Are We Making the Most of Community Pharmacies? Implementation of Antimicrobial Stewardship Measures in Community Pharmacies: A Narrative Review
Abstract
1. Introduction
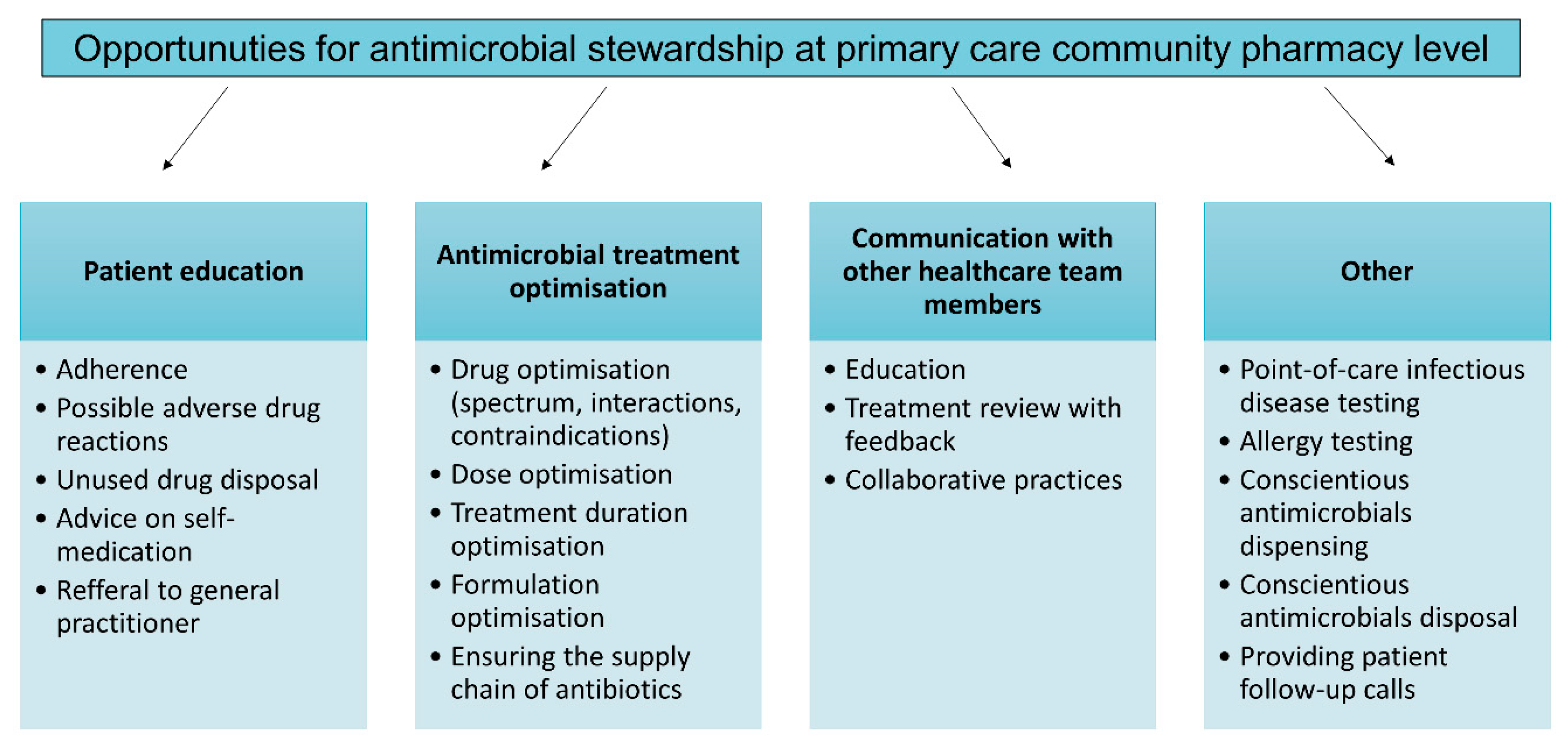
2. Antimicrobial Stewardship in Community Pharmacy
2.1. Awareness of and Barriers to Implementing Antimicrobial Stewardship Programs at the Primary Care Pharmacy Level
2.2. Common Primary Care Indications
2.2.1. Upper Respiratory Tract Infections
2.2.2. Uncomplicated Urinary Tract Infections
2.3. Pharmacist as a Member of a Multidisciplinary Team
2.4. Other Considerations
2.4.1. Penicillin Allergy
2.4.2. Veterinary Drugs
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Andersson, D.I. Evolutionary Trajectories to Antibiotic Resistance. Annu. Rev. Microbiol. 2017, 71, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C. What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, L.; Gauthier, T.; Heil, E.; Klepser, M.; Kelly, K.M.; Nailor, M.; Wei, W.; Suda, K. Outpatient Stewardship Working, G. The essential role of pharmacists in antibiotic stewardship in outpatient care: An official position statement of the Society of Infectious Diseases Pharmacists. J. Am. Pharm. Assoc. 2018, 58, 481–484. [Google Scholar] [CrossRef]
- Anderson, D.J.; Watson, S.; Moehring, R.W.; Komarow, L.; Finnemeyer, M.; Arias, R.M.; Huvane, J.; Bova Hill, C.; Deckard, N.; Sexton, D.J.; et al. Feasibility of Core Antimicrobial Stewardship Interventions in Community Hospitals. JAMA Netw. Open 2019, 2, e199369. [Google Scholar] [CrossRef]
- Resman, F. Antimicrobial stewardship programs; a two-part narrative review of step-wise design and issues of controversy Part I: Step-wise design of an antimicrobial stewardship program. Ther. Adv. Infect. Dis. 2020, 7, 2049936120933187. [Google Scholar] [CrossRef]
- Hall, J.W.; Bouchard, J.; Bookstaver, P.B.; Haldeman, M.S.; Kishimbo, P.; Mbwanji, G.; Mwakyula, I.; Mwasomola, D.; Seddon, M.; Shaffer, M.; et al. The Mbeya Antimicrobial Stewardship Team: Implementing Antimicrobial Stewardship at a Zonal-Level Hospital in Southern Tanzania. Pharmacy 2020, 8, 107. [Google Scholar] [CrossRef]
- Horumpende, P.G.; Sonda, T.B.; van Zwetselaar, M.; Antony, M.L.; Tenu, F.F.; Mwanziva, C.E.; Shao, E.R.; Mshana, S.E.; Mmbaga, B.T.; Chilongola, J.O. Prescription and non-prescription antibiotic dispensing practices in part I and part II pharmacies in Moshi Municipality, Kilimanjaro Region in Tanzania: A simulated clients approach. PLoS ONE 2018, 13, e0207465. [Google Scholar] [CrossRef]
- Koji, E.M.; Gebretekle, G.B.; Tekle, T.A. Practice of over-the-counter dispensary of antibiotics for childhood illnesses in Addis Ababa, Ethiopia: A simulated patient encounter study. Antimicrob. Resist. Infect. Control 2019, 8, 119. [Google Scholar] [CrossRef]
- Zawahir, S.; Lekamwasam, S.; Aslani, P. Community pharmacy staff’s response to symptoms of common infections: A pseudo-patient study. Antimicrob. Resist. Infect. Control 2019, 8, 60. [Google Scholar] [CrossRef]
- Hoxha, I.; Malaj, A.; Kraja, B.; Bino, S.; Oluka, M.; Markovic-Pekovic, V.; Godman, B. Are pharmacists’ good knowledge and awareness on antibiotics taken for granted? The situation in Albania and future implications across countries. J. Glob. Antimicrob. Resist. 2018, 13, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Peiffer-Smadja, N.; Poda, A.; Ouedraogo, A.S.; Guiard-Schmid, J.B.; Delory, T.; Le Bel, J.; Bouvet, E.; Lariven, S.; Jeanmougin, P.; Ahmad, R.; et al. Paving the Way for the Implementation of a Decision Support System for Antibiotic Prescribing in Primary Care in West Africa: Preimplementation and Co-Design Workshop With Physicians. J. Med. Internet Res. 2020, 22, e17940. [Google Scholar] [CrossRef] [PubMed]
- Markovic-Pekovic, V.; Grubisa, N. Self-medication with antibiotics in the Republic of Srpska community pharmacies: Pharmacy staff behavior. Pharmacoepidemiol. Drug Saf. 2012, 21, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Sabry, N.A.; Farid, S.F.; Dawoud, D.M. Antibiotic dispensing in Egyptian community pharmacies: An observational study. Res. Soc. Adm. Pharm. 2014, 10, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Darj, E.; Newaz, M.S.; Zaman, M.H. Pharmacists’ perception of their challenges at work, focusing on antimicrobial resistance: A qualitative study from Bangladesh. Glob. Health Action 2019, 12, 1735126. [Google Scholar] [CrossRef] [PubMed]
- Alhomoud, F.; Almahasnah, R.; Alhomoud, F.K. “You could lose when you misuse”—Factors affecting over-the-counter sale of antibiotics in community pharmacies in Saudi Arabia: A qualitative study. BMC Health Serv. Res. 2018, 18, 915. [Google Scholar] [CrossRef]
- Zakaa El-Din, M.; Samy, F.; Mohamed, A.; Hamdy, F.; Yasser, S.; Ehab, M. Egyptian community pharmacists’ attitudes and practices towards antibiotic dispensing and antibiotic resistance; a cross-sectional survey in Greater Cairo. Curr. Med. Res. Opin. 2019, 35, 939–946. [Google Scholar] [CrossRef]
- Auta, A.; Hadi, M.A.; Oga, E.; Adewuyi, E.O.; Abdu-Aguye, S.N.; Adeloye, D.; Strickland-Hodge, B.; Morgan, D.J. Global access to antibiotics without prescription in community pharmacies: A systematic review and meta-analysis. J. Infect. 2019, 78, 8–18. [Google Scholar] [CrossRef]
- Jamshed, S.; Padzil, F.; Shamsudin, S.H.; Bux, S.H.; Jamaluddin, A.A.; Bhagavathula, A.S.; Azhar, S.; Hassali, M.A. Antibiotic Stewardship in Community Pharmacies: A Scoping Review. Pharmacy 2018, 6, 92. [Google Scholar] [CrossRef]
- Feng, Z.; Hayat, K.; Huang, Z.; Shi, L.; Li, P.; Xiang, C.; Gong, Y.; Chang, J.; Jiang, M.; Yang, C.; et al. Knowledge, attitude, and practices of community pharmacy staff toward antimicrobial stewardship programs: A cross-sectional study from Northeastern China. Expert Rev. Anti Infect. Ther. 2020, 1–8. [Google Scholar] [CrossRef]
- Tonna, A.P.; Weidmann, A.E.; Sneddon, J.; Stewart, D. Views and experiences of community pharmacy team members on antimicrobial stewardship activities in Scotland: A qualitative study. Int. J. Clin. Pharm. 2020, 42, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, T.; Thompson, A.; Williams, M.; Zaidi, S.T.R. Validation and implementation of a national survey to assess antimicrobial stewardship awareness, practices and perceptions amongst community pharmacists in Australia. J. Glob. Antimicrob. Resist. 2020, 21, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.R.; Saqib, A.; Iftikhar, S.; Sadiq, T. Knowledge of community pharmacists about antibiotics, and their perceptions and practices regarding antimicrobial stewardship: A cross-sectional study in Punjab, Pakistan. Infect. Drug Resist. 2018, 11, 133–145. [Google Scholar] [CrossRef]
- Hayat, K.; Li, P.; Rosenthal, M.; Xu, S.; Chang, J.; Gillani, A.H.; Khan, F.U.; Sarwar, M.R.; Ji, S.; Shi, L.; et al. Perspective of community pharmacists about community-based antimicrobial stewardship programs. A multicenter cross-sectional study from China. Expert Rev. Anti Infect. Ther. 2019, 17, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Ashiru-Oredope, D.; Doble, A.; Thornley, T.; Saei, A.; Gold, N.; Sallis, A.; McNulty, C.A.M.; Lecky, D.; Umoh, E.; Klinger, C. Improving Management of Respiratory Tract Infections in Community Pharmacies and Promoting Antimicrobial Stewardship: A Cluster Randomised Control Trial with a Self-Report Behavioural Questionnaire and Process Evaluation. Pharmacy 2020, 8, 44. [Google Scholar] [CrossRef]
- Khan, M.U.; Hassali, M.A.; Ahmad, A.; Elkalmi, R.M.; Zaidi, S.T.; Dhingra, S. Perceptions and Practices of Community Pharmacists towards Antimicrobial Stewardship in the State of Selangor, Malaysia. PLoS ONE 2016, 11, e0149623. [Google Scholar] [CrossRef]
- Rizvi, T.; Thompson, A.; Williams, M.; Zaidi, S.T.R. Perceptions and current practices of community pharmacists regarding antimicrobial stewardship in Tasmania. Int. J. Clin. Pharm. 2018, 40, 1380–1387. [Google Scholar] [CrossRef]
- Del Fiol Fde, S.; Barberato-Filho, S.; Lopes, L.C.; Bergamaschi Cda, C.; Boscariol, R. Assessment of Brazilian pharmacists’ knowledge about antimicrobial resistance. J. Infect. Dev. Ctries 2015, 9, 239–243. [Google Scholar] [CrossRef]
- Jones, L.F.; Owens, R.; Sallis, A.; Ashiru-Oredope, D.; Thornley, T.; Francis, N.A.; Butler, C.; McNulty, C.A.M. Qualitative study using interviews and focus groups to explore the current and potential for antimicrobial stewardship in community pharmacy informed by the Theoretical Domains Framework. BMJ Open 2018, 8, e025101. [Google Scholar] [CrossRef]
- Rehman, I.U.; Asad, M.M.; Bukhsh, A.; Ali, Z.; Ata, H.; Dujaili, J.A.; Blebil, A.Q.; Khan, T.M. Knowledge and Practice of Pharmacists toward Antimicrobial Stewardship in Pakistan. Pharmacy 2018, 6, 116. [Google Scholar] [CrossRef]
- Waseem, H.; Ali, J.; Sarwar, F.; Khan, A.; Rehman, H.S.U.; Choudri, M.; Arif, N.; Subhan, M.; Saleem, A.R.; Jamal, A.; et al. Assessment of knowledge and attitude trends towards antimicrobial resistance (AMR) among the community members, pharmacists/pharmacy owners and physicians in district Sialkot, Pakistan. Antimicrob. Resist. Infect. Control 2019, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.; Asghar, S.; Mushtaq, I.; Malik, I. Community pharmacists as antibiotic stewards: A qualitative study exploring the current status of Antibiotic Stewardship Program in Bahawalpur, Pakistan. J. Infect. Public Health 2020, 13, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Z.; Hassali, M.A.; Hashmi, F.K.; Godman, B.; Saleem, F. Antimicrobial dispensing practices and determinants of antimicrobial resistance: A qualitative study among community pharmacists in Pakistan. Fam. Med. Community Health 2019, 7, e000138. [Google Scholar] [CrossRef] [PubMed]
- Atkins, L.; Chadborn, T.; Bondaronek, P.; Ashiru-Oredope, D.; Beech, E.; Herd, N.; de La Moriniere, V.; Gonzalez-Iraizoz, M.; Hopkins, S.; McNulty, C.; et al. Content and Mechanism of Action of National Antimicrobial Stewardship Interventions on Management of Respiratory Tract Infections in Primary and Community Care. Antibiotics 2020, 9, 512. [Google Scholar] [CrossRef]
- Revolinski, S.; Pawlak, J.; Beckers, C. Assessing Pharmacy Students’ and Preceptors’ Understanding of and Exposure to Antimicrobial Stewardship Practices on Introductory Pharmacy Practice Experiences. Pharmacy 2020, 8, 149. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Allison, R.; Jones, L.F.; Holmes, A.; Patel, P.; Lecky, D.M.; Ahmad, R.; McNulty, C.A.M. Preventing and Managing Urinary Tract Infections: Enhancing the Role of Community Pharmacists-A Mixed Methods Study. Antibiotics 2020, 9, 583. [Google Scholar] [CrossRef]
- Charani, E.; Castro-Sanchez, E.; Bradley, S.; Nathwani, D.; Holmes, A.H.; Davey, P. Implementation of antibiotic stewardship in different settings—Results of an international survey. Antimicrob. Resist. Infect. Control 2019, 8, 34. [Google Scholar] [CrossRef]
- Rusic, D.; Bozic, J.; Bukic, J.; Vilovic, M.; Tomicic, M.; Seselja Perisin, A.; Leskur, D.; Modun, D.; Cohadzic, T.; Tomic, S. Antimicrobial Resistance: Physicians’ and Pharmacists’ Perspective. Microb. Drug Resist. 2020. [Google Scholar] [CrossRef]
- Rusic, D.; Bozic, J.; Bukic, J.; Seselja Perisin, A.; Leskur, D.; Modun, D.; Tomic, S. Evaluation of accordance of antibiotics package size with recommended treatment duration of guidelines for sore throat and urinary tract infections. Antimicrob. Resist. Infect. Control 2019, 8, 30. [Google Scholar] [CrossRef]
- Jukic, I.; Rusic, D.; Vukovic, J.; Zivkovic, P.M.; Bukic, J.; Leskur, D.; Seselja Perisin, A.; Luksic, M.; Modun, D. Correlation of registered drug packs with Maastricht V/Florence Consensus Report and national treatment guidelines for management of Helicobacter pylori infection. Basic Clin. Pharmacol. Toxicol. 2020, 126, 212–225. [Google Scholar] [CrossRef]
- Jukic, I.; Rusic, D.; Vukovic, J.; Modun, D. Response to the Letter to the Editor entitled “Correlation of registered drug packs in Greece with Maastricht V/Florence and Hellenic Helicobacter pylori infection treatment Consensuses: A poor or a proper match?”. Basic Clin. Pharmacol. Toxicol. 2020, 127, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Dobson, E.L.; Klepser, M.E.; Pogue, J.M.; Labreche, M.J.; Adams, A.J.; Gauthier, T.P.; Turner, R.B.; Su, C.P.; Jacobs, D.M.; Suda, K.J.; et al. Outpatient antibiotic stewardship: Interventions and opportunities. J. Am. Pharm. Assoc. 2017, 57, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.; Yacoob, Z.; Knobloch, M.J.; Safdar, N. Community pharmacy interventions to improve antibiotic stewardship and implications for pharmacy education: A narrative overview. Res. Soc. Adm. Pharm. 2019, 15, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Parente, D.M.; Morton, J. Role of the Pharmacist in Antimicrobial Stewardship. Med. Clin. N. Am. 2018, 102, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.L.; Fleming-Dutra, K.E.; Shapiro, D.J.; Hyun, D.Y.; Hicks, L.A.; Outpatient Antibiotic Use Target-Setting Workgroup. Frequency of First-line Antibiotic Selection Among US Ambulatory Care Visits for Otitis Media, Sinusitis, and Pharyngitis. JAMA Intern. Med. 2016, 176, 1870–1872. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.; O’Doherty, J.; O’Regan, A.; Dunne, C. Antibiotic use for acute respiratory tract infections (ARTI) in primary care; what factors affect prescribing and why is it important? A narrative review. Ir. J. Med. Sci. 2018, 187, 969–986. [Google Scholar] [CrossRef]
- Klepser, M.E.; Dobson, E.L.; Pogue, J.M.; Labreche, M.J.; Adams, A.J.; Gauthier, T.P.; Turner, R.B.; Su, C.P.; Jacobs, D.M.; Suda, K.J.; et al. A call to action for outpatient antibiotic stewardship. J. Am. Pharm. Assoc. 2017, 57, 457–463. [Google Scholar] [CrossRef]
- Essack, S.; Bell, J.; Shephard, A. Community pharmacists-Leaders for antibiotic stewardship in respiratory tract infection. J. Clin. Pharm. Ther. 2018, 43, 302–307. [Google Scholar] [CrossRef]
- Wessels, M.R. Clinical practice. Streptococcal pharyngitis. N. Engl. J. Med. 2011, 364, 648–655. [Google Scholar] [CrossRef]
- Palla, A.H.; Khan, R.A.; Gilani, A.H.; Marra, F. Over prescription of antibiotics for adult pharyngitis is prevalent in developing countries but can be reduced using McIsaac modification of Centor scores: A cross-sectional study. BMC Pulm. Med. 2012, 12, 70. [Google Scholar] [CrossRef]
- Saengcharoen, W.; Jaisawang, P.; Udomcharoensab, P.; Buathong, K.; Lerkiatbundit, S. Appropriateness of diagnosis of streptococcal pharyngitis among Thai community pharmacists according to the Centor criteria. Int. J. Clin. Pharm. 2016, 38, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Demore, B.; Tebano, G.; Gravoulet, J.; Wilcke, C.; Ruspini, E.; Birge, J.; Boivin, J.M.; Henard, S.; Dieterling, A.; Munerol, L.; et al. Rapid antigen test use for the management of group A streptococcal pharyngitis in community pharmacies. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Essack, S.; Bell, J.; Burgoyne, D.; Tongrod, W.; Duerden, M.; Sessa, A.; Altiner, A.; Shephard, A. Point-of-Care Testing for Pharyngitis in the Pharmacy. Antibiotics 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.; Gallacher, D.; Achana, F.; Court, R.; Taylor-Phillips, S.; Nduka, C.; Stinton, C.; Willans, R.; Gill, P.; Mistry, H. Rapid antigen detection and molecular tests for group A streptococcal infections for acute sore throat: Systematic reviews and economic evaluation. Health Technol. Assess. 2020, 24, 1–232. [Google Scholar] [CrossRef]
- Mantzourani, E.; Evans, A.; Cannings-John, R.; Ahmed, H.; Hood, K.; Reid, N.; Howe, R.; Williams, E.; Way, C. Impact of a pilot NHS-funded sore throat test and treat service in community pharmacies on provision and quality of patient care. BMJ Open Qual. 2020, 9, e000833. [Google Scholar] [CrossRef]
- Klepser, D.G.; Klepser, M.E.; Dering-Anderson, A.M.; Morse, J.A.; Smith, J.K.; Klepser, S.A. Community pharmacist-physician collaborative streptococcal pharyngitis management program. J. Am. Pharm. Assoc. 2016, 56, 323–329. [Google Scholar] [CrossRef]
- Thornley, T.; Marshall, G.; Howard, P.; Wilson, A.P. A feasibility service evaluation of screening and treatment of group A streptococcal pharyngitis in community pharmacies. J. Antimicrob. Chemother. 2016, 71, 3293–3299. [Google Scholar] [CrossRef]
- Klepser, D.G.; Klepser, M.E.; Murry, J.S.; Borden, H.; Olsen, K.M. Evaluation of a community pharmacy-based influenza and group A streptococcal pharyngitis disease management program using polymerase chain reaction point-of-care testing. J. Am. Pharm. Assoc. 2019, 59, 872–879. [Google Scholar] [CrossRef]
- Hawker, J.I.; Smith, S.; Smith, G.E.; Morbey, R.; Johnson, A.P.; Fleming, D.M.; Shallcross, L.; Hayward, A.C. Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995-2011: Analysis of a large database of primary care consultations. J. Antimicrob. Chemother. 2014, 69, 3423–3430. [Google Scholar] [CrossRef]
- Schroeck, J.L.; Ruh, C.A.; Sellick, J.A., Jr.; Ott, M.C.; Mattappallil, A.; Mergenhagen, K.A. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrob. Agents Chemother. 2015, 59, 3848–3852. [Google Scholar] [CrossRef] [PubMed]
- Albasri, A.; Van den Bruel, A.; Hayward, G.; McManus, R.J.; Sheppard, J.P.; Verbakel, J.Y.J. Impact of point-of-care tests in community pharmacies: A systematic review and meta-analysis. BMJ Open 2020, 10, e034298. [Google Scholar] [CrossRef] [PubMed]
- Pulcini, C.; Pauvif, L.; Paraponaris, A.; Verger, P.; Ventelou, B. Perceptions and attitudes of French general practitioners towards rapid antigen diagnostic tests in acute pharyngitis using a randomized case vignette study. J. Antimicrob. Chemother. 2012, 67, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Klepser, M.E.; Adams, A.J. Pharmacy-based management of influenza: Lessons learned from research. Int. J. Pharm. Pract. 2018, 26, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Corn, C.E.; Klepser, D.G.; Dering-Anderson, A.M.; Brown, T.G.; Klepser, M.E.; Smith, J.K. Observation of a Pharmacist-Conducted Group A Streptococcal Pharyngitis Point-of-Care Test: A Time and Motion Study. J. Pharm. Pract. 2018, 31, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, E.; Hicks, R.; Evans, A.; Williams, E.; Way, C.; Deslandes, R. Community Pharmacist Views On The Early Stages Of Implementation Of A Pathfinder Sore Throat Test And Treat Service In Wales: An Exploratory Study. Integr. Pharm. Res. Pract. 2019, 8, 105–113. [Google Scholar] [CrossRef]
- Van der Velden, A.W.; Bell, J.; Sessa, A.; Duerden, M.; Altiner, A. Sore throat: Effective communication delivers improved diagnosis, enhanced self-care and more rational use of antibiotics. Int. J. Clin. Pract. Suppl. 2013, 67, 10–16. [Google Scholar] [CrossRef]
- Avent, M.L.; Fejzic, J.; van Driel, M.L. An underutilised resource for Antimicrobial Stewardship: A ‘snapshot’ of the community pharmacists’ role in delayed or ‘wait and see’ antibiotic prescribing. Int. J. Pharm. Pract. 2018, 26, 373–375. [Google Scholar] [CrossRef]
- Smieszek, T.; Pouwels, K.B.; Dolk, F.C.K.; Smith, D.R.M.; Hopkins, S.; Sharland, M.; Hay, A.D.; Moore, M.V.; Robotham, J.V. Potential for reducing inappropriate antibiotic prescribing in English primary care. J. Antimicrob. Chemother. 2018, 73, ii36–ii43. [Google Scholar] [CrossRef]
- McCormick, J.Z.; Cardwell, S.M.; Wheelock, C.; Wong, C.M.; Vander Weide, L.A. Impact of ambulatory antimicrobial stewardship on prescribing patterns for urinary tract infections. J. Clin. Pharm. Ther. 2020, 45, 1312–1319. [Google Scholar] [CrossRef]
- Gauld, N.J.; Zeng, I.S.; Ikram, R.B.; Thomas, M.G.; Buetow, S.A. Antibiotic treatment of women with uncomplicated cystitis before and after allowing pharmacist-supply of trimethoprim. Int. J. Clin. Pharm. 2017, 39, 165–172. [Google Scholar] [CrossRef]
- Beahm, N.P.; Smyth, D.J.; Tsuyuki, R.T. Outcomes of Urinary Tract Infection Management by Pharmacists (RxOUTMAP): A study of pharmacist prescribing and care in patients with uncomplicated urinary tract infections in the community. Can. Pharm. J. 2018, 151, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Ung, E.; Czarniak, P.; Sunderland, B.; Parsons, R.; Hoti, K. Assessing pharmacists’ readiness to prescribe oral antibiotics for limited infections using a case-vignette technique. Int. J. Clin. Pharm. 2017, 39, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.; Caldwell, G.; Cassells, K.; Burton, J.; Watson, A. Building capacity in primary care: The implementation of a novel ‘Pharmacy First’ scheme for the management of UTI, impetigo and COPD exacerbation. Prim. Health Care Res. Dev. 2018, 19, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, C.; Husereau, D.R.; Beahm, N.P.; Smyth, D.; Tsuyuki, R.T. Cost-effectiveness and budget impact of the management of uncomplicated urinary tract infection by community pharmacists. BMC Health Serv. Res. 2019, 19, 499. [Google Scholar] [CrossRef]
- Booth, J.L.; Mullen, A.B.; Thomson, D.A.; Johnstone, C.; Galbraith, S.J.; Bryson, S.M.; McGovern, E.M. Antibiotic treatment of urinary tract infection by community pharmacists: A cross-sectional study. Br. J. Gen. Pract. 2013, 63, e244–e249. [Google Scholar] [CrossRef]
- Park, S.; Kang, J.E.; Choi, H.J.; Kim, C.J.; Chung, E.K.; Kim, S.A.; Rhie, S.J. Antimicrobial Stewardship Programs in Community Health Systems Perceived by Physicians and Pharmacists: A Qualitative Study with Gap Analysis. Antibiotics 2019, 8, 252. [Google Scholar] [CrossRef]
- Waters, C.D. Pharmacist-driven antimicrobial stewardship program in an institution without infectious diseases physician support. Am. J. Health Syst. Pharm. 2015, 72, 466–468. [Google Scholar] [CrossRef]
- Thornley, T.; Ashiru-Oredope, D.; Beech, E.; Howard, P.; Kirkdale, C.L.; Elliott, H.; Harris, C.; Roberts, A. Antimicrobial use in UK long-term care facilities: Results of a point prevalence survey. J. Antimicrob. Chemother. 2019, 74, 2083–2090. [Google Scholar] [CrossRef]
- Thornley, T.; Ashiru-Oredope, D.; Normington, A.; Beech, E.; Howard, P. Antibiotic prescribing for residents in long-term-care facilities across the UK. J. Antimicrob. Chemother. 2019, 74, 1447–1451. [Google Scholar] [CrossRef]
- Takito, S.; Kusama, Y.; Fukuda, H.; Kutsuna, S. Pharmacist-supported antimicrobial stewardship in a retirement home. J. Infect. Chemother. 2020, 26, 858–861. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. beta-Lactams and beta-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed]
- Dolk, F.; Pouwels, K.B.; Smith, D.; Robotham, J.V.; Smieszek, T. Antibiotics in primary care in England: Which antibiotics are prescribed and for which conditions? J. Antimicrob. Chemother. 2018, 73, ii2–ii10. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.A., Jr.; Trubiano, J.; Coleman, D.T.; Rukasin, C.R.F.; Phillips, E.J. The challenge of de-labeling penicillin allergy. Allergy 2020, 75, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Sacco, K.A.; Bates, A.; Brigham, T.J.; Imam, J.S.; Burton, M.C. Clinical outcomes following inpatient penicillin allergy testing: A systematic review and meta-analysis. Allergy 2017, 72, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Devchand, M.; Kirkpatrick, C.M.J.; Stevenson, W.; Garrett, K.; Perera, D.; Khumra, S.; Urbancic, K.; Grayson, M.L.; Trubiano, J.A. Evaluation of a pharmacist-led penicillin allergy de-labelling ward round: A novel antimicrobial stewardship intervention. J. Antimicrob. Chemother. 2019, 74, 1725–1730. [Google Scholar] [CrossRef]
- Cheon, E.; Horowitz, H.W. New Avenues for Antimicrobial Stewardship: The Case for Penicillin Skin Testing by Pharmacists. Clin. Infect. Dis. 2019, 68, 2123–2124. [Google Scholar] [CrossRef]
- Gugkaeva, Z.; Crago, J.S.; Yasnogorodsky, M. Next step in antibiotic stewardship: Pharmacist-provided penicillin allergy testing. J. Clin. Pharm. Ther. 2017, 42, 509–512. [Google Scholar] [CrossRef]
- Park, M.A.; McClimon, B.J.; Ferguson, B.; Markus, P.J.; Odell, L.; Swanson, A.; Kloos-Olson, K.E.; Bjerke, P.F.; Li, J.T. Collaboration between allergists and pharmacists increases beta-lactam antibiotic prescriptions in patients with a history of penicillin allergy. Int. Arch. Allergy Immunol. 2011, 154, 57–62. [Google Scholar] [CrossRef]
- Macy, E.; Shu, Y.H. The Effect of Penicillin Allergy Testing on Future Health Care Utilization: A Matched Cohort Study. J. Allergy Clin. Immunol. Pract. 2017, 5, 705–710. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Li, Y.; Banerji, A.; Yun, B.J.; Long, A.A.; Walensky, R.P. The Cost of Penicillin Allergy Evaluation. J. Allergy Clin. Immunol. Pract. 2018, 6, 1019–1027. [Google Scholar] [CrossRef]
- McDowell, A.; Beard, R.; Brightmore, A.; Lu, L.W.; McKay, A.; Mistry, M.; Owen, K.; Swan, E.; Young, J. Veterinary Pharmaceutics: An Opportunity for Interprofessional Education in New Zealand? Pharmaceutics 2017, 9, 25. [Google Scholar] [CrossRef]
- Fredrickson, M.E.; Terlizzi, H.; Horne, R.L.; Dannemiller, S. The role of the community pharmacist in veterinary patient care: A cross-sectional study of pharmacist and veterinarian viewpoints. Pharm. Pract. 2020, 18, 1928. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial Resistance in Veterinary Medicine: An Overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [PubMed]
- Omwenga, I.; Aboge, G.O.; Mitema, E.S.; Obiero, G.; Ngaywa, C.; Ngwili, N.; Wamwere, G.; Wainaina, M.; Bett, B. Antimicrobial Usage and Detection of Multidrug-Resistant Staphylococcus aureus, Including Methicillin-Resistant Strains in Raw Milk of Livestock from Northern Kenya. Microb. Drug Resist. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lunha, K.; Leangapichart, T.; Jiwakanon, J.; Angkititrakul, S.; Sunde, M.; Jarhult, J.D.; Strom Hallenberg, G.; Hickman, R.A.; Van Boeckel, T.; Magnusson, U. Antimicrobial Resistance in Fecal Escherichia coli from Humans and Pigs at Farms at Different Levels of Intensification. Antibiotics 2020, 9, 662. [Google Scholar] [CrossRef]
- Andrew Selaledi, L.; Mohammed Hassan, Z.; Manyelo, T.G.; Mabelebele, M. The Current Status of the Alternative Use to Antibiotics in Poultry Production: An African Perspective. Antibiotics 2020, 9, 594. [Google Scholar] [CrossRef]
- Phares, C.A.; Danquah, A.; Atiah, K.; Agyei, F.K.; Michael, O.T. Antibiotics utilization and farmers’ knowledge of its effects on soil ecosystem in the coastal drylands of Ghana. PLoS ONE 2020, 15, e0228777. [Google Scholar] [CrossRef]
- Al-Mustapha, A.I.; Adetunji, V.O.; Heikinheimo, A. Risk Perceptions of Antibiotic Usage and Resistance: A Cross-Sectional Survey of Poultry Farmers in Kwara State, Nigeria. Antibiotics 2020, 9, 378. [Google Scholar] [CrossRef]
- Bergspica, I.; Kaprou, G.; Alexa, E.A.; Prieto, M.; Alvarez-Ordonez, A. Extended Spectrum beta-Lactamase (ESBL) Producing Escherichia coli in Pigs and Pork Meat in the European Union. Antibiotics 2020, 9, 678. [Google Scholar] [CrossRef]
- Pozza, G.; Pinto, A.; Crovato, S.; Mascarello, G.; Bano, L.; Dacasto, M.; Battisti, A.; Bartoli, B.; Ravarotto, L.; Marangon, S. Antimicrobial use and antimicrobial resistance: Standpoint and prescribing behaviour of Italian cattle and pig veterinarians. Ital. J. Anim. Sci. 2020, 19, 905–916. [Google Scholar] [CrossRef]
- Sommanustweechai, A.; Chanvatik, S.; Sermsinsiri, V.; Sivilaikul, S.; Patcharanarumol, W.; Yeung, S.; Tangcharoensathien, V. Antibiotic distribution channels in Thailand: Results of key-informant interviews, reviews of drug regulations and database searches. Bull. World Health Organ. 2018, 96, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.; Ebata, A.; MacGregor, H. Interventions to Reduce Antibiotic Prescribing in LMICs: A Scoping Review of Evidence from Human and Animal Health Systems. Antibiotics 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Afakye, K.; Kiambi, S.; Koka, E.; Kabali, E.; Dorado-Garcia, A.; Amoah, A.; Kimani, T.; Adjei, B.; Caudell, M.A. The Impacts of Animal Health Service Providers on Antimicrobial Use Attitudes and Practices: An Examination of Poultry Layer Farmers in Ghana and Kenya. Antibiotics 2020, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Anthony, F.; Acar, J.; Franklin, A.; Gupta, R.; Nicholls, T.; Tamura, Y.; Thompson, S.; Threlfall, E.J.; Vose, D.; van Vuuren, M.; et al. Antimicrobial resistance: Responsible and prudent use of antimicrobial agents in veterinary medicine. Rev. Sci. Tech. 2001, 20, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Nye, C.; Watson, T.; Kubasiewicz, L.; Raw, Z.; Burden, F. No Prescription, No Problem! A Mixed-Methods Study of Antimicrobial Stewardship Relating to Working Equines in Drug Retail Outlets of Northern India. Antibiotics 2020, 9, 295. [Google Scholar] [CrossRef]
- Martino, G.; Crovato, S.; Pinto, A.; Dorotea, T.; Mascarello, G.; Brunetta, R.; Fabrizio Agnoletti, F.; Bonfanti, L. Farmers’ attitudes towards antimicrobial use and awareness of antimicrobial resistance: A comparative study among turkey and rabbit farmers. Ital. J. Anim. Sci. 2019, 18, 194–201. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusic, D.; Bukić, J.; Seselja Perisin, A.; Leskur, D.; Modun, D.; Petric, A.; Vilovic, M.; Bozic, J. Are We Making the Most of Community Pharmacies? Implementation of Antimicrobial Stewardship Measures in Community Pharmacies: A Narrative Review. Antibiotics 2021, 10, 63. https://doi.org/10.3390/antibiotics10010063
Rusic D, Bukić J, Seselja Perisin A, Leskur D, Modun D, Petric A, Vilovic M, Bozic J. Are We Making the Most of Community Pharmacies? Implementation of Antimicrobial Stewardship Measures in Community Pharmacies: A Narrative Review. Antibiotics. 2021; 10(1):63. https://doi.org/10.3390/antibiotics10010063
Chicago/Turabian StyleRusic, Doris, Josipa Bukić, Ana Seselja Perisin, Dario Leskur, Darko Modun, Ana Petric, Marino Vilovic, and Josko Bozic. 2021. "Are We Making the Most of Community Pharmacies? Implementation of Antimicrobial Stewardship Measures in Community Pharmacies: A Narrative Review" Antibiotics 10, no. 1: 63. https://doi.org/10.3390/antibiotics10010063
APA StyleRusic, D., Bukić, J., Seselja Perisin, A., Leskur, D., Modun, D., Petric, A., Vilovic, M., & Bozic, J. (2021). Are We Making the Most of Community Pharmacies? Implementation of Antimicrobial Stewardship Measures in Community Pharmacies: A Narrative Review. Antibiotics, 10(1), 63. https://doi.org/10.3390/antibiotics10010063









