In Vitro and In Vivo SERS Biosensing for Disease Diagnosis
Abstract
1. Introduction
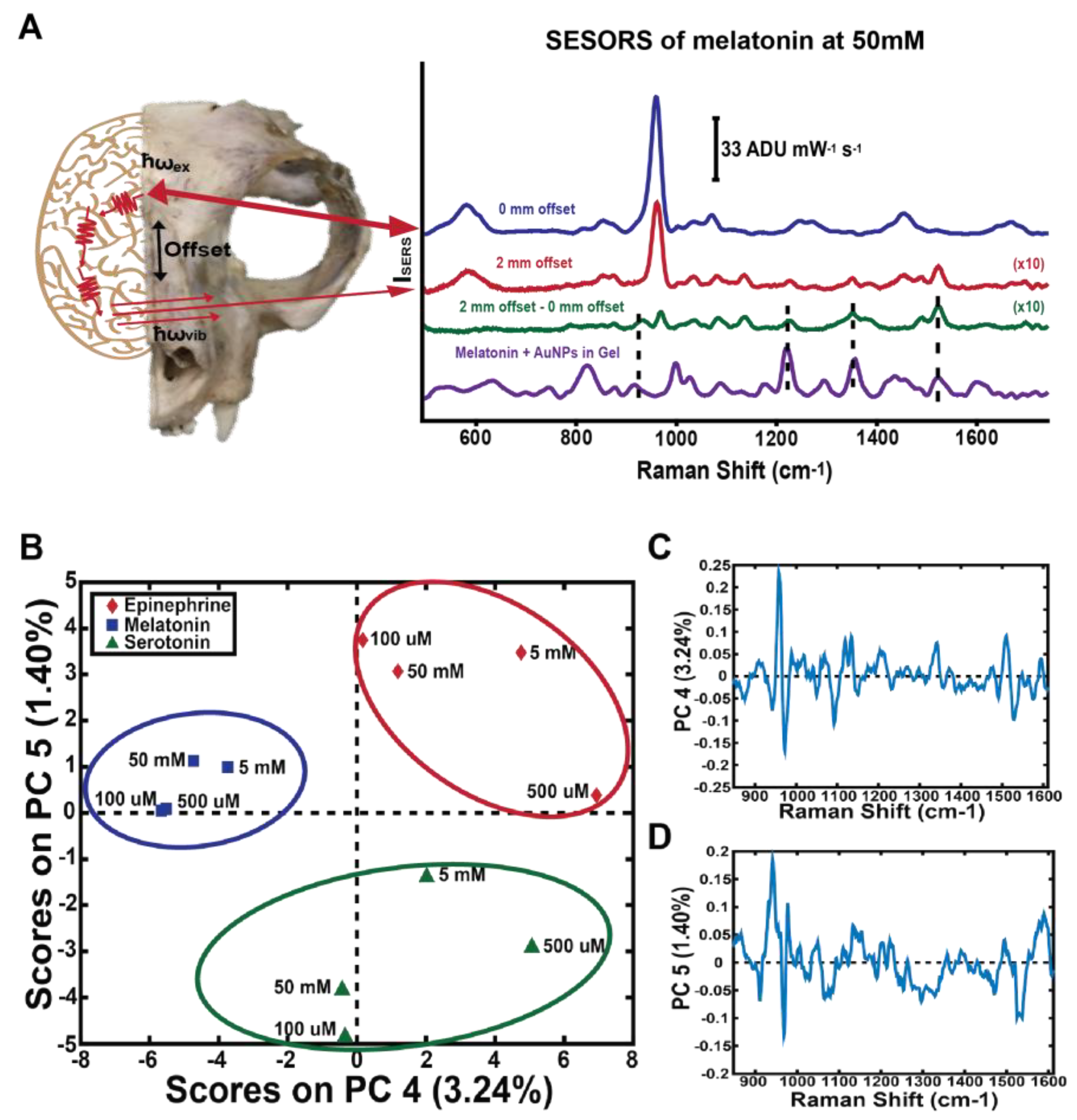
2. Neurological Diseases
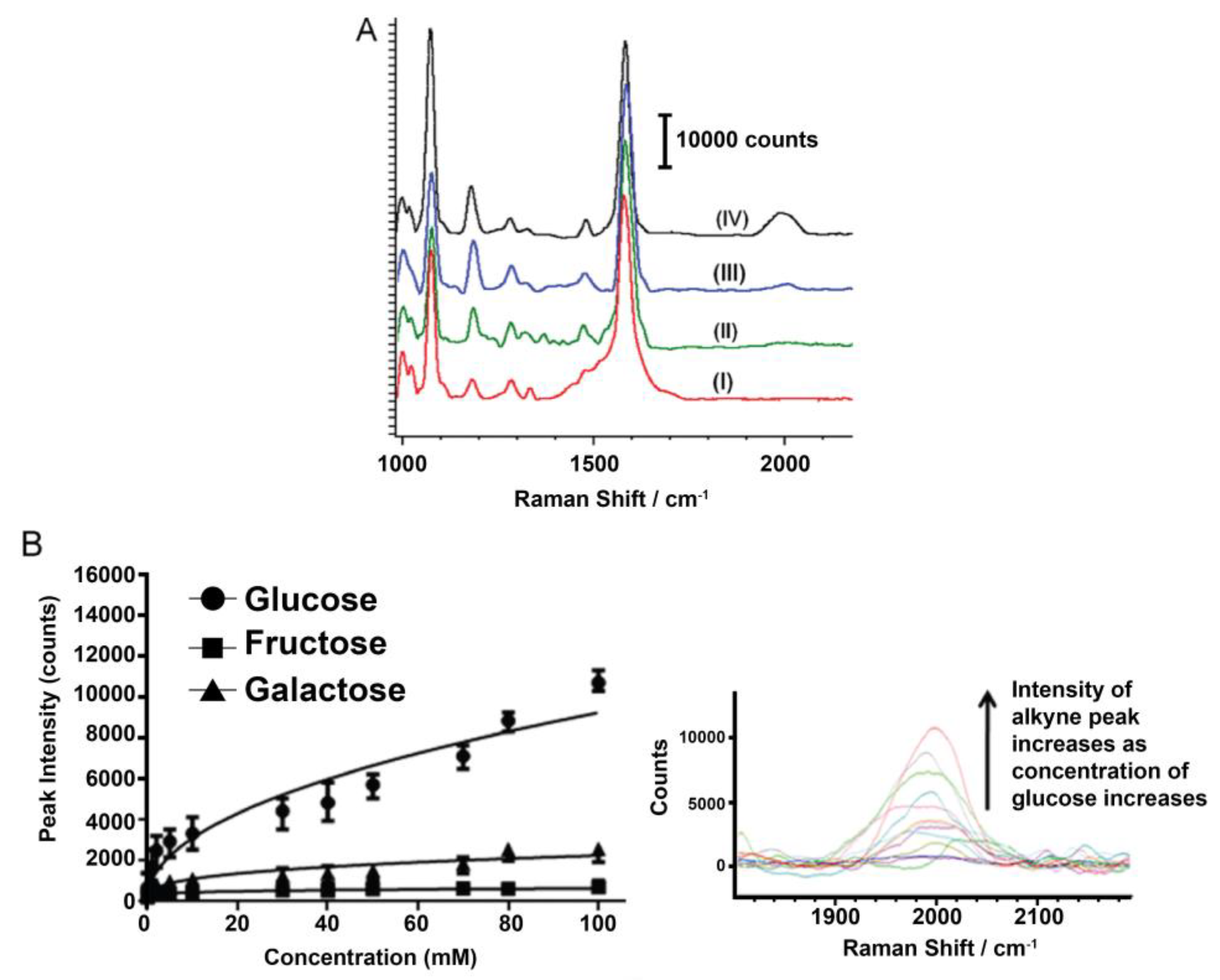
3. Diabetes
4. Cardiovascular Disease
5. Cancer
5.1. Breast Cancer
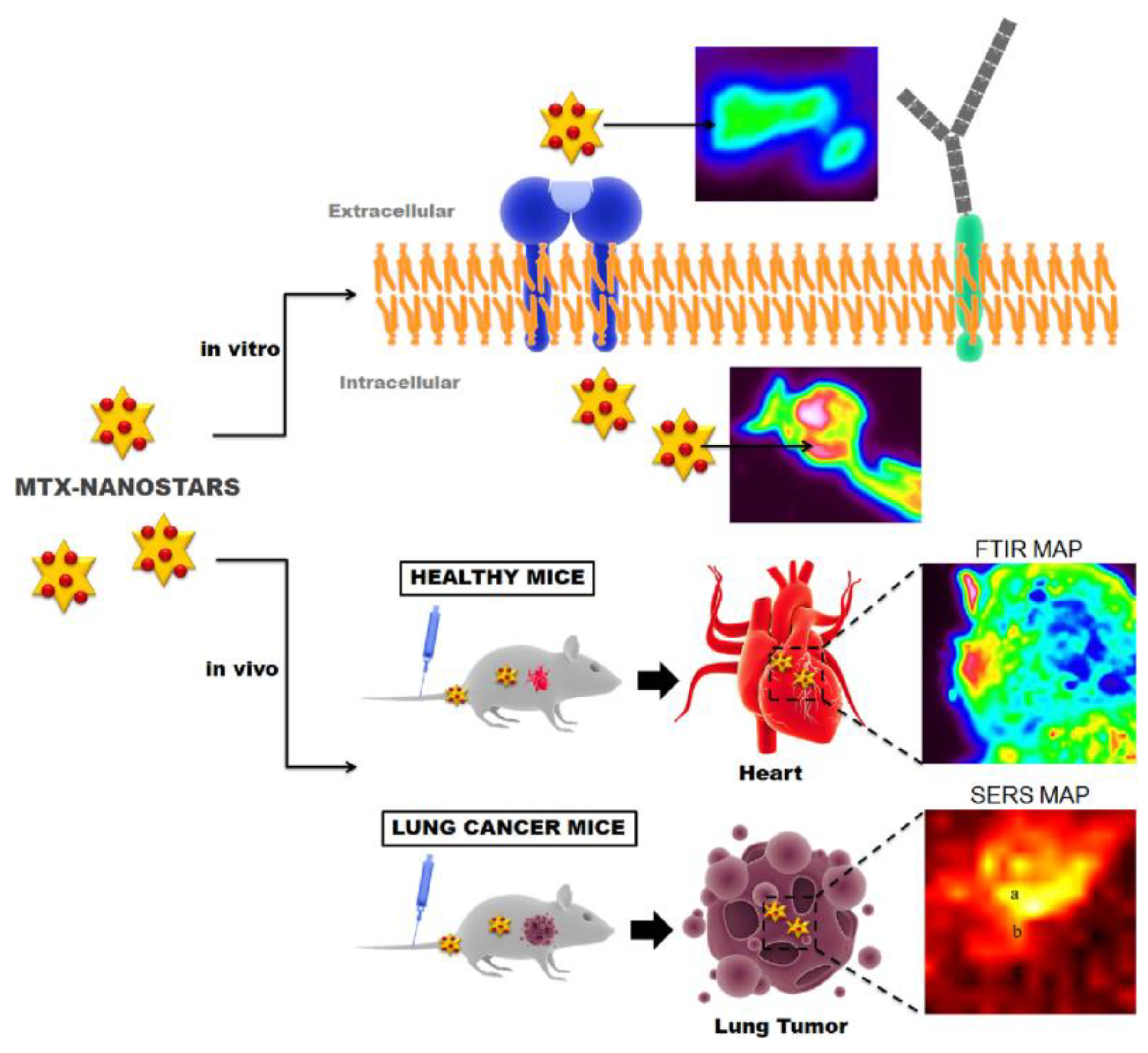
5.2. Lung Cancer
5.3. Colorectal Cancers
5.4. Prostate Cancer
5.5. Additional Cancers
6. Viral Diseases
7. Future Directions
Acknowledgments
Conflicts of Interest
References
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface Raman spectroelectrochemistry part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 1977, 8, 1–20. [Google Scholar] [CrossRef]
- Henry, A.-I.; Sharma, B.; Cardinal, M.F.; Kurouski, D.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy biosensing: In vivo diagnostics and multimodal imaging. Anal. Chem. 2016, 88, 6638–6647. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.X.; Li, J.M.; Liu, B.J.; Liu, D.Y.; Liu, J.X.; Terfort, A.; Xie, Z.X.; Tian, Z.Q.; Ren, B. Uniform gold spherical particles for single-particle surface-enhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 4130–4135. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Murphy, C.J. Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir 2004, 20, 6414–6420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, E. Nanoparticle SERS substrates. In Surface Enhanced Raman Spectroscopy; Schlücker, S., Ed.; Wiley-VCH: Weinheim, Germany, 2011; pp. 39–69. [Google Scholar]
- Liu, Z.; Cheng, L.; Zhang, L.; Jing, C.; Shi, X.; Yang, Z.B.; Long, Y.T.; Fang, J.X. Large-area fabrication of highly reproducible surface enhanced Raman substrate via a facile double sided tape-assisted transfer approach using hollow au-ag alloy nanourchins. Nanoscale 2014, 6, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.-X.; Tzeng, W.-C.; Huang, C.-L. One-pot synthesis of icosahedral silver nanoparticles by using a photoassisted tartrate reduction method under UV light with a wavelength of 310 nm. ChemPhysChem 2016, 17, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Agapov, R.L.; Srijanto, B.; Fowler, C.; Briggs, D.; Lavrik, N.V.; Sepaniak, M.J. Lithography-free approach to highly efficient, scalable SERS substrates based on disordered clusters of disc-on-pillar structures. Nanotechnology 2013, 24, 505302. [Google Scholar] [CrossRef] [PubMed]
- Dieringer, J.A.; McFarland, A.D.; Shah, N.C.; Stuart, D.A.; Whitney, A.V.; Yonzon, C.R.; Young, M.A.; Zhang, X.Y.; Van Duyne, R.P. Surface enhanced Raman spectroscopy: New materials, concepts, characterization tools, and applications. Faraday Discuss. 2006, 132, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Masango, S.S.; Hackler, R.A.; Large, N.; Henry, A.I.; McAnally, M.O.; Schatz, G.C.; Stair, P.C.; Van Duyne, R.P. High-resolution distance dependence study of surface-enhanced Raman scattering enabled by atomic layer deposition. Nano Lett. 2016, 16, 4251–4259. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.; Jamieson, L.E.; Faulds, K.; Graham, D. Surface-enhanced Raman spectroscopy for in vivo biosensing. Nat. Rev. Chem. 2017, 1, 0060. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Liz-Marzán, L.M. SERS-based diagnosis and biodetection. Small 2010, 6, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Radziuk, D.; Moehwald, H. Prospects for plasmonic hot spots in single molecule SERS towards the chemical imaging of live cells. Phys. Chem. Chem. Phys. 2015, 17, 21072–21093. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer. Available online: http://www.who.int/cancer/en/ (accessed on 20 March 2018).
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.-I.; Dieringer, J.A.; Van Duyne, R.P. Creating, characterizing, and controlling chemistry with SERS hot spots. Phys. Chem. Chem. Phys. 2013, 15, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Fernanda Cardinal, M.; Kleinman, S.L.; Greeneltch, N.G.; Frontiera, R.R.; Blaber, M.G.; Schatz, G.C.; Van Duyne, R.P. High-performance SERS substrates: Advances and challenges. MRS Bull. 2013, 38, 615–624. [Google Scholar] [CrossRef]
- Yigit, M.V.; Medarova, Z. In vivo and ex vivo applications of gold nanoparticles for biomedical SERS imaging. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 232–241. [Google Scholar] [PubMed]
- Goodacre, R.; Graham, D.; Faulds, K. Recent developments in quantitative SERS: Moving towards absolute quantification. TrAC Trends Anal. Chem. 2018, 102, 359–368. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Hussein, M.M.A. Neurotoxic effects of silver nanoparticles and the protective role of rutin. Biomed. Pharmacother. 2017, 90, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Huefner, A.; Septiadi, D.; Wilts, B.D.; Patel, I.I.; Kuan, W.L.; Fragniere, A.; Barker, R.A.; Mahajan, S. Gold nanoparticles explore cells: Cellular uptake and their use as intracellular probes. Methods 2014, 68, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Wang, Y.; Dasari, R.R.; Feld, M.S. Near-infrared surface-enhanced Raman scattering (NIR-SERS) of neurotransmitters in colloidal silver solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 481–487. [Google Scholar] [CrossRef]
- Lee, N.S.; Hsieh, Y.Z.; Paisley, R.F.; Morris, M.D. Surface-enhanced Raman spectroscopy of the catecholamine neurotransmitters and related compounds. Anal. Chem. 1988, 60, 442–446. [Google Scholar] [CrossRef] [PubMed]
- McGlashen, M.L.; Davis, K.L.; Morris, M.D. Surface enhanced Raman spectroscopy of neurotransmitters. AIP Conf. Proc. 1989, 191, 707–712. [Google Scholar]
- Tiwari, V.S.; Khetani, A.; Monfared, A.M.T.; Smith, B.; Anis, H.; Trudeau, V.L. Detection of amino acid neurotransmitters by surface enhanced Raman scattering and hollow core photonic crystal fiber. In Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications IV; International Society for Optics and Photonics: Washington, DC, USA, 2012; Volume 8233. [Google Scholar]
- Siek, M.; Kaminska, A.; Kelm, A.; Rolinski, T.; Holyst, R.; Opallo, M.; Niedzioka-Jonsson, J. Electrodeposition for preparation of efficient surface-enhanced Raman scattering-active silver nanoparticle substrates for neurotransmitter detection. Electrochim. Acta 2013, 89, 284–291. [Google Scholar] [CrossRef]
- El Alami, A.; Lagarde, F.; Tamer, U.; Baitoul, M.; Daniel, P. Enhanced Raman spectroscopy coupled to chemometrics for identification and quantification of acetylcholinesterase inhibitors. Vib. Spectrosc. 2016, 87, 27–33. [Google Scholar] [CrossRef]
- Moody, A.S.; Sharma, B. Multi-metal, multi-wavelength surface-enhanced Raman spectroscopy detection of neurotransmitters. ACS Chem. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.R.; Martin, R.S.; Schultz, Z.D. Role of surface adsorption in the surface-enhanced Raman scattering and electrochemical detection of neurotransmitters. J. Phys. Chem. C 2016, 120, 20624–20633. [Google Scholar] [CrossRef] [PubMed]
- Fleming, G.D.; Koch, R.; Perez, J.M.; Cabrera, J.L. Raman and SERS study of N-acetyl-5-methoxytryptamine, melatonin-the influence of the different molecular fragments on the SERS effect. Vib. Spectrosc. 2015, 80, 70–78. [Google Scholar] [CrossRef]
- Song, P.; Guo, X.Y.; Pan, Y.C.; Wen, Y.; Zhang, Z.R.; Yang, H.F. SERS and in situ SERS spectroelectrochemical investigations of serotonin monolayers at a silver electrode. J. Electroanal. Chem. 2013, 688, 384–391. [Google Scholar] [CrossRef]
- El-Said, W.A.; Choi, J.W. In-situ detection of neurotransmitter release from PC12 cells using surface enhanced Raman spectroscopy. Biotechnol. Bioprocess Eng. 2014, 19, 1069–1076. [Google Scholar] [CrossRef]
- Tang, L.J.; Li, S.; Han, F.; Liu, L.Q.; Xu, L.G.; Ma, W.; Kuang, H.; Li, A.K.; Wang, L.B.; Xu, C.L. SERS-active Au@Ag nanorod dimers for ultrasensitive dopamine detection. Biosens. Bioelectron. 2015, 71, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, L.; Cui, G.; Xu, L.; Wu, X.; Kuang, H.; Xu, C. Regioselective plasmonic nano-assemblies for bimodal sub-femtomolar dopamine detection. Nanoscale 2017, 9, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xia, M.; Liang, O.; Sun, K.; Cipriano, A.F.; Schroeder, T.; Liu, H.N.; Xie, Y.H. Label-free SERS selective detection of dopamine and serotonin using graphene-Au nanopyramid heterostructure. Anal. Chem. 2015, 87, 10255–10261. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.L.; Wyss, A.; Esser, N.; Dittrich, P.S. Label-free biosensors based on in situ formed and functionalized microwires in microfluidic devices. Analyst 2015, 140, 7896–7901. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.X.; Chen, Z.P.; Chen, Y.; Liu, Q.; Yu, R.Q. Quantification of dopamine in biological samples by surface-enhanced Raman spectroscopy: Comparison of different calibration models. Chemom. Intell. Lab. Syst. 2017, 169, 87–93. [Google Scholar] [CrossRef]
- An, J.H.; Choi, D.K.; Lee, K.J.; Choi, J.W. Surface-enhanced Raman spectroscopy detection of dopamine by DNA targeting amplification assay in Parkison’s model. Biosens. Bioelectron. 2015, 67, 739–746. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Lee, K.-J.; Choi, J.-W. Gold nanoparticles-based barcode analysis for detection of norepinephrine. J. Biomed. Nanotechnol. 2016, 12, 357–365. [Google Scholar] [CrossRef]
- Masson, J.F.; Breault-Turcot, J.; Faid, R.; Poirier-Richard, H.P.; Yockell-Lelievre, H.; Lussier, F.; Spatz, J.P. Plasmonic nanopipette biosensor. Anal. Chem. 2014, 86, 8998–9005. [Google Scholar] [CrossRef] [PubMed]
- Battey, J.; Wada, E. Two distinct receptor subtypes for mammalian bombesin-like peptides. Trends Neurosci. 1991, 14, 524–528. [Google Scholar] [CrossRef]
- Tata, A.; Szkudlarek, A.; Kim, Y.; Proniewicz, E. Adsorption of bombesin and its carboxyl terminal fragments onto the colloidal gold nanoparticles: SERS studies. Vib. Spectrosc. 2016, 84, 1–6. [Google Scholar] [CrossRef]
- Tąta, A.; Gralec, B.; Proniewicz, E. Unsupported platinum nanoparticles as effective sensors of neurotransmitters and possible drug curriers. Appl. Surf. Sci. 2018, 435, 256–264. [Google Scholar] [CrossRef]
- Tata, A.; Szkudlarek, A.; Kim, Y.; Proniewicz, E. Interaction of bombesin and its fragments with gold nanoparticles analyzed using surface-enhanced Raman spectroscopy. Spectrochim. Acta A 2017, 173, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Domin, H.; Pieta, E.; Piergies, N.; Swiech, D.; Kim, Y.; Proniewicz, L.M.; Proniewicz, E. Neuropeptide Y and its C-terminal fragments acting on Y-2 receptor: Raman and SERS spectroscopy studies. J. Colloid Interface Sci. 2015, 437, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Domin, H.; Swiech, D.; Piergies, N.; Pieta, E.; Kim, Y.; Proniewicz, E. Characterization of the surface geometry of acetyl-[leu(28,31)]-npy(24–36), a selective Y-2 receptor agonist, onto the Ag and Au surfaces. Vib. Spectrosc. 2016, 85, 1–6. [Google Scholar] [CrossRef]
- daFonseca, B.G.; Costa, L.A.S.; Sant’Ana, A.C. Insights of adsorption mechanisms of TRP-peptides on plasmonic surfaces by SERS. Spectrochim. Acta A 2018, 190, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Monfared, A.M.T.; Tiwari, V.S.; Trudeau, V.L.; Anis, H. Surface-enhanced Raman scattering spectroscopy for the detection of glutamate and gamma-aminobutyric acid in serum by partial least squares analysis. IEEE Photonics J. 2015, 7, 1–16. [Google Scholar] [CrossRef]
- Lussier, F.; Brule, T.; Vishwakarma, M.; Das, T.; Spatz, J.P.; Masson, J.F. Dynamic-SERS optophysiology: A nanosensor for monitoring cell secretion events. Nano Lett. 2016, 16, 3866–3871. [Google Scholar] [CrossRef] [PubMed]
- Lussier, F.; Brule, T.; Bourque, M.J.; Ducrot, C.; Trudeau, L.E.; Masson, J.F. Dynamic SERS nanosensor for neurotransmitter sensing near neurons. Faraday Discuss. 2017, 205, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Scaramuzza, S.; Litti, L.; Meneghetti, M.; Zuccolotto, G.; Rosato, A.; Nicolato, E.; Marzola, P.; Fracasso, G.; Anselmi, C.; et al. Magneto-plasmonic Au-Fe alloy nanoparticles designed for multimodal SERS-MRI-CT imaging. Small 2014, 10, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
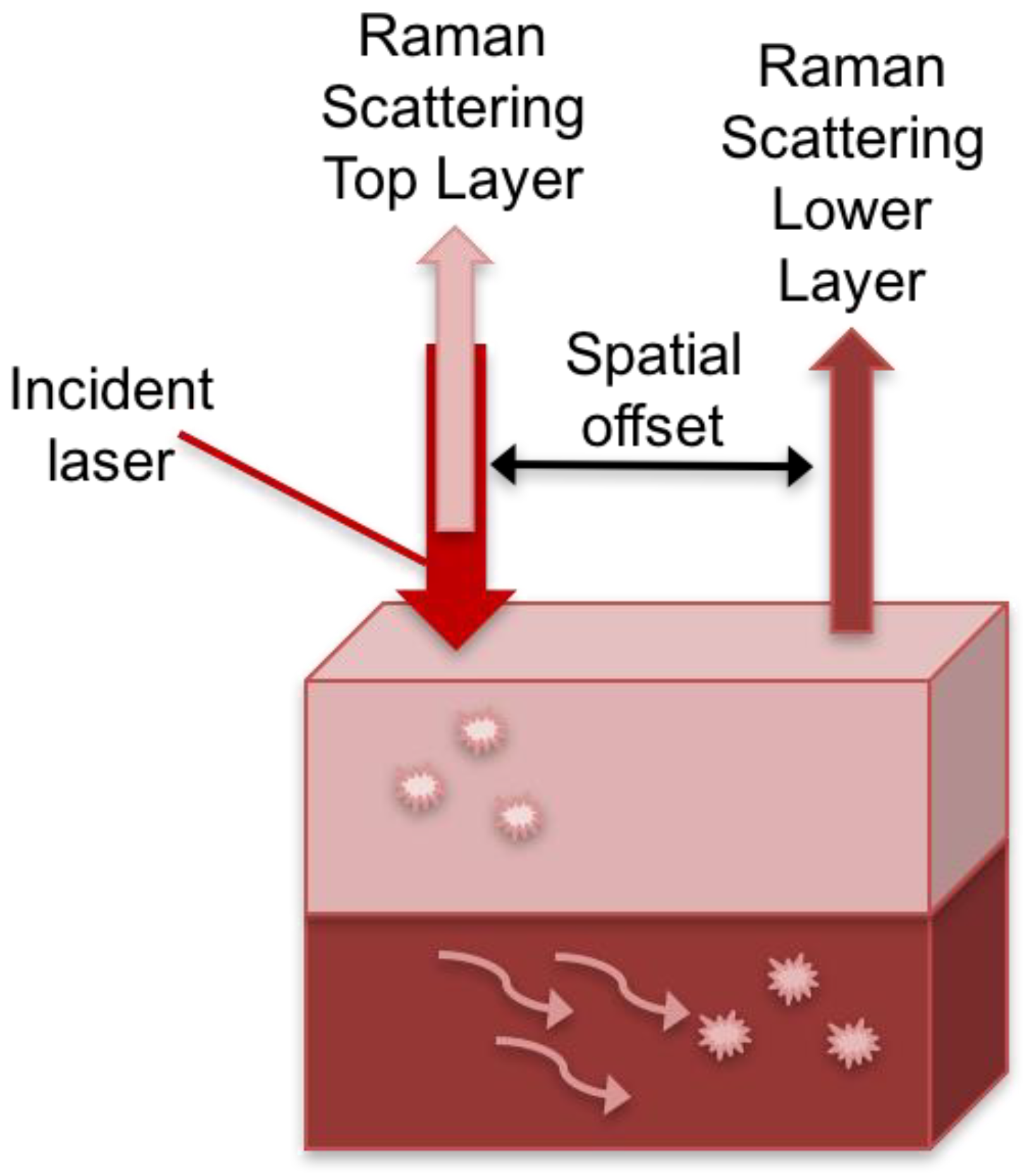
- Matousek, P.; Clark, I.P.; Draper, E.R.; Morris, M.D.; Goodship, A.E.; Everall, N.; Towrie, M.; Finney, W.F.; Parker, A.W. Subsurface probing in diffusely scattering media using spatially offset Raman spectroscopy. Appl. Spectrosc. 2005, 59, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Moody, A.S.; Baghernejad, P.C.; Webb, K.R.; Sharma, B. Surface enhanced spatially offset Raman spectroscopy detection of neurochemicals through the skull. Anal. Chem. 2017, 89, 5688–5692. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 6. Glycemic targets: Standards of medical care in diabetes—2018. Diabetes Care 2017, 41, S55–S64. [Google Scholar]
- Jensen, M.H.; Christensen, T.F.; Tarnow, L.; Mahmoudi, Z.; Johansen, M.D.; Hejlesen, O.K. Professional continuous glucose monitoring in subjects with type 1 diabetes: Retrospective hypoglycemia detection. J. Diabetes Sci. Technol. 2013, 7, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Cappon, G.; Acciaroli, G.; Vettoretti, M.; Facchinetti, A.; Sparacino, G. Wearable continuous glucose monitoring sensors: A revolution in diabetes treatment. Electronics 2017, 6, 65. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas. Available online: http://www.diabetesatlas.org (accessed on 20 January 2018).
- Birech, Z.; Mwangi, P.W.; Bukachi, F.; Mandela, K.M. Application of Raman spectroscopy in type 2 diabetes screening in blood using leucine and isoleucine amino-acids as biomarkers and in comparative anti-diabetic drugs efficacy studies. PLoS ONE 2017, 12, e0185130. [Google Scholar] [CrossRef] [PubMed]
- Dingari, N.C.; Horowitz, G.L.; Kang, J.W.; Dasari, R.R.; Barman, I. Raman spectroscopy provides a powerful diagnostic tool for accurate determination of albumin glycation. PLoS ONE 2012, 7, e32406. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Wang, Y.; Zhang, B.; Sun, D.; Fu, C.; Xu, W.; Xu, S. Glucose oxidase probe as a surface-enhanced Raman scattering sensor for glucose. Anal. Bioanal. Chem. 2016, 408, 7513–7520. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, H.; Schultz, Z.D.; Camden, J.P. Sensing glucose in urine and serum and hydrogen peroxide in living cells by use of a novel boronate nanoprobe based on surface-enhanced Raman spectroscopy. Anal. Chem. 2016, 88, 7191–7197. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, W.; Wang, M. Sensing of salivary glucose using nano-structured biosensors. Biosensors 2016, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Al-Ogaidi, I.; Gou, H.; Al-kazaz, A.K.A.; Aguilar, Z.P.; Melconian, A.K.; Zheng, P.; Wu, N. A gold@silica core–shell nanoparticle-based surface-enhanced Raman scattering biosensor for label-free glucose detection. Anal. Chim. Acta 2014, 811, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Yuen, J.M.; Shah, N.C.; Walsh, J.T.; Glucksberg, M.R.; Van Duyne, R.P. In vivo, transcutaneous glucose sensing using surface-enhanced spatially offset Raman spectroscopy: Multiple rats, improved hypoglycemic accuracy, low incident power, and continuous monitoring for greater than 17 days. Anal. Chem. 2011, 83, 9146–9152. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Bugga, P.; Madison, L.R.; Henry, A.-I.; Blaber, M.G.; Greeneltch, N.G.; Chiang, N.; Mrksich, M.; Schatz, G.C.; Van Duyne, R.P. Bisboronic acids for selective, physiologically relevant direct glucose sensing with surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2016, 138, 13952–13959. [Google Scholar] [CrossRef] [PubMed]
- Fossey, J.S.; James, T.D. Boronic acid-based receptors. Supramol. Chem. 2012. [Google Scholar] [CrossRef]
- Kong, K.V.; Lam, Z.; Lau, W.K.O.; Leong, W.K.; Olivo, M. A transition metal carbonyl probe for use in a highly specific and sensitive SERS-based assay for glucose. J. Am. Chem. Soc. 2013, 135, 18028–18031. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.V.; Ho, C.J.H.; Gong, T.; Lau, W.K.O.; Olivo, M. Sensitive SERS glucose sensing in biological media using alkyne functionalized boronic acid on planar substrates. Biosens. Bioelectron. 2014, 56, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Atar, N.; Yola, M.L.; Eryılmaz, M.; Torul, H.; Tamer, U.; Boyacı, İ.H.; Üstündağ, Z. A novel glucose biosensor platform based on Ag@AuNPs modified graphene oxide nanocomposite and SERS application. J. Colloid Interface Sci. 2013, 406, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Huang, M.; Wang, K.; Song, B.; Wang, Y.; Chen, J.; Liu, X.; Li, X.; Lin, L.; Huang, G. Urine surface-enhanced Raman spectroscopy for non-invasive diabetic detection based on a portable Raman spectrometer. Laser Phys. Lett. 2016, 13, 065604. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Z.; Feng, S.; Lin, J.; Liu, N.; Wang, J.; Li, L.; Zeng, Y.; Li, B.; Zeng, H.; et al. Label-free optical detection of type ii diabetes based on surface-enhanced Raman spectroscopy and multivariate analysis. J. Raman Spectrosc. 2014, 45, 884–889. [Google Scholar] [CrossRef]
- Pucetaite, M.; Velicka, M.; Pilipavicius, J.; Beganskiene, A.; Ceponkus, J.; Sablinskas, V. Uric acid detection by means of SERS spectroscopy on dried Ag colloidal drops. J. Raman Spectrosc. 2016, 47, 681–686. [Google Scholar] [CrossRef]
- Guo, W.; Hu, Y.; Wei, H. Enzymatically activated reduction-caged SERS reporters for versatile bioassays. Analyst 2017, 142, 2322–2326. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Yang, X.; Xie, S.; Yuan, R.; Chai, Y. Metal organic frameworks combining CoFe2O4 magnetic nanoparticles as highly efficient SERS sensing platform for ultrasensitive detection of N-terminal pro-brain natriuretic peptide. ACS Appl. Mater. Interfaces 2016, 8, 7683–7690. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Fouad, D.M.; El-Safty, S.A. Ultrasensitive label-free detection of cardiac biomarker myoglobin based on surface-enhanced Raman spectroscopy. Sens. Actuators B Chem. 2016, 228, 401–409. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Lin, J.; Lai, W.; Huang, W.; Chen, H.; Weng, G. Label-free optical detection of acute myocardial infarction based on blood plasma surface-enhanced Raman spectroscopy. J. Appl. Spectrosc. 2016, 83, 798–804. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, P.; Guo, R.; Wang, H.; Li, T. Exonuclease III-boosted cascade reactions for ultrasensitive SERS detection of nucleic acids. Biosens. Bioelectron. 2018, 104, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, C.; Li, R.; Shen, C.; Cai, X.; Sun, L.; Luo, C.; Yin, Y. Noninvasive and prospective diagnosis of coronary heart disease with urine using surface-enhanced Raman spectroscopy. Analyst 2018. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.; Lee, S.; Yoon, S.-Y.; Lee, E.K.; Chang, S.-I.; Choo, J. SERS-based competitive immunoassay of troponin I and CK-MB markers for early diagnosis of acute myocardial infarction. Chem. Commun. 2014, 50, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, L.; Liu, B.; Ni, H.; Sun, L.; Su, E.; Chen, H.; Gu, Z.; Zhao, X. Quantitative and ultrasensitive detection of multiplex cardiac biomarkers in lateral flow assay with core-shell SERS nanotags. Biosens. Bioelectron. 2018, 106, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Attree, S.; Horgan, A.; Knight, A.; Kumarswami, N.; Porter, R.; Worsley, G. Optical scattering artifacts observed in the development of multiplexed surface enhanced Raman spectroscopy nanotag immunoassays. Anal. Chem. 2012, 84, 8246–8252. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Conde, J.; Bao, C.; Chen, Y.; Curtin, J.; Cui, D. Gold nanostars for efficient in vitro and in vivo real-time SERS detection and drug delivery via plasmonic-tunable Raman/FTIR imaging. Biomaterials 2016, 106, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Bowey, K.; Tanguay, J.-F.; Sandros, M.G.; Tabrizian, M. Microwave-assisted synthesis of surface-enhanced Raman scattering nanoprobes for cellular sensing. Colloids Surf. B Biointerfaces 2014, 122, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, P.; Habeeb Muhammed, M.A.; Alsaiari, S.K.; Moosa, B.; Almalik, A.; Kumar, A.; Ringe, E.; Khashab, N.M. Tunable and linker free nanogaps in core–shell plasmonic nanorods for selective and quantitative detection of circulating tumor cells by SERS. ACS Appl. Mater. Interfaces 2017, 9, 37597–37605. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Liu, X.; Pei, Y.; Ling, Y.; Wu, P.; Cai, C. Leakage-free polypyrrole–Au nanostructures for combined Raman detection and photothermal cancer therapy. J. Mater. Chem. B 2017, 5, 7949–7962. [Google Scholar] [CrossRef]
- Pal, S.; Harmsen, S.; Oseledchyk, A.; Hsu, H.-T.; Kircher, M.F. MUC1 aptamer targeted SERS nanoprobes. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef] [PubMed]
- Eom, G.; Kim, H.; Hwang, A.; Son, H.-Y.; Choi, Y.; Moon, J.; Kim, D.; Lee, M.; Lim, E.-K.; Jeong, J.; et al. Nanogap-rich Au nanowire SERS sensor for ultrasensitive telomerase activity detection: Application to gastric and breast cancer tissues diagnosis. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Feng, J.; Chen, L.; Xia, Y.; Xing, J.; Li, Z.; Qian, Q.; Wang, Y.; Wu, A.; Zeng, L.; Zhou, Y. Bioconjugation of gold nanobipyramids for SERS detection and targeted photothermal therapy in breast cancer. ACS Biomater. Sci. Eng. 2017, 3, 608–618. [Google Scholar] [CrossRef]
- Wang, Y.W.; Reder, N.P.; Kang, S.; Glaser, A.K.; Yang, Q.; Wall, M.A.; Javid, S.H.; Dintzis, S.M.; Liu, J.T.C. Raman-encoded molecular imaging with topically applied SERS nanoparticles for intraoperative guidance of lumpectomy. Cancer Res. 2017, 77, 4506–4516. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.A.; Ou, Y.-C.; Faley, S.; Paul, E.P.; Hittinger, J.P.; Cutright, C.C.; Lin, E.C.; Bellan, L.M.; Bardhan, R. Theranostic gold nanoantennas for simultaneous multiplexed Raman imaging of immunomarkers and photothermal therapy. ACS Omega 2017, 2, 3583–3594. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Arteaga, A.; de Jesús Zermeño Nava, J.; Kolosovas-Machuca, E.S.; Velázquez-Salazar, J.J.; Vinogradova, E.; José-Yacamán, M.; Navarro-Contreras, H.R. Diagnosis of breast cancer by analysis of sialic acid concentrations in human saliva by surface-enhanced Raman spectroscopy of silver nanoparticles. Nano Res. 2017, 10, 3662–3670. [Google Scholar] [CrossRef]
- Cervo, S.; Mansutti, E.; Del Mistro, G.; Spizzo, R.; Colombatti, A.; Steffan, A.; Sergo, V.; Bonifacio, A. SERS analysis of serum for detection of early and locally advanced breast cancer. Anal. Bioanal. Chem. 2015, 407, 7503–7509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.-P.; Perner, S.; Bankfalvi, A.; Schlücker, S. Effect of antigen retrieval methods on nonspecific binding of antibody–metal nanoparticle conjugates on formalin-fixed paraffin-embedded tissue. Anal. Chem. 2017, 90, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Parchur, A.K.; Li, Q.; Zhou, A. Near-infrared photothermal therapy of prussian-blue-functionalized lanthanide-ion-doped inorganic/plasmonic multifunctional nanostructures for the selective targeting of HER2-expressing breast cancer cells. Biomater. Sci. 2016, 4, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, F.; Jamieson, L.E.; Mabbott, S.; Plakas, K.; Shand, N.; Detty, M.; Graham, D.; Faulds, K. Through tissue imaging of a live breast cancer tumour model using handheld surface enhanced spatially offset resonance Raman spectroscopy. Chem. Sci. 2018, 9, 3788–3792. [Google Scholar] [CrossRef]
- Li, Q.; Parchur, A.K.; Zhou, A. In vitro biomechanical properties, fluorescence imaging, surface-enhanced Raman spectroscopy, and photothermal therapy evaluation of luminescent functionalized CaMoO4:Eu@Au hybrid nanorods on human lung adenocarcinoma epithelial cells. Sci. Technol. Adv. Mater. 2016, 17, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zheng, J.; Tang, P.; Xu, W.; Qing, Z.; Yang, S.; Li, J.; Yang, R. Quantitative monitoring of hypoxia-induced intracellular acidification in lung tumor cells and tissues using activatable surface-enhanced Raman scattering nanoprobes. Anal. Chem. 2016, 88, 11852–11859. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Hwang, M.; Choi, B.; Jeong, H.; Jung, J.-H.; Kim, H.K.; Hong, S.; Park, J.-H.; Choi, Y. Exosome classification by pattern analysis of surface-enhanced Raman spectroscopy data for lung cancer diagnosis. Anal. Chem. 2017, 89, 6695–6701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, X.; Xu, G.; Jin, X.; Luan, M.; Lou, J.; Wang, L.; Huang, C.; Ye, J. Identification and distinction of non-small-cell lung cancer cells by intracellular SERS nanoprobes. RSC Adv. 2016, 6, 5401–5407. [Google Scholar] [CrossRef]
- Song, C.; Yang, Y.; Yang, B.; Min, L.; Wang, L. Combination assay of lung cancer associated serum markers using surface-enhanced Raman spectroscopy. J. Mater. Chem. B 2016, 4, 1811–1817. [Google Scholar] [CrossRef]
- Song, C.Y.; Yang, Y.J.; Yang, B.Y.; Sun, Y.Z.; Zhao, Y.P.; Wang, L.H. An ultrasensitive SERS sensor for simultaneous detection of multiple cancer-related miRNAs. Nanoscale 2016, 8, 17365–17373. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Su, B.; Liu, C.; Song, Q.; Luo, D.; Mo, G.; Wang, T. Selective surface enhanced Raman scattering for quantitative detection of lung cancer biomarkers in superparticle@MOF structure. Adv. Mater. 2017, 30, 1702275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, W.; Wang, J.; Luo, D.; Qiao, X.; Qin, X.; Wang, T. Ultrasensitive surface-enhanced Raman scattering sensor of gaseous aldehydes as biomarkers of lung cancer on dendritic Ag nanocrystals. Anal. Chem. 2017, 89, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Deng, D.; Deng, Q.; Wu, P.; Zhang, H.; Cai, C. Synthesis of magnetic Fe3O4–Au hybrids for sensitive SERS detection of cancer cells at low abundance. J. Mater. Chem. B 2015, 3, 4487–4495. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Li, S.; Zhang, S.; Jin, L. Discrimination of rectal cancer through human serum using surface-enhanced Raman spectroscopy. Appl. Phys. B 2015, 119, 393–398. [Google Scholar] [CrossRef]
- Lin, D.; Huang, H.; Qiu, S.; Feng, S.; Chen, G.; Chen, R. Diagnostic potential of polarized surface enhanced Raman spectroscopy technology for colorectal cancer detection. Opt. Express 2016, 24, 2222–2234. [Google Scholar] [CrossRef] [PubMed]
- Tirinato, L.; Gentile, F.; Di Mascolo, D.; Coluccio, M.L.; Das, G.; Liberale, C.; Pullano, S.A.; Perozziello, G.; Francardi, M.; Accardo, A.; et al. SERS analysis on exosomes using super-hydrophobic surfaces. Microelectron. Eng. 2012, 97, 337–340. [Google Scholar] [CrossRef]
- Chang, H.; Kang, H.; Ko, E.; Jun, B.-H.; Lee, H.-Y.; Lee, Y.-S.; Jeong, D.H. PSA detection with femtomolar sensitivity and a broad dynamic range using SERS nanoprobes and an area-scanning method. ACS Sens. 2016, 1, 645–649. [Google Scholar] [CrossRef]
- Yang, K.; Hu, Y.; Dong, N.; Zhu, G.; Zhu, T.; Jiang, N. A novel SERS-based magnetic aptasensor for prostate specific antigen assay with high sensitivity. Biosens. Bioelectron. 2017, 94, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Choi, N.; Wang, R.; Lee, S.; Moon, K.C.; Yoon, S.-Y.; Chen, L.; Choo, J. Simultaneous detection of dual prostate specific antigens using surface-enhanced Raman scattering-based immunoassay for accurate diagnosis of prostate cancer. ACS Nano 2017, 11, 4926–4933. [Google Scholar] [CrossRef] [PubMed]
- Nima, Z.A.; Alwbari, A.M.; Dantuluri, V.; Hamzah, R.N.; Sra, N.; Motwani, P.; Arnaoutakis, K.; Levy, R.A.; Bohliqa, A.F.; Nedosekin, D.; et al. Targeting nano drug delivery to cancer cells using tunable, multi-layer, silver-decorated gold nanorods. J. Appl. Toxicol. 2017, 37, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, M.; Rüger, J.; Kirchberger-Tolstik, T.; Schie, I.W.; Henkel, T.; Weber, K.; Cialla-May, D.; Krafft, C.; Popp, J. A droplet-based microfluidic chip as a platform for leukemia cell lysate identification using surface-enhanced Raman scattering. Anal. Bioanal. Chem. 2017, 410, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Wee, E.J.H.; Wang, Y.; Tsao, S.C.-H.; Trau, M. Simple, sensitive and accurate multiplex detection of clinically important melanoma DNA mutations in circulating tumour DNA with SERS nanotags. Theranostics 2016, 6, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Kanchanapally, R.; Fan, Z.; Singh, A.K.; Sinha, S.S.; Ray, P.C. Multifunctional hybrid graphene oxide for label-free detection of malignant melanoma from infected blood. J. Mater. Chem. B 2014, 2, 1934–1937. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Liao, J.-D.; You, J.-W.; Wu, C.-L. Focused-ion-beam-fabricated Au/Ag multilayered nanorod array as SERS-active substrate for virus strain detection. Sens. Actuators B Chem. 2013, 181, 361–367. [Google Scholar] [CrossRef]
- Negri, P.; Dluhy, R.A. Detection of genetic markers related to high pathogenicity in influenza by SERS. Analyst 2013, 138, 4877–4884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maneeprakorn, W.; Bamrungsap, S.; Apiwat, C.; Wiriyachaiporn, N. Surface-enhanced Raman scattering based lateral flow immunochromatographic assay for sensitive influenza detection. RSC Adv. 2016, 6, 112079–112085. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Park, H.J.; Yang, S.C.; Choo, J. Early diagnosis of influenza virus a using surface-enhanced Raman scattering-based lateral flow assay. Bull. Korean Chem. Soc. 2016, 37, 2019–2024. [Google Scholar] [CrossRef]
- Moon, J.; Yi, S.Y.; Hwang, A.; Eom, G.; Sim, J.; Jeong, J.; Lim, E.-K.; Chung, B.H.; Kim, B.; Jung, J.; et al. Facile and sensitive detection of influenza viruses using SERS antibody probes. RSC Adv. 2016, 6, 84415–84419. [Google Scholar] [CrossRef]
- Karn-orachai, K.; Sakamoto, K.; Laocharoensuk, R.; Bamrungsap, S.; Songsivilai, S.; Dharakul, T.; Miki, K. Extrinsic surface-enhanced Raman scattering detection of influenza A virus enhanced by two-dimensional gold@silver core–shell nanoparticle arrays. RSC Adv. 2016, 6, 97791–97799. [Google Scholar] [CrossRef]
- Zengin, A.; Tamer, U.; Caykara, T. SERS detection of hepatitis B virus DNA in a temperature-responsive sandwich-hybridization assay. J. Raman Spectrosc. 2017, 48, 668–672. [Google Scholar] [CrossRef]
- Kamińska, A.; Witkowska, E.; Winkler, K.; Dzięcielewski, I.; Weyher, J.L.; Waluk, J. Detection of hepatitis B virus antigen from human blood: SERS immunoassay in a microfluidic system. Biosens. Bioelectron. 2015, 66, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Cheng, Z.; Yu, J.; Choo, P.; Chen, L.; Choo, J. A SERS-based lateral flow assay biosensor for highly sensitive detection of HIV-1 DNA. Biosens. Bioelectron. 2016, 78, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Purrà, M.; Carré-Camps, M.; de Puig, H.; Bosch, I.; Gehrke, L.; Hamad-Schifferli, K. Surface-enhanced Raman spectroscopy-based sandwich immunoassays for multiplexed detection of zika and dengue viral biomarkers. ACS Infect. Dis. 2017, 3, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.M.; Fan, Z.; Sinha, S.S.; Shi, Y.; Le, L.; Bai, F.; Ray, P.C. Bioconjugated gold nanoparticle based SERS probe for ultrasensitive identification of mosquito-borne viruses using Raman fingerprinting. J. Phys. Chem. C 2015, 119, 23669–23675. [Google Scholar] [CrossRef] [PubMed]
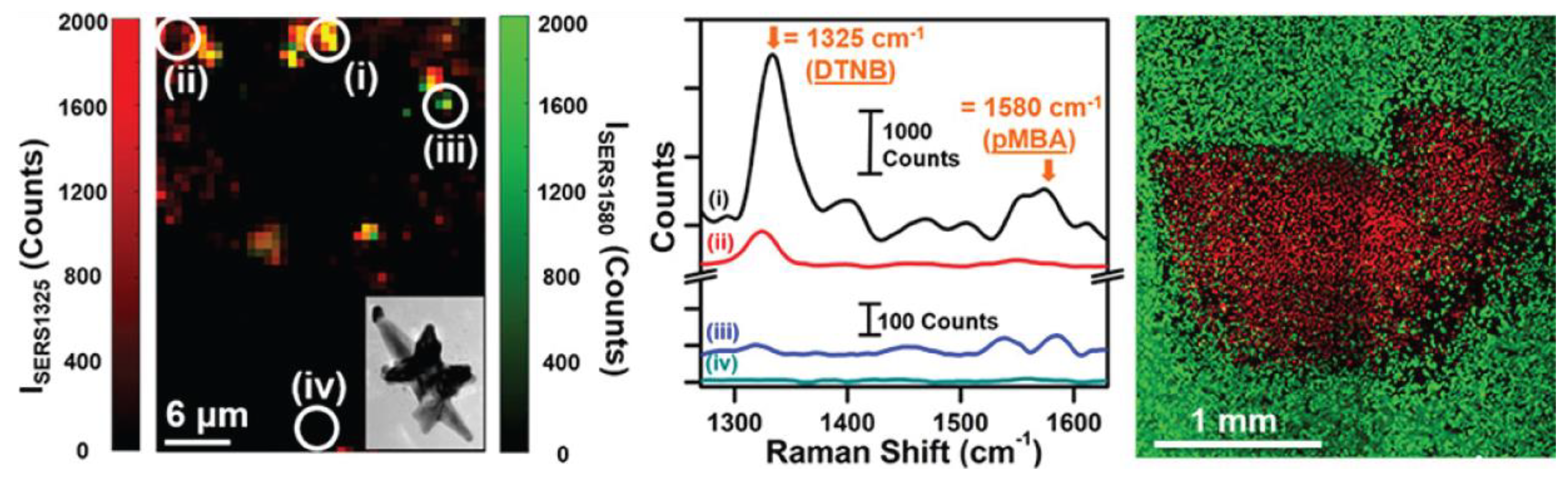
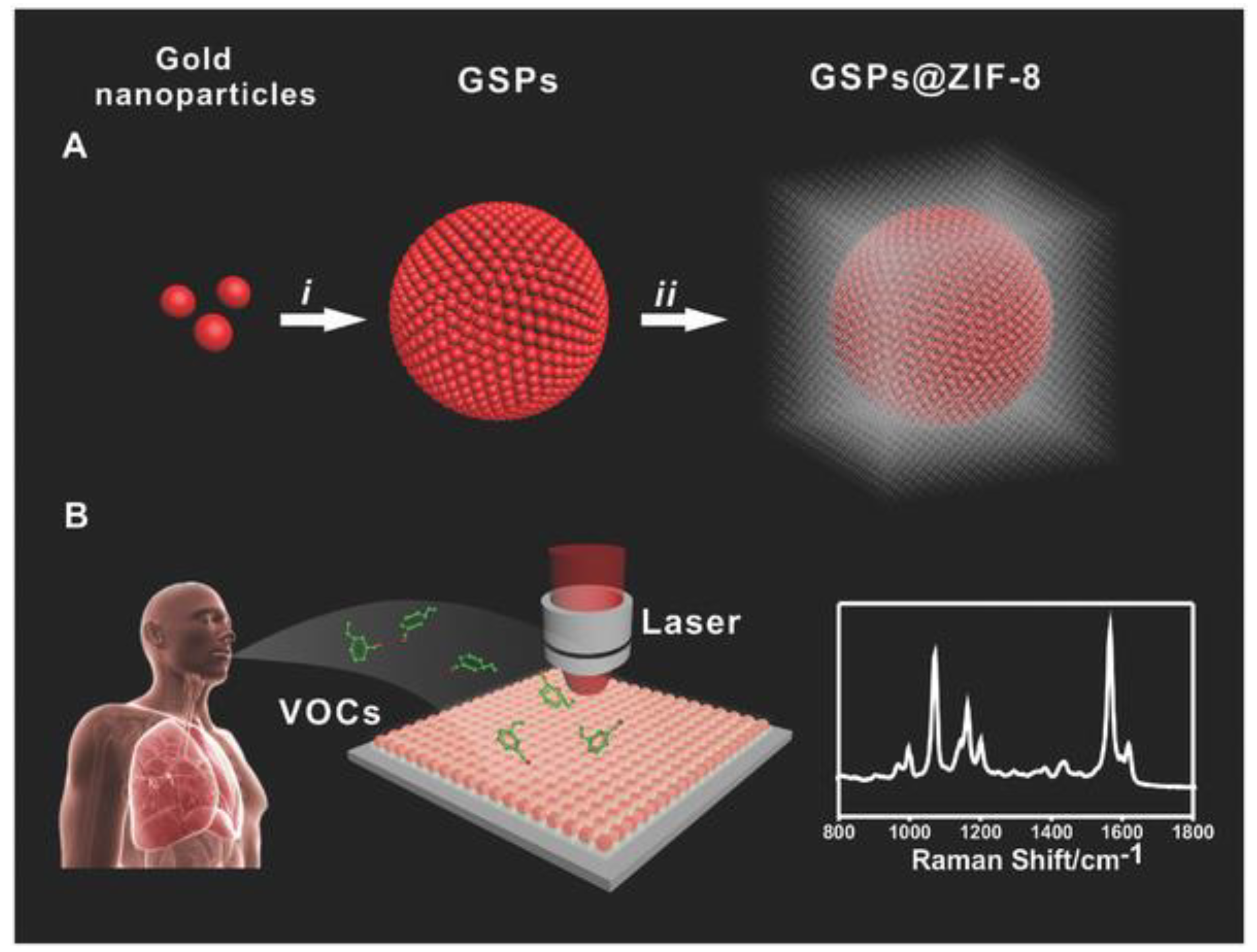
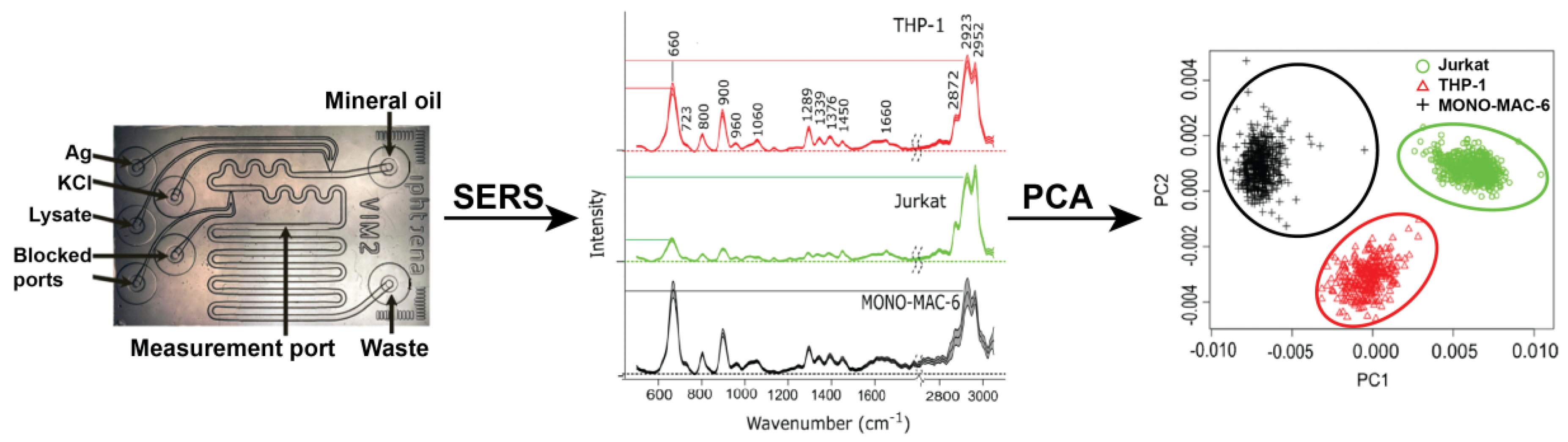

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, T.J.; Moody, A.S.; Payne, T.D.; Sarabia, G.M.; Daniel, A.R.; Sharma, B. In Vitro and In Vivo SERS Biosensing for Disease Diagnosis. Biosensors 2018, 8, 46. https://doi.org/10.3390/bios8020046
Moore TJ, Moody AS, Payne TD, Sarabia GM, Daniel AR, Sharma B. In Vitro and In Vivo SERS Biosensing for Disease Diagnosis. Biosensors. 2018; 8(2):46. https://doi.org/10.3390/bios8020046
Chicago/Turabian StyleMoore, T. Joshua, Amber S. Moody, Taylor D. Payne, Grace M. Sarabia, Alyssa R. Daniel, and Bhavya Sharma. 2018. "In Vitro and In Vivo SERS Biosensing for Disease Diagnosis" Biosensors 8, no. 2: 46. https://doi.org/10.3390/bios8020046
APA StyleMoore, T. J., Moody, A. S., Payne, T. D., Sarabia, G. M., Daniel, A. R., & Sharma, B. (2018). In Vitro and In Vivo SERS Biosensing for Disease Diagnosis. Biosensors, 8(2), 46. https://doi.org/10.3390/bios8020046




