. Agents Chemother. 2017, 61, e01889-16. [Google Scholar] [CrossRef] [PubMed]
- Okoche, D.; Asiimwe, B.B.; Katabazi, F.A.; Kato, L.; Najjuka, C.F. Prevalence and Characterization of Carbapenem-Resistant Enterobacteriaceae Isolated from Mulago National Referral Hospital, Uganda. PLoS ONE 2015, 10, e0135745. [Google Scholar] [CrossRef] [PubMed]
- Boutal, H.; Vogel, A.; Bernabeu, S.; Devilliers, K.; Creton, E.; Cotellon, G.; Plaisance, M.; Oueslati, S.; Dortet, L.; Jousset, A.; et al. A multiplex lateral flow immunoassay for the rapid identification of NDM-, KPC-, IMPand VIM-type and OXA-48-like carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 909–915. [Google Scholar] [CrossRef]
- De Jonge, B.L.; Karlowsky, J.A.; Kazmierczak, K.M.; Biedenbach, D.J.; Sahm, D.F.; Nichols, W.W. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae isolates collected during the INFORM Global Surveillance Study (2012 to 2014). Antimicrob. Agents Chemother. 2016, 60, 3163–3169. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Lob, S.H.; Kazmierczak, K.M.; Badal, R.E.; Young, K.; Motyl, M.R.; Sahm, D.F. In vitro activity of imipenem against carbapenemasepositive Enterobacteriaceae isolates collected by the SMART global surveillance program from 2008 to 2014. J. Clin. Microbiol. 2017, 55, 1638–1649. [Google Scholar] [CrossRef]
- Pitout, J.; Peirano, G.; Kock, M.M.; Strydom, K.A.; Matsumura, Y. The Global Ascendency of OXA-48-Type Carbapenemases. Clin. Microbiol. Rev. 2019, 33, e00102-19. [Google Scholar] [CrossRef]
- Aquino-Andrade, A.; Merida-Vieyra, J.; de la Garza, E.A.; Arzate-Barbosa, P.; De Colsa Ranero, A. Carbapenemase-producing Enterobacteriaceae in Mexico: Report of seven non-clonal cases in a pediatric hospital. BMC Microbiol. 2018, 18, 38. [Google Scholar] [CrossRef]
- Hernandez-Garcia, M.; Leon-Sampedro, R.; Perez-Viso, B.; Morosini, M.I.; Lopez-Fresnena, N.; Diaz-Agero, C.; Coque, T.M.; Ruiz-Garbajosa, P.; Canton, R. First report of an OXA-48- and CTX-M-213-producing Kluyvera species clone recovered from patients admitted in a university hospital in Madrid, Spain. Antimicrob. Agents Chemother. 2018, 62, e01238-18. [Google Scholar] [CrossRef]
- Gauthier, L.; Dortet, L.; Cotellon, G.; Creton, E.; Cuzon, G.; Ponties, V.; Bonnin, R.A.; Naas, T. Diversity of carbapenemase-producing Escherichia coli isolates in France in 2012–2013. Antimicrob. Agents Chemother. 2018, 62, e00266-18. [Google Scholar] [CrossRef]
- Chen, L.; Al Laham, N.; Chavda, K.D.; Mediavilla, J.R.; Jacobs, M.R.; Bonomo, R.A.; Kreiswirth, B.N. First report of an OXA-48-producing multidrugresistant Proteus mirabilis strain from Gaza, Palestine. Antimicrob. Agents Chemother. 2015, 59, 4305–4307. [Google Scholar] [CrossRef] [PubMed]
- Regev-Yochay, G.; Smollan, G.; Tal, I.; Zade, N.P.; Haviv, Y.; Nudelman, V.; Gal-Mor, O.; Jaber, H.; Zimlichman, E.; Keller, N.; et al. Sink traps as the source of transmission of OXA-48-producing Serratia marcescens in an intensive care unit. Infect. Control Hosp. Epidemiol. 2018, 39, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Peirano, G.; Matsumura, Y.; Adams, M.D.; Bradford, P.; Motyl, M.; Chen, L.; Kreiswirth, B.N.; Pitout, J. Genomic epidemiology of global carbapenemase-producing Enterobacter spp., 2008–2014. Emerg. Infect. Dis. 2018, 24, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Bedenic, B.; Slade, M.; Starcevic, L.Ž.; Sardelic, S.; Vranic-Ladavac, M.; Bencic, A.; Atalic, V.Z.; Bogdan, M.; Bubonja-Šonje, M.; Tomic-Paradžik, M.; et al. Epidemic spread of OXA-48 beta-lactamase in Croatia. J. Med. Microbiol. 2018, 6, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, S.N.; Perreten, V.; Johannes, S.; Droz, S.; Bodmer, T.; Endimiani, A. OXA-48 carbapenemase-producing Salmonella enterica serovar Kentucky isolate of sequence type 198 in a patient transferred from Libya to Switzerland. Antimicrob. Agents Chemother. 2014, 58, 2446–2449. [Google Scholar] [CrossRef] [PubMed]
- Woerther, P.-L.; Jardak, T.; Ben Hassine, I.; Forget, S.; Chachaty, E.; Arlet, G.; Decré, D. A long-term study of the diversity of OXA-48-like carbapenemase-producing bacterial strains in infected patients and carriers. Microb. Drug Resist. 2018, 24, 181–189. [Google Scholar] [CrossRef]
- Kidd, J.M.; Livermore, D.M.; Nicolau, D.P. The difficulties of identifying and treating Enterobacterales with OXA-48-like carbapenemases. Clin. Microbiol. Infect. 2020, 26, 401–403. [Google Scholar] [CrossRef]
- Carrër, A.; Poirel, L.; Eraksoy, H.; Cagatay, A.A.; Badur, S.; Nordmann, P. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob. Agents Chemother. 2008, 52, 2950–2954. [Google Scholar] [CrossRef]
- Azap, O.; Otlu, B.; Yeşilkaya, A.; Yakupoğulları, Y. Detection of OXA-48-like Carbapenemase-Producing Klebsiella pneumoniae in a Tertiary Care Center in Turkey: Molecular Characterization and Epidemiology. Balk. Med. J. 2013, 30, 259–260. [Google Scholar] [CrossRef]
- Djahmi, N.; Dunyach-Remy, C.; Pantel, A.; Dekhil, M.; Sotto, A.; Lavigne, J.-P. Epidemiology of carbapenemase-producing Enterobacteriaceae and Acinetobacter baumannii in Mediterranean Countries. Biomed. Res. 2014, 2014, 305784. [Google Scholar]
- Cuzon, G.; Naas, T.; Bogaerts, P.; Glupczynski, Y.; Huang, T.D.; Nordmann, P. Plasmid-encoded carbapenem-hydrolyzing beta-lactamase OXA-48 in an imipenem-susceptible Klebsiella pneumoniae strain from Belgium. Antimicrob. Agents Chemother. 2008, 52, 3463–3464. [Google Scholar] [CrossRef] [PubMed]
- Cuzon, G.; Naas, T.; Lesenne, A.; Benhamou, M.; Nordmann, P. Plasmid-mediated carbapenem-hydrolysing OXA-48 betalactamase in Klebsiella pneumoniae from Tunisia. Int. J. Antimicrob. Agents 2010, 36, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Carbonnelle, E.; Bernabeu, S.; Gutmann, L.; Rotimi, V.; Nordmann, P. Importation of OXA-48-producing Klebsiella pneumoniae from Kuwait. J. Antimicrob. Chemother. 2012, 67, 2051–2052. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lascols, C.; Peirano, G.; Hackel, M.; Laupland, K.B.; Pitout, J.D. Surveillance and molecular epidemiology of Klebsiella pneumoniae isolates that produce carbapenemases: First report of OXA-48-like enzymes in North America. Antimicrob. Agents Chemother. 2013, 57, 130–136. [Google Scholar] [CrossRef]
- Taggar, G.; Attiq Rheman, M.; Boerlin, P.; Diarra, M.S. Molecular Epidemiology of Carbapenemases in Enterobacteriales from Humans, Animals, Food and the Environment. Antibiotics 2020, 9, 693. [Google Scholar] [CrossRef]
- Oteo, J.; Saez, D.; Bautista, V.; Fernández-Romero, S.; Molina, J.M.H.; Pérez-Vázquez, M.; Aracil, B.; Campos, J.; Spanish Collaborating Group for the Antibiotic Resistance Surveillance Program. Carbapenemase producing Enterobacteriaceae in Spain in 2012. Antimicrob. Agents Chemother. 2013, 57, 6344–6347. [Google Scholar] [CrossRef]
- Estudio EPINE-EPSS n°30, 2019: Estudio de Prevalencia de las Infecciones Nosocomiales en España [EPINE-EPSS Study, n°30, 2019: Study of Prevalence of Nosocomial Infections in Spain]. 2019. Available online: https://epine.es/api/documento-publico/2019%20EPINE%20Informe%20Espa%C3%B1a%2027112019.pdf/reports-esp (accessed on 1 December 2020).
- Rivera-Izquierdo, M.; Valero-Ubierna, M.; Martínez-Diz, S.; Fernández-García, M.Á.; Martín-Romero, D.T.; Maldonado-Rodríguez, F.; Sánchez-Pérez, M.R.; Martín-delosReyes, L.M.; Martínez-Ruiz, V.; Lardelli-Claret, P.; et al. Clinical Factors, Preventive Behaviours and Temporal Outcomes Associated with COVID-19 Infection in Health Professionals at a Spanish Hospital. Int. J. Environ. Res. Public Health 2020, 17, 4305. [Google Scholar] [CrossRef]
- Rivera-Izquierdo, M.; Valero-Ubierna, M.D.; R-delAmo, J.L.; Fernández-García, M.Á.; Martínez-Diz, S.; Tahery-Mahmoud, A.; Rodríguez-Camacho, M.; Gámiz-Molina, A.B.; Barba-Gyengo, N.; Gámez-Baeza, P.; et al. Sociodemographic, clinical and laboratory factors on admission associated with COVID-19 mortality in hospitalized patients: A retrospective observational study. PLoS ONE 2020, 15, e0235107. [Google Scholar] [CrossRef]
- El-Kazzaz, W.; Metwally, L.; Yahia, R.; Al-Harbi, N.; El-Taher, A.; Hetta, H.F. Antibiogram, Prevalence of OXA Carbapenemase Encoding Genes, and RAPD-Genotyping of Multidrug-Resistant Acinetobacter baumannii Incriminated in Hidden Community-Acquired Infections. Antibiotics 2020, 9, 603. [Google Scholar] [CrossRef]
- Oteo, J.; Ortega, A.; Bartolomé, R.; Bou, G.; Conejo, C.; Fernández-Martínez, M.; González-López, J.J.; Martínez-García, L.; Martínez-Martínez, L.; Merino, M.; et al. GEIH-GEMARA (SEIMC) and REIPI. Prospective multicenter study of carbapenemase-producing Enterobacteriaceae from 83 hospitals in Spain reveals high in vitro susceptibility to colistin and meropenem. Antimicrob. Agents Chemother. 2015, 59, 3406–3412. [Google Scholar] [CrossRef]
- Hernández-García, M.; Pérez-Viso, B.; Turrientes, M.C.; Díaz-Agero, C.; López-Fresneña, N.; Bonten, M.; Malhotra-Kumar, S.; Ruiz-Garbajosa, P.; Cantón, R. Characterization of carbapenemase-producing Enterobacteriaceae from colonized patients in a university hospital in Madrid, Spain, during the R-GNOSIS project depicts increased clonal diversity over time with maintenance of high-risk clones. J. Antimicrob. Chemother. 2018, 73, 3039–3043. [Google Scholar] [CrossRef] [PubMed]
- Lázaro-Perona, F.; Rodríguez-Tejedor, M.; Ruiz-Carrascoso, G.; Díaz-Pollán, B.; Loeches, B.; Ramos-Ramos, J.C.; Mingorance, J. Intestinal loads of OXA-48-producing Klebsiella pneumoniae in colonized patients determined from surveillance rectal swabs. Clin. Microbiol. Infect. 2020, in press. [Google Scholar] [CrossRef]
- Mateos, M.; Hernández-García, M.; Del Campo, R.; Martínez-García, L.; Gijón, D.; Morosini, M.I.; Ruiz-Garbajosa, P.; Cantón, R. Emergence and Persistence over Time of Carbapenemase-Producing Enterobacter Isolates in a Spanish University Hospital in Madrid, Spain (2005–2018). Microb. Drug Resist. 2020. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Izquierdo, M.; Benavente-Fernández, A.; López-Gómez, J.; Láinez-Ramos-Bossini, A.J.; Rodríguez-Camacho, M.; Valero-Ubierna, M.; Martín-delosReyes, L.M.; Jiménez-Mejías, E.; Moreno-Roldán, E.; Lardelli-Claret, P.; et al. Prevalence of Multi-Resistant Microorganisms and Antibiotic Stewardship among Hospitalized Patients Living in Residential Care Homes in Spain: A Cross-Sectional Study. Antibiotics 2020, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, H.; Glasner, C.; Albiger, B.; Aanensen, D.M.; Tomlinson, C.T.; Andrasević, A.T.; Cantón, R.; Carmeli, Y.; Friedrich, A.W.; Giske, C.G.; et al. European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): A prospective, multinational study. Lancet Infect. Dis. 2017, 17, 153–163. [Google Scholar]
- Mora-Guzmán, I.; Rubio-Perez, I.; Domingo-Garcia, D.; Martín-Pérez, E. Infecciones asociadas a enterobacterias productoras de carbapenemasas OXA-48 en pacientes quirúrgicos: Consumo de antibióticos y evolución de sensibilidades [Infections by OXA-48 carbapenemase-producing Enterobacteriaceae in surgical patients: Antibiotic consumption and susceptibility patterns]. Rev. Esp. Quimioter. 2020, 33, 448–452. [Google Scholar] [PubMed]
- Ministerio de Sanidad. Proyecto Infección Quirúrgica Zero [Zero Surgical Infection]. Available online: https://infeccionquirurgicazero.es/es/ (accessed on 10 December 2020).
- Bartsch, S.M.; McKinnell, J.A.; Mueller, L.E.; Miller, L.G.; Gohil, S.K.; Huang, S.S.; Lee, B.Y. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin. Microbiol. Infect. 2017, 23, 48.e9–48.e16. [Google Scholar] [CrossRef]
- García-Fernández, S.; García-Castillo, M.; Bou, G.; Calvo, J.; Cercenado, E.; Delgado, M.; Pitart, C.; Mulet, X.; Tormo, N.; López Mendoza, D.; et al. Activity of ceftolozane/tazobactam against Pseudomonas aeruginosa and Enterobacterales isolates recovered from intensive care unit patients in Spain: The SUPERIOR multicentre study. Int. J. Antimicrob. Agents 2019, 53, 682–688. [Google Scholar] [CrossRef]
- López-González, L.; Viñuela-Prieto, J.M.; Rodriguez-Avial, I.; Manzano, R.; Candel, F.J. Description of carbapenemase-producing Enterobacteriaceae isolates in a Spanish tertiary hospital. Epidemiological analysis and clinical impact. Rev. Esp. Quimioter. 2019, 32, 254–262. [Google Scholar]
- Ministerio de Sanidad. Proyecto Resistencia Zero [Zero Resistance Project]. Available online: https://www.seguridaddelpaciente.es/es/practicas-seguras/seguridad-pacientes-criticos/proyectoresistencia-zero/ (accessed on 10 December 2020).
- Maseda, E.; Salgado, P.; Anillo, V.; Ruiz-Carrascoso, G.; Gómez-Gil, R.; Martín-Funke, C.; Gimenez, M.J.; Granizo, J.J.; Aguilar, L.; Gilsanz, F. Risk factors for colonization by carbapenemase-producing enterobacteria at admission to a Surgical ICU: A retrospective study. Enferm. Infect. Microbiol. Clin. 2017, 35, 333–337. [Google Scholar] [CrossRef]
- Robustillo-Rodela, A.; Pérez-Blanco, V.; Ruiz, M.A.E.; Carrascoso, G.R.; Iglesias, J.C.F.; Martín, D.A. Successful control of 2 simultaneous outbreaks of OXA-48 carbapenemase-producing Enterobacteriaceae and multidrug-resistant Acinetobacter baumannii in an intensive care unit. Am. J. Infect. Control. 2017, 45, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Infection Control Isolation Precautions. Available online: https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html (accessed on 10 December 2020).
- Peirano, G.; Chen, L.; Kreiswirth, B.N.; Pitout, J. Emerging Antimicrobial-Resistant High-Risk Klebsiella pneumoniae Clones ST307 and ST147. Antimicrob. Agents Chemother. 2020, 64, e01148-20. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, L.; de Been, M.; Rogers, M.; Schürch, A.C.; Scharringa, J.; van der Zee, A.; Bonten, M.; Fluit, A.C. Sequence-based epidemiology of an OXA-48 plasmid during a hospital outbreak. Antimicrob. Agents Chemother. 2019, 63, e01204-19. [Google Scholar] [CrossRef] [PubMed]
- Solgi, H.; Nematzadeh, S.; Giske, C.G.; Badmasti, F.; Westerlund, F.; Lin, Y.L.; Goyal, G.; Nikbin, V.S.; Nemati, A.H.; Shahcheraghi, F. Molecular Epidemiology of OXA-48 and NDM-1 Producing Enterobacterales Species at a University Hospital in Tehran, Iran, Between 2015 and 2016. Front. Microbiol. 2020, 11, 936. [Google Scholar] [CrossRef]
- Kopotsa, K.; Sekyere, J.O.; Mbelle, N.M. Plasmid evolution in carbapenemase-producing Enterobacteriaceae: A review. Ann. N. Y. Acad. Sci. 2019, 1457, 61–91. [Google Scholar] [CrossRef]
- Datta, N.; Hedges, R.W. R factors of compatibility goup A. J. Gen. Microbiol. 1973, 74, 335–336. [Google Scholar] [CrossRef][Green Version]
- Lee, T.D.; Adie, K.; McNabb, A.; Purych, D.; Mannan, K.; Azana, R.; Ng, C.; Tang, P.; Hoang, L.M. Rapid Detection of KPC, NDM, and OXA-48-Like Carbapenemases by Real-Time PCR from Rectal Swab Surveillance Samples. J. Clin. Microbiol. 2015, 53, 2731–2733. [Google Scholar] [CrossRef]
- Mittal, G.; Gaind, R.; Kumar, D.; Kaushik, G.; Gupta, K.B.; Verma, P.K.; Deb, M. Risk factors for fecal carriage of carbapenemase producing Enterobacteriaceae among intensive care unit patients from a tertiary care center in India. BMC Microbiol. 2016, 16, 138. [Google Scholar] [CrossRef]
- Kim, Y.A.; Lee, S.J.; Park, Y.S.; Lee, Y.J.; Yeon, J.H.; Seo, Y.H.; Lee, K. Risk Factors for Carbapenemase-Producing Enterobacterales Infection or Colonization in a Korean Intensive Care Unit: A Case–Control Study. Antibiotics 2020, 9, 680. [Google Scholar] [CrossRef]
- Reuland, E.A.; Al Naiemi, N.; Kaiser, A.M.; Heck, M.; Kluytmans, J.A.; Savelkoul, P.H.; Elders, P.J.M.; Vandebroucke-Grauls, C.M.J.E. Prevalence and risk factors for carriage of ESBL-producing Enterobacteriaceae in Amsterdam. J. Antimicrob. Chemother. 2016, 71, 1076–1082. [Google Scholar] [CrossRef]
- Ruppé, E.; Armand-Lefèvre, L.; Estellat, C.; El-Mniai, A.; Boussadia, Y.; Consigny, P.H. Acquisition of carbapenemaseproducing Enterobacteriaceae by healthy travellers to India, France, February 2012 to March 2013. Eurosurveillance 2014, 19, 20768. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Burns, K.; Baño, J.R.; Borg, M.; Daikos, G.; Dumpis, U.; Lucet, J.C.; Moro, M.L.; Tacconelli, E.; Simonsen, G.S.; et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: Guidance from the European Centre for Disease Prevention and Control. Antimicrob. Resist. Infect. Control. 2017, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Grau, S.; Fondevilla, E.; Echeverría-Esnal, D.; Alcorta, A.; Limon, E.; Gudiol, F.; VINCat Program Group. Widespread increase of empirical carbapenem use in acute care hospitals in Catalonia, Spain. Enferm. Infecc. Microbiol. Clin. 2019, 37, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Rodriguez-Bano, J. The Use of Noncarbapenem β-Lactams for the Treatment of Extended-Spectrum β-Lactamase Infections. Clin. Infect. Dis. 2017, 64, 972–980. [Google Scholar] [CrossRef]
- Plan Nacional de Resistencia a Antibióticos (PRAN). Programs of Optimization of Antibiotic Use (PROA). Available online: https://www.resistenciaantibioticos.es/es/programas-de-optimizacion-de-uso-de-los-antibioticos-proa (accessed on 10 December 2020).
- Rodríguez-Baño, J.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Pascual, A. Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018, 31, e00079-17. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.F.; Bassetti, M.; Cremer, O.; Daikos, G.; de Waele, J.; Kallil, A.; Kipnis, E.; Kollef, M.; Laupland, K.; Paiva, J.-A.; et al. Rationalizing antimicrobial therapy in the ICU: A narrative review. Intensive Care Med. 2019, 45, 172–189. [Google Scholar] [CrossRef]
- Napolitano, F.; Della Polla, G.; De Simone, C.; Lambiase, C.; Pelullo, C.P.; Angelillo, I.F. The Knowledge, Attitudes, and Practices of Community Pharmacists in their Approach to Antibiotic Use: A Nationwide Survey in Italy. Antibiotics 2019, 8, 177. [Google Scholar] [CrossRef]
- Evren, E.; Azap, O.K.; Colakoglu, S.; Arslan, H. In vitro activity of fosfomycin in combination with imipenem, meropenem, colistin and tigecycline against OXA 48-positive Klebsiella pneumoniae strains. Diagn. Microbiol. Infect. Dis. 2013, 76, 335–338. [Google Scholar] [CrossRef]
- Tumbarello, M.; Losito, A.R.; Giamarellou, H. Optimizing therapy in carbapenem-resistant Enterobacteriaceae infections. Curr. Opin. Infect. Dis. 2018, 31, 566–577. [Google Scholar] [CrossRef]
- Shaidullina, E.; Shelenkov, A.; Yanushevich, Y.; Mikhaylova, Y.; Shagin, D.; Alexandrova, I.; Ershova, O.; Akimkin, V.; Kozlov, R.; Edelstein, M. Antimicrobial Resistance and Genomic Characterization of OXA-48- and CTX-M-15-Co-Producing Hypervirulent Klebsiella pneumoniae ST23 Recovered from Nosocomial Outbreak. Antibiotics 2020, 9, 862. [Google Scholar] [CrossRef]
- Stewart, A.; Harris, P.; Henderson, A.; Paterson, D. Treatment of Infections by OXA-48-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e01195-18. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, L.; González-López, J.J. Carbapenemases in Enterobacteriaceae: Types and molecular epidemiology. Enferm. Infecc. Microbiol. Clin. 2014, 32, 4–9. [Google Scholar] [CrossRef]
- Gill, C.M.; Asempa, T.E.; Nicolau, D.P. Efficacy of human-simulated exposures of meropenem/vaborbactam and meropenem against OXA-48 β-lactamase-producing Enterobacterales in the neutropenic murine thigh infection model. J. Antimicrob. Chemother. 2021, 76, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Lob, S.H.; Kazmierczak, K.M.; Hawser, S.P.; Magnet, S.; Young, K.; Motyl, M.R.; Sahm, D.F. In vitro activity of imipenem/relebactam against Gram-negative ESKAPE pathogens isolated in 17 European countries: 2015 SMART surveillance programme. J. Antimicrob. Chemother. 2018, 73, 1872–1879. [Google Scholar] [CrossRef]
- Clark, J.A.; Kulengowski, B.; Burgess, D.S. In vitro activity of eravacycline compared with tigecycline against carbapenem-resistant Enterobacteriaceae. Int. J. Antimicrob. Agents. 2020, 56, 106178. [Google Scholar] [CrossRef]
- Livermore, D.M.; Warner, M.; Mushtaq, S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2013, 68, 2286–2290. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis 2020. online ahead of print. [Google Scholar] [CrossRef]
- Ito, A.; Nishikawa, T.; Matsumoto, S.; Yoshizawa, H.; Sato, T.; Nakamura, R.; Tsuji, M.; Yamano, Y. Siderophore Cephalosporin Cefiderocol Utilizes Ferric Iron Transporter Systems for Antibacterial Activity against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 7396–7401. [Google Scholar]
- Fredborg, M.; Sondergaard, T.E.; Wang, M. Synergistic activities of meropenem double and triple combinations against carbapenemase-producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2017, 88, 355–360. [Google Scholar] [CrossRef]
- Sánchez-López, J.; Cantón, R. Current status of ESKAPE microorganisms in Spain: Epidemiology and resistance phenotypes. Rev. Esp. Quimioter. 2019, 32, 27–31. [Google Scholar]
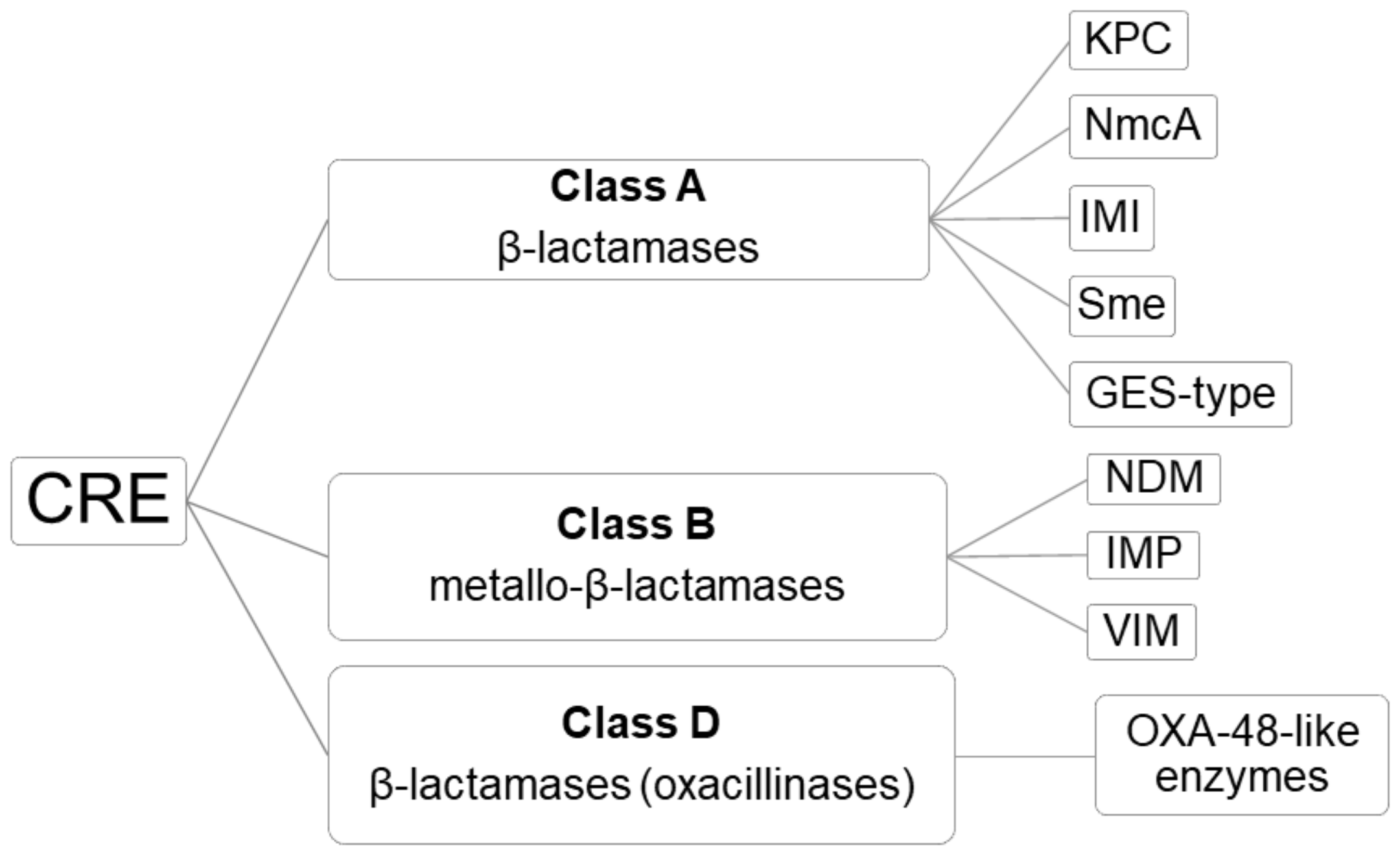
| Enzyme | First Clinical Identification | Reference |
|---|---|---|
| OXA-48 | Turkey, 2001 | [18] |
| OXA-54 | France, 2003 | [19] |
| OXA-162 | Turkey, 2012 | [20] |
| OXA-163 | Argentina, 2008 | [21] |
| OXA-181 | India, 2010 | [22] |
| OXA-199 | China, 2012 | [23] |
| OXA-204 | Tunisia/France, 2012 | [24] |
| OXA-232 | India/France, 2011 | [25] |
| OXA-244 | Spain, 2012 | [26] |
| OXA-245 | Spain, 2012 | [26] |
| OXA-247 | Argentina, 2010 | [27] |
| OXA-252 | Canada, 2014 | [28] |
| OXA-370 | Brazil, 2013 | [29] |
| OXA-405 | France, 2014 | [30] |
| OXA-416 | Italy, 2013 | [31] |
| OXA-436 | Denmark, 2015 | [32] |
| OXA-438 | Argentina, 2020 | [33] |
| OXA-484 | United Kingdom, 2015 | [34] |
| OXA-505 | USA, 2018 | [35] |
| OXA-519 | Belgium, 2015 | [36] |
| OXA-535 | France, 2018 | [37] |
| OXA-566 | New Zealand, 2017 | [38] |
| First Clinical Identification | References |
|---|---|
| Klebsiella pneumoniae | [44,45,46,47,48] |
| Klebsiella oxytoca | [44,45,48,49] |
| Kluyvera spp. | [48,50] |
| Escherichia coli | [44,45,48,51] |
| Proteus mirabilis | [44,45,48,52] |
| Serratia marcescens | [53] |
| Enterobacter cloacae | [44,45,48,54] |
| Enterobacter aerogenes | [44,45,48] |
| Enterobacter sakasakii | [15] |
| Citrobacter freundii | [44,45,48,55] |
| Citrobacter koseri | [44,45,48] |
| Citrobacter braakii | [15] |
| Salmonella enterica | [44,45,48,56] |
| Morganella morganii | [44,45,48] |
| Providencia rettgeri | [15] |
| Raoultella planticola | [15] |
| CRE Species | Nosocomial Infections | Community Infections | ||||
|---|---|---|---|---|---|---|
| IM | CR-IM | %CR | IM | CR-IM | %CR | |
| Escherichia coli | 607 | 10 | 2.1 | 1341 | 12 | 1.1 |
| Klebsiella pneumoniae | 316 | 30 | 11.2 | 305 | 30 | 11.5 |
| Klebsiella oxytoca | 60 | 2 | 4.1 | 79 | 3 | 5.0 |
| Klebsiella spp., other | 12 | 1 | 10.0 | 15 | 0 | 0.0 |
| Enterobacter aerogenes | 45 | 3 | 8.3 | 23 | 0 | 0.0 |
| Enterobacter cloacae | 126 | 9 | 8.3 | 92 | 2 | 2.3 |
| Enterobacter spp., other | 17 | 1 | 7.7 | 15 | 1 | 12.5 |
| Citrobacter spp. | 58 | 4 | 10.8 | 49 | 0 | 0.0 |
| Proteus spp. | 127 | 3 | 3.4 | 218 | 4 | 2.4 |
| Serratia marcescens | 66 | 5 | 9.6 | 58 | 1 | 2.1 |
| Serratia spp., other | 3 | 0 | 0.0 | 6 | 0 | 0.0 |
| Morganella spp. | 53 | 6 | 12.8 | 58 | 0 | 0.0 |
| Other Enterobacterales | 3 | 0 | 0.0 | 8 | 0 | 0.0 |
| Antibiotic Group | % |
| Penicillins including β-lactamase inhibitors | 27.5 |
| Fluoroquinolones | 15.4 |
| Third generation cephalosporins | 12.4 |
| Carbapenems | 7.0 |
| Macrolides | 3.1 |
| First generation cephalosporins | 2.3 |
| Antibiotic | % |
| Amoxicillin and clavulanic acid | 14.4 |
| Ceftriaxone | 9.8 |
| Piperacillin and tazobactam | 8.5 |
| Levofloxacin | 7.8 |
| Cefazolin | 7.4 |
| Meropenem | 5.7 |
| Ciprofloxacin | 4.7 |
| Linezolid | 2.8 |
| Cotrimoxazole | 2.1 |
| Vancomycin | 2.0 |
| Metronidazole | 2.0 |
| Clindamycin | 1.9 |
| Cefuroxime | 1.8 |
| Azithromycin | 1.7 |
| Gentamycin | 1.7 |
| Ampicillin | 1.4 |
| Cefotaxime | 1.2 |
| Ertapenem | 1.2 |
| Daptomycin | 1.1 |
| Ceftazidime | 1.0 |
| Imipenem and cilastatin | 1.0 |
| Cloxacillin | 0.9 |
| Amikacin | 0.9 |
| Amoxicillin | 0.8 |
| Fosfomycin | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Izquierdo, M.; Láinez-Ramos-Bossini, A.J.; Rivera-Izquierdo, C.; López-Gómez, J.; Fernández-Martínez, N.F.; Redruello-Guerrero, P.; Martín-delosReyes, L.M.; Martínez-Ruiz, V.; Moreno-Roldán, E.; Jiménez-Mejías, E. OXA-48 Carbapenemase-Producing Enterobacterales in Spanish Hospitals: An Updated Comprehensive Review on a Rising Antimicrobial Resistance. Antibiotics 2021, 10, 89. https://doi.org/10.3390/antibiotics10010089
Rivera-Izquierdo M, Láinez-Ramos-Bossini AJ, Rivera-Izquierdo C, López-Gómez J, Fernández-Martínez NF, Redruello-Guerrero P, Martín-delosReyes LM, Martínez-Ruiz V, Moreno-Roldán E, Jiménez-Mejías E. OXA-48 Carbapenemase-Producing Enterobacterales in Spanish Hospitals: An Updated Comprehensive Review on a Rising Antimicrobial Resistance. Antibiotics. 2021; 10(1):89. https://doi.org/10.3390/antibiotics10010089
Chicago/Turabian StyleRivera-Izquierdo, Mario, Antonio Jesús Láinez-Ramos-Bossini, Carlos Rivera-Izquierdo, Jairo López-Gómez, Nicolás Francisco Fernández-Martínez, Pablo Redruello-Guerrero, Luis Miguel Martín-delosReyes, Virginia Martínez-Ruiz, Elena Moreno-Roldán, and Eladio Jiménez-Mejías. 2021. "OXA-48 Carbapenemase-Producing Enterobacterales in Spanish Hospitals: An Updated Comprehensive Review on a Rising Antimicrobial Resistance" Antibiotics 10, no. 1: 89. https://doi.org/10.3390/antibiotics10010089
APA StyleRivera-Izquierdo, M., Láinez-Ramos-Bossini, A. J., Rivera-Izquierdo, C., López-Gómez, J., Fernández-Martínez, N. F., Redruello-Guerrero, P., Martín-delosReyes, L. M., Martínez-Ruiz, V., Moreno-Roldán, E., & Jiménez-Mejías, E. (2021). OXA-48 Carbapenemase-Producing Enterobacterales in Spanish Hospitals: An Updated Comprehensive Review on a Rising Antimicrobial Resistance. Antibiotics, 10(1), 89. https://doi.org/10.3390/antibiotics10010089






