Real-World Use of Generic Meropenem: Results of an Observational Study
Abstract
1. Introduction
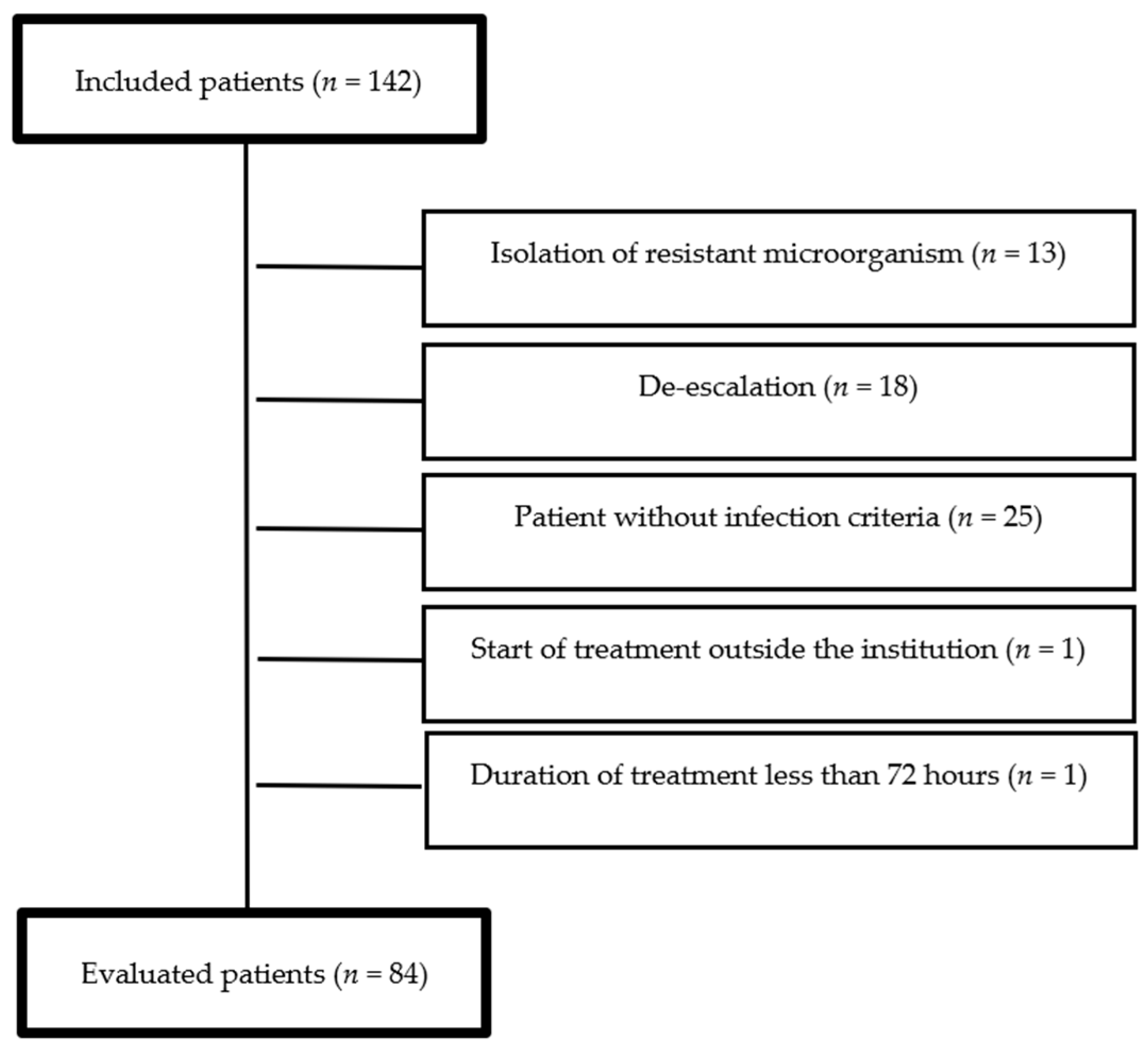
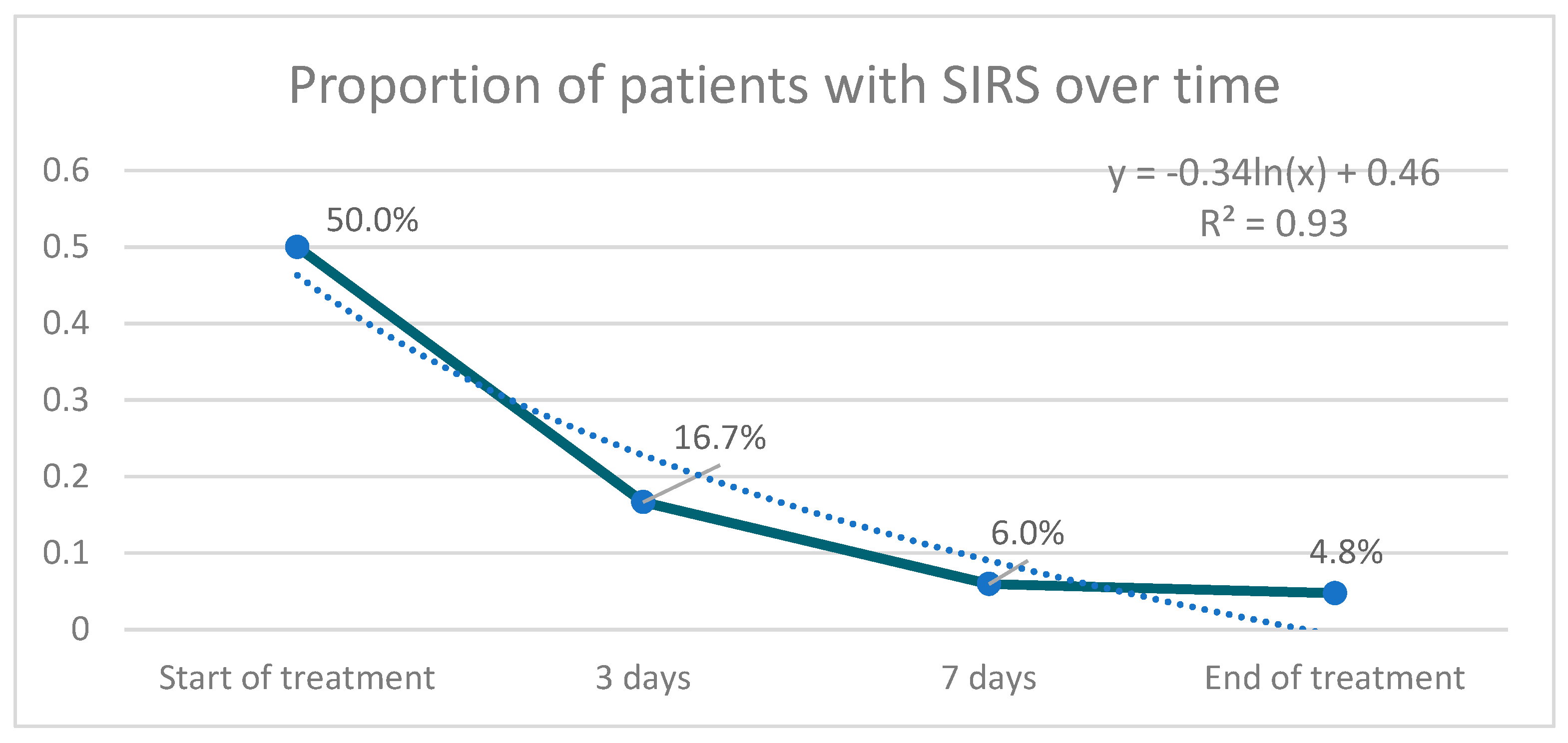
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministerio de Salud y Protección Social, Dirección de Medicamentos y Tecnologías en Salud. Plan Nacional de Respuesta a la Resistencia a Antimicrobianos—Plan Estratégico. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/MET/plan-respuesta-resistencia-antimicrobianos.pdf (accessed on 5 August 2018).
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Mohr, J.F., III. Update on the efficacy and tolerability of meropenem in the treatment of serious bacterial infections. Clin. Infect. Dis. 2008, 47 (Suppl. 1), S41–S51. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.M.; Lyseng-Williamson, K.A.; Keam, S.J. Meropenem: A review of its use in the treatment of serious bacterial infections. Drugs 2008, 68, 803–838. [Google Scholar] [CrossRef] [PubMed]
- Linden, P. Safety profile of meropenem: An updated review of over 6,000 patients treated with meropenem. Drug Saf. 2007, 30, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-World Evidence—What Is It and What Can It Tell Us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Blonde, L.; Khunti, K.; Harris, S.B.; Meizinger, C.; Skolnik, N.S. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv. Ther. 2018, 35, 1763–1774. [Google Scholar] [CrossRef]
- Tattevin, P.; Crémieux, A.C.; Rabaud, C.; Gauzit, R. Efficacy and quality of antibacterial generic products approved for human use: A systematic review. Clin. Infect. Dis. 2014, 58, 458–469. [Google Scholar] [CrossRef]
- Tattevin, P.; Saleh-Mghir, A.; Davido, B.; Ghout, I.; Massias, L.; Garcia de la Maria, C.; Miro, J.M.; Perronne, C.; Laurent, F.; Cremieux, A.C. Comparison of six generic vancomycin products for treatment of methicillin-resistant Staphylococcus aureus experimental endocarditis in rabbits. Antimicrob. Agents Chemother. 2013, 57, 1157–1162. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Jan, I.-S.; Cheng, S.-H. Comparative analysis of the cost and effectiveness of generic and brand-name antibiotics: The case of uncomplicated urinary tract infection. Pharmacoepidemiol. Drug Saf. 2017, 26, 301–309. [Google Scholar] [CrossRef]
- Machado-Alba, J.E.; Gaviria-Mendoza, A.; Machado-Duque, M.E. Results of the effectiveness of two piperacillin-tazobactam molecules in the real world. Int. J. Infect. Dis. 2018, 76, 91–96. [Google Scholar] [CrossRef]
- Tansuphasawadikul, S.; Simaroj, S.; Chantarothorn, S.; Nuntachit, N.; Jutivorakool, K.; Munsakul, W.; Yomtem, K.; Tangkosakul, T.; Wannasunthornchai, S. Therapeutic effectiveness of a generic versus original meropenem in serious infections. J. Med. Assoc. Thai. 2011, 94, 172–178. [Google Scholar] [PubMed]
- Leelarasamee, A.; Rongrungruang, Y.; Trakulsomboon, S.; Pongpech, P.; Thanawattanawanich, P.; Jithavech, P. Bioequivalence, antibacterial activity and therapeutic outcome of a generic meropenem (Mapenem). J. Med. Assoc. Thai. 2008, 91, 980–988. [Google Scholar] [PubMed]
- Mer, M.; Snyman, J.R.; van Rensburg, C.E.; van Tonder, J.J.; Laurens, I. A prospective, observational study comparing the PK/PD relationships of generic Meropenem (Mercide®) to the innovator brand in critically ill patients. Clin. Pharmacol. 2016, 8, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Angkasekwinai, N.; Werarak, P.; Tongsai, S.; Thamlikitkul, V. Effectiveness and safety of generic formulation of meropenem (Penem) for treatment of infections at Siriraj Hospital. J. Med. Assoc. Thai. 2012, 95 (Suppl. 2), S34–S41. [Google Scholar]
- Serafim, R.; Gomes, J.; Salluh, J.; Póvoa, P. A comparison of the quick SOFA and systemic inflammatory response syndrome criteria for the diagnosis of sepsis and prediction mortality. A systematic review and meta-analysis. Chest 2018, 153, 646–655. [Google Scholar] [CrossRef]
- Luna, C.M.; Palma, I.; Niederman, M.S.; Membriani, E.; Giovini, V.; Wiemken, T.; Peyrani, P.; Ramirez, J.A. The Impact of Age and Comorbidities on the Mortality of Patients of Different Age Groups Admitted with Community-acquired Pneumonia. Ann. Am. Thorac. Soc. 2016, 13, 1519–1526. [Google Scholar] [CrossRef]
- Nair, G.B.; Niederman, M.S. Nosocomial pneumonia: Lessons learned. Crit. Care Clin. 2013, 29, 521–546. [Google Scholar] [CrossRef]
- Loukides, S.; Polyzogopoulos, D. The effect of diabetes mellitus on the outcome of patients with chronic obstructive pulmonary disease exacerbated due to respiratory infections. Respiration 1996, 63, 170–173. [Google Scholar] [CrossRef]
- Nitzan, O.; Elias, M.; Chazan, B.; Saliba, W. Urinary tract infections in patients with type 2 diabetes mellitus: Review of prevalence, diagnosis, and management. Diabetes Metab. Syndr. Obes. 2015, 8, 129–136. [Google Scholar]
- Stapleton, A. Urinary tract infections in patients with diabetes. Am. J. Med. 2002, 113 (Suppl. 1A), 80S–84S. [Google Scholar] [CrossRef]
- Wright, S.W.; Wrenn, K.D.; Haynes, M.; Haas, D.W. Prevalence and risk factors for multidrug resistant uropathogens in ED patients. Am. J. Emerg. Med. 2000, 18, 143–146. [Google Scholar] [CrossRef]
- Thomsen, R.W.; Hundborg, H.H.; Lervang, H.-H.; Johnsen, S.P.; Schønheyder, H.C.; Sørensen, H.T. Diabetes mellitus as a risk and prognostic factor for community-acquired bacteremia due to enterobacteria: A 10-year, population-based study among adults. Clin. Infect. Dis. 2005, 40, 628–631. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Livermore, D.M. beta-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 1995, 8, 557–584. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Phillips, C.; Vanstone, J.R. Educational intervention to reduce treatment of asymptomatic bacteriuria in long-term care. BMJ Open Qual. 2018, 7, e000483. [Google Scholar] [CrossRef]
- Thalhammer, F.; Horl, W.H. Pharmacokinetics of meropenem in patients with renal failure and patients receiving renal replacement therapy. Clin. Pharmacokinet. 2000, 39, 271–279. [Google Scholar] [CrossRef]
- Ramon Azanza, J.; Garcia, E.; Sadaba, B.; Manubens, A. Antibiotic use in patients with renal or hepatic failure. Enferm. Infecc. Microbiol. Clin. 2009, 27, 593–599. [Google Scholar]
- Kula, B.; Djordjevic, G.; Robinson, J.L. A systematic review: Can one prescribe carbapenems to patients with IgE-mediated allergy to penicillins or cephalosporins? Clin. Infect. Dis. 2014, 59, 1113–1122. [Google Scholar] [CrossRef]
- Vesga, O.; Agudelo, M.; Salazar, B.E.; Rodriguez, C.A.; Zuluaga, A.F. Generic vancomycin products fail in vivo despite being pharmaceutical equivalents of the innovator. Antimicrob. Agents Chemother. 2010, 54, 3271–3279. [Google Scholar] [CrossRef]
- Cisneros-Herreros, J.M.; Cobo-Reinoso, J.; Pujol-Rojo, M.; Rodríguez-Baño, J.; Salavert-Lletí, M. Guía para el diagnóstico y tratamiento del paciente con bacteriemia. Guías de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC). Enferm. Infecc. Microbiol. Clín. 2007, 25, 111–130. [Google Scholar] [CrossRef]
- Solomkin, J.S.; Mazuski, J.; Bradley, J.S.; Rodvold, K.A.; Goldstein, E.J.C.; Baron, E.J.; O’Neill, P.J.; Chow, A.W.; Dellinger, E.P.; Eachempati, S.R.; et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 133–164. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.G.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef]
- Martinez, E.; Osorio, J.; Delgado, J.; Esparza, G.; Motoa, G.; Blanco, V.; Hernández, C.; Agudelo, A.; Aluma, L.; Betancurt, C.; et al. Infecciones del tracto urinario bajo en adultos y embarazadas: Consenso para el manejo empírico. Infectio 2013, 17, 122–135. [Google Scholar] [CrossRef]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clinl Infectl. Dis. 2019, 68, e83–e110. [Google Scholar]
- Asociación Colombiana de Neumología y Cirugía de Tórax (ACNCT); Asociación Colombiana de Medicina Crítica y Cuidado Intensivo (AMCI); Asociación Colombiana de Medicina Interna (ACMI); Asociación Colombiana de Infectología (ACIN). Recomendaciones para el diagnóstico, tratamiento y prevención de la neumonía adquirida en la comunidad en adultos inmunocompetentes. Infectio 2013, 17, S1–S38. [Google Scholar] [CrossRef][Green Version]
- Alí Munive, A.; Ortiz Ruiz, G.; Dueñas Castell, C. Consenso colombiano de neumonía nosocomial 2013. Infectio 2013, 17, 6–18. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Balk, R.A. Systemic inflammatory response syndrome (SIRS): Where did it come from and is it still relevant today? Virulence 2014, 5, 20–26. [Google Scholar] [CrossRef]
- The Importance of Pharmacovigilance—Safety Monitoring of Medicinal Products. Available online: https://apps.who.int/iris/handle/10665/42493 (accessed on 5 November 2020).
- Drug Interaction Checker. Available online: https://www.drugs.com/drug_interactions.html (accessed on 10 October 2015).
| Variable | n | % |
|---|---|---|
| Age | ||
| 18–65 | 39 | 46.4 |
| ≥65 | 45 | 53.6 |
| Sex | ||
| Female | 38 | 45.2 |
| Male | 46 | 54.8 |
| Comorbidities | ||
| Hypertension | 75 | 89.2 |
| Diabetes mellitus | 42 | 50.0 |
| Chronic obstructive pulmonary disease | 28 | 33.3 |
| Renal disease | 28 | 33.3 |
| Cancer | 19 | 22.6 |
| Malnutrition | 19 | 22.6 |
| HIV infection | 4 | 4.7 |
| Indication | ||
| Urinary tract infection | 21 | 25.0 |
| Pneumonia | 17 | 20.2 |
| Skin and soft tissue infection | 13 | 15.5 |
| Intra-abdominal infection | 10 | 11.9 |
| Operative site infection | 6 | 7.1 |
| Primary bacteremia | 5 | 6.0 |
| Another type of infection * | 12 | 14.3 |
| Etiological agent | ||
| Pseudomonas aeruginosa | 18 | 32.1 |
| Escherichia coli | 16 | 28.6 |
| Klebsiella pneumoniae | 10 | 17.9 |
| Another etiological agent ** | 12 | 21.4 |
| Variable | n | % |
|---|---|---|
| Dose | ||
| 1 g every 8 h | 72 | 85.7 |
| 1 g every 12 h | 5 | 6.0 |
| 2 g every 8 h | 3 | 3.6 |
| 0.5 g every 12 h | 2 | 2.4 |
| 1 g every 24 h | 2 | 2.4 |
| Duration of treatment | ||
| 4–6 days | 10 | 11.9 |
| 7 days | 23 | 27.4 |
| 8–13 days | 32 | 38.1 |
| 14 days | 14 | 16.7 |
| >14 days | 5 | 5.9 |
| Prescriber specialty | ||
| Internal Medicine | 45 | 53.6 |
| Infectology | 9 | 10.7 |
| Urology | 8 | 9.5 |
| Intensive care | 8 | 9.5 |
| Others | 14 | 16.8 |
| Variable | n (%) | n (%) | p Value |
|---|---|---|---|
| Dysthermia | 29 (34.5) | 0 (0.0) | 0.000 |
| Tachycardia | 47 (56.0) | 13 (15.5) | 0.050 |
| Tachypnea | 27 (32.1) | 7 (8.3) | 0.832 |
| Leukocytosis | 39 (46.4) | 15 (17.9) | 0.008 |
| Response Type | n | % |
|---|---|---|
| Complete response (clinical + microbiological) | 20 | 23.8 |
| Clinical response | 62 | 73.8 |
| Microbiological response | 1 | 1.2 |
| Total responding patients | 83 | 98.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garnica-Velandia, S.; Aristizábal-Ruiz, L.A.; Alvarez-Moreno, C.A. Real-World Use of Generic Meropenem: Results of an Observational Study. Antibiotics 2021, 10, 62. https://doi.org/10.3390/antibiotics10010062
Garnica-Velandia S, Aristizábal-Ruiz LA, Alvarez-Moreno CA. Real-World Use of Generic Meropenem: Results of an Observational Study. Antibiotics. 2021; 10(1):62. https://doi.org/10.3390/antibiotics10010062
Chicago/Turabian StyleGarnica-Velandia, Santiago, Luz Adriana Aristizábal-Ruiz, and Carlos Arturo Alvarez-Moreno. 2021. "Real-World Use of Generic Meropenem: Results of an Observational Study" Antibiotics 10, no. 1: 62. https://doi.org/10.3390/antibiotics10010062
APA StyleGarnica-Velandia, S., Aristizábal-Ruiz, L. A., & Alvarez-Moreno, C. A. (2021). Real-World Use of Generic Meropenem: Results of an Observational Study. Antibiotics, 10(1), 62. https://doi.org/10.3390/antibiotics10010062





