Design and Fabrication of a BiCMOS Dielectric Sensor for Viscosity Measurements: A Possible Solution for Early Detection of COPD
Abstract
1. Introduction
2. Materials and Methods
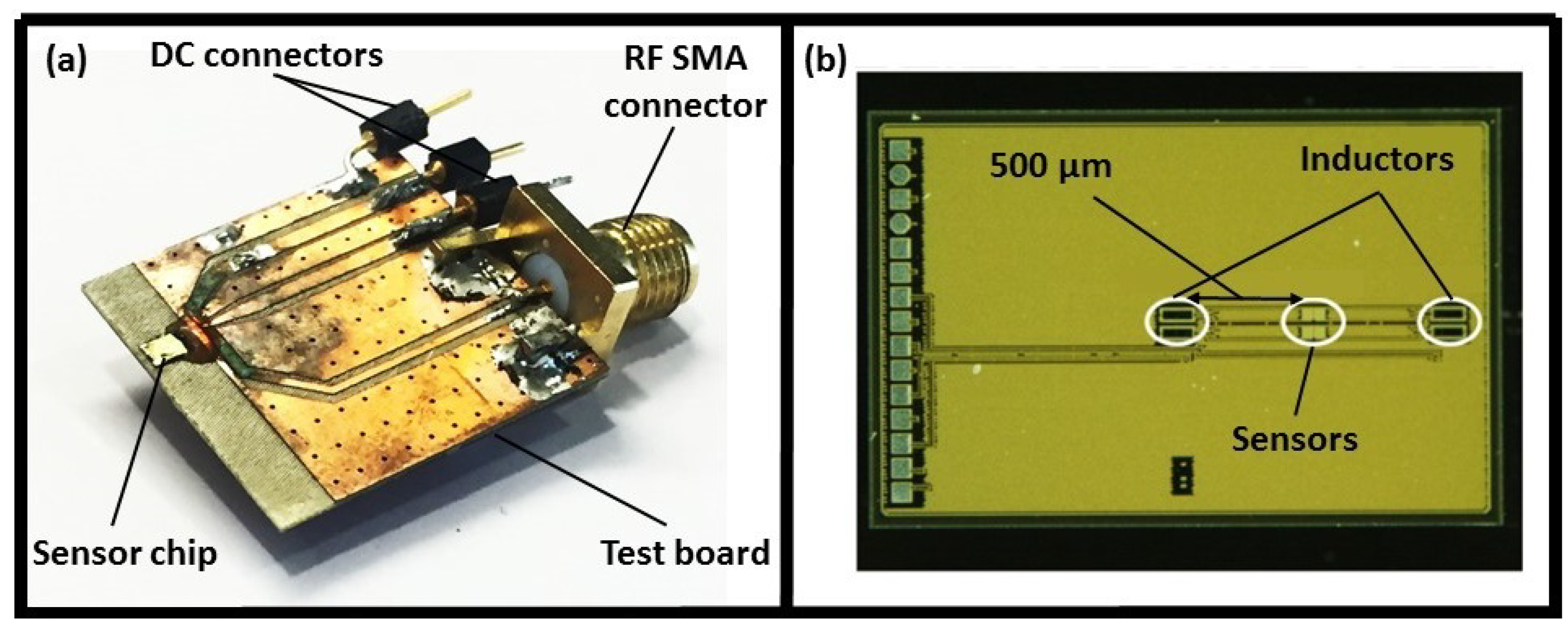
2.1. First Generation of the Sensor
2.1.1. Sensor Design and Operation Principle
2.1.2. Sensor Performance and Required Modifications
- Even though the RF output of the first-generation sensor was useful for conducting pilot experiments and evaluating the system during preliminary studies, its numerous drawbacks made it an unfavourable choice for out-of-the-lab applications. The main drawback of the RF output was the necessity of having costly and bulky spectrum analyzers for the signal acquisition which is an unrealistic intention for the development of POC devices. In addition, the RF signal is generally very sensitive to external distortions which causes a considerable amount of noise on the sensor outcome.
- Due to the lack of an adequate packaging, the sensor was extremely susceptible to ESD-caused damages. As a consequence, handling the system during the wire bonding process, soldering of PCB elements, and running experiments were significantly inefficient and complicated. Moreover, the spreading of conductive liquids on the PCB surface, especially during the characterization of biological samples containing water, caused the sensor to short-circuit. Hence, a proper packaging for ESD protection and short-circuit prevention of the system was required.
- Considering the inhomogeneous nature of biological liquids, including mucin and saliva, a series of sensing elements were required to increase the overall sensing ability of the system and improve its repeatability.
- Since the sensor measures the dielectric constant of a sample, electrical features of the sample determine the sensor outcome rather than its mechanical features. For instance, increasing the concentration of ethanol in an ethanol–glycerol mixture decreases both the permittivity and the viscosity of the mixture. Conversely, increasing the concentration of water in a water-glycerol mixture increases the permittivity of the mixture while decreasing its viscosity. Thus, the measured permittivity for a given viscous sample is dependent on the constituents of that sample. In other words, it is only the permittivity of the solution which is detected by the sensor rather than its absolute viscosity values. This is due to the fact that there is no direct mathematical correlation between the viscosity and any electrical quantity. Consequently, different calibrations are required based on the intrinsic characteristics of the tested samples. This issue causes calibration complexity and makes the viscosity detection of unknown samples impractical. Therefore, a more reliable calibration and validation method is necessary to improve sensor outcome. For this purpose, the direct calibration of the sensor using a commercialized viscometer is recommended.
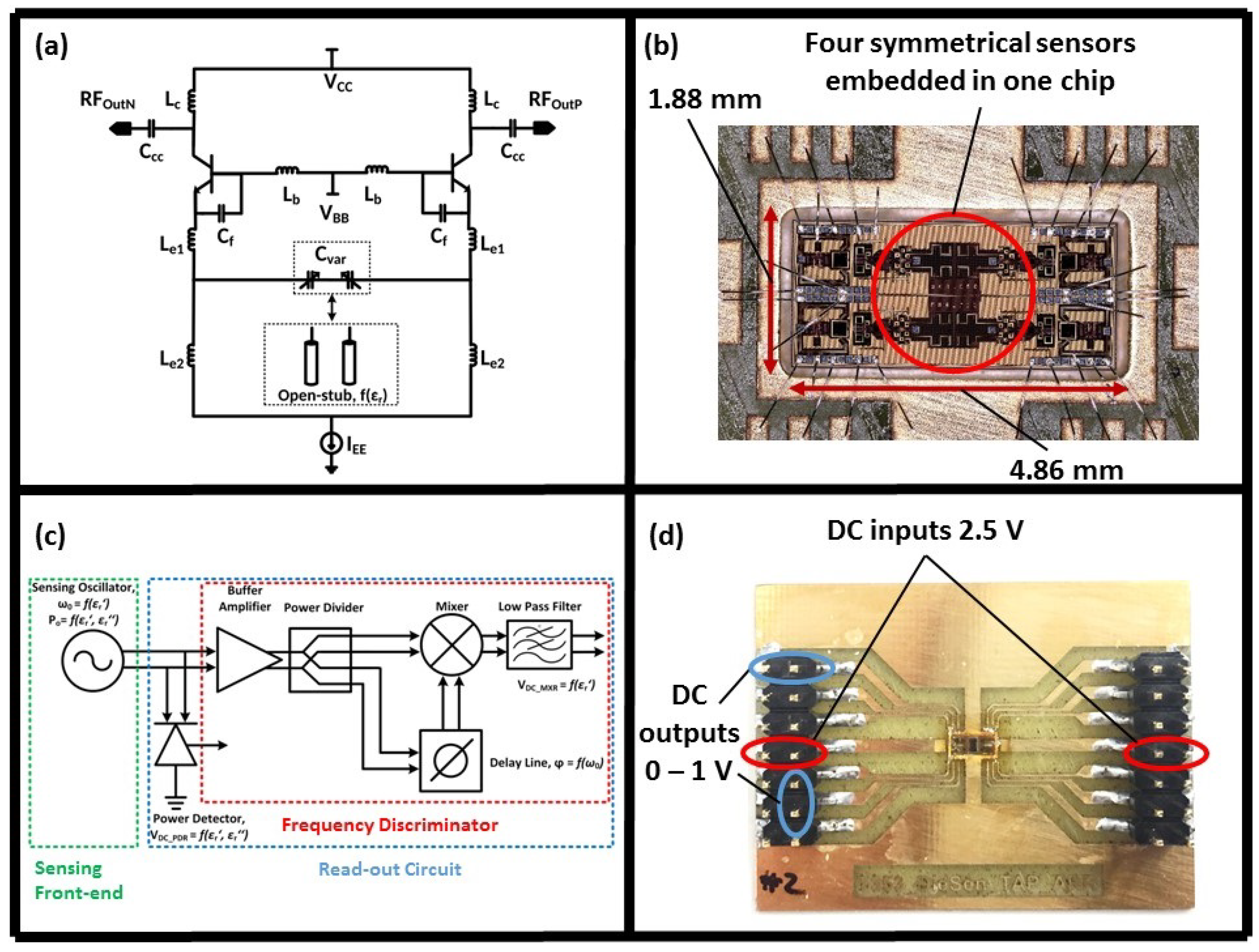
2.2. Second Generation of the Sensor
2.2.1. Sensor Design and Functionality
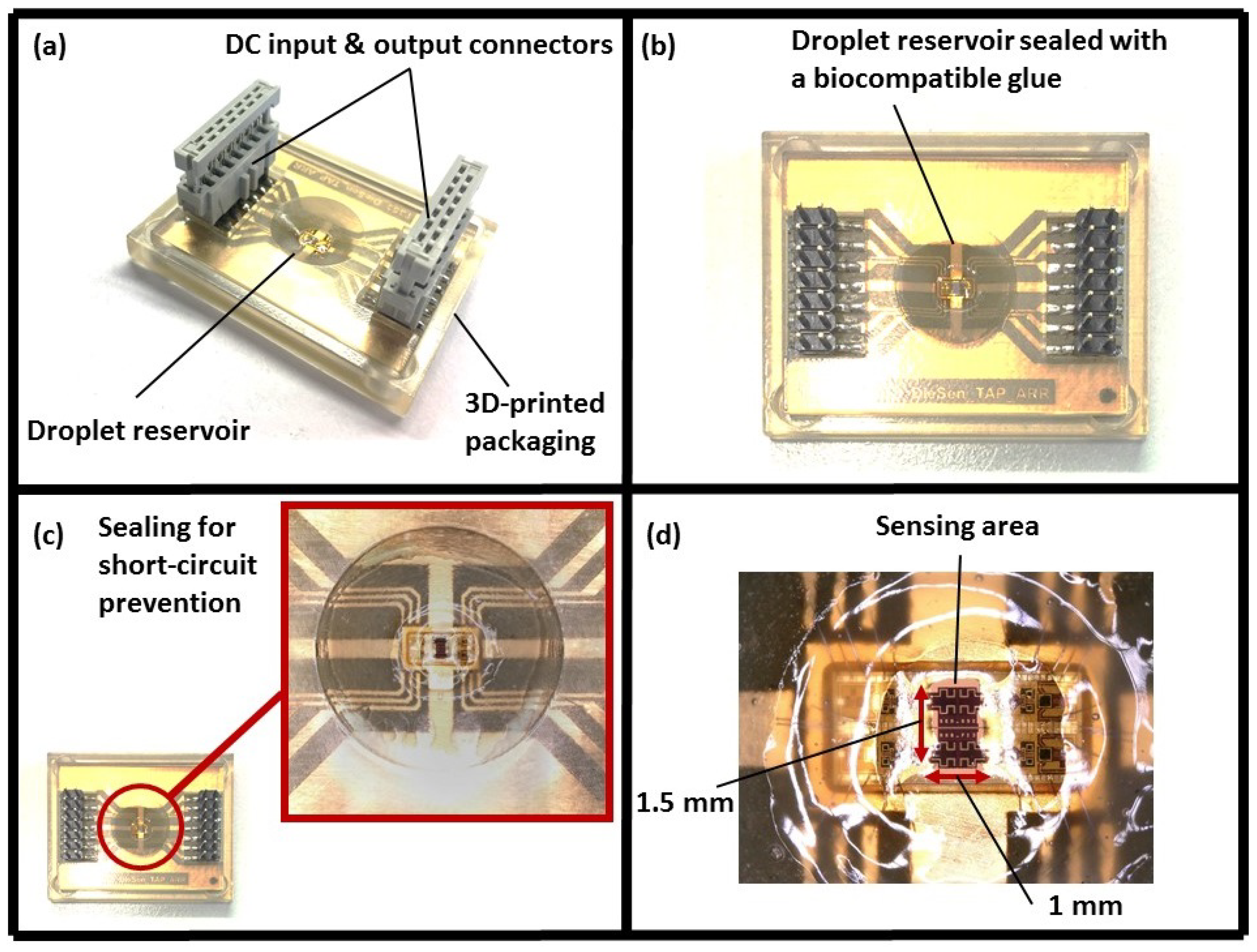
2.2.2. System Packaging
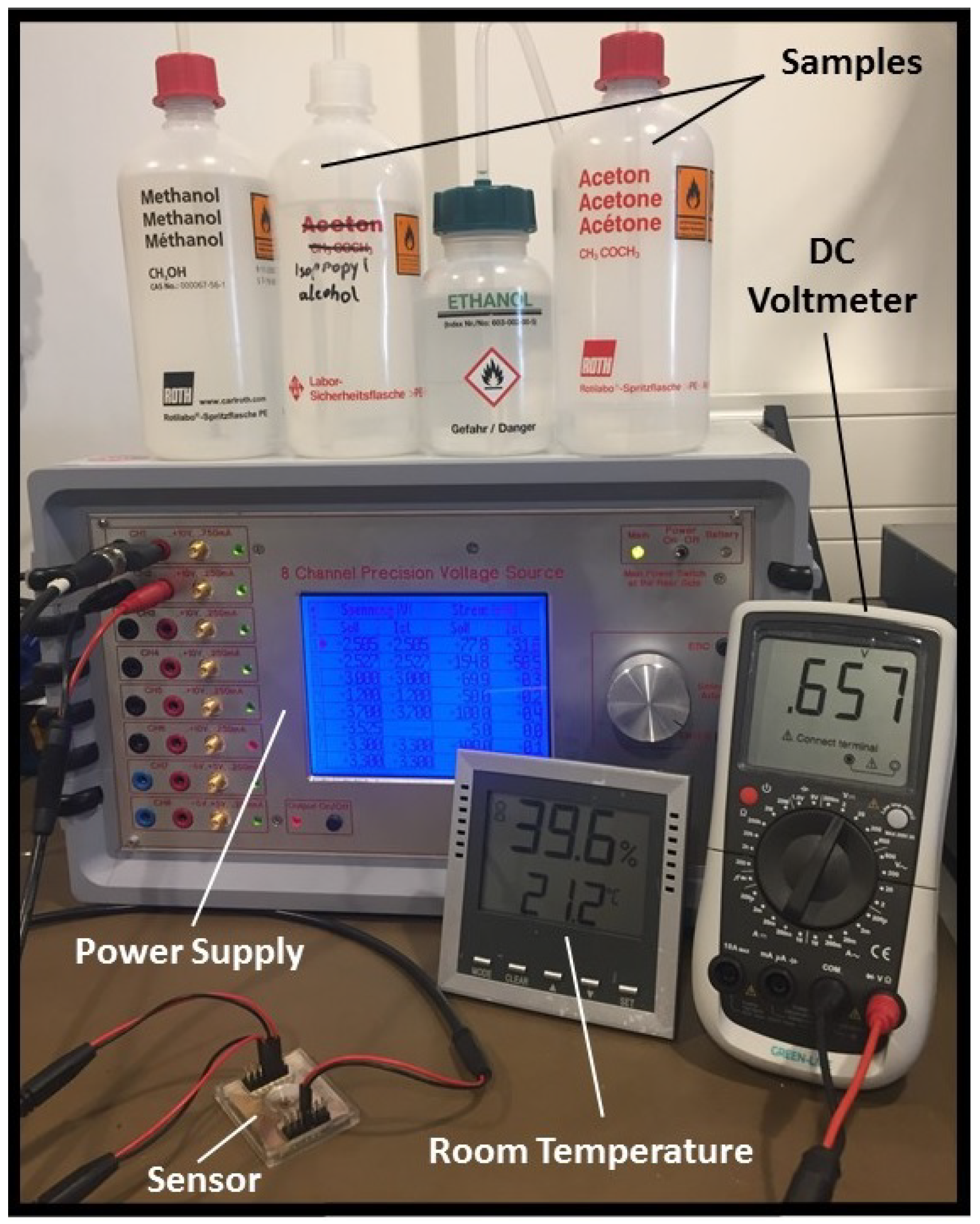
2.2.3. Experimental Setup
3. Results and Discussions
3.1. Calibration
3.2. Performance Assessment
- Accuracy: calculated as the difference between the actual and the measured value divided by the actual value (relative error). The total error of all three sets of measurements is reported.
- Repeatability: presented as the maximum standard deviation of the errors observed during three experiments.
- Hysteresis: calculated as the difference between the sensor initial measurements before and after performing an experiment. The highest value of all trials is reported.
- Drift: the sensor output with no MUT was recorded from the initiation of the system for a time period of 10 minutes. Drift was calculated as the difference between the lowest and the highest dielectric constant value measured during the first and last 10 seconds.
- Noise: calculated as the difference between the lowest and the highest dielectric constant value acquired in a 10 second data set with no MUT.
4. Conclusions and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnes, P.J. Mechanisms in COPD: Differences from asthma. J. Chest 2000, 117, 10S–14S. [Google Scholar] [CrossRef]
- Csikesz, N.G.; Gartman, E.J. New developments in the assessment of COPD: Early diagnosis is key. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 277–286. [Google Scholar]
- Robinson, W.; Woolley, P.; Altounyan, R.E.C. Reduction of sputum viscosity in chronic bronchitis. Lancet 1958, 272, 819–821. [Google Scholar] [CrossRef]
- Lopez-Vidriero, M.T.; Reid, L. Chemical markers of mucous and serum glycoproteins and their relation to viscosity in mucoid and purulent sputum from various hypersecretory diseases. Am. Rev. Respir. Dis. 1978, 117, 465–477. [Google Scholar] [PubMed]
- Boss, C.; Meurville, E.; Sallese, J.M.; Ryser, P. A viscosity-dependent affinity sensor for continuous monitoring of glucose in biological fluids. Biosens. Bioelectron. 2011, 30, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Lee, S.Y.; Jee, S.; Atajanov, A.; Yang, S. Micro-Viscometer for Measuring Shear-Varying Blood Viscosity over a Wide-Ranging Shear Rate. Sensors 2017, 17, 1442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, S.; Davidson, A.; Yang, B.; Wang, Q.; Lin, Q. A MEMS viscometric sensor for continuous glucose monitoring. J. Micromech. Microeng. 2007, 17, 2528. [Google Scholar] [CrossRef]
- Cakmak, O.; Ermek, E.; Kılınc, N.; Barıs, I.; Kavakli, I.H.; Yaralioglu, G.; Urey, H. Microcantilever based LoC system for coagulation measurements. In Proceedings of the 18th International Conference on Miniaturized Systems for Chemistry and Life Sciences, San Antonio, TX, USA, 26–30 October 2014; pp. 2050–2052. [Google Scholar]
- Beyer, U.; Schafer, D.; Thomas, A.; Aulich, H.; Haueter, U.; Reihl, B.; Ehwald, R. Recording of subcutaneous glucose dynamics by a viscometric affinity sensor. Diabetologia 2001, 44, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.M.; Cui, X.; Yang, C. The application of on-chip optofluidic microscopy for imaging Giardia lamblia trophozoites and cysts. Biomed. Microdevices 2009, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Zarrin, P.S.; Escoto, A.; Xu, R.; Patel, R.V.; Naish, M.D.; Trejos, A.L. Development of an optical fiber-based sensor for grasping and axial force sensing. In Proceedings of the International Conference on Robotics and Automation (ICRA), Singapore, 29 May–3 June 2017; pp. 939–944. [Google Scholar]
- Daniels, J.S.; Pourmand, N. Label-free impedance biosensors: Opportunities and challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Zarrin, P.S.; Escoto, A.; Xu, R.; Patel, R.V.; Naish, M.D.; Trejos, A.L. Development of a 2-DOF Sensorized Surgical Grasper for Grasping and Axial Force Measurements. IEEE Sens. J. 2018, 18, 2816–2826. [Google Scholar] [CrossRef]
- Kuenzi, S. Implantable Glucose Sensor: An Approach Based on a Rotating Microviscometer Combined with a Sensitive Liquid Containing Dextran and Concanavalin A; EPFL University: Lausanne, Switzerland, 2007. [Google Scholar]
- Ghallab, Y.H.; Ismail, Y. CMOS based lab-on-a-chip: Applications, challenges and future trends. IEEE Circuits Syst. Mag. 2014, 14, 27–47. [Google Scholar] [CrossRef]
- Gulari, M.N.; Ghannad-Rezaie, M.; Chronis, N. A compact, optofluidic system for measuring red blood cell concentration. In Proceedings of the 17th International Conference on Solid-State Sensors, Actuators and Microsystems, Barcelona, Spain, 16–20 June 2013; pp. 2552–2555. [Google Scholar]
- Guha, S.; Jamal, F.I.; Wenger, C. A Review on Passive and Integrated Near-Field Microwave Biosensors. Biosensors 2017, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Entesari, K.; Helmy, A.A.; Moslehi-Bajestan, M. Integrated Systems for Biomedical Applications: Silicon-Based RFVMicrowave Dielectric Spectroscopy and Sensing. IEEE Microwave Mag. 2017, 18, 57–72. [Google Scholar] [CrossRef]
- Stagni, C.; Guiducci, C.; Benini, L.; Ricco, B.; Carrara, S.; Samori, B.; Paulus, C.; Schienle, M.; Augustyniak, M.; Thewes, R. CMOS DNA sensor array with integrated A/D conversion based on label-free capacitance measurement. IEEE J. Solid-State Circuits 2006, 41, 2956–2964. [Google Scholar] [CrossRef]
- Ghafar-Zadeh, E.; Sawan, M. A hybrid microfluidic/CMOS capacitive sensor dedicated to lab-on-chip applications. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Ghafar-Zadeh, E.; Sawan, M. Charge-based capacitive sensor array for CMOS-based laboratory-on-chip applications. IEEE Sens. J. 2008, 8, 325–332. [Google Scholar] [CrossRef]
- Kim, J.W. Development of Interdigitated Capacitor Sensors for Direct and Wireless Measurements of the Dielectric Properties of Liquids; The University of Texas at Austin: Austin, TX, USA, 2008. [Google Scholar]
- Guha, S.; Schmalz, K.; Meliani, C.; Wenger, C. CMOS based sensor for dielectric spectroscopy of biological cell suspension. J. Phys. Conf. Ser. 2013, 434, 012017. [Google Scholar] [CrossRef]
- Guha, S.; Jamal, F.I.; Schmalz, K.; Wenger, C.; Meliani, C. CMOS lab on a chip device for dielectric characterization of cell suspensions based on a 6 GHz oscillator. In Proceedings of the European Microwave Conference (EuMC), Nuremberg, Germany, 6–10 October 2013; pp. 471–474. [Google Scholar]
- Lonappan, A.; Kumar, A.V.; Bindu, G.; Thomas, V.; Mathew, K.T. Qualitative analysis of human semen using microwaves. In Proceedings of the Progress in Electromagnetics Research Symposium, Cambridge, MA, USA, 26–29 March 2006; pp. 110–114. [Google Scholar]
- Helmy, A.; Jeon, H.J.; Lo, Y.C.; Larsson, A.J.; Kulkarni, R.; Kim, J.; Silva-Martinez, J.; Entesari, K. A self-sustained CMOS microwave chemical sensor using a frequency synthesizer. IEEE J. Solid-State Circuits 2012, 47, 2467–2483. [Google Scholar] [CrossRef]
- Guha, S.; Ramaker, K.; Krause, T.; Wenger, C. A CMOS radio frequency biosensor for rapid detection and screening of sputum-mucin viscosity. In Proceedings of the SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Guha, S.; Wenger, C. Radio frequency CMOS chem-bio Viscosity sensors based on dielectric spectroscopy. In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC), Porto, Portugal, 21–23 February 2017; pp. 142–148. [Google Scholar]
- Guha, S.; Schmalz, K.; Wenger, C.; Herzel, F. Self-calibrating highly sensitive dynamic capacitance sensor: Towards rapid sensing and counting of particles in laminar flow systems. Analyst 2015, 140, 3262–3272. [Google Scholar] [CrossRef] [PubMed]
- Jamal, F.I.; Guha, S.; Eissa, M.; Wessel, J.; Kissinger, D. A Fully Integrated Low-Power 30 GHz Complex Dielectric Sensor in a 0.25-μm BiCMOS Technology. IEEE J. Electromagn. RF Microw. Med. Biol. 2018. [Google Scholar] [CrossRef]
- Jamal, F.I.; Guha, S.; Eissa, M.; Borngräber, J.; Meliani, C.; Jalli, H.; Kissinger, D.; Wessel, J. Low-Power Miniature K-Band Sensors for Dielectric Characterization of Biomaterials. IEEE Trans. Microw. Theory Tech. 2017, 65, 1012–1023. [Google Scholar] [CrossRef]
- Hughes, J.V.; Armstrong, H.L. The dielectric constant of dry air. J. Appl. Phys. 1952, 23, 501–504. [Google Scholar] [CrossRef]
- Gregory, A.P.; Clarke, R.N. Tables of the Complex Permittivity of Dielectric Reference Liquids at Frequencies Up to 5 GHz; National Physical Laboratory: Teddington, UK, 2001.
- Buckley, F.; Maryott, A. Tables of Dielectric Dispersion Data for Pure Liquids and Dilute Solutions; Volume 589 of National Bureau of Standards circular, U.S. Dept. of Commerce: Ann Arbor, MI, USA, November 1958. [Google Scholar]
- Megriche, A.; Belhadj, A.; Mgaidi, A. Microwave dielectric properties of binary solvent wateralcohol, alcohol-alcohol mixtures at temperatures between −35 ∘C and +35 ∘C and dielectric relaxation studies. Mediterr. J. Chem. 2012, 1, 200–209. [Google Scholar] [CrossRef]
- Sihvola, A. Mixing rules with complex dielectric coefficients. Subsurf. Sens. Technol. Appl. 2000, 1, 393–415. [Google Scholar] [CrossRef]

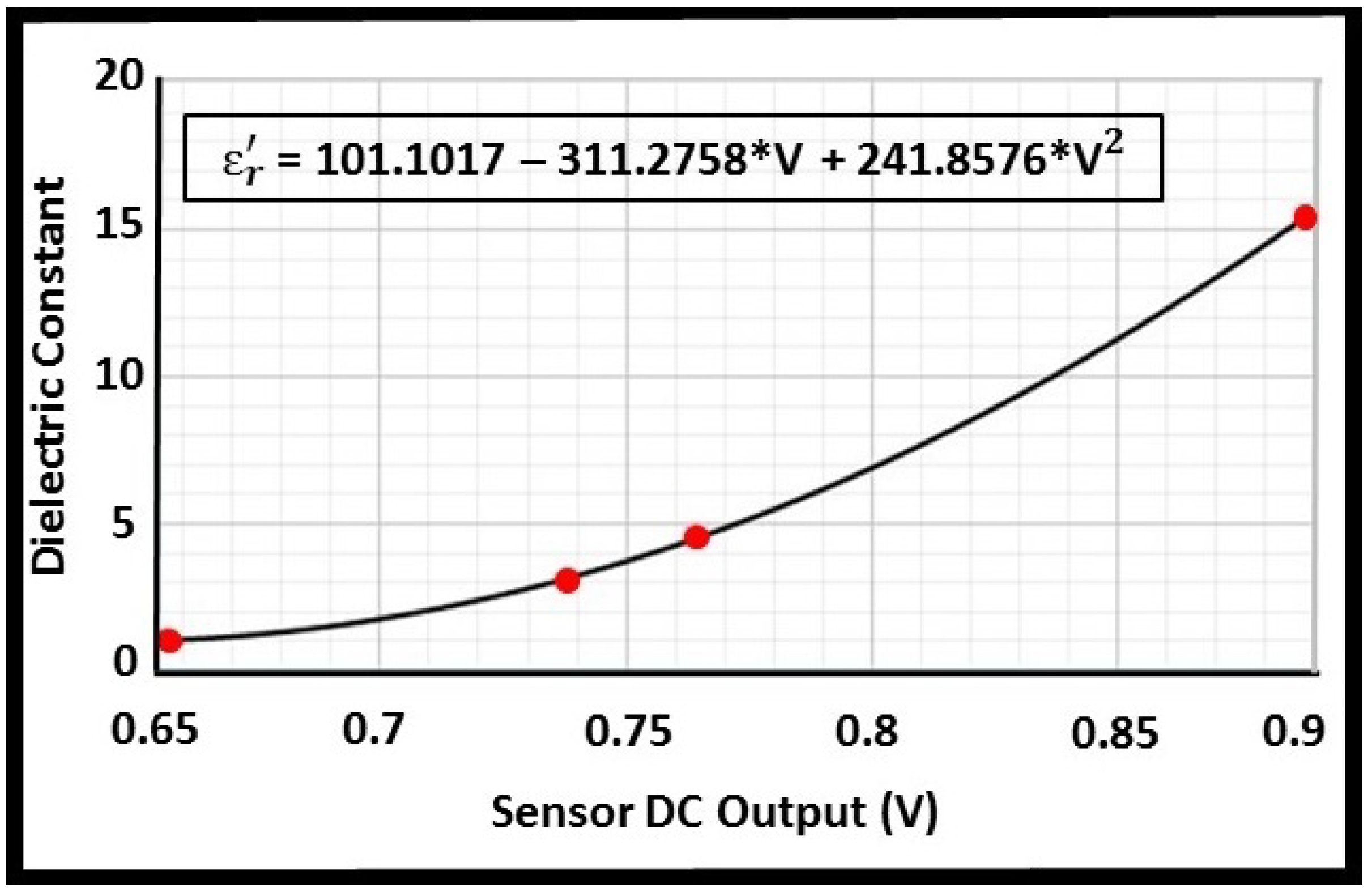
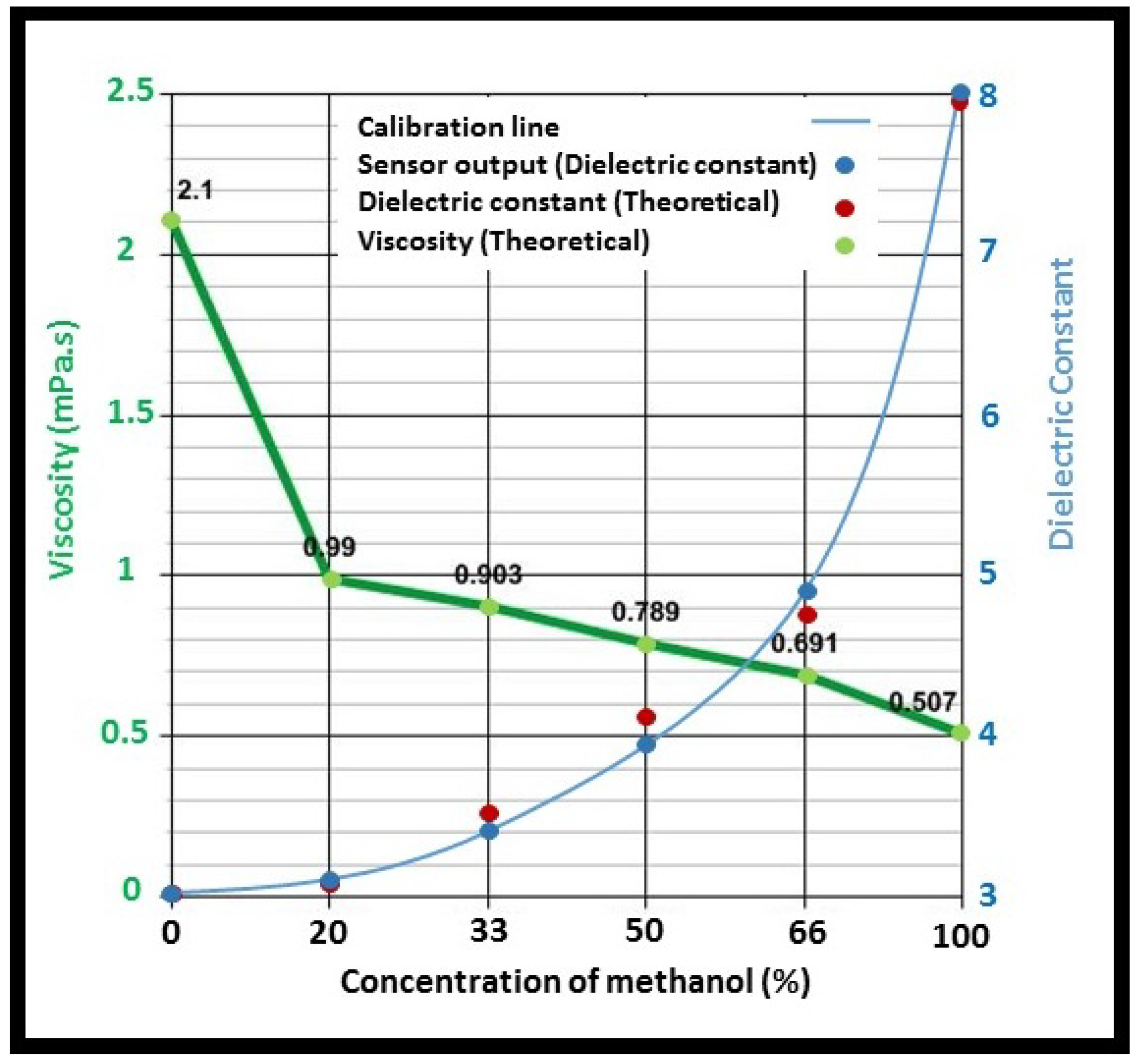
| Material | Dielectric Constant |
|---|---|
| Air | 1 |
| Isopropanol | 3.08 |
| Ethanol | 4.51 |
| Methanol | 8.2 |
| Acetone | 15.4 |
| Methanol | Actual Value | Exp. 1 | Exp. 2 | Exp. 3 |
|---|---|---|---|---|
| Dielectric Constant () | 8.2 | 8.82 | 8.14 | 8.65 |
| Accuracy | Repeatability | Hysteresis | Drift | Noise | |
|---|---|---|---|---|---|
| /(mV) | 4.17% | 5.36% | 0.014 (2 mV) | 0.038 (5 mV) | 0.006 (1 mV) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soltani Zarrin, P.; Jamal, F.I.; Guha, S.; Wessel, J.; Kissinger, D.; Wenger, C. Design and Fabrication of a BiCMOS Dielectric Sensor for Viscosity Measurements: A Possible Solution for Early Detection of COPD. Biosensors 2018, 8, 78. https://doi.org/10.3390/bios8030078
Soltani Zarrin P, Jamal FI, Guha S, Wessel J, Kissinger D, Wenger C. Design and Fabrication of a BiCMOS Dielectric Sensor for Viscosity Measurements: A Possible Solution for Early Detection of COPD. Biosensors. 2018; 8(3):78. https://doi.org/10.3390/bios8030078
Chicago/Turabian StyleSoltani Zarrin, Pouya, Farabi Ibne Jamal, Subhajit Guha, Jan Wessel, Dietmar Kissinger, and Christian Wenger. 2018. "Design and Fabrication of a BiCMOS Dielectric Sensor for Viscosity Measurements: A Possible Solution for Early Detection of COPD" Biosensors 8, no. 3: 78. https://doi.org/10.3390/bios8030078
APA StyleSoltani Zarrin, P., Jamal, F. I., Guha, S., Wessel, J., Kissinger, D., & Wenger, C. (2018). Design and Fabrication of a BiCMOS Dielectric Sensor for Viscosity Measurements: A Possible Solution for Early Detection of COPD. Biosensors, 8(3), 78. https://doi.org/10.3390/bios8030078





