Effect of a Berry Polyphenolic Fraction on Biofilm Formation, Adherence Properties and Gene Expression of Streptococcus mutans and Its Biocompatibility with Oral Epithelial Cells
Abstract
:1. Introduction
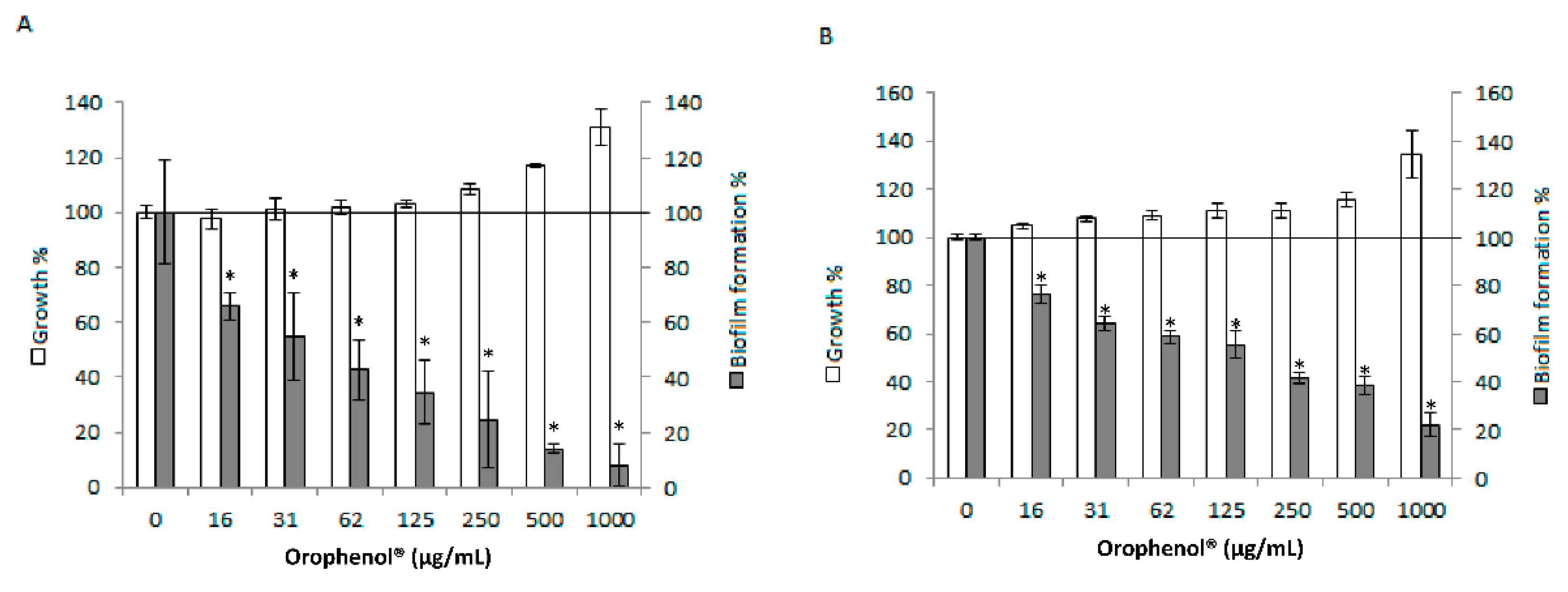
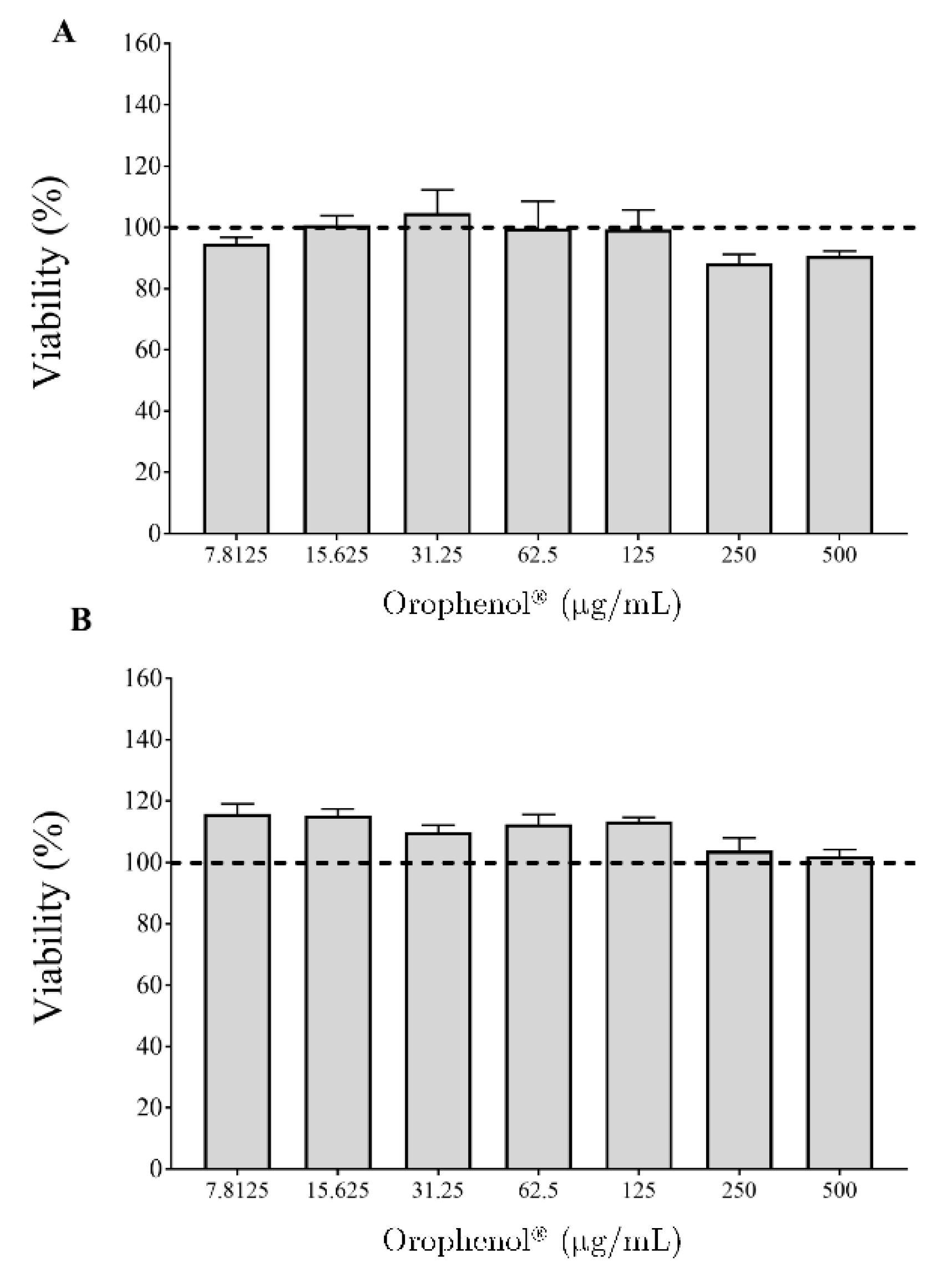
2. Results
3. Discussion
4. Materials and Methods
4.1. Berry Polyphenolic Fraction (Orophenol®) and Phenolic Characterization
4.2. Bacteria and Growth Conditions
4.3. Antibacterial Assay
4.4. Biofilm Formation Assay
4.5. Adherence Assays
4.5.1. Saliva Collection
4.5.2. Fluorescent Labeling of Bacteria
4.5.3. Adherence to Saliva-Coated Hydroxyapatite
4.5.4. Adherence to Saliva-Coated Nickel–Chrome Alloy
4.6. qRT-PCR Analysis
4.7. In Vitro Biocompatibility Assay with Oral Epithelial Cells
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cummins, D. Dental caries: A disease which remains a public health concern in the 21st century-the exploration of a breakthrough technology for caries prevention. J. Clin. Dent. 2013, 24, 1–14. [Google Scholar]
- Lamont, R.J.; Egland, P.G. Dental caries. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 945–955. [Google Scholar]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- Klein, M.I.; Hwang, G.; Campanella, O.H.; Santos, P.H.S.; Koo, H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front. Cell. Infect. Microbiol. 2015, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.; Xiao, J.; Klein, M.I. Extracellular polysaccharides matrix–an often forgotten virulence factor in oral biofilm research. Int. J. Oral Sci. 2009, 1, 229–234. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Chinsembu, K.C. Plants and natural products used in the management of oral infections and improvement of oral health. Acta Trop. 2016, 154, 6–18. [Google Scholar] [CrossRef]
- Feghali, K.; Feldman, M.; La, V.D.; Santos, J.; Grenier, D. Cranberry proanthocyanidins: Natural weapons against periodontal diseases. J. Agric. Food Chem. 2012, 60, 5728–5735. [Google Scholar] [CrossRef]
- USDA. USDA Database for the Flavonoid Content of Selected Foods (Release 3.0); U.S. Department of Agriculture, Agricultural Research Service Press: Beltsville, MD, USA, 2011.
- Bonifait, L.; Grenier, D. Cranberry polyphenols: Potential benefits for dental caries and periodontal disease. J. Can. Dent. Ass. 2011, 76, 130. [Google Scholar]
- Philip, N.; Walsh, L.J. Cranberry polyphenols: Natural weapons against dental caries. Dent. J. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Lagha, A.; Dudonné, S.; Desjardins, Y.; Grenier, D. Wild blueberry (Vaccinium angustifolium Ait.) polyphenols target Fusobacterium nucleatum and the host inflammatory response: Potential innovative molecules for treating periodontal diseases. J. Agric. Food Chem. 2015, 63, 6999–7008. [Google Scholar] [CrossRef]
- Duarte, S.; Gregoire, S.; Singh, A.P.; Vorsa, N.; Schaich, K.; Bowen, W.H.; Koo, H. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol. Lett. 2006, 257, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, A.; Kimizuka, R.; Kato, T.; Okuda, K. Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation. Oral Microbiol. Immunol. 2004, 19, 150–154. [Google Scholar] [CrossRef]
- Steinberg, D.; Feldman, M.; Ofek, I.; Weiss, E.I. Effect of a high-molecular-weight component of cranberry on constituents of dental biofilm. J. Antimicrob. Chemother. 2004, 54, 86–89. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bossier, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nature Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J. Communication among human oral plaque bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Tang, N.; Aspiras, M.B.; Lau, P.C.; Lee, J.H.; Ellen, R.P.; Cvitkovitch, D.G. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 2002, 184, 2699–2708. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, A.; Kuramitsu, H.K. Multiple Streptococcus mutans genes are involved in biofilm formation. Appl. Environ. Microbiol. 2002, 68, 2372–2380. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, A.; Ansai, T.; Takehara, T.; Kuramitsu, H.K. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl. Environ. Microbiol. 2005, 71, 2372–2380. [Google Scholar] [CrossRef] [Green Version]
- Merritt, J.; Qi, F.; Goodman, S.D.; Anderson, M.H.; Shi, W. Mutation of luxS affects biofilm formation in Streptococcus mutans. Infect. Immun. 2003, 71, 1972–1979. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.T.; Burne, R.A. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J. Bacteriol. 2004, 186, 2682–2691. [Google Scholar] [CrossRef] [Green Version]
- Sztajer, H.; Lemme, A.; Vilchez, R.; Schulz, S.; Geffers, R.; Yip, C.Y.Y.; Levesque, C.M.; Cvitkovitch, D.G.; Wagner-Döbler, I. Autoinducer-2-regulated genes in Streptococcus mutans UA159 and global metabolic effect on the luxS mutation. J. Bacteriol. 2008, 190, 401–415. [Google Scholar] [CrossRef] [Green Version]
- Philip, N.; Bandara, H.M.H.N.; Leishman, S.J.; Walsh, L.J. Inhibitory effects of fruit berry extracts on Streptococcus mutans biofilms. Eur. J. Oral Sci. 2019, 127, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Dudonne, S.; Dube, P.; Pilon, G.; Marette, A.; Jacques, H.; Weisnagel, J.; Desjardins, Y. Modulation of strawberry/cranberry phenolic compounds glucuronidation by co-supplementation with onion: Characterization of phenolic metabolites in rat plasma using an optimized muSPE-UHPLC-MS/MS method. J. Agric. Food Chem. 2014, 62, 3244–3256. [Google Scholar] [CrossRef]
- LeBel, G.; Haas, B.; Adam, A.A.; Veilleux, M.P.; Ben Lagha, A.; Grenier, D. Effect of cinnamon (Cinnamum verum) bark essential oil on the halitosis-associated bacterium Solobacterium moorei and in vitro cytotoxicity. Arch. Oral Biol. 2017, 83, 97–104. [Google Scholar] [CrossRef]
- Bedran, T.B.L.; Grignon, L.; Spolidorio, D.P.; Grenier, D. Subinhibitory concentrations of triclosan promote Streptococcus mutans biofilm formation and adherence to oral epithelial cells. PLoS ONE 2014, 9, e89059. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, M.; Millhouse, E.; Culshaw, S.; Edwards, C.A.; Ramage, G.; Comber, E. Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2015, 6, 719–729. [Google Scholar] [CrossRef] [Green Version]
- Groeger, S.; Michel, J.; Meyle, J. Establishment and characterization of immortalized human gingival keratinocyte cell lines. J. Periodontal Res. 2008, 43, 604–614. [Google Scholar] [CrossRef]


| Composition | Amount (mg/100 g Dry Weight) |
|---|---|
| PHENOLIC ACIDS | 10,707 |
| Caffeic acid | 611 |
| Chlorogenic acid | 1060 |
| Cinnamic acid | 551 |
| p-coumaric acid | 4517 |
| Cryptochlorogenic acid | 7 |
| Ferulic acid | 249 |
| Gallic acid | 117 |
| p-hydroxybenzoic acid | 948 |
| Isoferulic acid | 27 |
| Neochlorogenic | 64 |
| Protocatechuic acid | 1525 |
| Salicylic acid | 986 |
| Sinapic acid | 45 |
| FLAVONOIDS | 19,756 |
| Flavonols | 18,769 |
| Isorhamnetin | 902 |
| Kaempferol | 184 |
| Kaempferol glucoside | 693 |
| Myricetin | 145 |
| Myricetin glucoside | 486 |
| Phlorizin | 617 |
| Quercetin | 4917 |
| Quercetin galactoside | 3556 |
| Quercetin glucoside | 1627 |
| Quercetin rhamnoside | 3794 |
| Quercetin rutinoside | 1848 |
| Anthocyanins | 450 |
| Flavan-3-ols | 537 |
| Catechin | 393 |
| Epicatechin | 144 |
| PROCYANIDINS | 5286 |
| Monomers | 1530 |
| Dimers | 1721 |
| Trimers | 589 |
| Tetramers | 240 |
| Pentamers | 92 |
| Hexamers | 56 |
| Heptamers | 20 |
| Polymers (DP > 10) | 1038 |
| Genes | Primer Sequences | Product Size | |
|---|---|---|---|
| 16S rRNA | Sense | 5′ CCATGTGTAGCGGTGAAATGC 3′ | 144 |
| Antisense | 5′ TCATCGTTTACGGCGTGGAC 3′ | ||
| comD | Sense | 5′ TTCCTGCAAACTCGATCATATAGG 3′ | 113 |
| Antisense | 5′ TGCCAGTTCTGACTTGTTTAGGC 3′ | ||
| gtfC | Sense | 5′ TTCCGTCCCTTATTGATGACATG 3′ | 122 |
| Antisense | 5′ AATTGAAGCGGACTGGTTGCT 3′ | ||
| luxS | Sense | 5′ CCAGGGACATCTTTCCATGAGAT 3′ | 147 |
| Antisense | 5′ ACGGGATGATTGACTGTTCCC 3′ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souissi, M.; Ben Lagha, A.; Chaieb, K.; Grenier, D. Effect of a Berry Polyphenolic Fraction on Biofilm Formation, Adherence Properties and Gene Expression of Streptococcus mutans and Its Biocompatibility with Oral Epithelial Cells. Antibiotics 2021, 10, 46. https://doi.org/10.3390/antibiotics10010046
Souissi M, Ben Lagha A, Chaieb K, Grenier D. Effect of a Berry Polyphenolic Fraction on Biofilm Formation, Adherence Properties and Gene Expression of Streptococcus mutans and Its Biocompatibility with Oral Epithelial Cells. Antibiotics. 2021; 10(1):46. https://doi.org/10.3390/antibiotics10010046
Chicago/Turabian StyleSouissi, Mariem, Amel Ben Lagha, Kamel Chaieb, and Daniel Grenier. 2021. "Effect of a Berry Polyphenolic Fraction on Biofilm Formation, Adherence Properties and Gene Expression of Streptococcus mutans and Its Biocompatibility with Oral Epithelial Cells" Antibiotics 10, no. 1: 46. https://doi.org/10.3390/antibiotics10010046
APA StyleSouissi, M., Ben Lagha, A., Chaieb, K., & Grenier, D. (2021). Effect of a Berry Polyphenolic Fraction on Biofilm Formation, Adherence Properties and Gene Expression of Streptococcus mutans and Its Biocompatibility with Oral Epithelial Cells. Antibiotics, 10(1), 46. https://doi.org/10.3390/antibiotics10010046





