Solid-State rGO-PEDOT:PSS Transducing Material for Cost-Effective Enzymatic Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Material and Reagents
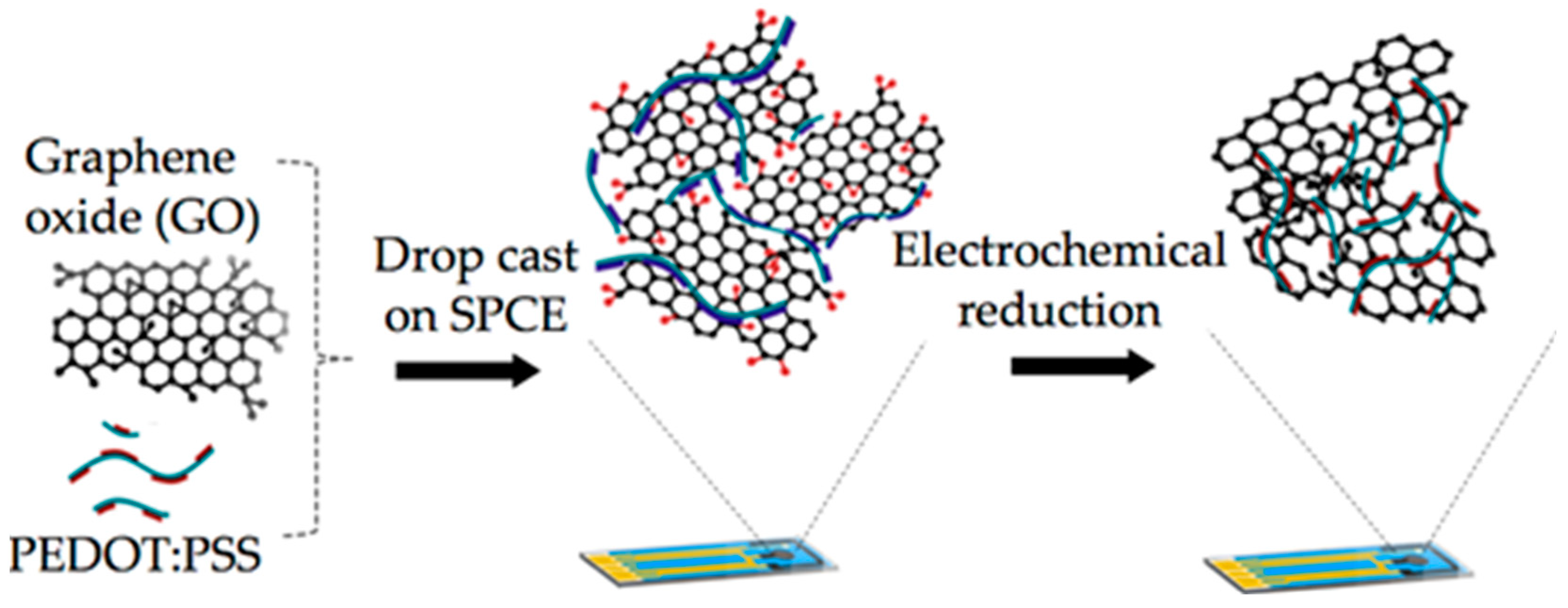
2.3. Preparation of rGO and rGO-PEDOT:PSS Electrodes
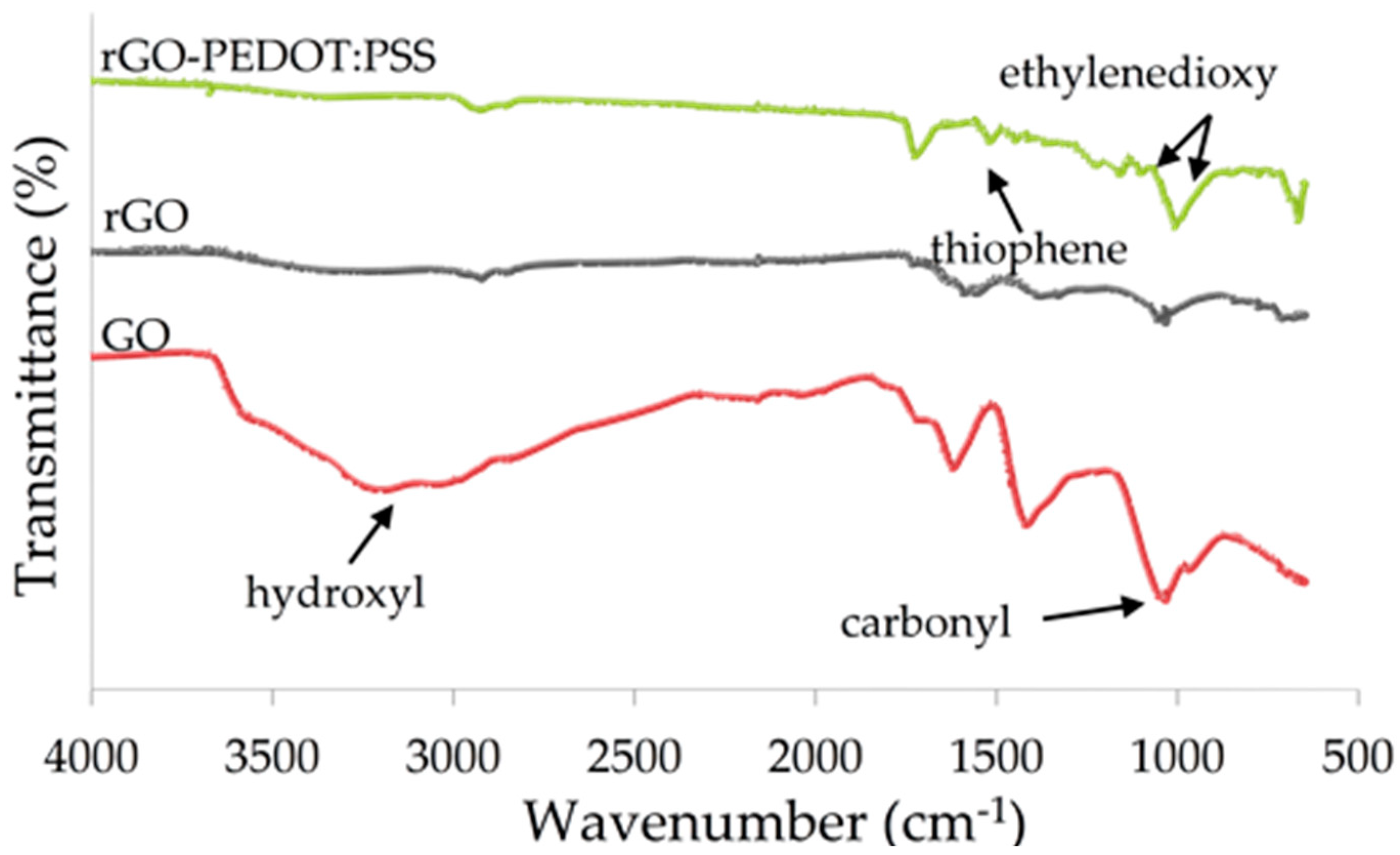
2.4. Surface Characterization
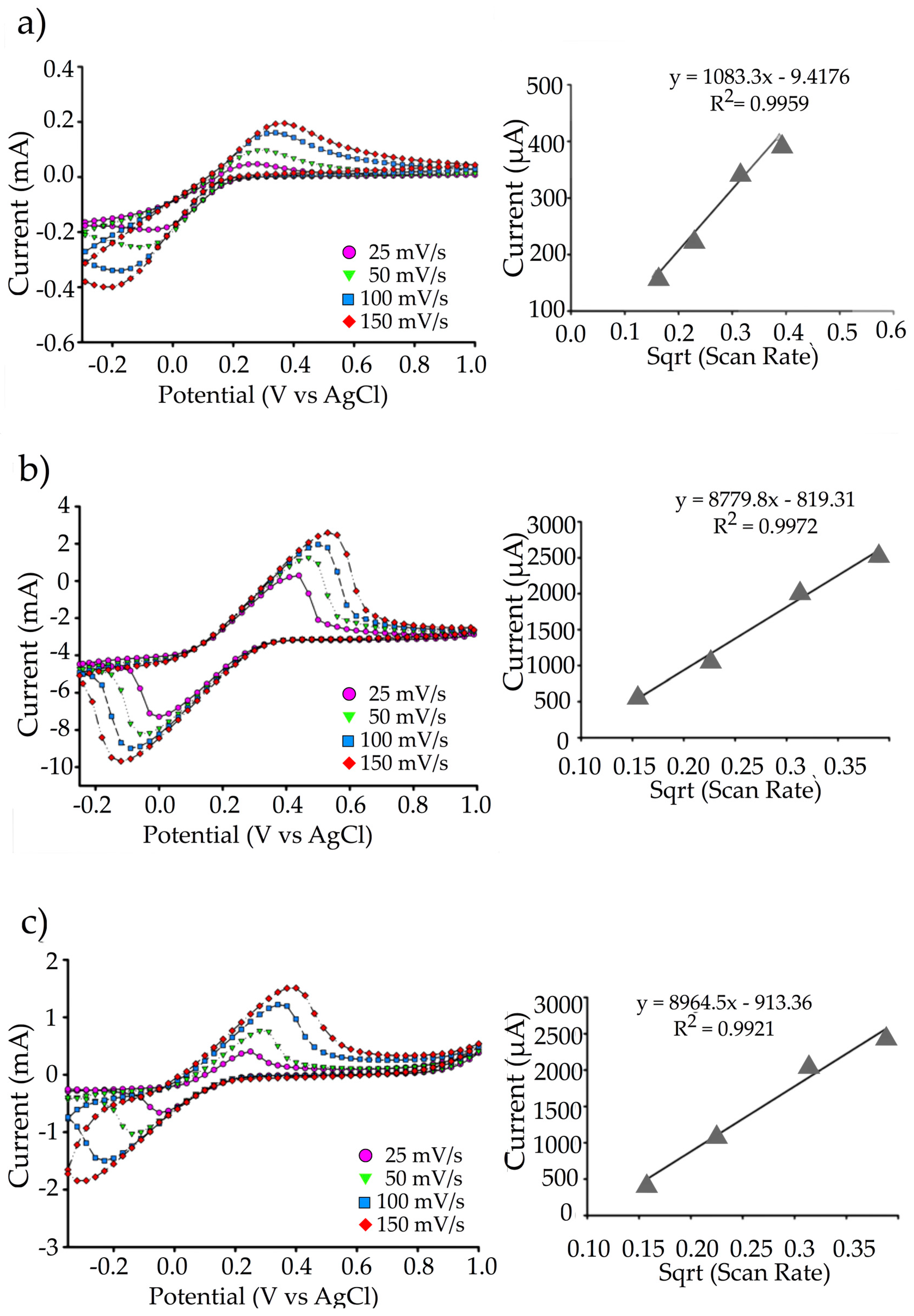
2.5. Cyclic Voltammetry of rGO/SPCE and rGO-PEDOT:PSS/SPCE
3. Results and Discussion
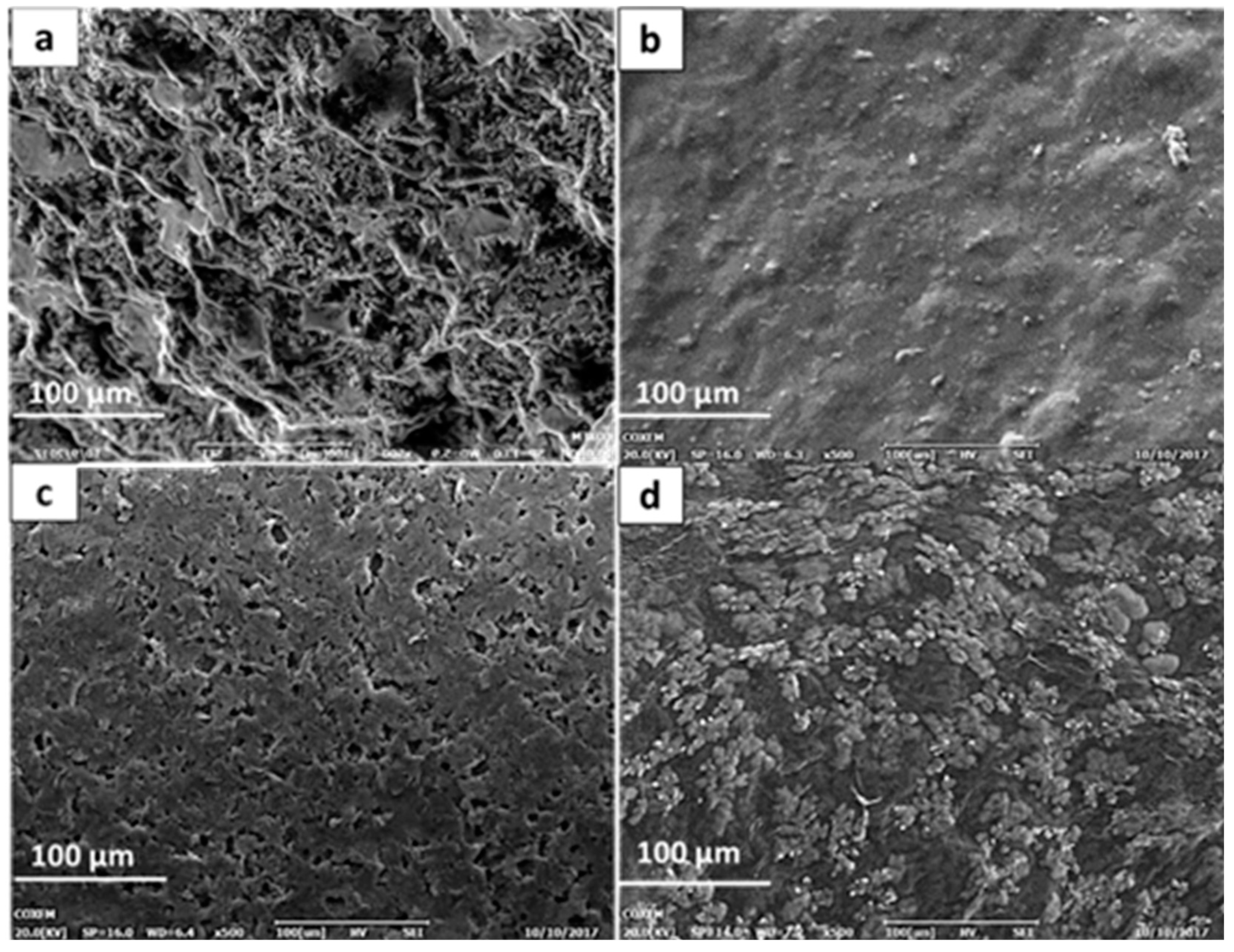
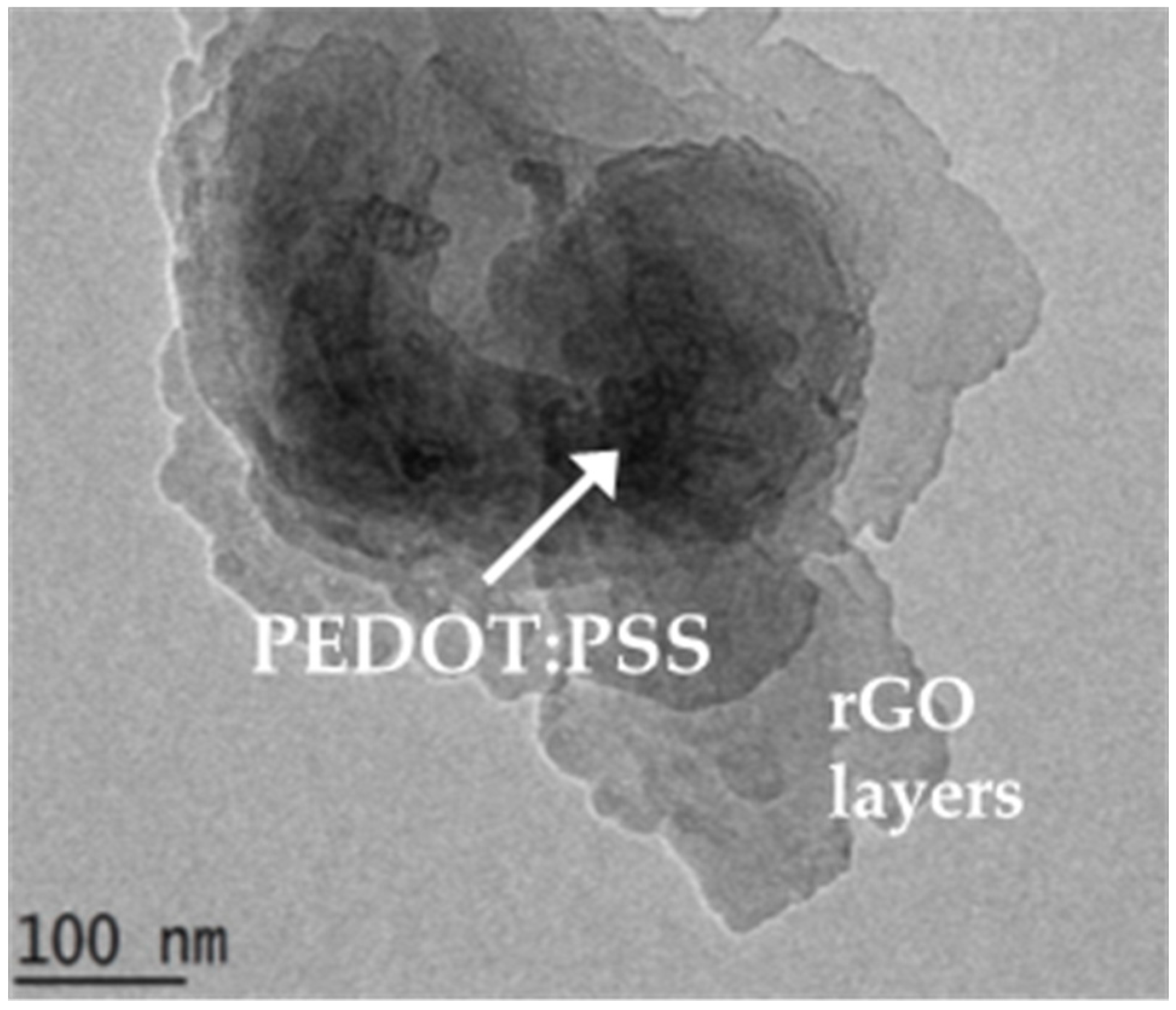
3.1. Surface Morphologies and Characterization
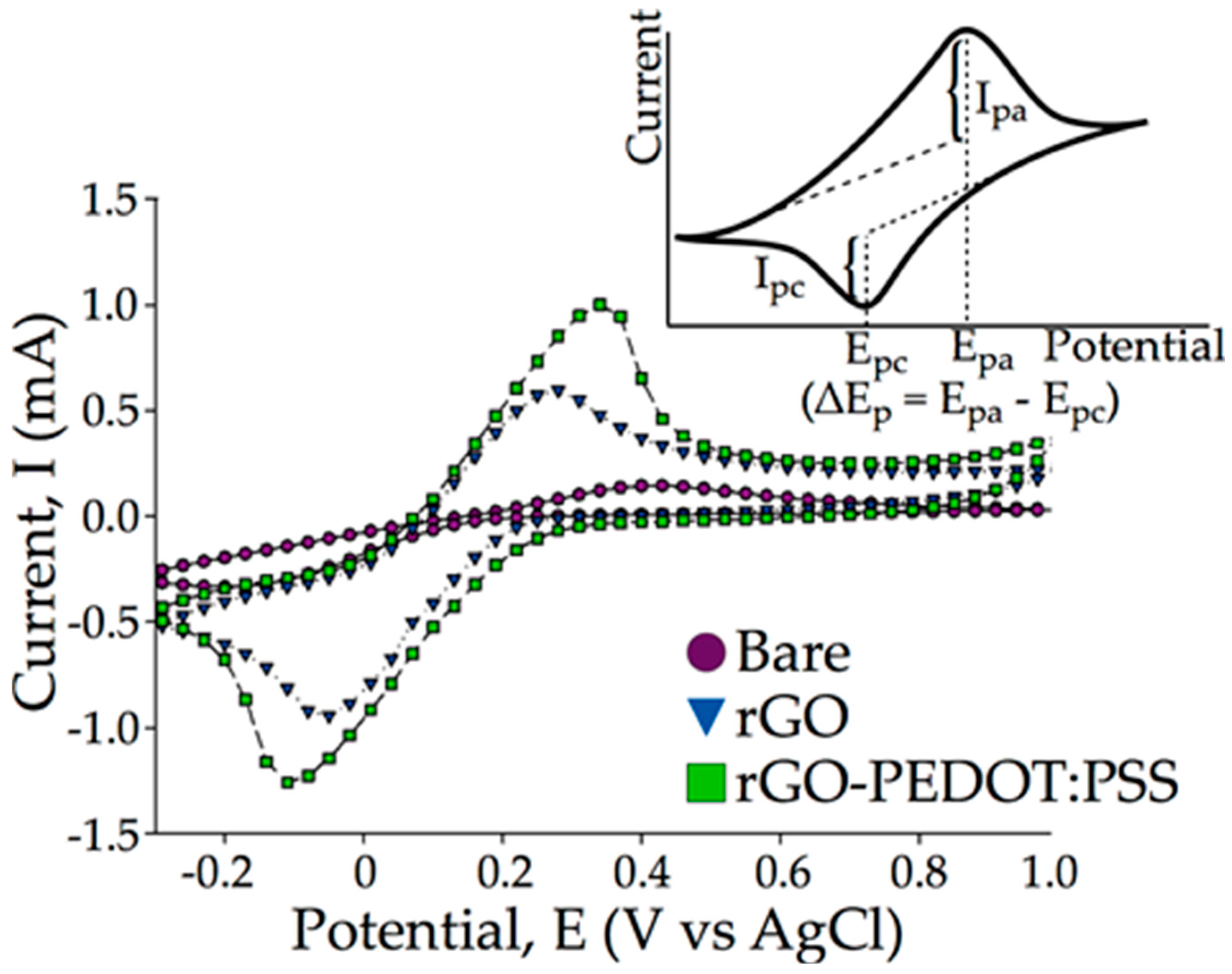
3.2. Cyclic Voltammetric Comparison between rGO and rGO-PEDOT:PSS
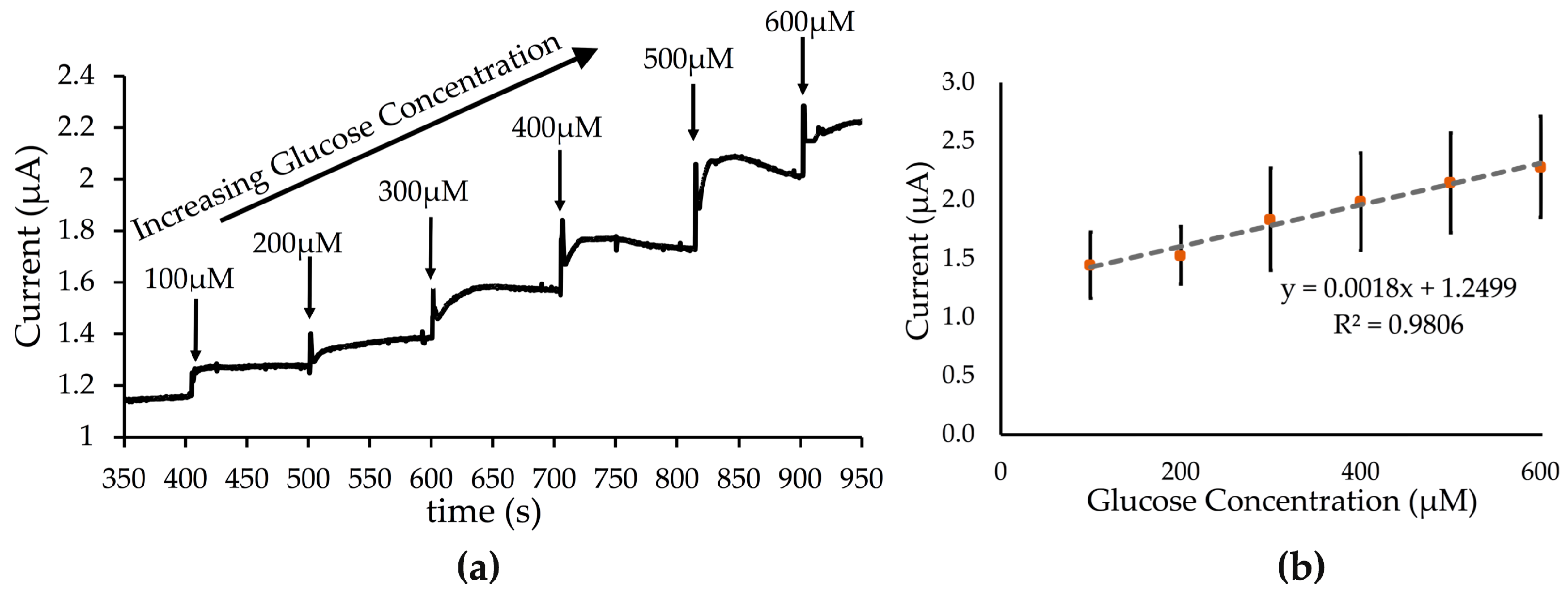
3.3. rGO-PEDOT:PSS as a Transducer in Enzymatic Sensing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hayat, A.; Marty, J.L. Disposable Screen Printed Electrochemical Sensors: Tools for Environmental Monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [PubMed]
- González-Sánchez, M.I.; Gómez-Monedero, B.; Agrisuelas, J.; Iniesta, J.; Valero, E. Highly Activated Screen-Printed Carbon Electrodes by Electrochemical Treatment with Hydrogen Peroxide. Electrochem. Commun. 2018, 91, 36–40. [Google Scholar] [CrossRef]
- Cui, G.; Yoo, J.H.; Lee, J.S.; Yoo, J.; Uhm, J.H.; Cha, G.S.; Nam, H. Effect of Pre-Treatment on the Surface and Electrochemical Properties of Screen-Printed Carbon Paste Electrodes. Analyst 2001, 126, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Guth, U.; Zosel, J.; Riedel, J.; Tran, T.N.; Berthold, M.; Vonau, C.; Sasum, U. New Developments in Electrode Materials for Electrochemical Sensors. In Advancement in Sensing Technology. Smart Sensors, Measurement and Instrumentation; Mukhopadhyay, S., Jayasundera, K., Fuchs, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 181–189. [Google Scholar]
- Pumera, M. Graphene in Biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- Sriprachuabwong, C.; Karuwan, C.; Wisitsorrat, A.; Phokharatkul, D.; Lomas, T.; Sritongkham, P.; Tuantranont, A. Inkjet-Printed Graphene-PEDOT:PSS Modified Screen Printed Carbon Electrode for Biochemical Sensing. J. Mater. Chem. 2012, 22, 5478–5485. [Google Scholar] [CrossRef]
- Jo, G.; Choe, M.; Lee, S.; Park, W.; Kahng, Y.; Lee, T. The Application of Graphene as Electrodes in Electrical And Optical Devices. Nanotechnology 2012, 23, 112001. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct Electrodeposition of Reduced Graphene Oxide on Glassy Carbon Electrode and its Electrochemical Application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Clark, L.C., Jr. The Enzyme Electrode. In Biosensors: Fundamentals and Applications; Turner, A., Karube, I., Wilson, G.S., Eds.; Oxford University Press: New York, NY, USA, 1987; Volume 1, Chapter 1; pp. 3–12. ISBN 0-19-854724-2. [Google Scholar]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Prusty, S. Review on Conducting Polymers and Their Applications. Polym. Plast. Technol. Eng. 2012, 51, 1487–1500. [Google Scholar] [CrossRef]
- Huang, X.; Guo, H.; Yang, J.; Wang, K.; Niu, X.; Liu, X. Moderately Reduced Graphene Oxide/PEDOT:PSS as Hole Transport Layer to Fabricate Efficient Perovskite Hybrid Solar Cells. Org. Electron. Phys. Mater. Appl. 2016, 39, 288–295. [Google Scholar] [CrossRef]
- Park, Y.; Soon Choi, K.; Young Kim, S. Graphene Oxide/PEDOT:PSS and Reduced Graphene Oxide/PEDOT:PSS Hole Extraction Layers in Organic Photovoltaic Cells. Phys. Status Solidi Appl. Mater. Sci. 2012, 209, 1363–1368. [Google Scholar] [CrossRef]
- Bobacka, J. Conducting Polymer-Based Solid-State Ion-Selective Electrodes. Electroanalysis 2006, 18, 7–18. [Google Scholar] [CrossRef]
- Groenendaal, B.L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and Its Derivatives-Past, Present, and Future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, J.H.; Lee, D.E.; Joo, J. Enhancement of Electrical Conductivity of Poly(3, 4-Ethylenedioxythiophene)/Poly(4-Styrenesulfonate) by a Change of Solvents. Synth. Met. 2002, 126, 311–316. [Google Scholar] [CrossRef]
- Gerhard, H.; Jonas, F. Poly (Alkylenedioxythiophene)S—New, Very Stable Conducting Polymers. J. Phys. Org. Chem. 1992, 4, 116–118. [Google Scholar]
- Pathak, C.S.; Singh, J.P.; Singh, R. Preparation of Novel Graphene-PEDOT:PSS Nanocomposite Films and Fabrication of Heterojunction Diodes with n-Si. Chem. Phys. Lett. 2018, 694, 75–81. [Google Scholar] [CrossRef]
- Kirchmeyer, S.; Reuter, K. Scientific Importance, Properties and Growing Applications of Poly(3,4-Ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077–2088. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, B.; Razal, J.M.; Xu, Q.; Zhao, C.; Hou, Y.; Seyedin, S.; Jalili, R.; Wallace, G.G.; Chen, J. High-Performance Flexible All-Solid-State Supercapacitor from Large Free-Standing Graphene-PEDOT/PSS Films. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Gao, S.; Wu, J.; Lin, J. High-performing Dye-Sensitized Solar Cells Based on Reduced Graphene Oxide/PEDOT-PSS Counter Electrodes with Sulfuric Acid Post-Treatment. J. Appl. Polym. Sci. 2015, 132, 1–5. [Google Scholar] [CrossRef]
- Yan, D.; Liu, Y.; Li, Y.; Zhuo, R.; Wu, Z.; Ren, P.; Li, S.; Wang, J.; Yan, P.; Geng, Z. Synthesis and Electrochemical Properties of MnO2/rGO/PEDOT:PSS Ternary Composite Electrode Material for Supercapacitors. Mater. Lett. 2014, 127, 53–55. [Google Scholar] [CrossRef]
- Islam, M.M.; Chidembo, A.T.; Aboutalebi, S.H.; Cardillo, D.; Liu, H.K.; Konstantinov, K.; Dou, S.X. Liquid Crystalline Graphene Oxide/PEDOT:PSS Self-Assembled 3D Architecture for Binder-Free Supercapacitor Electrodes. Front. Energy Res. 2014, 2, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Zhang, J. Interactions of Graphene and Graphene Oxide with Proteins and Peptides. Nanotechnol. Rev. 2013, 2, 27–45. [Google Scholar] [CrossRef]
- Pandit, S.; De, M. Interaction of Amino Acids and Graphene Oxide: Trends in Thermodynamic Properties. J. Phys. Chem. C 2017, 121, 600–608. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.K.; Son, Y. Glucose Biosensor Constructed from Capped Conducting Microtubules of PEDOT. Sens. Actuators B Chem. 2008, 133, 244–250. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, S.M.; Park, N.I.; Lee, S.; Chung, D. Preparation and Characterization of Conducting Polymer Nanocomposite with Partially Reduced Graphene Oxide. Synth. Met. 2015, 201, 61–66. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The Reduction of Graphene Oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Berendjchi, A.; Khajavi, R.; Yousefi, A.A.; Yazdanshenas, M.E. Improved Continuity of Reduced Graphene Oxide on Polyester Fabric by Use of Polypyrrole to Achieve a Highly Electro-Conductive and Flexible Substrate. Appl. Surf. Sci. 2016, 363, 264–272. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, J.; Zhu, K.; Li, J.; Wang, J.; Li, S.; Wang, X. Ultra High Permittivity and Significantly Enhanced Electric Field Induced Strain in PEDOT:PSS-RGO@PU Intelligent Shape-Changing Electro-Active Polymers. RSC Adv. 2014, 4, 64061–64067. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Seop Kim, H. Fast and Fully-Scalable Synthesis of Reduced Graphene Oxide. Sci. Rep. 2015, 5, 10160. [Google Scholar] [CrossRef] [PubMed]
- Obreja, A.C.; Iordanescu, S.; Gavrila, R.; Dinescu, A.; Comanescu, F.; Matei, A.; Danila, M.; Dragoman, M.; Iovu, H. Flexible Films Based on Graphene/Polymer Nanocomposite with Improved Electromagnetic Interference Shielding. In Proceedings of the 2015 International Semiconductor Conference (CAS), Sinaia, Romania, 12–14 October 2015; pp. 49–52. [Google Scholar]
- Seekaew, Y.; Lokavee, S.; Phokharatkul, D.; Wisitsoraat, A.; Kerdcharoen, T.; Wongchoosuk, C. Low-Cost and Flexible Printed Graphene–PEDOT:PSS Gas Sensor for Ammonia Detection. Org. Electron. 2014, 15, 2971–2981. [Google Scholar] [CrossRef]
- Nguyen Huynh, N.M.; Boeva, Z.A.; Smått, J.H.; Pesonen, M.; Lindfors, T. Reduced Graphene Oxide as a Water, Carbon Dioxide and Oxygen Barrier in Plasticized Poly(Vinyl Chloride) Films. RSC Adv. 2018, 8, 17645–17655. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, J.; Kim, J.H. Direct Synthesis of Highly Conductive Poly(3,4-Ethylenedioxythiophene):Poly(4-Styrenesulfonate) (PEDOT:PSS)/Graphene Composites and Their Applications in Energy Harvesting Systems. Nano Res. 2014, 7, 717–730. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Collins, B.A.; Sessolo, M.; Stavrinidou, E.; Strakosas, X.; Tassone, C.; Delongchamp, D.M.; Malliaras, G.G. Structural Control of Mixed Ionic and Electronic Transport in Conducting Polymers. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Lacey, S.D.; Xu, L.; Xie, H.; Li, T.; Danner, V.A.; Hu, L. Reduced Graphene Oxide Film with Record-High Conductivity And Mobility. Mater. Today 2018, 21, 186–192. [Google Scholar] [CrossRef]
- Cui, L.; Gao, J.; Xu, T.; Zhao, Y.; Qu, L. Polymer/Graphene Hybrids for Advanced Energy-Conversion and -Storage Materials. Chem. Asian J. 2016, 11, 1151–1168. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M. Pancake Bonding: An Unusual Pi-Stacking Interaction. Chem. Eur. J. 2019, 25, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Turner, A.P.F. Glucose Oxidase: An Ideal Enzyme. Biosens. Bioelectron. 1992, 7, 165–185. [Google Scholar] [CrossRef]
- Analytical Methods Committee. Recommendations for the Definition, Estimation and Use of the Detection Limit. Analyst 1987, 112, 199–204. [Google Scholar] [CrossRef]
- Lavín, Á.; de Vicente, J.; Holgado, M.; Laguna, M.F.; Casquel, R.; Santamaría, B.; Maigler, M.V.; Hernández, A.L.; Ramírez, Y. On the Determination of Uncertainty and Limit of Detection in Label-Free Biosensors. Sensors 2018, 18, 2038. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Gorton, L.; Ludwig, R.; Antiochia, R. A Third Generation Glucose Biosensor Based on Cellobiose Dehydrogenase Immobilized on a Glassy Carbon Electrode Decorated with Electrodeposited Gold Nanoparticles: Characterization and Application in Human Saliva. Sensors (Switzerland) 2017, 17, 1912. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.; Wang, Y.; Fan, K.; Wu, J.; Ying, Y. Direct Electrochemical Reduction of Graphene Oxide on Ionic Liquid Doped Screen-Printed Electrode and its Electrochemical Biosensing Application. Biosens. Bioelectron. 2011, 28, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, L.; Li, W.J. Graphene-Based Glucose Sensors: A Brief Review. IEEE Trans. Nanobiosci. 2015, 14, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Hartono, A.; Sanjaya, E.; Ramli, R. Glucose Sensing Using Capacitive Biosensor Based on Polyvinylidene Fluoride Thin Film. Biosensors 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Rungsawang, T.; Punrat, E.; Adkins, J.; Henry, C.; Chailapakul, O. Development of Electrochemical Paper-Based Glucose Sensor Using Cellulose-4-Aminophenylboronic Acid-Modified Screen-Printed Carbon Electrode. Electroanalysis 2016, 28, 462–468. [Google Scholar] [CrossRef]
- Ridhuan, N.S.; Abdul Razak, K.; Lockman, Z. Fabrication and Characterization of Glucose Biosensors by Using Hydrothermally Grown ZnO Nanorods. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abellán-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Morallón, E. A Stretchable and Screen-Printed Electrochemical Sensor for Glucose Determination in Human Perspiration. Biosens. Bioelectron. 2017, 91, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Gu, S.X.; Li, W.W.; Chen, T.T.; Xu, Q.; Wang, W. A Paper Disk Equipped with Graphene/Polyaniline/Au Nanoparticles/Glucose Oxidase Biocomposite Modified Screen-Printed Electrode: Toward Whole Blood Glucose Determination. Biosens. Bioelectron. 2014, 56, 77–82. [Google Scholar] [CrossRef] [PubMed]


| Electrode | Method | Sensitivity (µA/(mM·cm2)) | LoD (mM) | References | |
|---|---|---|---|---|---|
| 1 | GOx/rGO/IL- SPE | CV | 22.78 | 0.001 | [47] |
| 2 | PVDF/Ag/GOx | Capacitive | N/D | 13 | [49] |
| 3 | (µPAD)GOx/4-APBA/cellulose-modified SPCE | CV | N/D | 0.86 | [50] |
| 4 | Nafion/GOx/ZnO NRs/ITO | CV | 48.75 | 0.06 | [51] |
| 5 | GOx/Pt-graphite SPE | CV | 105 | 0.01 | [52] |
| 6 | Gr/PANI/AuNPs/GOx/SPCE | DPV | 20.32 | 0.1 | [53] |
| 7 | rGO-PEDOT:PSS-GOx/SPCE | CV | 57.3 | 0.0868 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Wahab, F.; Abdul Guthoos, H.F.; Wan Salim, W.W.A. Solid-State rGO-PEDOT:PSS Transducing Material for Cost-Effective Enzymatic Sensing. Biosensors 2019, 9, 36. https://doi.org/10.3390/bios9010036
Abd-Wahab F, Abdul Guthoos HF, Wan Salim WWA. Solid-State rGO-PEDOT:PSS Transducing Material for Cost-Effective Enzymatic Sensing. Biosensors. 2019; 9(1):36. https://doi.org/10.3390/bios9010036
Chicago/Turabian StyleAbd-Wahab, Firdaus, Habibah Farhana Abdul Guthoos, and Wan Wardatul Amani Wan Salim. 2019. "Solid-State rGO-PEDOT:PSS Transducing Material for Cost-Effective Enzymatic Sensing" Biosensors 9, no. 1: 36. https://doi.org/10.3390/bios9010036
APA StyleAbd-Wahab, F., Abdul Guthoos, H. F., & Wan Salim, W. W. A. (2019). Solid-State rGO-PEDOT:PSS Transducing Material for Cost-Effective Enzymatic Sensing. Biosensors, 9(1), 36. https://doi.org/10.3390/bios9010036





