Assessing the Potential Deployment of Biosensors for Point-of-Care Diagnostics in Developing Countries: Technological, Economic and Regulatory Aspects
Abstract
1. Introduction
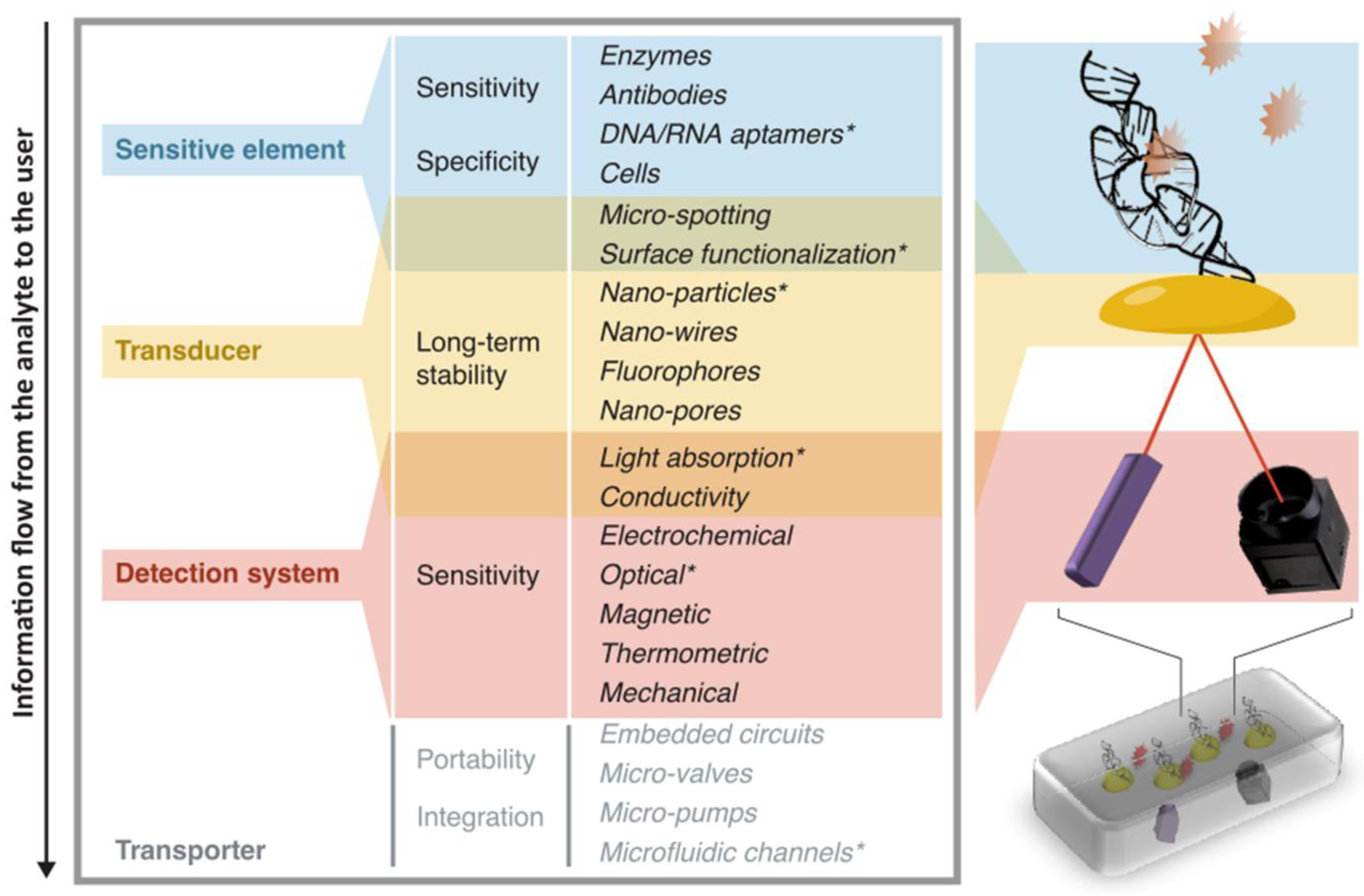
2. Part I. A Biosensor “Shell by Shell”
- a sensitive element is used to create an interaction between the target and the device. This interaction should involve the target as selectively as possible compared to other components of the sample, in order for the information to be specific to the target. For this part, biological molecules such as DNA strands, aptamers, and antibodies are a particularly adapted option, given their high specificity to the most widely demanded analytes;
- a transducer is used to convert the interaction effect into a readable parameter. Fluorescent molecules and functionalized surfaces (where usually organic molecules are bound to solid materials) are examples of such components, used in a large variety of biosensors;
- a detection system is used to measure the signal coming from the interaction between the device and the target. For instance, elements such as filters, lens, photodiodes and CCD cameras are widely used when the readout is optical, whereas silicon or metal components are common when the readout is electrical;
- a transporter includes the whole structure in which the other elements are contained. This part may also integrate steps for sample pre-processing (centrifugation, extraction, sorting, etc.) and data analysis which does not involve the biosensing step stricto sensu. The transporter can be very bulky like a fully integrated genome sequencer [18], down to simple assemblies contained in cm-sized tools [19].
- In the case of a portable SPR sensor as proposed by Guiducci and co-workers [19], the goal is to quantify the concentration of tobramycin (an antibiotics) filtered from undiluted blood serum. The sensitive element is a DNA-based aptamer. Many copies of this DNA strand cover isolated gold nano-islands, which change their refractive index upon binding of the complementary strands, and thus act as transducers. The detection system can then measure this change by using a light source for excitation and a photo-detector. The transporter is then a glass slide for the microfluidic handling of the sample and a plastic box in which all these elements are assembled. A schematic view of the functional parts of this portable biosensor is illustrated in Figure 1.
- In the case of the PacBio RS II by Pacific Biosciences [18], the goal is to sequence long DNA strands, for mutation detection or gene expression quantification. The sensitive element is composed of polymerase proteins trapped into nanowells. The transducer is a set of dyes with which single nucleotides are tagged. The detection system consists of an evanescent excitation system able to measure the fluorescence of single nucleotides in the nanowell, which changes over time during their interaction with the polymerase. The transporter is a large structure that integrates all the opto-electronics for the sensing and the hardware for the analysis.
3. Part II. Technological Challenges in Low-Resource Settings
4. Part III. Tackling Financing and Regulations
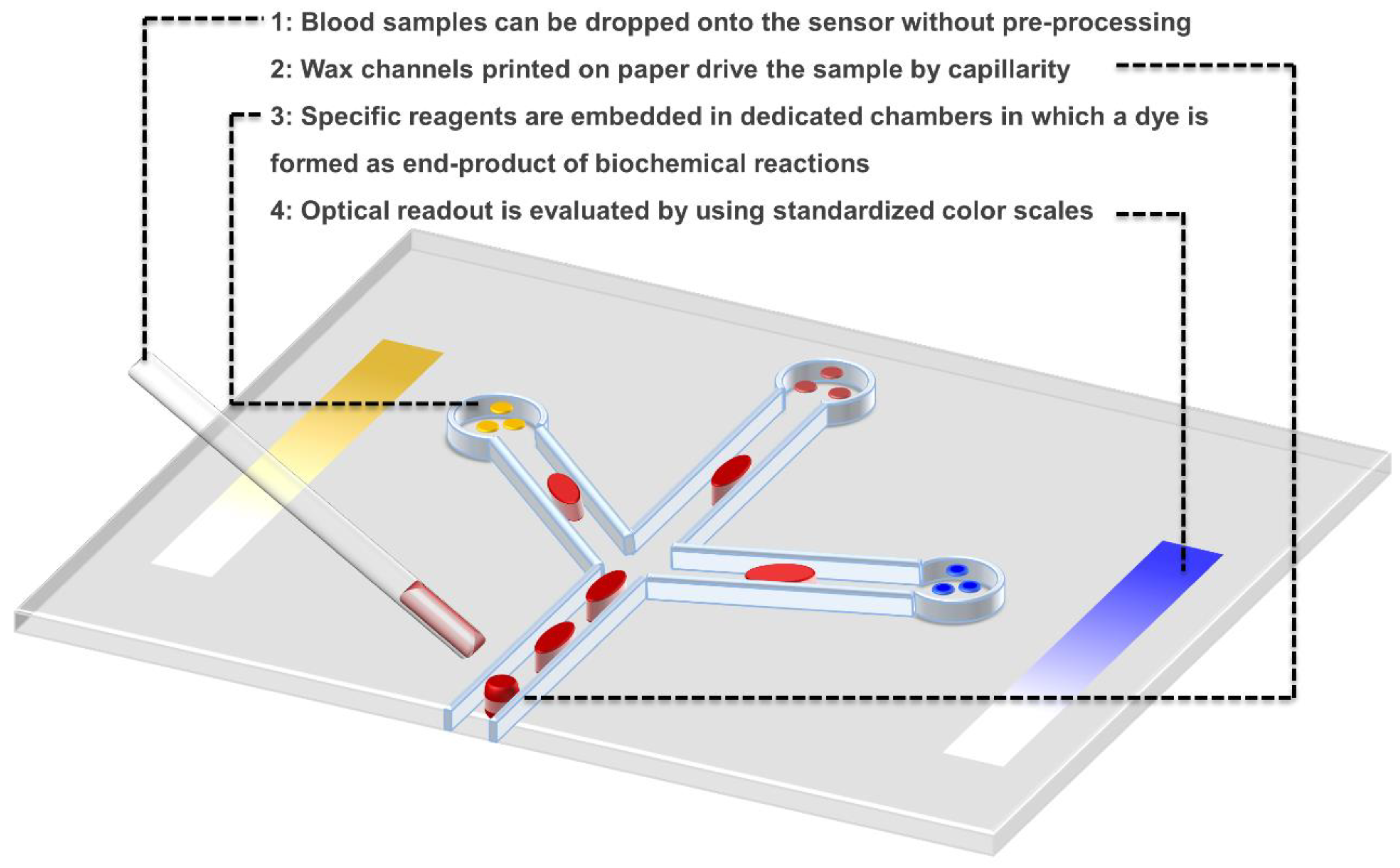
5. Part IV. A Technological Breakthrough as a Promising Case
- 5.
- the sensitive element is L-alanine, which is converted to pyruvate by ALT;
- 6.
- the transducer is a red dye formed as an end-product of a series of enzymatic reactions starting by the pyruvate;
- 7.
- the transporter is a piece of paper treated with wax to integrate channels for sample handling and chambers for reaction detection;
- 8.
- the detection system is merely the eye of the operator, who analyses the result with the help of a simple color-scale, and positive/negative controls integrated on the paper chip.
6. Perspectives
- to model infectious disease spreading,
- to understand drug resistance surging,
- to efficiently introduce vaccinations,
- and to define strategies for the healthcare system (for medical intervention in specific areas, for sanitary reimbursements, etc.).
- the research in breakthrough inexpensive technologies;
- the sharing of open-source technical solutions;
- the improvement of low-cost fabrication processes and scalability;
- the rising engagement of regulatory agencies in the clinical validation of the promising devices;
- and the commitment of local medical staff to modify their practices towards innovative diagnostic methods.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, G.; Steketee, R.W.; Black, R.E.; Bhutta, Z.A.; Morris, S.S. How many child deaths can we prevent this year? Lancet 2003, 362, 65–71. [Google Scholar] [CrossRef]
- Seddon, J.A.; Warren, R.M.; Enarson, D.A.; Beyers, N.; Schaaf, H.S. Drug-resistant tuberculosis transmission and resistance amplification within families. Emerg. Infect. Dis. 2012, 18, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Okeke, I.N.; Laxminarayan, R.; Bhutta, Z.A.; Duse, A.G.; Jenkins, P.; O’Brien, T.F.; Pablos-Mendez, A.; Klugman, K.P. Antimicrobial resistance in developing countries. Part I: Recent trends and current status. Lancet Infect. Dis. 2005, 5, 481–493. [Google Scholar] [CrossRef]
- Yeung, S.; Van Damme, W.; Socheat, D.; White, N.J.; Mills, A. Cost of increasing access to artemisinin combination therapy: The Cambodian experience. Malar. J. 2008, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Kilian, A.H.D.; Metzger, W.G.; Mutschelknauss, E.J.; Kabagambe, G.; Langi, P.; Korte, R.; von Sonnenburg, F. Reliability of malaria microscopy in epidemiological studies: Results of quality control. Trop. Med. Int. Health 2000, 5, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kain, K.C.; Harrington, M.A.; Tennyson, S.; Keystone, J.S. Imported malaria: Prospective analysis of problems in diagnosis and management. Clin. Infect. Dis. 1998, 27, 142–149. [Google Scholar] [CrossRef] [PubMed]
- WHO–Regional Office for the Western Pacific/TDR. Evaluation of Rapid Diagnostic Tests: Malaria. Available online: http://www.who.int/malaria/areas/diagnosis/rapid_diagnostic_tests/en/ (accessed on 28 November 2018).
- Petti, C.A.; Polage, C.R.; Quinn, T.C.; Ronald, A.R.; Sande, M.A. Laboratory medicine in Africa: A barrier to effective health care. Clin. Infect. Dis. 2006, 42, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Ridderhof, J.C.; van Deun, A.; Kam, K.M.; Narayanan, P.; Aziz, M.A. Roles of laboratories and laboratory systems in effective tuberculosis programmes. Bull. World Health Organ. 2007, 85, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Plowe, C.V.; Roper, C.; Barnwell, J.W.; Happi, C.T.; Joshi, H.H.; Mbacham, W.; Meshnick, S.R.; Mugittu, K.; Naidoo, I.; Price, R.N.; et al. World Antimalarial Resistance Network (WARN) III: Molecular markers for drug resistant malaria. Malar. J. 2007, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Field, S.P. Donation in populations with high endemic virus infection. Vox Sang. 2004, 87, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Allain, J.-P.; Candotti, D.; Soldan, K.; Sarkodie, F.; Phelps, B.; Giachetti, C.; Shyamala, V.; Yeboah, F.; Anokwa, M.; Owusu-Ofori, S.; et al. The risk of hepatitis B virus infection by transfusion in Kumasi, Ghana. Blood 2003, 101, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, Y.; Ying, C.; Wang, D.; Du, C. Nanopore-based fourth-generation DNA sequencing technology. Genom. Proteom. Bioinform. 2015, 13, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Asphahani, F.; Zhang, M. Cellular impedance biosensors for drug screening and toxin detection. Analyst 2007, 132, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Lin, C.-W.; Wei, K.-C.; Huang, C.-Y.; Hsu, P.-H.; Liu, H.-L.; Lu, Y.-J.; Lin, S.-C.; Yang, H.-W.; Ma, C.-C.M. Non-invasive screening for early Alzheimer’s disease diagnosis by a sensitively immunomagnetic biosensor. Sci. Rep. 2016, 6, 25155. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.-H.; Lee, S.-Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- PacBio, R.S., II. Pacific Biosciences 2016. Available online: http://www.pacb.com/products-and-services/pacbio-systems/rsii/ (accessed on 28 November 2018).
- Cappi, G.; Spiga, F.M.; Moncada, Y.; Ferretti, A.; Beyeler, M.; Bianchessi, M.; Decosterd, L.; Buclin, T.; Guiducci, C. Label-free detection of tobramycin in serum by transmission-localized surface plasmon resonance. Anal. Chem. 2015, 87, 5278–5285. [Google Scholar] [CrossRef] [PubMed]
- Waddie, A.J.; Taghizadeh, M.R. Scalability and miniaturization of optoelectronic Hopfield networks based around micro- and diffractive optical elements. Algorithms Syst. Opt. Inf. Process. 2001, 4471, 136–146. [Google Scholar]
- Solgaard, O. Miniaturization of free space optical systems. Appl. Opt. 2010, 49, F18–F31. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Morrow, T.J.; Keating, C.D. Nanowire sensors for multiplexed detection of biomolecules. Curr. Opin. Chem. Biol. 2008, 12, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, Y.; Zhou, S.; Wang, G.; Guan, X. Nanopore biosensor for label-free and real-time detection of anthrax lethal factor. ACS Appl. Mater. Interfaces 2014, 6, 7334–7339. [Google Scholar] [CrossRef] [PubMed]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Shumaker-Parry, J.S.; Zareie, M.H.; Aebersold, R.; Campbell, C.T. Microspotting streptavidin and double-stranded DNA arrays on gold for high-throughput studies of protein-DNA interactions by surface plasmon resonance microscopy. Anal. Chem. 2004, 76, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S.; Ali, M.A.; Anand, P.; Agrawal, V.V.; John, R.; Maji, S.; Malhotra, B.D. Microfluidic-integrated biosensors: Prospects for point-of-care diagnostics. Biotechnol. J. 2013, 8, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Carrilho, E.; Thomas, S.W.; Sindi, H.; Whitesides, G.M. Simple telemedicine for developing regions: Camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Volpetti, F.; Garcia-Cordero, J.; Maerkl, S.J. A microfluidic platform for high-throughput multiplexed protein quantitation. PLoS ONE 2015, 10, e0117744. [Google Scholar] [CrossRef] [PubMed]
- Mardis, E.R. A decade/’s perspective on DNA sequencing technology. Nature 2011, 470, 198–203. [Google Scholar] [CrossRef] [PubMed]
- DNA Sequencing Costs: Data. Available online: https://www.genome.gov/sequencingcostsdata/ (accessed on 28 November 2018).
- Rolland, J.P.; Mourey, D.A. Paper as a novel material platform for devices. MRS Bull. 2013, 38, 299–305. [Google Scholar] [CrossRef]
- Niezen, G.; Eslambolchilar, P.; Thimbleby, H. Open-source hardware for medical devices. BMJ Innov. 2016, 2, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Gibney, E. ‘Open-hardware’ pioneers push for low-cost lab kit. Nat. News 2016, 531, 147. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.M. Maximizing returns for public funding of medical research with open-source hardware. Health Policy Technol. 2017, 6, 381–382. [Google Scholar] [CrossRef]
- Kong, D.S.; Thorsen, T.A.; Babb, J.; Wick, S.T.; Gam, J.J.; Weiss, R.; Carr, P.A. Open-source, community-driven microfluidics with Metafluidics. Nat. Biotechnol. 2017, 35, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Zoëga Andreasen, S.; Wolff, A.; Duong Bang, D. From lab on a chip to point of care devices: The role of open source microcontrollers. Micromachines 2018, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Pitrone, P.G.; Schindelin, J.; Stuyvenberg, L.; Preibisch, S.; Weber, M.; Eliceiri, K.W.; Huisken, J.; Tomancak, P. OpenSPIM: An open-access light-sheet microscopy platform. Nat. Methods 2013, 10, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Conroy, J. Developing biodefense IVDs still a priority. IVD Technol. 2006, 12, 37–46. [Google Scholar]
- Walker, E.P. Medical News: Device Makers Grumble over FDA Processes. Available online: http://www.medpagetoday.com/PublicHealthPolicy/FDAGeneral/27035 (accessed on 28 November 2018).
- Our Technology—DFA. Available online: http://dfa.org/our-technology/ (accessed on 28 November 2018).
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding Wax Printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-M.; Martinez, A.W.; Gong, J.; Mace, C.R.; Phillips, S.T.; Carrilho, E.; Mirica, K.A.; Whitesides, G.M. Paper-Based ELISA. Angew. Chem. 2010, 122, 4881–4884. [Google Scholar] [CrossRef]
- Pollock, N.R.; Rolland, J.P.; Kumar, S.; Beattie, P.D.; Jain, S.; Noubary, F.; Wong, V.L.; Pohlmann, R.A.; Ryan, U.S.; Whitesides, G.M. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci. Transl. Med. 2012, 4, 152ra129. [Google Scholar] [CrossRef] [PubMed]
- Vella, S.J.; Beattie, P.; Cademartiri, R.; Laromaine, A.; Martinez, A.W.; Phillips, S.T.; Mirica, K.A.; Whitesides, G.M. Measuring markers of liver function using a micropatterned paper device designed for blood from a fingerstick. Anal. Chem. 2012, 84, 2883–2891. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.R.; McGray, S.; Colby, D.J.; Noubary, F.; Nguyen, H.; Nguyen, T.A.; Khormaee, S.; Jain, S.; Hawkins, K.; Kumar, S.; et al. Field evaluation of a prototype paper-based point-of-care fingerstick transaminase test. PLoS ONE 2013, 8, e75616. [Google Scholar] [CrossRef] [PubMed]
- Liver Function–DFA. Available online: http://dfa.org/liver-function/ (accessed on 28 November 2018).
Strengths—Internal Positive Factors
| Weaknesses—Internal Negative Factors
|
Opportunities—External Positive Factors
| Threats—External Negative Factors
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliozzi, D.; Guibentif, T. Assessing the Potential Deployment of Biosensors for Point-of-Care Diagnostics in Developing Countries: Technological, Economic and Regulatory Aspects. Biosensors 2018, 8, 119. https://doi.org/10.3390/bios8040119
Migliozzi D, Guibentif T. Assessing the Potential Deployment of Biosensors for Point-of-Care Diagnostics in Developing Countries: Technological, Economic and Regulatory Aspects. Biosensors. 2018; 8(4):119. https://doi.org/10.3390/bios8040119
Chicago/Turabian StyleMigliozzi, Daniel, and Thomas Guibentif. 2018. "Assessing the Potential Deployment of Biosensors for Point-of-Care Diagnostics in Developing Countries: Technological, Economic and Regulatory Aspects" Biosensors 8, no. 4: 119. https://doi.org/10.3390/bios8040119
APA StyleMigliozzi, D., & Guibentif, T. (2018). Assessing the Potential Deployment of Biosensors for Point-of-Care Diagnostics in Developing Countries: Technological, Economic and Regulatory Aspects. Biosensors, 8(4), 119. https://doi.org/10.3390/bios8040119





