Computational Study of Sensitivity Enhancement in Surface Plasmon Resonance (SPR) Biosensors by Using the Inclusion of the Core-Shell for Biomaterial Sample Detection
Abstract
:1. Introduction
2. Materials and Methods
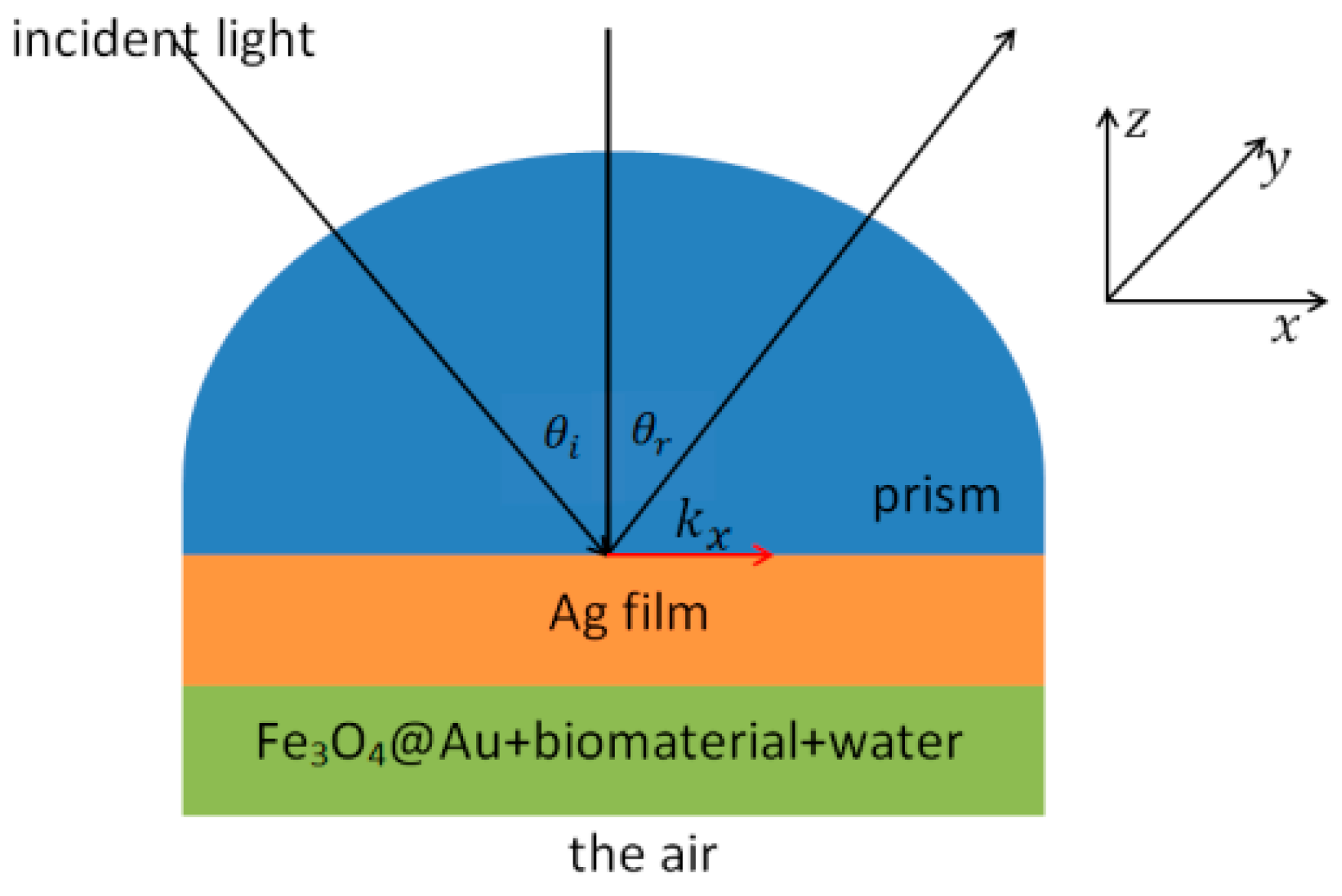
2.1. Kretschmann Configuration with Four Layers
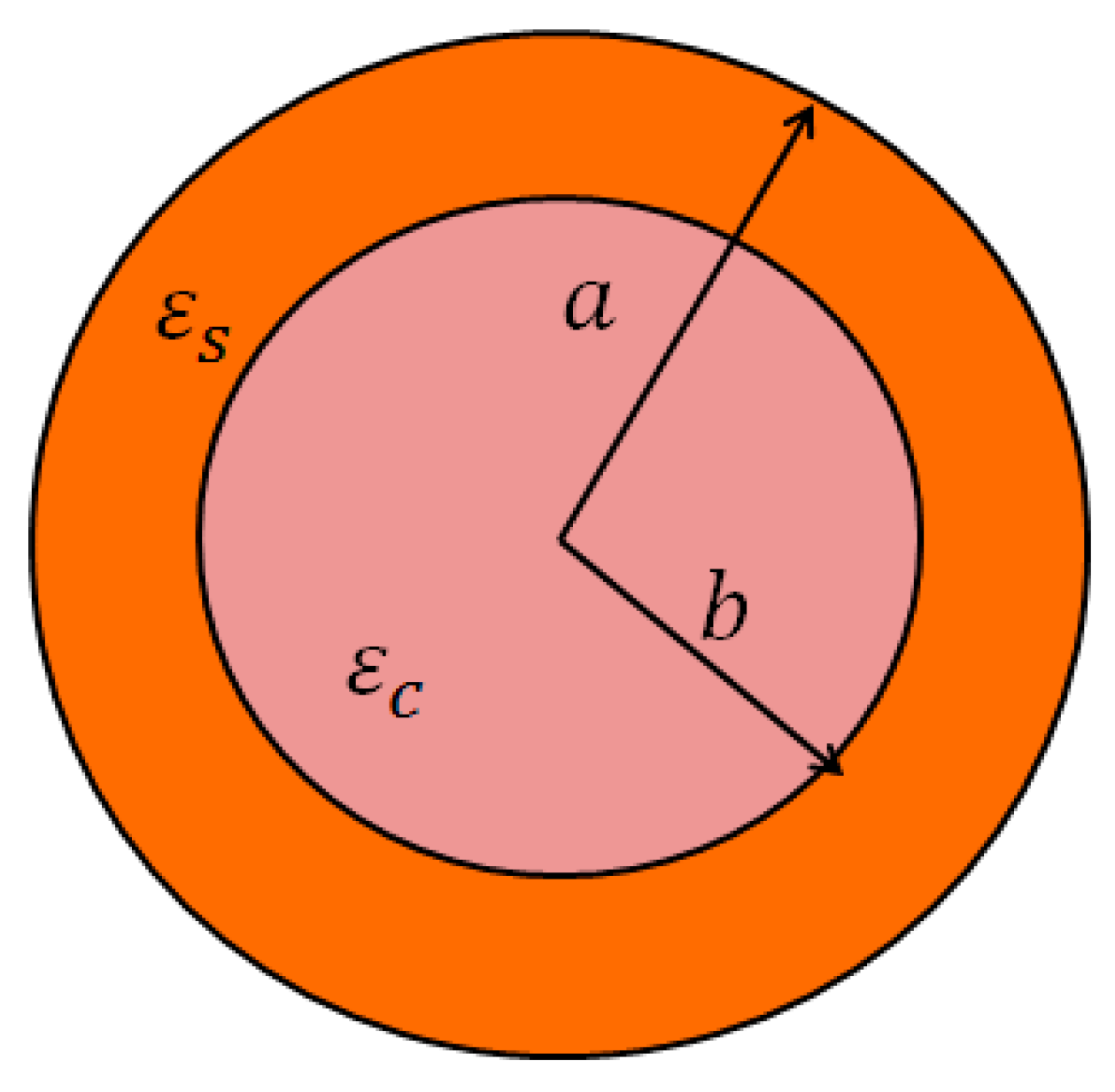
2.2. The Effective Permittivity of the Spherical Core-Shell
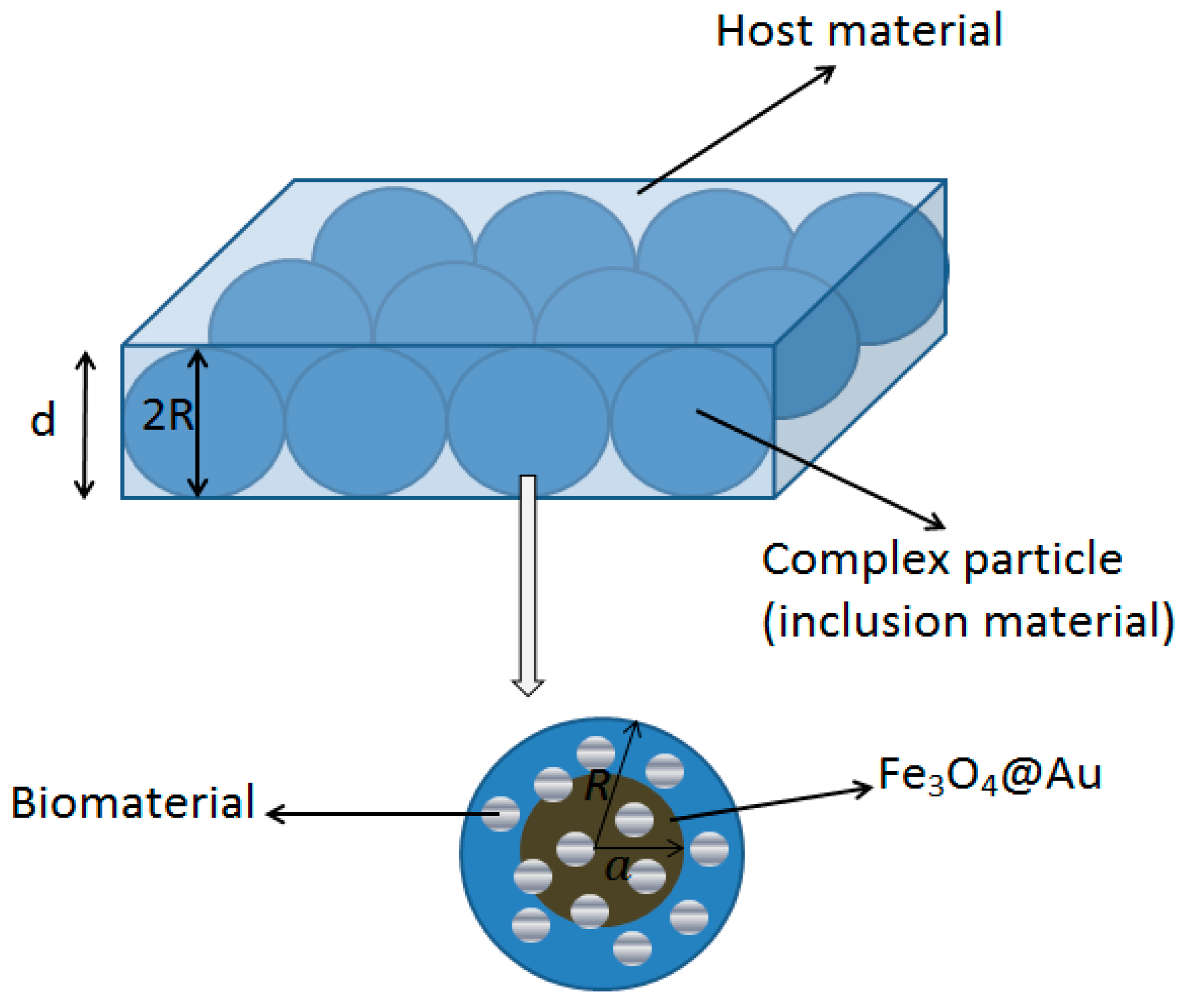
2.3. The Effective Permittivity of the Composite
2.4. Sensitivity from ATR Spectrum
3. Results and Discussion
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
References
- Stafford, S.; Garcia, R.S.; Gun’ko, Y.K. Multimodal Magnetic-Plasmonic Nanoparticles for Biomedical Applications. Appl. Sci. 2018, 8, 97. [Google Scholar] [CrossRef]
- Agbor, N.E.; Cresswell, J.P.; Petty, M.C.; Monkman, A.P. An optical gas sensor based on polyaniline Langmuir-Blodgett films. Sens. Actuators B Chem. 1997, 41, 137–141. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Notizen: Radiative Decay of Non Radiative Surface Plasmons Excited by Light. Z. Naturforsh 1968, 23A, 2135–2136. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators 1983, 4, 299–304. [Google Scholar] [CrossRef]
- He, L.; Musick, M.D.; Nicewarner, S.R.; Salinas, F.G.; Benkovic, S.J.; Natan, M.J.; Keating, C.D. Colloidal Au-Enhanced Surface Plasmon Resonance for Ultrasensitive Detection of DNA Hybridization. J. Am. Chem. Soc. 2000, 122, 9071–9077. [Google Scholar] [CrossRef]
- Milkani, E.; Lambert, C.R.; Mc Gimpsey, W.G. Direct detection of acetylcholinesterase inhibitor binding with an enzyme-based surface plasmon resonance sensor. Anal. Biochem. 2011, 408, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Salamon, Z.; Macleod, H.A.; Tollin, G. Surface plasmon resonance spectroscopy as a tool for investigating the biochemical and biophysical properties of membrane protein systems. II: Applications to biological systems. Biochim. Biophys. Acta 1997, 1331, 131–152. [Google Scholar] [CrossRef]
- Sharma, A.K. Plasmonic biosensor for detection of hemoglobin concentration in human blood: Design considerations. J. Appl. Phys. 2013, 114, 044701. [Google Scholar] [CrossRef]
- Wu, L.; Chu, H.S.; Koh, W.S.; Li, E.P. Highly sensitive graphene biosensors based on surface plasmon resonance. Opt. Express 2010, 18, 14395–14400. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.P.; Yao, G.H.; Fan, L.X.; Qiu, J.D. Magnetic Fe3O4@Au composite-enhanced surface plasmon resonance for ultrasensitive detection of magnetic nanoparticle-enriched α-fetoprotein. Anal. Chim. Acta 2012, 737, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Frasconi, M.; Tortolini, C.; Botrè, F.; Mazzei, F. Multifunctional Au Nanoparticle Dendrimer-Based Surface Plasmon Resonance Biosensor and Its Application for Improved Insulin Detection. Anal. Chem. 2010, 82, 7335–7342. [Google Scholar] [CrossRef] [PubMed]
- Baida, H.; Billaud, P.; Marhaba, S.; Christofilos, D.; Cottancin, E.; Crut, A.; Lermé, J.; Maioli, P.; Pellarin, M.; Broyer, M.; et al. Quantitative Determination of the Size Dependence of Surface Plasmon Resonance Damping in Single Ag@SiO2 Nanoparticles. Nano Lett. 2009, 9, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Surface Plasmon Resonance from Bimetallic Interface in Au–Ag Core-Shell Structure Nanowires. Nanoscale Res. Lett. 2009, 4, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Ji, A.; Sharma, R.P. Tunable Properties of Surface Plasmon Resonances: The Influence of Core–Shell Thickness and Dielectric Environment. Plasmonics 2014, 9, 651–657. [Google Scholar] [CrossRef]
- Ahmadi, N.; Poursalehi, R.; Farshi, M.K.M. The Interparticle Coupling Effect on Plasmon Resonance Properties of Magnetite@Au Magnetoplasmonic Nanoparticles. Procedia Mater. Sci. 2015, 11, 254–258. [Google Scholar] [CrossRef]
- Liu, Y.; Han, T.; Chen, C.; Bao, N.; Yu, C.M.; Gu, H.Y. A novel platform of hemoglobin on core–shell structurally Fe3O4@Au nanoparticles and its direct electrochemistry. Electrochim. Acta 2011, 56, 3238–3247. [Google Scholar] [CrossRef]
- Wang, J.; Song, D.; Zhang, H.; Zhang, J.; Jin, Y.; Zhang, H.; Zhou, H.; Sun, Y. Studies of Fe3O4/Ag/Au composites for immunoassay based on surface plasmon resonance biosensor. Colloids Surf. B Biointerfaces 2013, 102, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J.; Park, T.J.; Lee, S.; Park, J. Ultrasensitive DNA monitoring by Au-Fe3O4 nanocomplex. Sens. Actuators B Chem. 2012, 163, 224–232. [Google Scholar] [CrossRef]
- Chen, H.; Qi, F.; Zhou, H.; Jia, S.; Gao, Y.; Koh, K.; Yin, Y. Fe3O4@Au nanoparticles as a means of signal enhancement in surface plasmon resonance spectroscopy for thrombin detection. Sens. Actuators B Chem. 2015, 212, 505–511. [Google Scholar] [CrossRef]
- Guo, X. Fe3O4@Au nanoparticles enhanced surface plasmon resonance for ultrasensitive immunoassay. Sens. Actuators B Chem. 2014, 205, 276–280. [Google Scholar] [CrossRef]
- Sharma, A.K.; Jha, R.; Pattanaik, H.S. Design considerations for surface plasmon resonance based detection of human blood group in near infrared. J. Appl. Phys. 2010, 107, 034701. [Google Scholar] [CrossRef]
- Stuart, D.A.; Haes, A.J.; Yonzon, C.R.; Hicks, E.M.; Van Duyne, R.P. Biological applications of localized surface plasmonic phenomenae. IEE Proc. Nanobiotechnol. 2005, 152, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; El Sayed, I.H.; El-Sayed, M.A. Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems’. Plasmonics 2007, 2, 107–118. [Google Scholar] [CrossRef]
- Jin, Y.L.; Chen, J.Y.; Xu, L.; Wang, P.N. Refractive index measurement for biomaterial samples by total internal reflection. Phys. Med. Biol. 2006, 51. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Rather, H. Surface Plasmons on Smooth and Rough Surfaces and Gratings; Springer: Berlin, Germany, 1986; ISBN 978-3-540-47441-8. [Google Scholar]
- Schlegel, A.; Alvarado, S.F.; Wachter, P. Optical properties of magnetite (Fe3O4). J. Phys. C Solid State Phys. 1979, 12, 1157–1164. [Google Scholar] [CrossRef]
- Chettiar, U.K.; Engheta, N. Internal homogenization: Effective permittivity of a coated sphere. Opt. Express 2012, 20, 22976–22986. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q. Effective-medium theory for two-phase random composites with an interfacial shell. J. Mater. Sci. Technol. 2000, 16, 367–369. [Google Scholar]
- Verma, R.; Gupta, B.D.; Jha, R. Sensitivity enhancement of a surface plasmon resonance based biomolecules sensor using graphene and silicon layers. Sens. Actuators B Chem. 2011, 160, 623–631. [Google Scholar] [CrossRef]
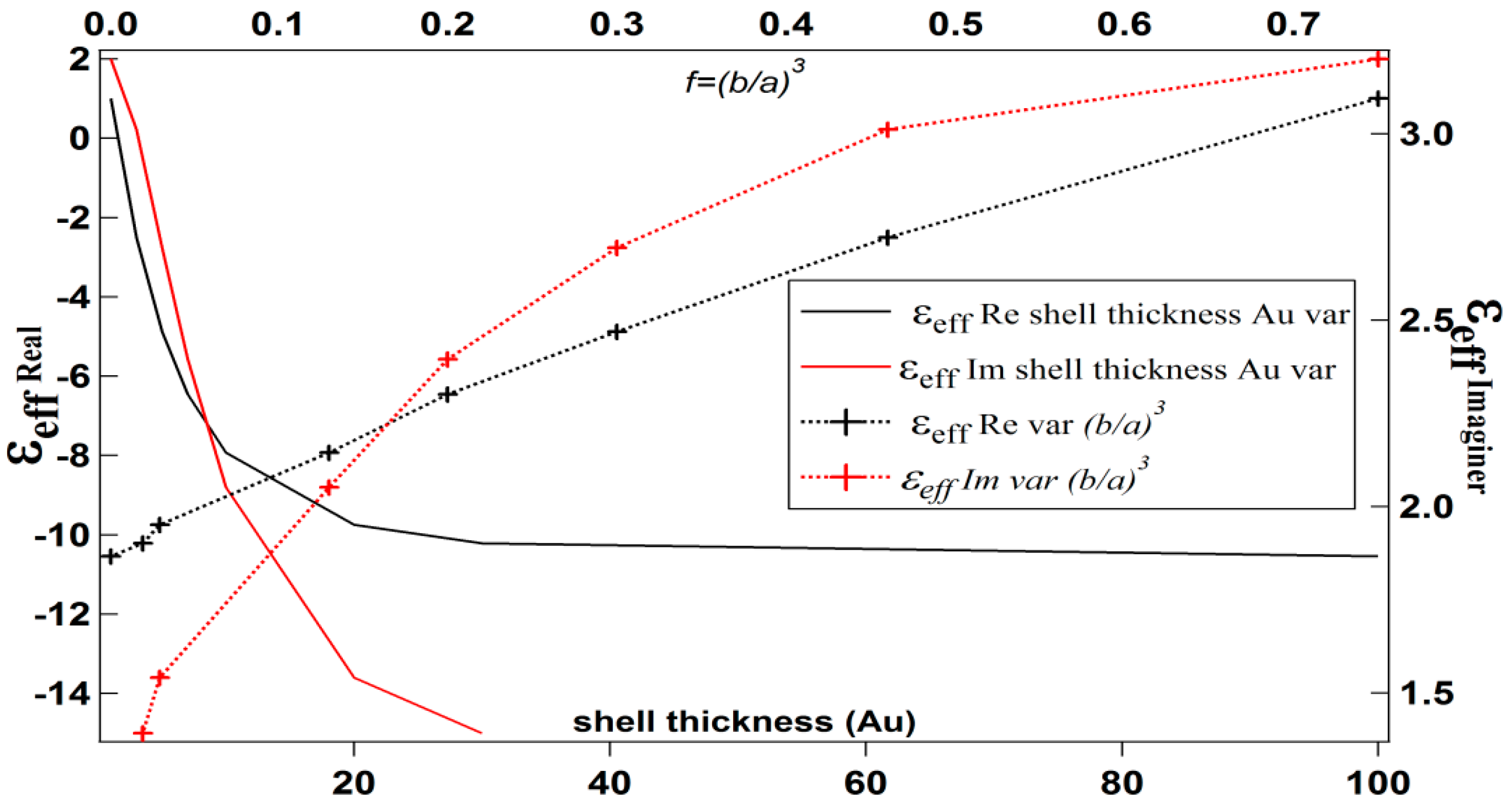
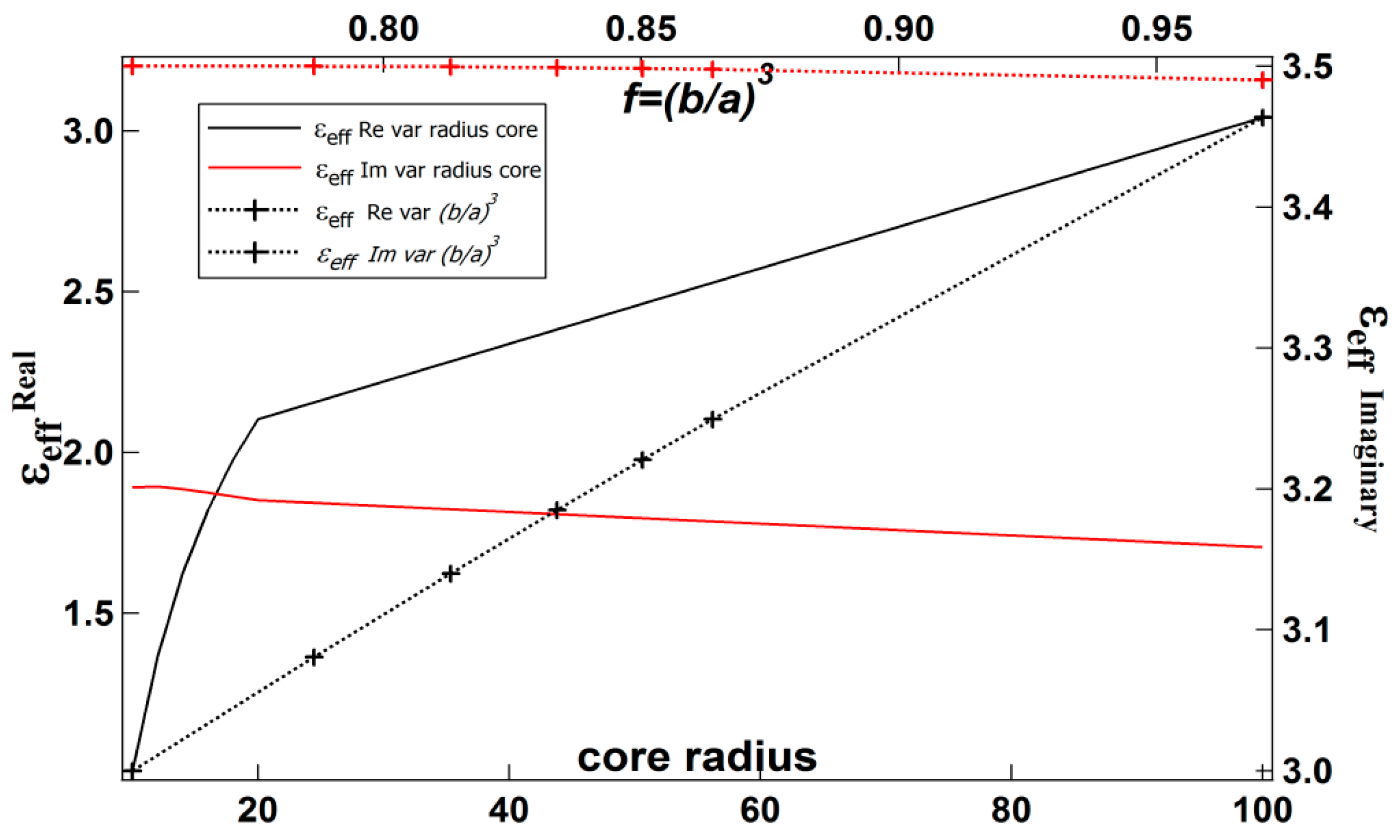
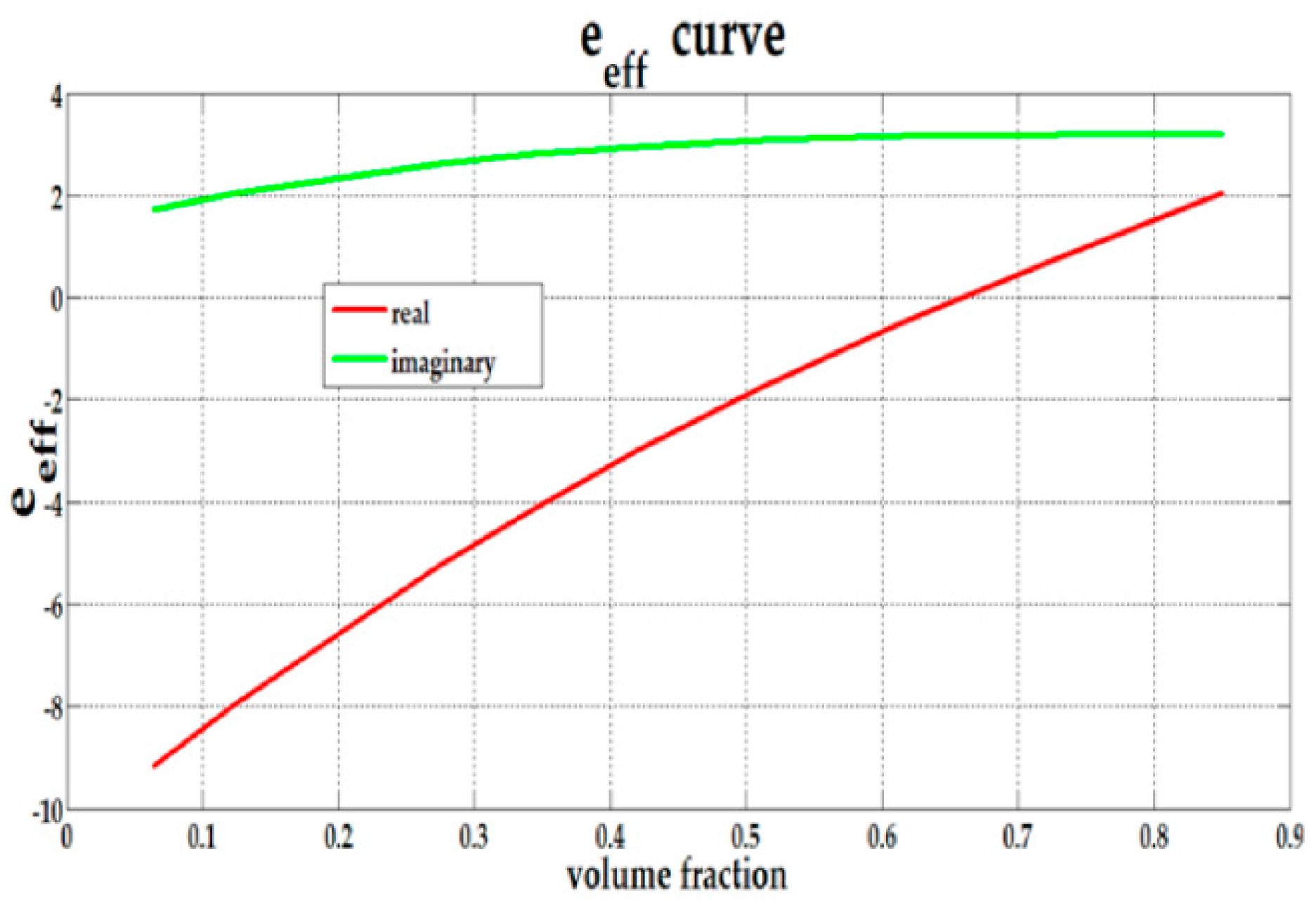
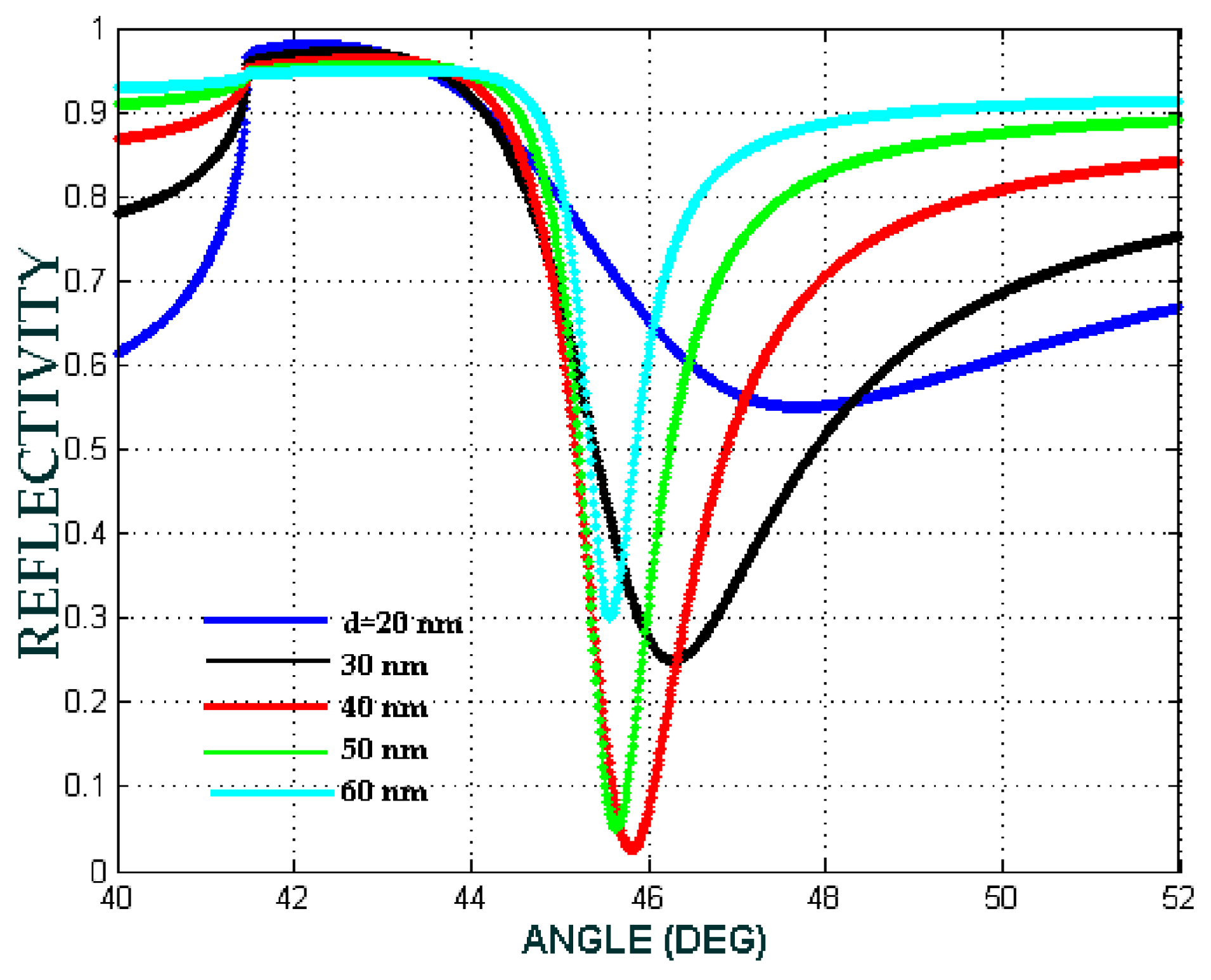

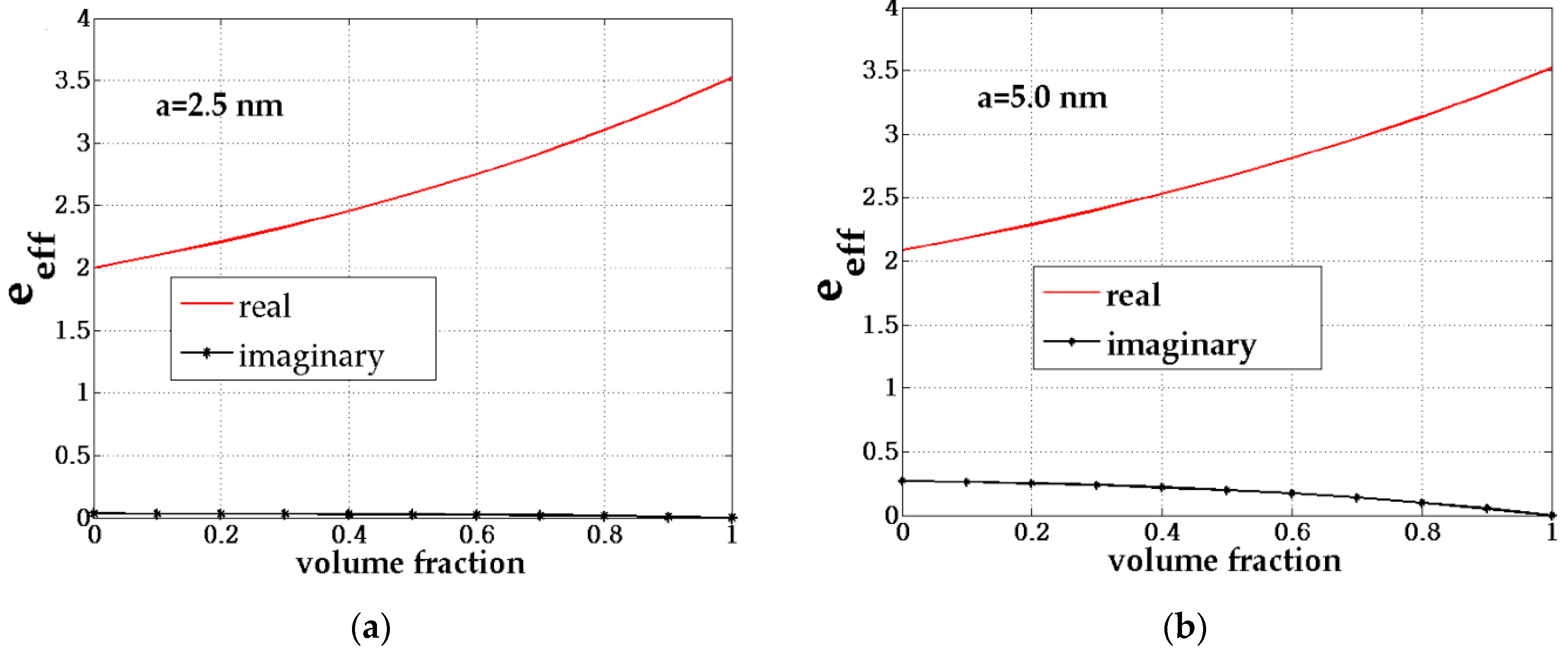
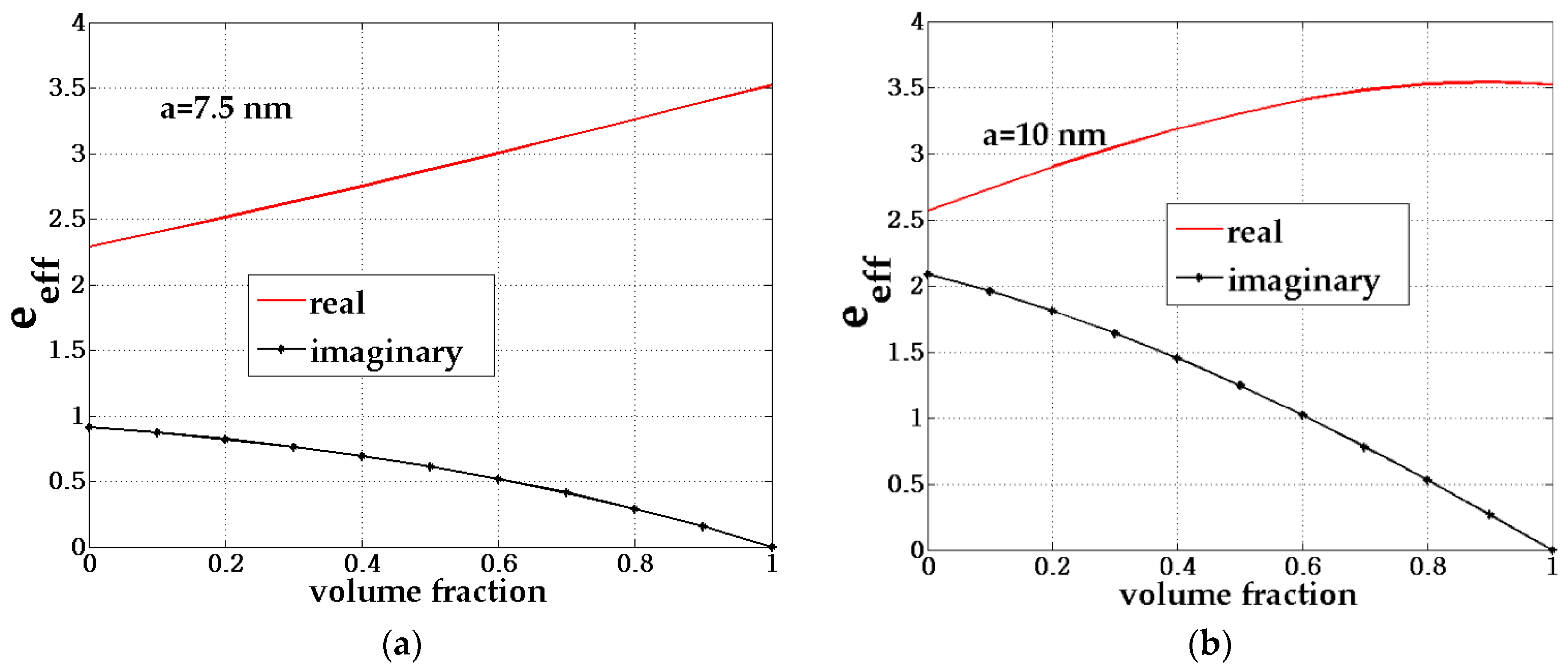


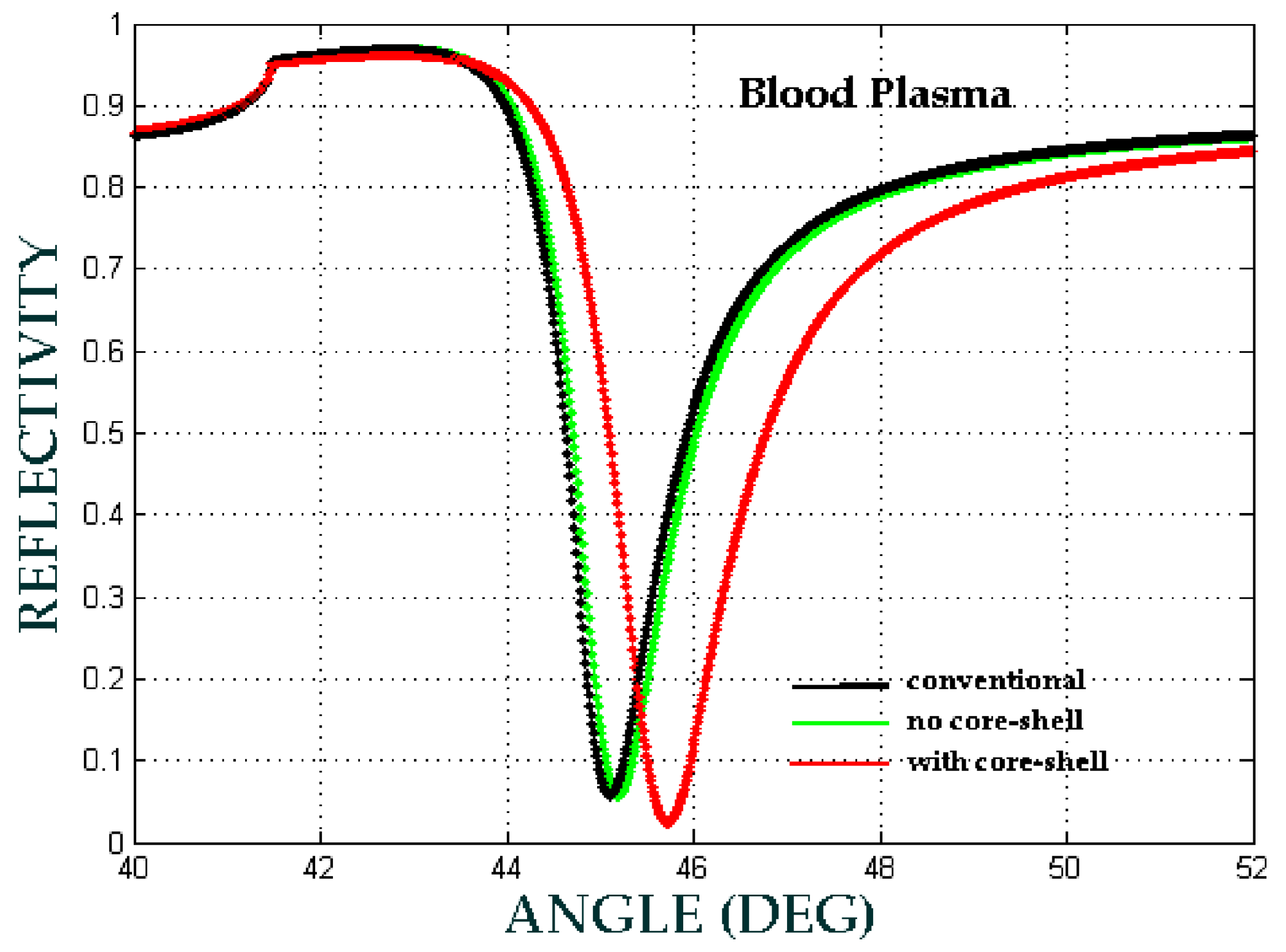
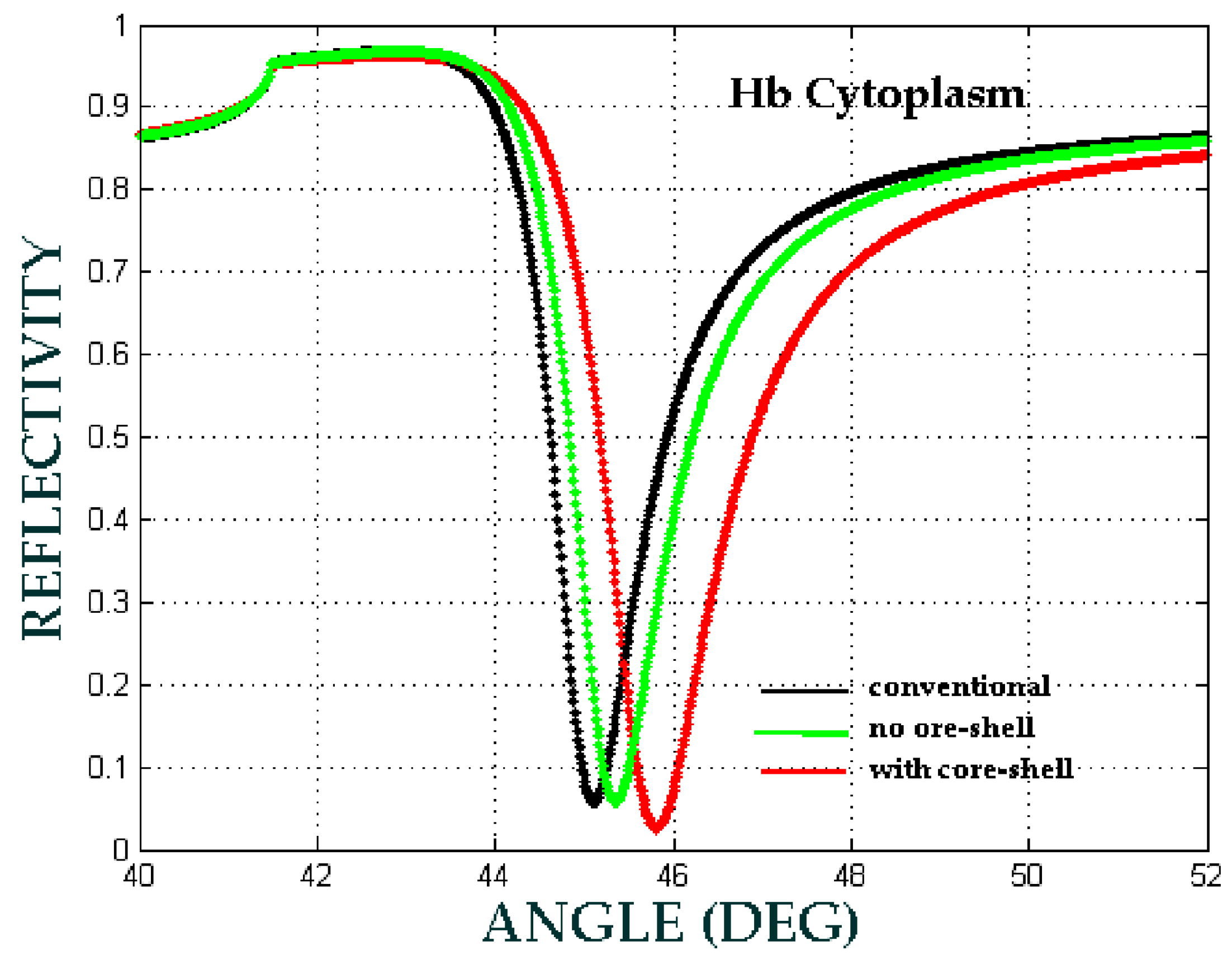
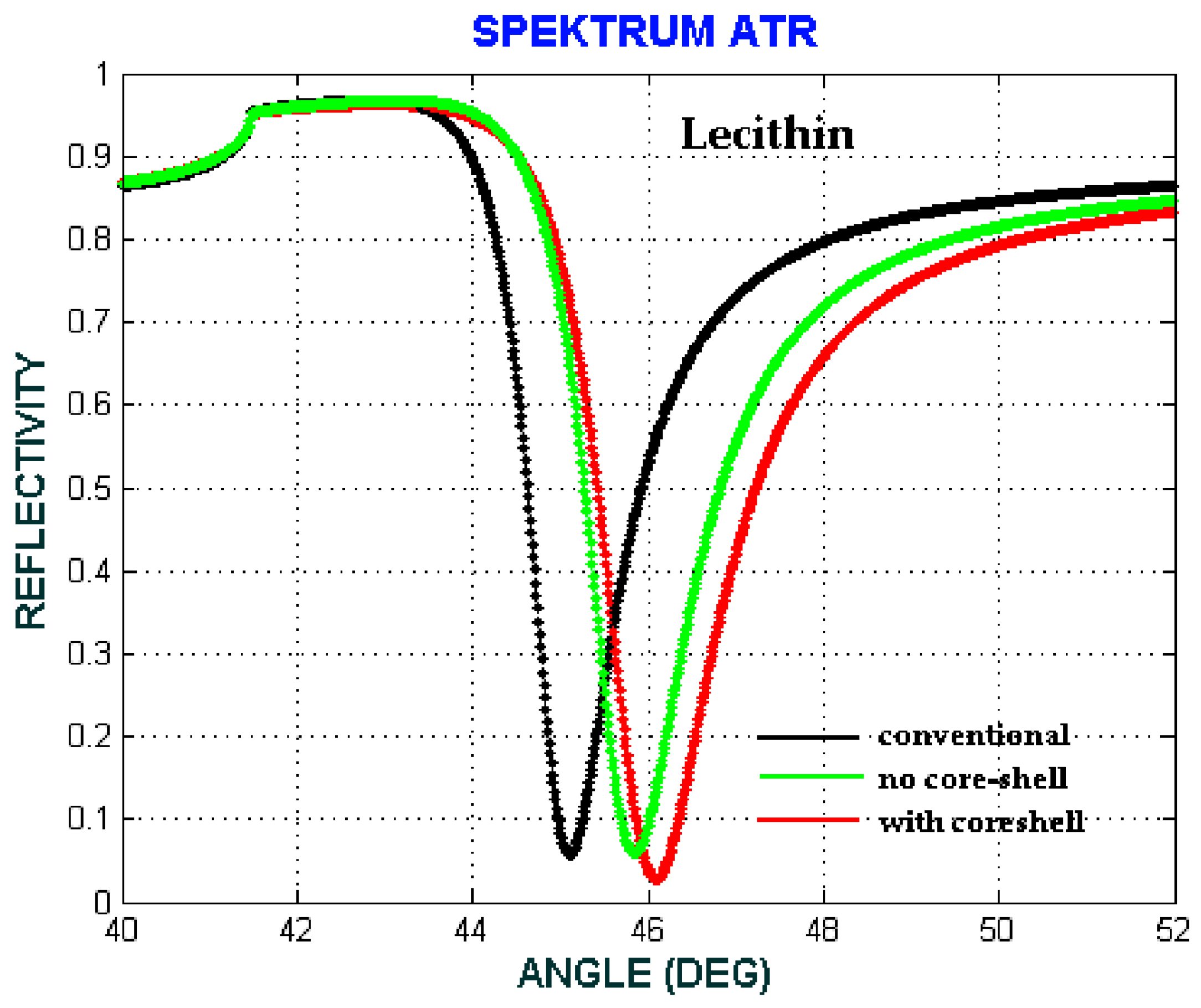
| b (nm) | a (nm) | Shell Thickness (nm) | (Real, Imag) | |
|---|---|---|---|---|
| 10 | 11 | 1 | 0.75 | 1.0092, 3.2011 |
| 10 | 13 | 3 | 0.46 | −2.5021, 3.0123 |
| 10 | 15 | 5 | 0.30 | −4.8721, 2.6948 |
| 10 | 17 | 7 | 0.20 | −6.4556, 2.3952 |
| 10 | 20 | 10 | 0.13 | −7.9297, 2.0516 |
| 10 | 30 | 20 | 0.03 | −9.7428, 1.5407 |
| 10 | 40 | 30 | 0.02 | −10.212, 1.3921 |
| 10 | 100 | 90 | 0.001 | −10.539, 1.2845 |
| b (nm) | a (nm) | (Real, Imag) | |
|---|---|---|---|
| 10 | 11 | 0.75 | 1.0092, 3.2011 |
| 12 | 13 | 0.78 | 1.3637, 3.2017 |
| 14 | 15 | 0.81 | 1.6230, 3.2000 |
| 16 | 17 | 0.83 | 1.8209, 3.1975 |
| 18 | 19 | 0.85 | 1.9768, 3.1947 |
| 20 | 21 | 0.86 | 2.1028, 3.1921 |
| 100 | 101 | 0.97 | 3.0427, 3.1587 |
| b (nm) | a (nm) | (Real, Imag) | |
|---|---|---|---|
| 9.5 | 10 | 0.85 | 2.04008, 3.19339 |
| 9.0 | 10 | 0.73 | 0.77837, 3.19889 |
| 8.5 | 10 | 0.61 | −0.49070, 3.16118 |
| 8.0 | 10 | 0.51 | −1.74550, 3.08115 |
| 7.5 | 10 | 0.42 | −2.96636, 2.96227 |
| 7.0 | 10 | 0.34 | −4.13333, 2.81043 |
| 6.5 | 10 | 0.27 | −5.22780, 2.63368 |
| Biomaterial Sample | |||
|---|---|---|---|
| Blood Plasma | 0.05 | 0.50 | 0.55 |
| Hb Cytoplasm | 0.21 | 0.44 | 0.65 |
| Lecithin | 0.71 | 0.77 | 0.94 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widayanti; Abraha, K.; Bambang Setio Utomo, A. Computational Study of Sensitivity Enhancement in Surface Plasmon Resonance (SPR) Biosensors by Using the Inclusion of the Core-Shell for Biomaterial Sample Detection. Biosensors 2018, 8, 75. https://doi.org/10.3390/bios8030075
Widayanti, Abraha K, Bambang Setio Utomo A. Computational Study of Sensitivity Enhancement in Surface Plasmon Resonance (SPR) Biosensors by Using the Inclusion of the Core-Shell for Biomaterial Sample Detection. Biosensors. 2018; 8(3):75. https://doi.org/10.3390/bios8030075
Chicago/Turabian StyleWidayanti, Kamsul Abraha, and Agung Bambang Setio Utomo. 2018. "Computational Study of Sensitivity Enhancement in Surface Plasmon Resonance (SPR) Biosensors by Using the Inclusion of the Core-Shell for Biomaterial Sample Detection" Biosensors 8, no. 3: 75. https://doi.org/10.3390/bios8030075
APA StyleWidayanti, Abraha, K., & Bambang Setio Utomo, A. (2018). Computational Study of Sensitivity Enhancement in Surface Plasmon Resonance (SPR) Biosensors by Using the Inclusion of the Core-Shell for Biomaterial Sample Detection. Biosensors, 8(3), 75. https://doi.org/10.3390/bios8030075





