Transistors for Chemical Monitoring of Living Cells
Abstract
Featured Application
Abstract
1. Introduction
2. Chemicals to be Sensed
2.1. Components of Cell Culture Media
2.2. Metabolites
3. Substrates for Cell Culture
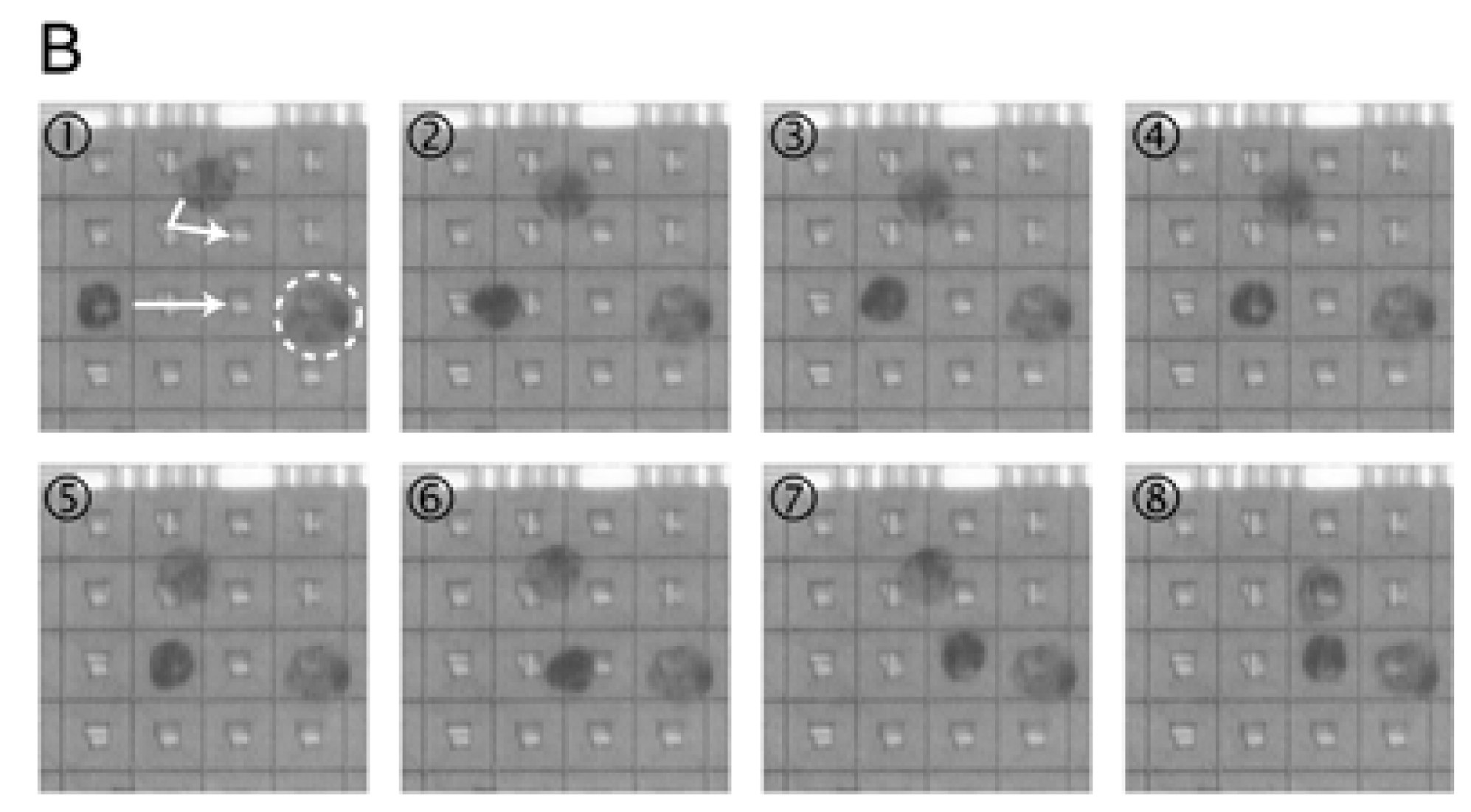
3.1. Approaches for Cells Localization
3.1.1. Passive Methods
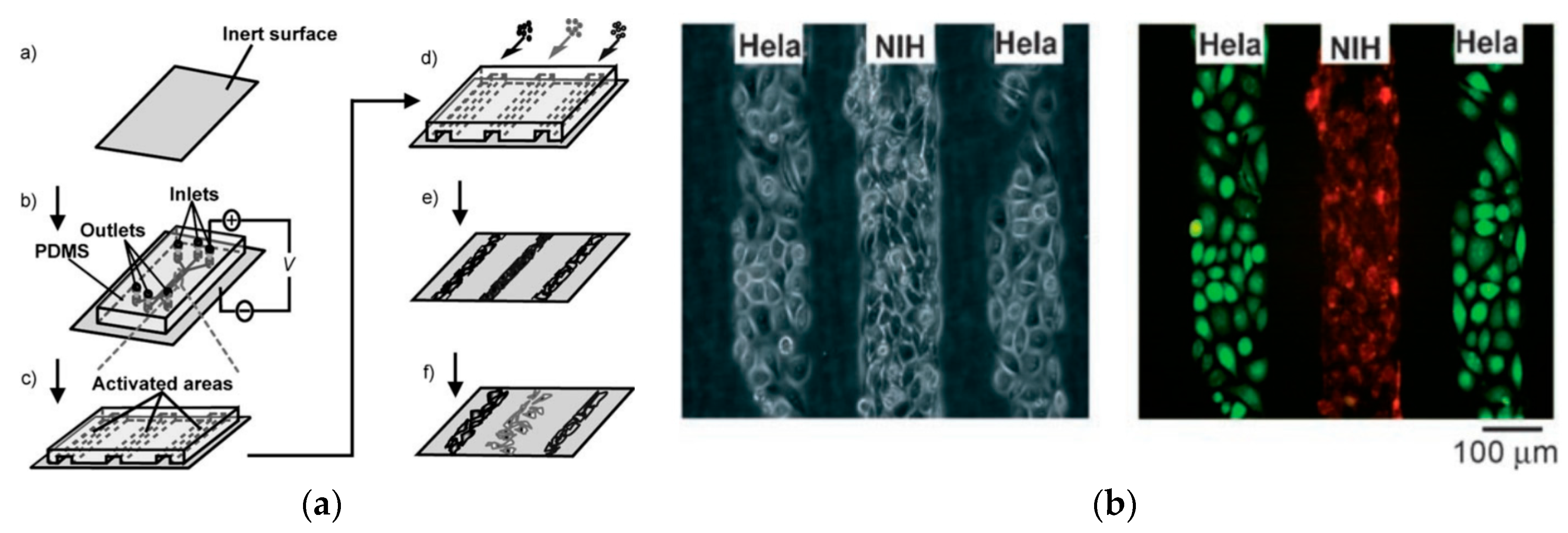
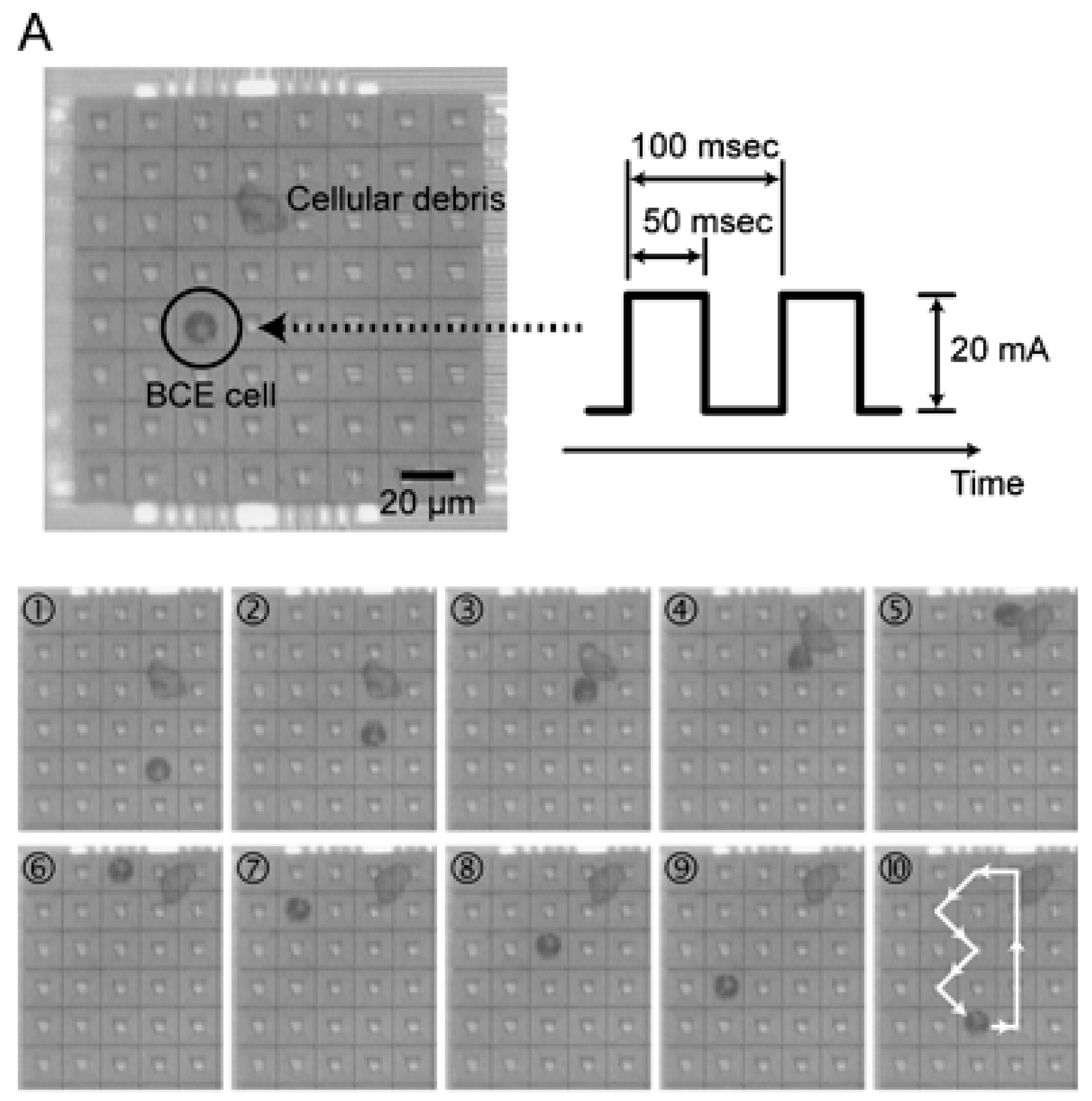
3.1.2. Active Methods
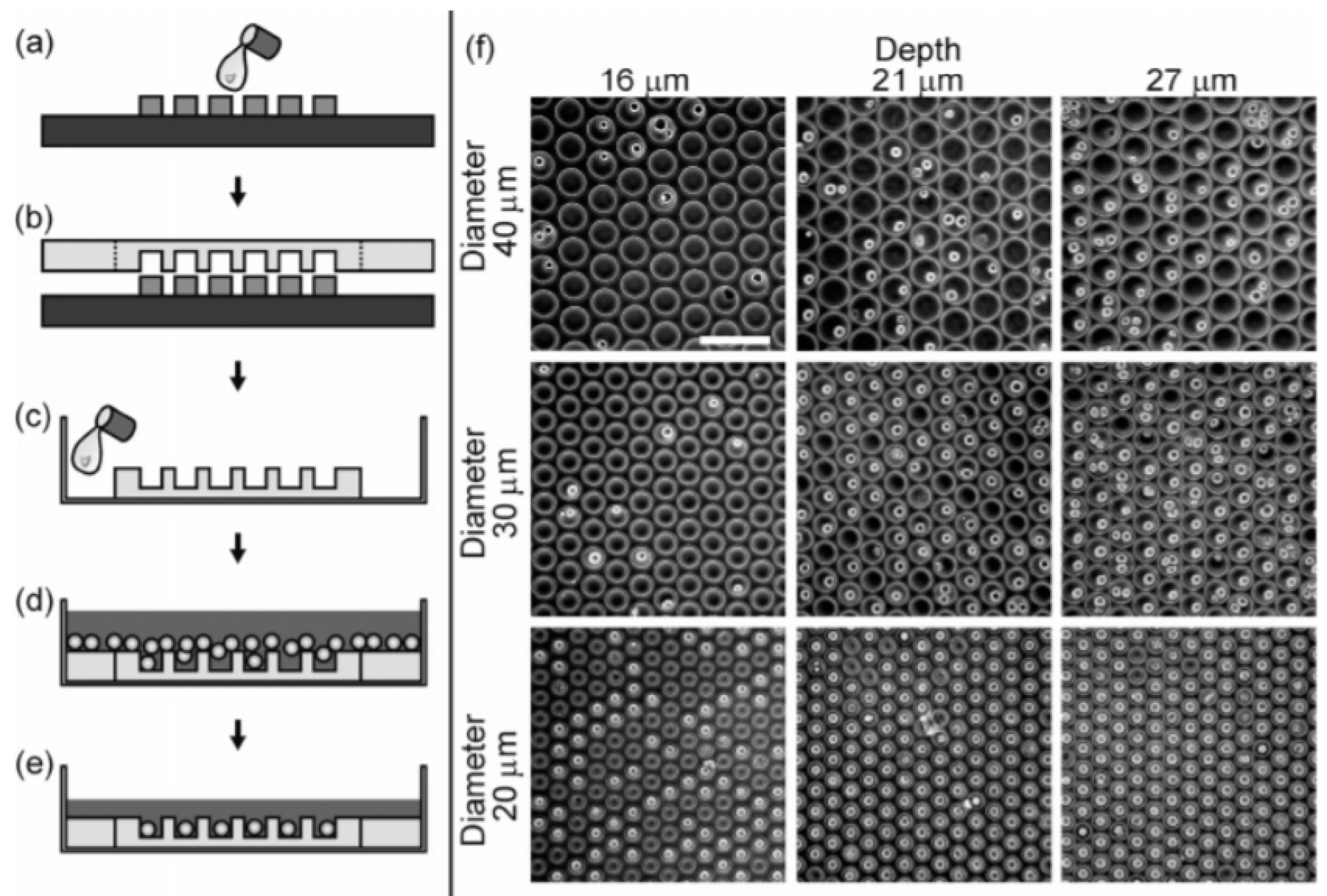
3.1.3. Printing
3.2. Application of Cells Localization to Electrochemical Sensors, then to Transistor-Based Sensors
4. Devices
4.1. Inorganic Transistors
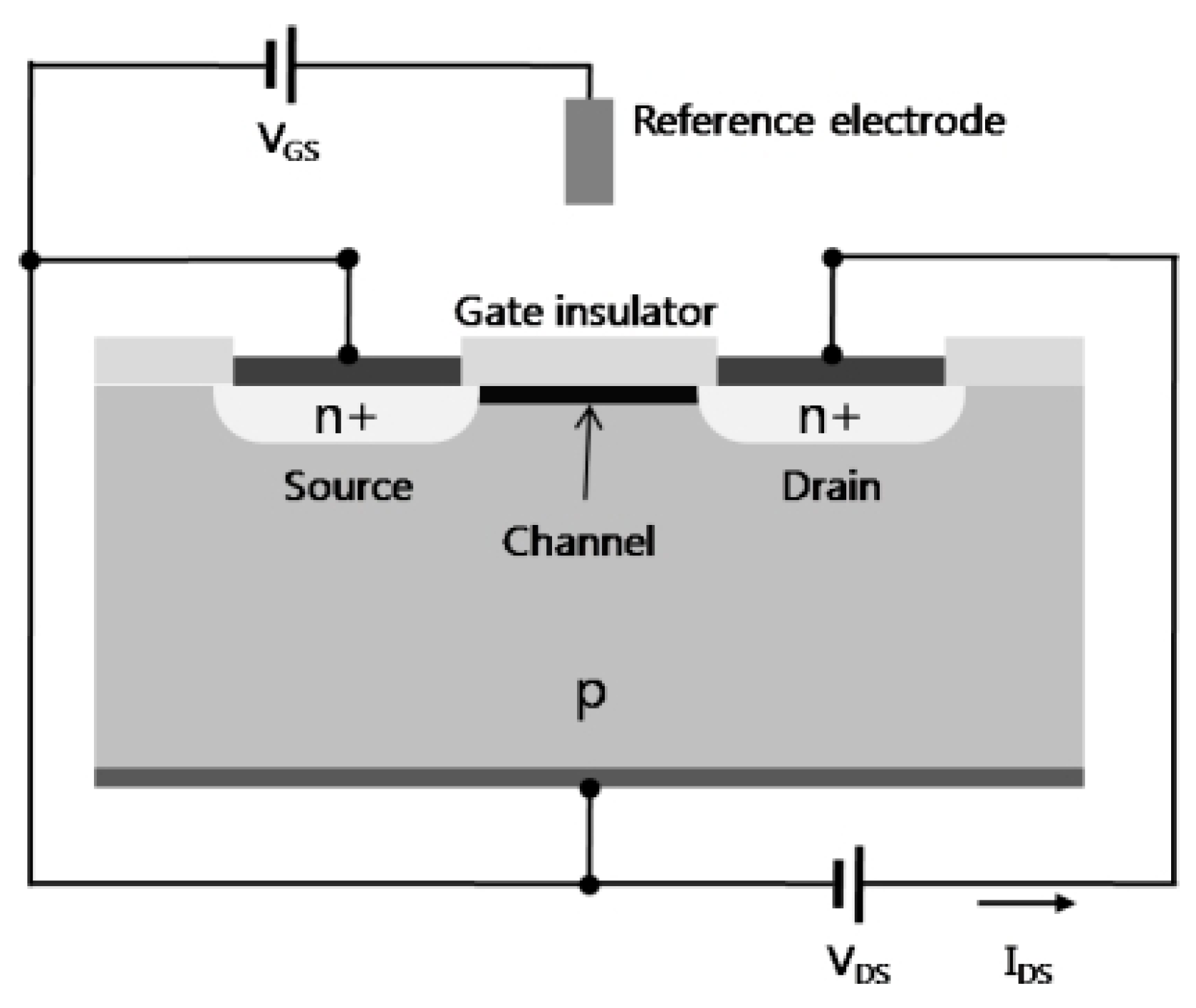
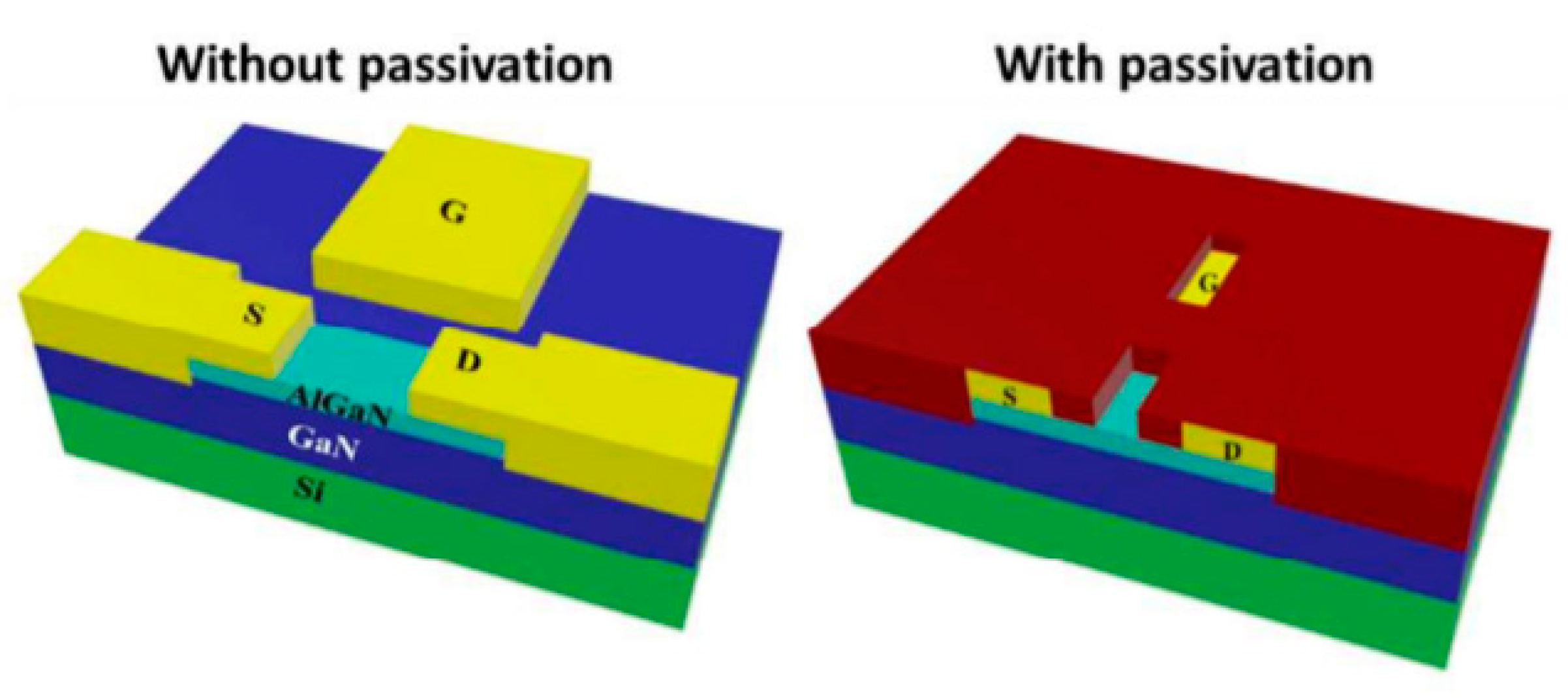
4.1.1. Field Effect Transistors
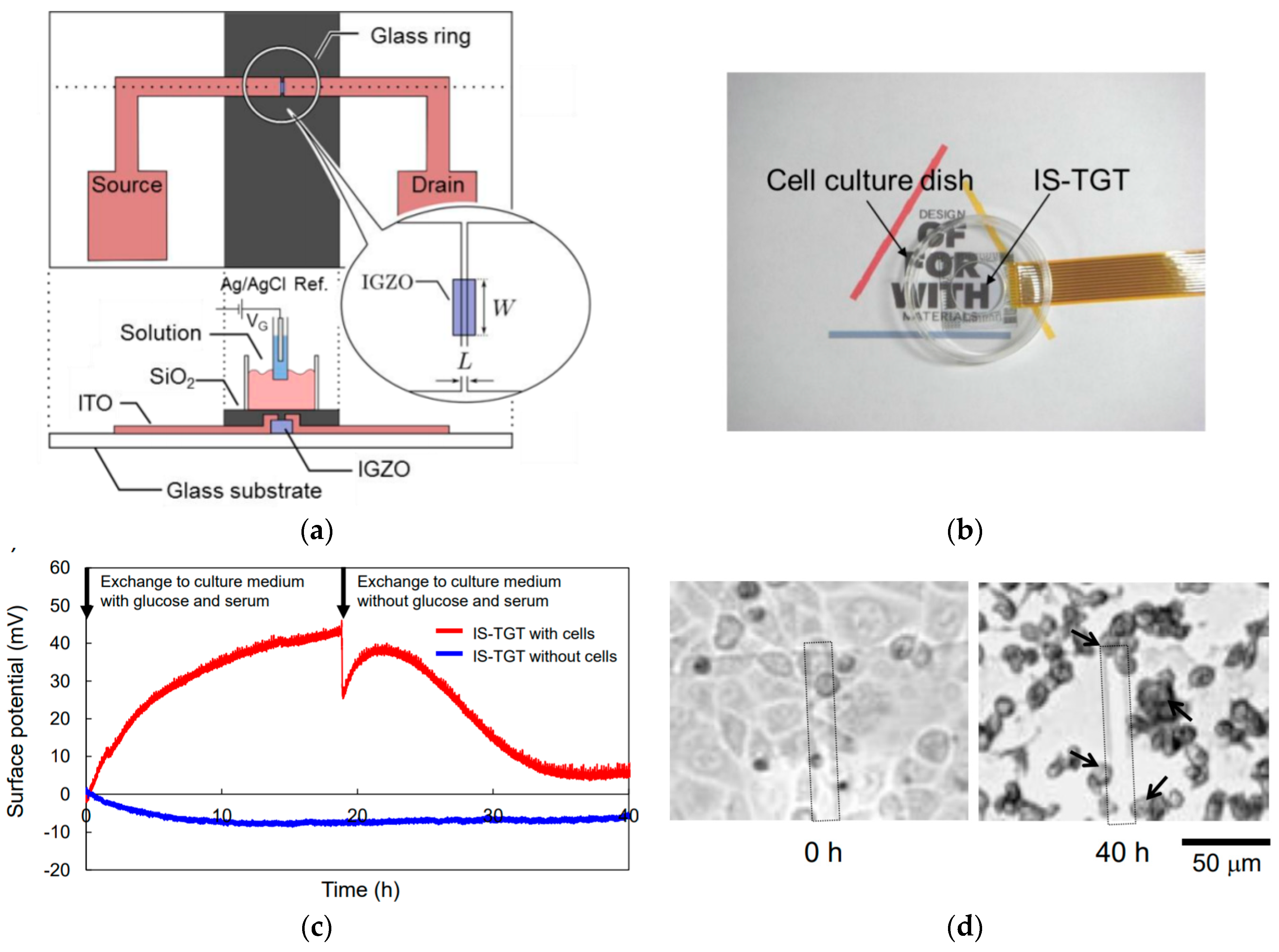
4.1.2. Electrolyte-Gated Field-Effect Transistors
4.2. Organic Transistors
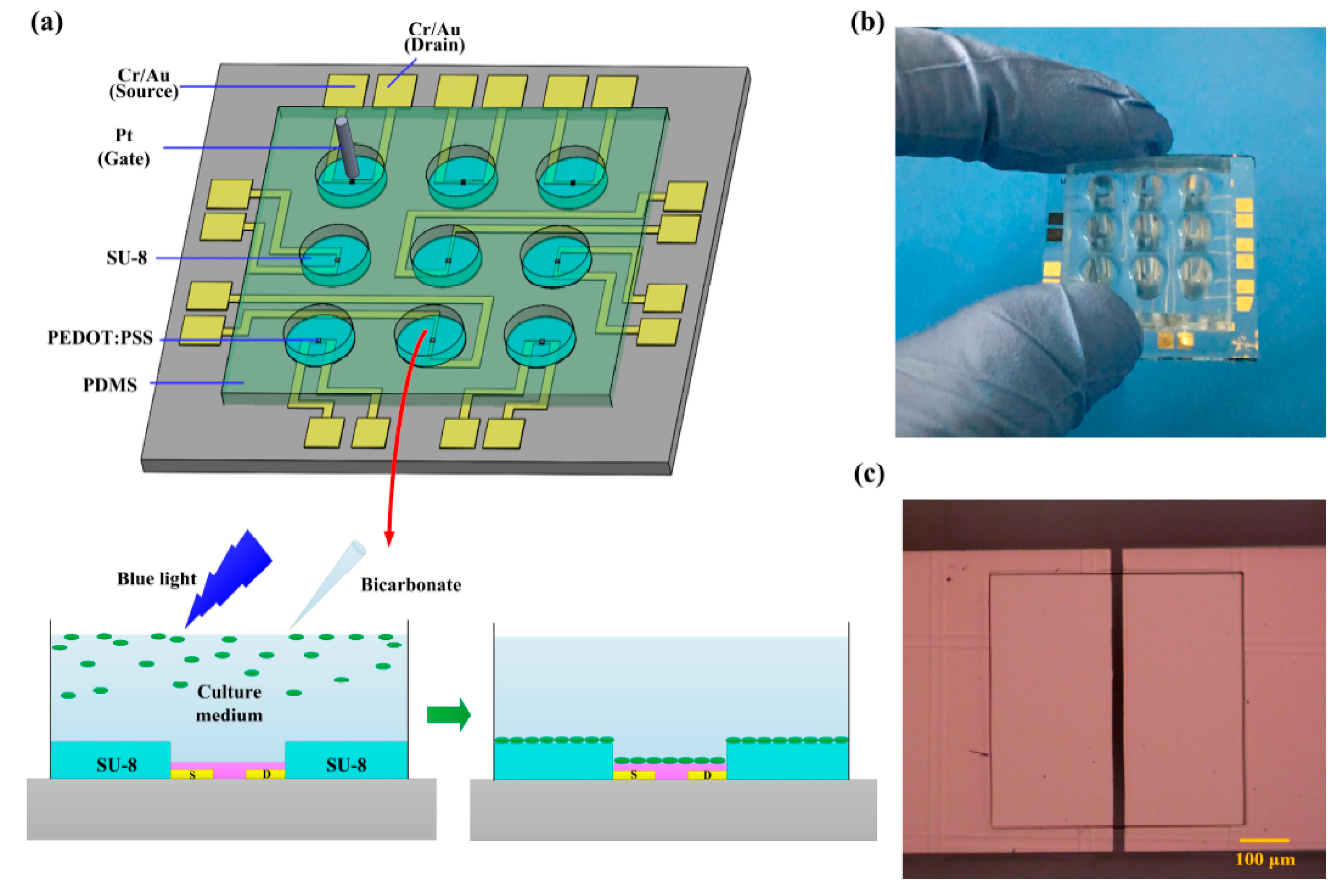
4.2.1. Organic Electrochemical Transistors
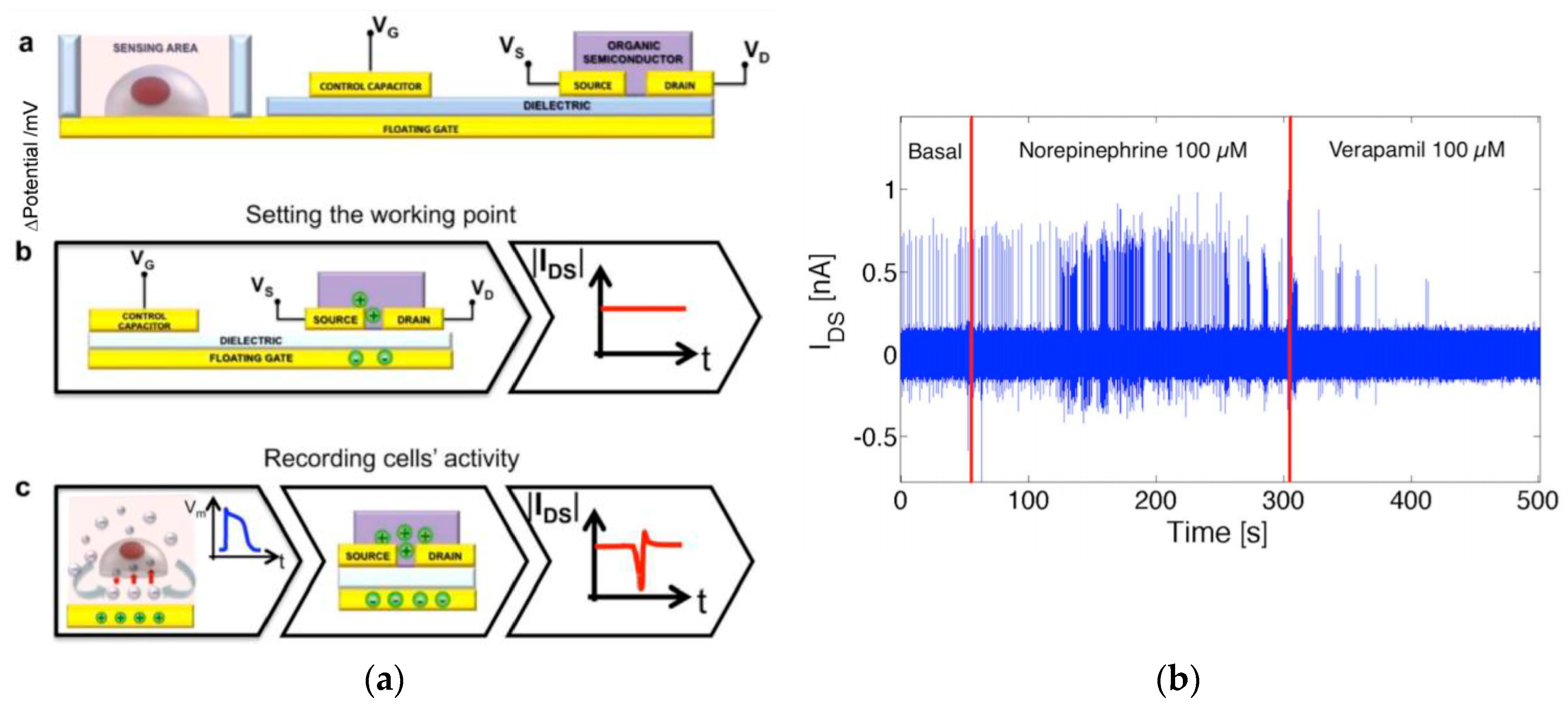
4.2.2. Organic Field-Effect Transistors
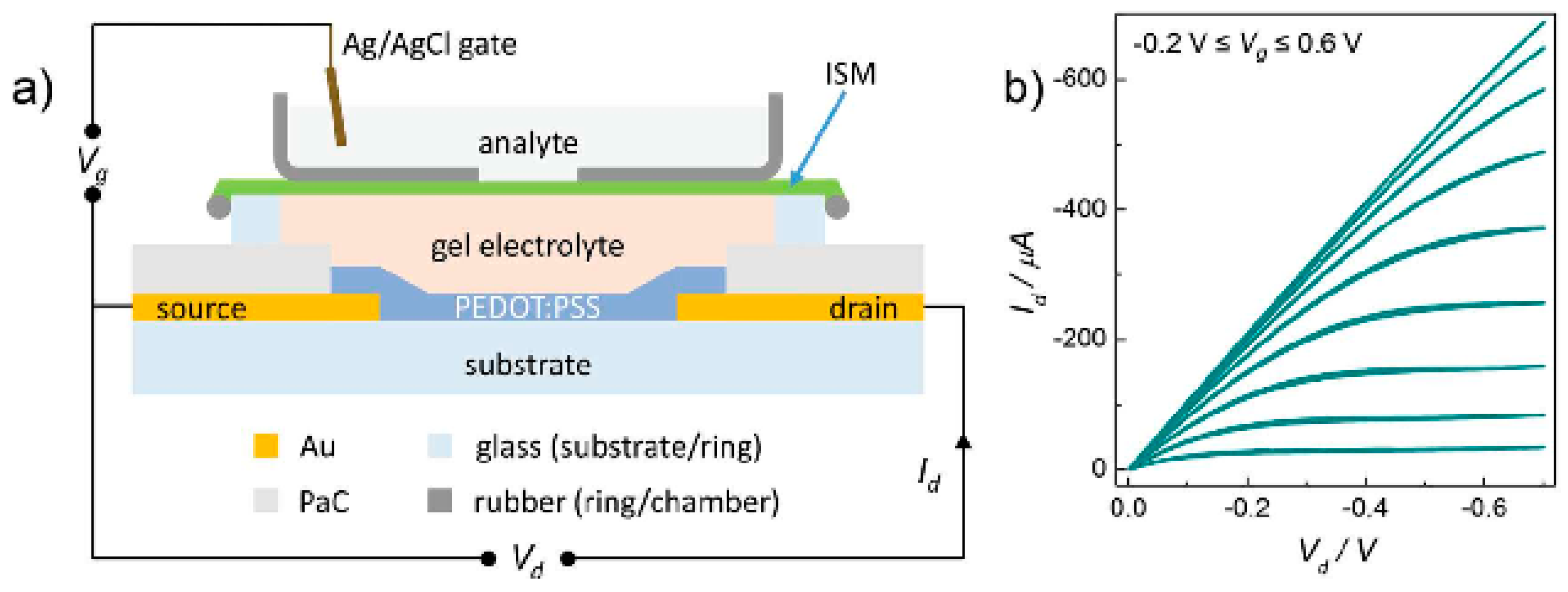
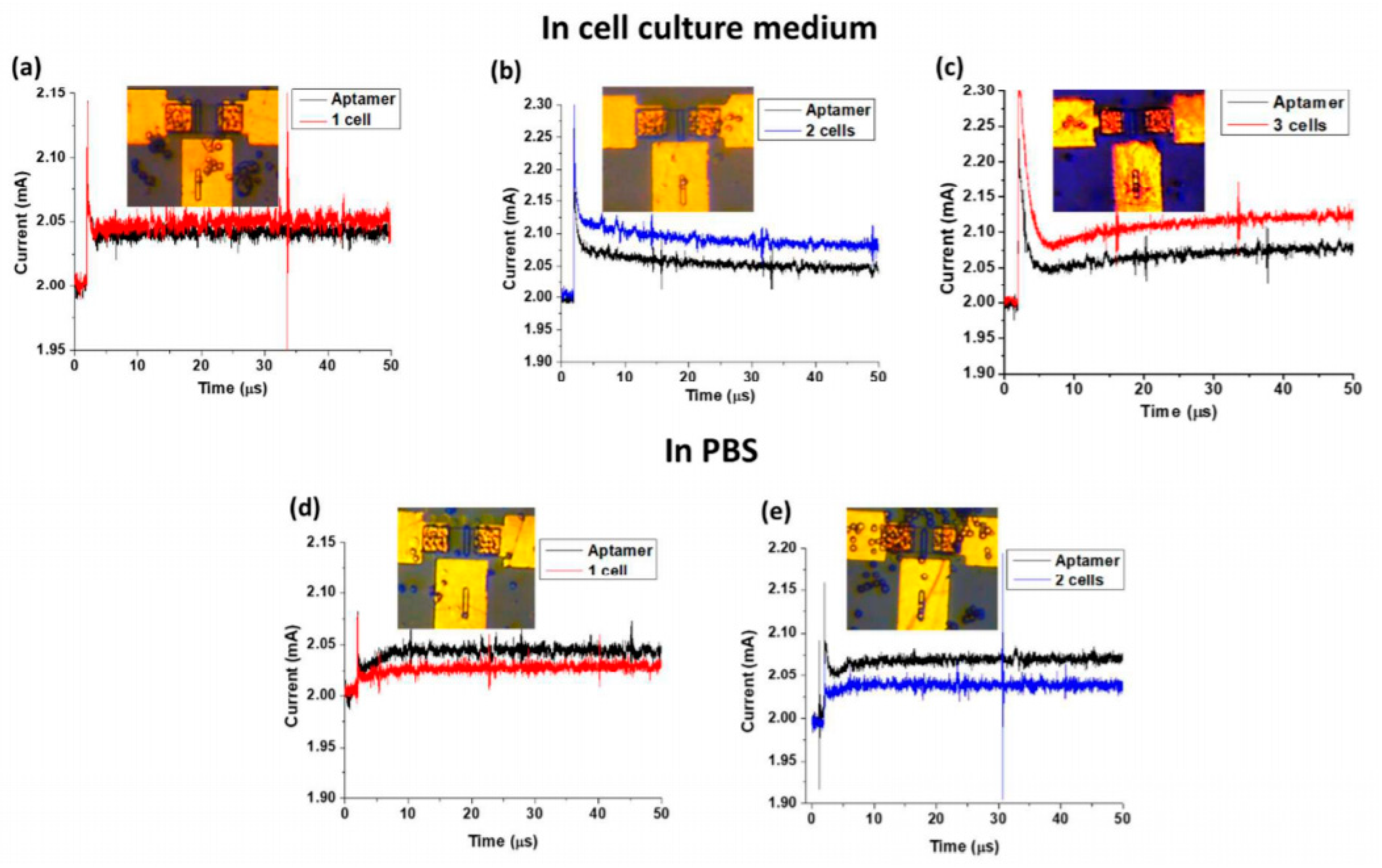
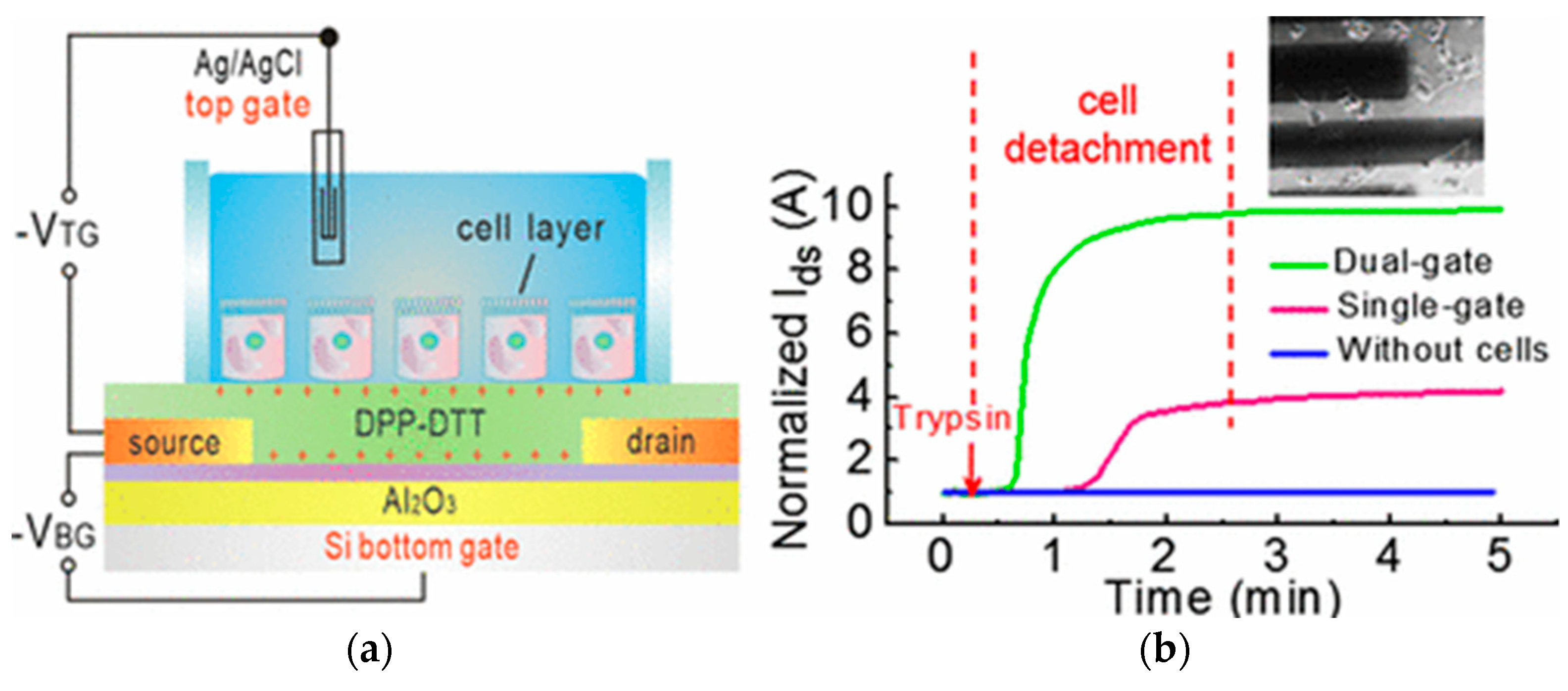
4.2.3. Electrolyte-Gated Organic Field Effect Transistors
5. Conclusions
Funding
Conflicts of Interest
References
- McConnell, H.M.; Owicki, J.C.; Parce, J.W.; Miller, D.L.; Baxter, G.T.; Wada, H.G.; Pitchford, S. The cytosensor microphysiometer: Biological applications of silicon technology. Science 1992, 257, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Nishimura, K.; Miyazawa, Y.; Saito, A.; Abe, H.; Kajisa, T. Ion Sensitive Transparent-Gate Transistor for Visible Cell Sensing. Anal. Chem. 2017, 89, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
- Nabovati, G.; Ghafar-Zadeh, E.; Letourneau, A.; Sawan, M. Towards High Throughput Cell Growth Screening: A New CMOS Biosensor Array for Life Science Applications. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Xiao, K.; Tao, M.; Nie, L.; Liu, D.; Ke, S.; Zeng, X.; Hu, Z.; Lin, P.; Zhang, Y. A Novel Organic Electrochemical Transistor-Based Platform for Monitoring the Senescent Green Vegetative Phase of Haematococcus pluvialis Cells. Sensors 2017, 17, 1997. [Google Scholar] [CrossRef] [PubMed]
- Ramuz, M.; Hama, A.; Rivnay, J.; Leleux, P.; Owens, R.M. Monitoring of cell layer coverage and differentiation with the organic electrochemical transistor. J. Mater. Chem. B 2015, 3, 5971–5977. [Google Scholar] [CrossRef]
- Nabovati Khormazard, G. A Label Free CMOS-Based Smart Petri Dish for Cellular Analysis. Ph.D. Thesis, École Polytechnique de Montréal, Montréal, QC, Canada, 20 June 2016. [Google Scholar]
- Braendlein, M.; Pappa, A.M.; Ferro, M.; Lopresti, A.; Acquaviva, C.; Mamessier, E.; Malliaras, G.G.; Owens, R.M. Lactate detection in tumor cell cultures using organic transistor circuits. Adv. Mater. 2017, 29, 1605744. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, I.; Tonelli, D.; Mariani, F.; Scavetta, E.; Marzocchi, M.; Fraboni, B. Selective detection of dopamine with an all PEDOT: PSS Organic Electrochemical Transistor. Sci. Rep. 2016, 6, 35419. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Arbault, S.; Guille, M.; Lemaître, F. The nature and efficiency of neurotransmitter exocytosis also depend on physicochemical parameters. ChemPhysChem 2007, 8, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meunier, A.; Fulcrand, R.; Sella, C.; Amatore, C.; Thouin, L.; Lemaitre, F.; Guille-Collignon, M. Multi-Chambers Microsystem for Simultaneous and Direct Electrochemical Detection of Reactive Oxygen and Nitrogen Species Released by Cell Populations. Electroanalysis 2016, 28, 1865–1872. [Google Scholar] [CrossRef]
- Spanu, A.; Lai, S.; Cosseddu, P.; Tedesco, M.; Martinoia, S.; Bonfiglio, A. An organic transistor-based system for reference-less electrophysiological monitoring of excitable cells. Sci. Rep. 2015, 5, 8807. [Google Scholar] [CrossRef] [PubMed]
- Stett, A.; Egert, U.; Guenther, E.; Hofmann, F.; Meyer, T.; Nisch, W.; Haemmerle, H. Biological application of microelectrode arrays in drug discovery and basic research. Anal. Bioanal. Chem. 2003, 377, 486–495. [Google Scholar] [CrossRef] [PubMed]
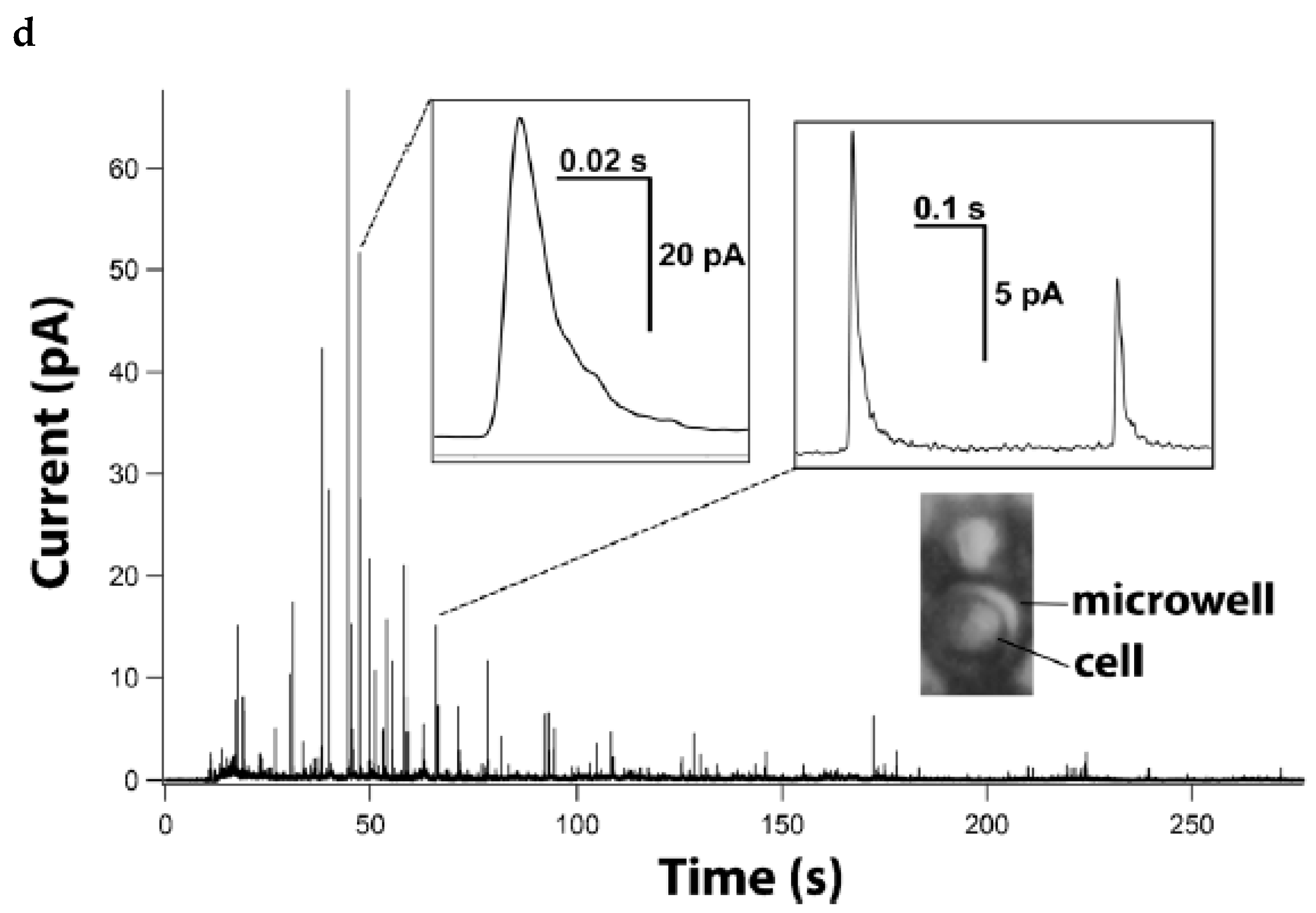
- Huang, M.; Delacruz, J.B.; Ruelas, J.C.; Rathore, S.S.; Lindau, M. Surface-modified CMOS IC electrochemical sensor array targeting single chromaffin cells for highly parallel amperometry measurements. Pflugers Arch. Eur. J. Physiol. 2018, 470, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Curto, V.F.; Marchiori, B.; Hama, A.; Pappa, A.M.; Ferro, M.P.; Braendlein, M.; Rivnay, J.; Fiocchi, M.; Malliaras, G.G.; Ramuz, M.; et al. Organic transistor platform with integrated microfluidics for in-line multi-parametric in vitro cell monitoring. Microsyst. Nanoeng. 2017, 3, 17028. [Google Scholar] [CrossRef]
- Schwartz, M.P.; Derfus, A.M.; Alvarez, S.D.; Bhatia, S.N.; Sailor, M.J. The smart petri dish: A nanostructured photonic crystal for real-time monitoring of living cells. Langmuir 2006, 22, 7084–7090. [Google Scholar] [CrossRef] [PubMed]
- Veliev, F.; Han, Z.; Kalita, D.; Briançon-Marjollet, A.; Bouchiat, V.; Delacour, C. Recording Spikes Activity in Cultured Hippocampal Neurons Using Flexible or Transparent Graphene Transistors. Front. Neurosci. 2017, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, G.; Idzko, A.L.; Yadav, A.; Martin, E.; Thalhammer, S. Toward Cheap Disposable Sensing Devices for Biological Assays. IEEE Trans. Nanotechnol. 2010, 9, 527–532. [Google Scholar] [CrossRef]
- Lin, P.; Yan, F.; Yu, J.; Chan, H.L.; Yang, M. The Application of Organic Electrochemical Transistors in Cell-Based Biosensors. Adv. Mater. 2010, 22, 3655–3660. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, F. Organic Thin-Film Transistors for Chemical and Biological Sensing. Adv. Mater. 2012, 24, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Strakosas, X.; Bongo, M.; Owens, R.M. The organic electrochemical transistor for biological applications. J. Appl. Polym. Sci. 2015, 132, 41735. [Google Scholar] [CrossRef]
- Jimison, L.H.; Tria, S.A.; Khodagholy, D.; Gurfinkel, M.; Lanzarini, E.; Hama, A.; Malliaras, G.G.; Owens, R.M. Measurement of barrier tissue integrity with an organic electrochemical transistor. Adv. Mater. 2012, 24, 5919–5923. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C., Jr.; Wolf, R.; Granger, D.; Taylor, Z. Continuous recording of blood oxygen tensions by polarography. J. Appl. Physiol. 1953, 6, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Yao, T. A chemically-modified enzyme membrane electrode as an amperometric glucose sensor. Anal. Chim. Acta 1983, 148, 27–33. [Google Scholar] [CrossRef]
- Cass, A.E.G.; Davis, G.; Francis, G.D.; Hill, H.A.O.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.L.; Turner, A.P.F. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, G.; Idzko, A.L.; Yadav, A.; Thalhammer, S. Organic ISFET Based on Poly (3-hexylthiophene). Sensors 2010, 10, 2262. [Google Scholar] [CrossRef] [PubMed]
- Sessolo, M.; Rivnay, J.; Bandiello, E.; Malliaras, G.G.; Bolink, H.J. Ion-Selective Organic Electrochemical Transistors. Adv. Mater. 2014, 26, 4803–4807. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Rai, P.; Jung, S.; Varadan, V.K. In vitro evaluation of flexible pH and potassium ion-sensitive organic field effect transistor sensors. Appl. Phys. Lett. 2008, 92, 233304. [Google Scholar] [CrossRef]
- Johnson, I.S.; Boder, G.B. Metabolites from Animal and Plant Cell Culture. Adv. Appl. Microbiol. 1972, 15, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Mak, C.H.; Liao, C.; Fu, Y.; Zhang, M.; Tang, C.Y.; Tsang, Y.H.; Chan, H.L.W.; Yan, F. Highly-sensitive epinephrine sensors based on organic electrochemical transistors with carbon nanomaterial modified gate electrodes. J. Mater. Chem. C 2015, 3, 6532–6538. [Google Scholar] [CrossRef]
- Tang, H.; Lin, P.; Chan, H.L.; Yan, F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. Biosens. Bioelectron. 2011, 26, 4559–4563. [Google Scholar] [CrossRef] [PubMed]
- Drummond, T.G.; Hill, M.G.; Barton, J.K. Electrochemical DNA sensors. Nat. Biotechnol. 2003, 21, 1192. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Kerman, K.; Tamiya, E. An Overview of Label-Free Electrochemical Protein Sensors. Sensors 2007, 7, 3442–3458. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Electrochemical biosensors for hormone analyses. Biosens. Bioelectron. 2015, 68, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, R.; Kolusheva, S. Carbohydrate biosensors. Chem. Rev. 2004, 104, 5987–6016. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Chandra, P.; Goyal, R.N.; Shim, Y.B. A review on determination of steroids in biological samples exploiting nanobio-electroanalytical methods. Anal. Chim. Acta 2013, 762, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Reder-Christ, K.; Bendas, G. Biosensor applications in the field of antibiotic research—A review of recent developments. Sensors 2011, 11, 9450–9466. [Google Scholar] [CrossRef] [PubMed]
- Folch, A.; Toner, M. Microengineering of Cellular Interactions. Ann. Rev. Biomed. Eng. 2000, 2, 227–256. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.; Voldman, J. Dielectrophoretic Traps for Single-Particle Patterning. Biophys. J. 2005, 88, 2193–2205. [Google Scholar] [CrossRef] [PubMed]
- Walt, D.R. Imaging optical sensor arrays. Curr. Opin. Chem. Biol. 2002, 6, 689–695. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, G.H.; Park, J.Y. Microwell Fabrication Methods and Applications for Cellular Studies. Biomed. Eng. Lett. 2013, 3, 131–137. [Google Scholar] [CrossRef]
- Revzin, A.; Tompkins, R.G.; Toner, M. Surface Engineering with Poly(ethylene glycol) Photolithography to Create High-Density Cell Arrays on Glass. Langmuir 2003, 19, 9855–9862. [Google Scholar] [CrossRef]
- Rettig, J.R.; Folch, A. Large-Scale Single-Cell Trapping and Imaging Using Microwell Arrays. Anal. Chem. 2005, 77, 5628–5634. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shah, P.; Phillips, C.; Sims, C.E.; Allbritton, N.L. Trapping cells on a stretchable microwell array for single-cell analysis. Anal. Bioanal. Chem. 2012, 402, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.S.; Takayama, S.; Ostuni, E.; Ingber, D.E.; Whitesides, G.M. Patterning proteins and cells using soft lithography. Biomaterials 1999, 20, 2363–2376. [Google Scholar] [CrossRef]
- Nilsson, J.; Evander, M.; Hammarström, B.; Laurell, T. Review of cell and particle trapping in microfluidic systems. Anal. Chim. Acta 2009, 649, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Lindström, S.; Andersson-Svahn, H. Overview of single-cell analyses: Microdevices and applications. Lab Chip 2010, 10, 3363–3372. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.G.; Revzin, A.; Simonian, A.; Reeves, T.; Pishko, M. Control of Mammalian Cell and Bacteria Adhesion on Substrates Micropatterned with Poly(ethylene glycol) Hydrogels. Biomed. Microdevices 2003, 5, 11–19. [Google Scholar] [CrossRef]
- Bolin, M.H.; Svennersten, K.; Nilsson, D.; Sawatdee, A.; Jager, E.W.; Richter-Dahlfors, A.; Berggren, M. Active Control of Epithelial Cell-Density Gradients Grown Along the Channel of an Organic Electrochemical Transistor. Adv. Mater. 2009, 21, 4379–4382. [Google Scholar] [CrossRef] [PubMed]
- Shaik, F.A.; Cathcart, G.; Ihida, S.; Lereau-Bernier, M.; Leclerc, E.; Sakai, Y.; Toshiyoshi, H.; Tixier-Mita, A. Thin-film-transistor array: An exploratory attempt for high throughput cell manipulation using electrowetting principle. J. Micromech. Microeng. 2017, 27, 054001. [Google Scholar] [CrossRef]
- Yousaf, M.N.; Houseman, B.T.; Mrksich, M. Using electroactive substrates to pattern the attachment of two different cell populations. Proc. Natl. Acad. Sci. USA 2001, 98, 5992–5996. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, B.; Ji, H.; Han, D.; Chen, S.; Tian, F.; Jiang, X. A Method for Patterning Multiple Types of Cells by Using Electrochemical Desorption of Self-Assembled Monolayers within Microfluidic Channels. Angew. Chem. Int. Ed. 2007, 46, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Liu, Y.; Ham, D.; Westervelt, R.M. Integrated cell manipulation system—CMOS/microfluidic hybrid. Lab Chip 2007, 7, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Seddon, B.J.; Shao, Y.; Girault, H.H. Printed microelectrode array and amperometric sensor for environmental monitoring. Electrochim. Acta 1994, 39, 2377–2386. [Google Scholar] [CrossRef]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Choi, J.S.; Kim, B.S.; Choi, Y.C.; Cho, Y.W. Piezoelectric inkjet printing of polymers: Stem cell patterning on polymer substrates. Polymer 2010, 51, 2147–2154. [Google Scholar] [CrossRef]
- Liberski, A.R.; Delaney, J.T., Jr.; Schubert, U.S. One Cell−One Well: A New Approach to Inkjet Printing Single Cell Microarrays. ACS Comb. Sci. 2010, 13, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Stybayeva, G.; Silangcruz, J.; Yan, J.; Ramanculov, E.; Dandekar, S.; George, M.D.; Revzin, A. Detecting cytokine release from single T-cells. Anal. Chem. 2009, 81, 8150–8156. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ueda, E.; Levkin, P.A. Droplet Microarrays: From Surface Patterning to High-Throughput Applications. Adv. Mater. 2018, 1706111. [Google Scholar] [CrossRef] [PubMed]
- Barbulovic-Nad, I.; Lucente, M.; Sun, Y.; Zhang, M.; Wheeler, A.R.; Bussmann, M. Bio-Microarray Fabrication Techniques—A Review. Crit. Rev. Biotechnol. 2008, 26, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Onuki-Nagasaki, R.; Fukuda, J.; Enomoto, J.; Yamaguchi, S.; Miyake, M. Development of super-dense transfected cell microarrays generated by piezoelectric inkjet printing. Lab Chip 2013, 13, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, H.; Tanabe, T. Cell micropatterning on an albumin-based substrate using an inkjet printing technique. J. Biomed. Mater. Res. 2009, 91A, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Ueno, A.; Akiyama, Y.; Morishima, K. Cell patterning through inkjet printing of one cell per droplet. Biofabrication 2012, 4, 045005. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.W.; Smith, P. Inkjet Printing for Biomedical Applications. In Cell-Based Microarrays. Methods in Molecular Biology; Ertl, P., Rothbauer, M., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1771. [Google Scholar]
- Thomas, C.A., Jr.; Springer, P.A.; Loeb, G.E.; Berwald-Netter, Y.; Okun, L.M. A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp. Cell Res. 1972, 74, 61–66. [Google Scholar] [CrossRef]
- Siu, W.; Cobbold, R.S.C. Characteristics of a multicathode polarographic oxygen electrode. Med. Biol. Eng. 1976, 14, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Shiku, H.; Matsue, T. Bioelectrochemical applications of microelectrode arrays in cell analysis and engineering. Curr. Opin. Electrochem. 2017, 5, 146–151. [Google Scholar] [CrossRef]
- Lilienfeld, J.E. Method and Apparatus for Controlling Electric Currents. U.S. Patent 1,745,175, 18 January 1930. [Google Scholar]
- Horowitz, G. Organic Field-Effect Transistors. Adv. Mater. 1998, 10, 365–377. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, S.K.; Kim, M. Ion-Sensitive Field-Effect Transistor for Biological Sensing. Sensors 2009, 9, 7111–7131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Koley, G.; Walsh, K.; Galloway, A.; Ortinski, P. Application of ion-senstitive field effect transistors for measuring glial cell K+ transport. Sensors 2016, 1–3. [Google Scholar] [CrossRef]
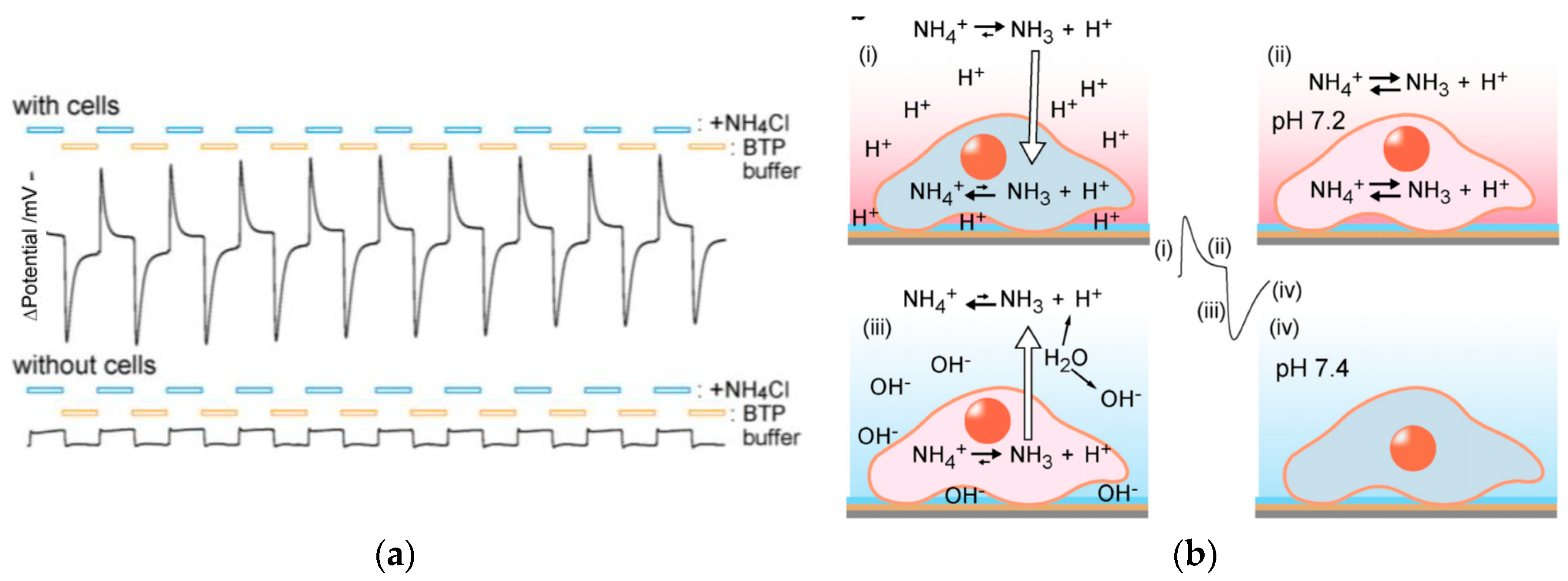
- Imaizumi, Y.; Goda, T.; Schaffhauser, D.F.; Okada, J.I.; Matsumoto, A.; Miyahara, Y. Proton-sensing transistor systems for detecting ion leakage from plasma membranes under chemical stimuli. Acta Biomater. 2017, 50, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, G.; Baur, B.; Wrobel, G.; Ingebrandt, S.; Offenhäusser, A.; Dadgar, A.; Krost, A.; Stutzmann, M.; Eickhoff, M. Recording of cell action potentials with AlGaN/GaN field-effect transistors. Appl. Phys. Lett. 2005, 86, 033901. [Google Scholar] [CrossRef]
- Ižák, T.; Procházka, V.; Sakata, T.; Rezek, B.; Kromka, A. Real-time monitoring of cell activities by diamond solution-gated field effect transistors. Proc. Eng. 2016, 168, 469–472. [Google Scholar] [CrossRef]
- Procházka, V.; Cifra, M.; Kulha, P.; Ižák, T.; Rezek, B.; Kromka, A. Influence of non-adherent yeast cells on electrical characteristics of diamond-based field-effect transistors. Appl. Surf. Sci. 2016, 395, 214–219. [Google Scholar] [CrossRef]
- Pulikkathodi, A.K.; Sarangadharan, I.; Chen, Y.; Lee, G.; Chyi, J.; Lee, G.; Wang, Y. Dynamic Monitoring of Transmembrane Potential Changes: Study of Ion Channels using Electrical Double Layer gated FET Biosensor. Lab Chip 2018, 18, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Coropceanu, V.; Cornil, J.; Da Silva Filho, D.A.; Olivier, Y.; Silbey, R.; Brédas, J.L. Charge transport in organic semiconductors. Chem. Rev. 2007, 107, 926–952. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 17086. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Giovannitti, A.; Sbircea, D.T.; Bandiello, E.; Niazi, M.R.; Hanifi, D.A.; Sessolo, M.; Amassian, A.; Malliaras, G.G.; Rivnay, J.; et al. Molecular Design of Semiconducting Polymers for High-Performance Organic Electrochemical Transistors. J. Am. Chem. Soc. 2016, 138, 10252–10259. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, F.; Chan, H.L. Ion-Sensitive Properties of Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 2010, 2, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Scheiblin, G.; Coppard, R.; Owens, R.M.; Mailley, P.; Malliaras, G.G. Referenceless pH Sensor using Organic Electrochemical Transistors. Adv. Mater. Technol. 2017, 2, 1600141. [Google Scholar] [CrossRef]
- Strakosas, X.; Huerta, M.; Donahue, M.J.; Hama, A.; Pappa, A.M.; Ferro, M.; Ramuz, M.; Rivnay, J.; Owens, R.M. Catalytically enhanced organic transistors for in vitro toxicology monitoring through hydrogel entrapment of enzymes. J. Appl. Polym. Sci. 2016, 134, 44483. [Google Scholar] [CrossRef]
- Yao, C.; Xie, C.; Lin, P.; Yan, F.; Huang, P.; Hsing, I. Organic Electrochemical Transistor Array for Recording Transepithelial Ion Transport of Human Airway Epithelial Cells. Adv. Mater. 2013, 25, 6575–6580. [Google Scholar] [CrossRef] [PubMed]
- Salyk, O.; Víteček, J.; Omasta, L.; Šafaříková, E.; Stříteský, S.; Vala, M.; Weiter, M. Organic Electrochemical Transistor Microplate for Real-Time Cell Culture Monitoring. Appl. Sci. 2017, 7, 998. [Google Scholar] [CrossRef]
- Bystrenova, E.; Jelitai, M.; Tonazzini, I.; Lazar, N.A.; Huth, M.; Stoliar, P.; Dionigi, C.; Cacace, M.G.; Nickel, B.; Madarasz, E.; et al. Neural Networks Grown on Organic Semiconductors. Adv. Funct. Mater. 2008, 18, 1751–1756. [Google Scholar] [CrossRef]
- Caserta, S.; Barra, M.; Manganelli, G.; Tomaiuolo, G.; Filosa, S.; Cassinese, A.; Guido, S. Cardiomyocyte Differentiation of Embryonic Stem Cells on the Surface of Organic Semiconductors. Int. J. Artif. Organs 2013, 36, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.T.; Bonfiglio, A.; Spanu, A.; Lai, S.; Martinooia, S.; Cosseddu, P. An Organic Transistor-Based System for Electrophysiological Monitoring of Cells and Method for the Monitoring of the Cells. Patent Application WO2016124714A1, 11 August 2016. [Google Scholar]
- Zhang, Y.; Li, J.; Li, R.; Sbircea, D.T.; Giovannitti, A.; Xu, J.; Xu, H.; Zhou, G.; Bian, L.; McCulloch, I.; et al. Liquid–Solid Dual-Gate Organic Transistors with Tunable Threshold Voltage for Cell Sensing. ACS Appl. Mater. Int. 2017, 9, 38687–38694. [Google Scholar] [CrossRef] [PubMed]
- Kergoat, L.; Piro, B.; Berggren, M.; Horowitz, G.; Pham, M.C. Advances in organic transistor-based biosensors: From organic electrochemical transistors to electrolyte-gated organic field-effect transistors. Anal. Bioanal. Chem. 2012, 402, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Noël, V.; Piro, B. Electrolytic gated organic field-effect transistors for application in biosensors—A Review. Electronics 2016, 5, 9. [Google Scholar] [CrossRef]
- Piro, B.; Wang, D.; Benaoudia, D.; Tibaldi, A.; Anquetin, G.; Noël, V.; Reisberg, S.; Mattana, G.; Jackson, B. Versatile transduction scheme based on electrolyte-gated organic field-effect transistor used as immunoassay readout system. Biosens. Bioelectron. 2017, 92, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Di Lauro, M.; Casalini, S.; Berto, M.; Campana, A.; Cramer, T.; Murgia, M.; Geoghegan, M.; Bortolotti, C.A.; Biscarini, F. The Substrate is a pH-Controlled Second Gate of Electrolyte-Gated Organic Field-Effect Transistor. ACS Appl. Mater. Interfaces 2016, 8, 31783–31790. [Google Scholar] [CrossRef] [PubMed]
- Fillaud, L.; Petenzi, T.; Pallu, J.; Piro, B.; Mattana, G.; Noel, V. Switchable Hydrogel-Gated Organic Field-Effect Transistors. Langmuir 2018, 34, 3686–3693. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.K.; Nguyen, T.N.; Anquetin, G.; Reisberg, S.; Noël, V.; Mattana, G.; Touzeau, J.; Barbault, F.; Pham, M.C.; Piro, B. Triggering the Electrolyte-Gated Organic Field-Effect Transistor output characteristics through gate functionalization using diazonium chemistry: Application to biodetection of 2, 4-dichlorophenoxyacetic acid. Biosens. Bioelectron. 2018, 113, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Desgranges, C.; Delhommelle, J. Modeling antigen-antibody nanoparticle bioconjugates and their polymorphs. J. Chem. Phys. 2018, 148, 124507. [Google Scholar] [CrossRef] [PubMed]
- Diacci, C.; Berto, M.; Di Lauro, M.; Bianchini, E.; Pinti, M.; Simon, D.T.; Biscarini, F.; Bortolotti, C.A. Label-free detection of interleukin-6 using electrolyte gated organic field effect transistors. Biointerphases 2017, 12, 05F401. [Google Scholar] [CrossRef] [PubMed]
- Berto, M.; Casalini, S.; Di Lauro, M.; Marasso, S.L.; Cocuzza, M.; Perrone, D.; Pinti, M.; Cossarizza, A.; Pirri, C.F.; Simon, D.T.; et al. Biorecognition in Organic Field Effect Transistors Biosensors: The Role of the Density of States of the Organic Semiconductor. Anal. Chem. 2016, 88, 12330–12338. [Google Scholar] [CrossRef] [PubMed]
- Mattana, G.; Loi, A.; Woytasik, M.; Barbaro, M.; Noël, V.; Piro, B. Inkjet-Printing: A New Fabrication Technology for Organic Transistors. Adv. Mater. Technol. 2017, 2, 1700063. [Google Scholar] [CrossRef]
- Cramer, T.; Chelli, B.; Murgia, M.; Barbalinardo, M.; Bystrenova, E.; de Leeuw, D.M.; Biscarini, F. Organic ultra-thin film transistors with a liquid gate for extracellular stimulation and recording of electric activity of stem cell-derived neuronal networks. Phys. Chem. Chem. Phys. 2013, 15, 3897–3905. [Google Scholar] [CrossRef] [PubMed]
- Tarabella, G.; D’Angelo, P.; Cifarelli, A.; Dimonte, A.; Romeo, A.; Berzina, T.; Erokhin, V.; Iannotta, S. A hybrid living/organic electrochemical transistor based on the Physarum polycephalum cell endowed with both sensing and memristive properties. Chem. Sci. 2015, 6, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Stavrinidou, E.; Gabrielsson, R.; Nilsson, K.P.R.; Singh, S.K.; Franco-Gonzalez, J.F.; Volkov, A.V.; Jonsson, M.P.; Grimoldi, A.; Elgland, M.; Zozoulenko, I.V.; et al. In vivo polymerization and manufacturing of wires and supercapacitors in plants. Proc. Natl. Acad. Sci. USA 2017, 201616456. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piro, B.; Mattana, G.; Reisberg, S. Transistors for Chemical Monitoring of Living Cells. Biosensors 2018, 8, 65. https://doi.org/10.3390/bios8030065
Piro B, Mattana G, Reisberg S. Transistors for Chemical Monitoring of Living Cells. Biosensors. 2018; 8(3):65. https://doi.org/10.3390/bios8030065
Chicago/Turabian StylePiro, Benoît, Giorgio Mattana, and Steeve Reisberg. 2018. "Transistors for Chemical Monitoring of Living Cells" Biosensors 8, no. 3: 65. https://doi.org/10.3390/bios8030065
APA StylePiro, B., Mattana, G., & Reisberg, S. (2018). Transistors for Chemical Monitoring of Living Cells. Biosensors, 8(3), 65. https://doi.org/10.3390/bios8030065




