Fluorescent and Colorimetric Electrospun Nanofibers for Heavy-Metal Sensing
Abstract
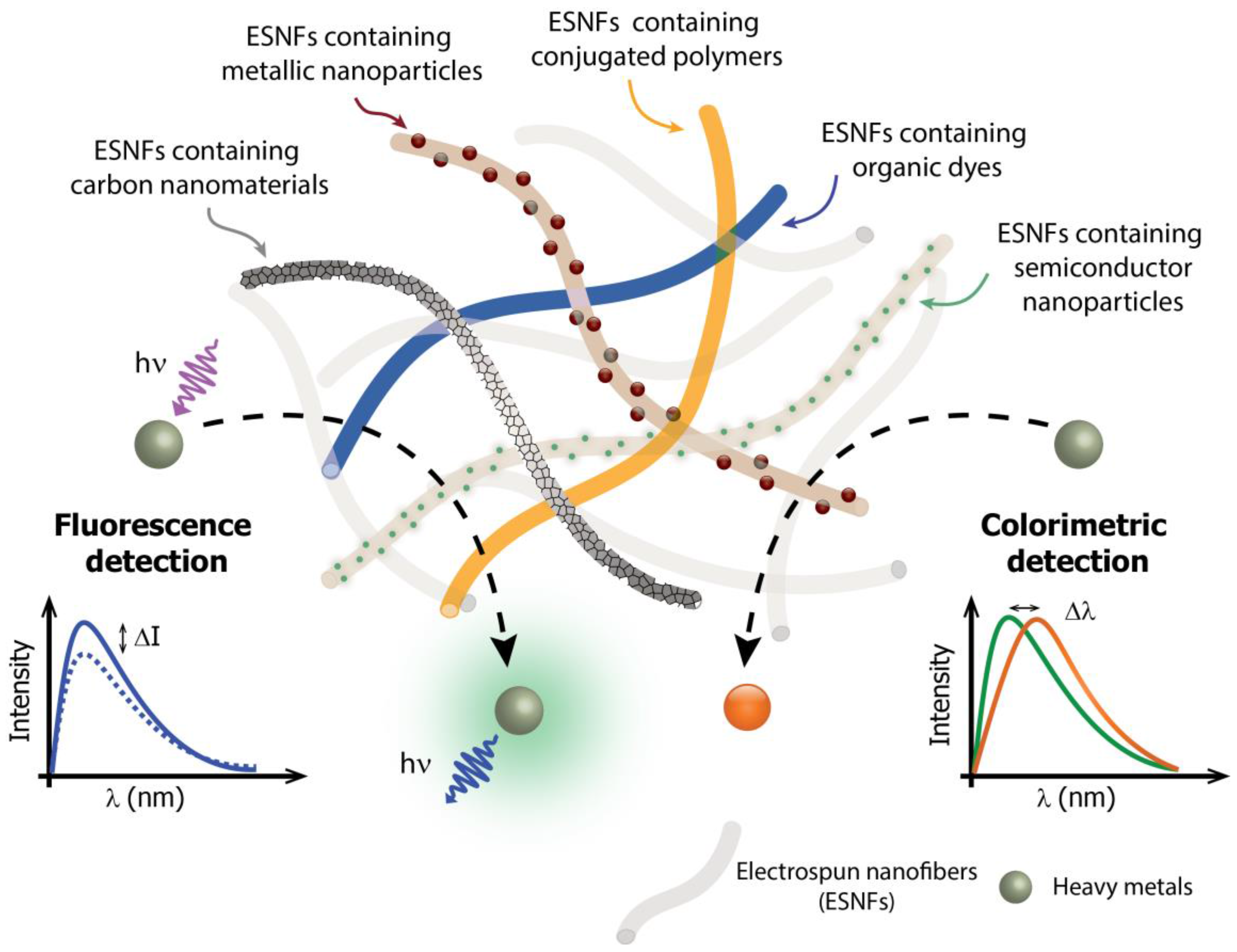
1. Introduction
2. Electrospun Nanofiber-Based Optical Sensors for Heavy-Metal Detection
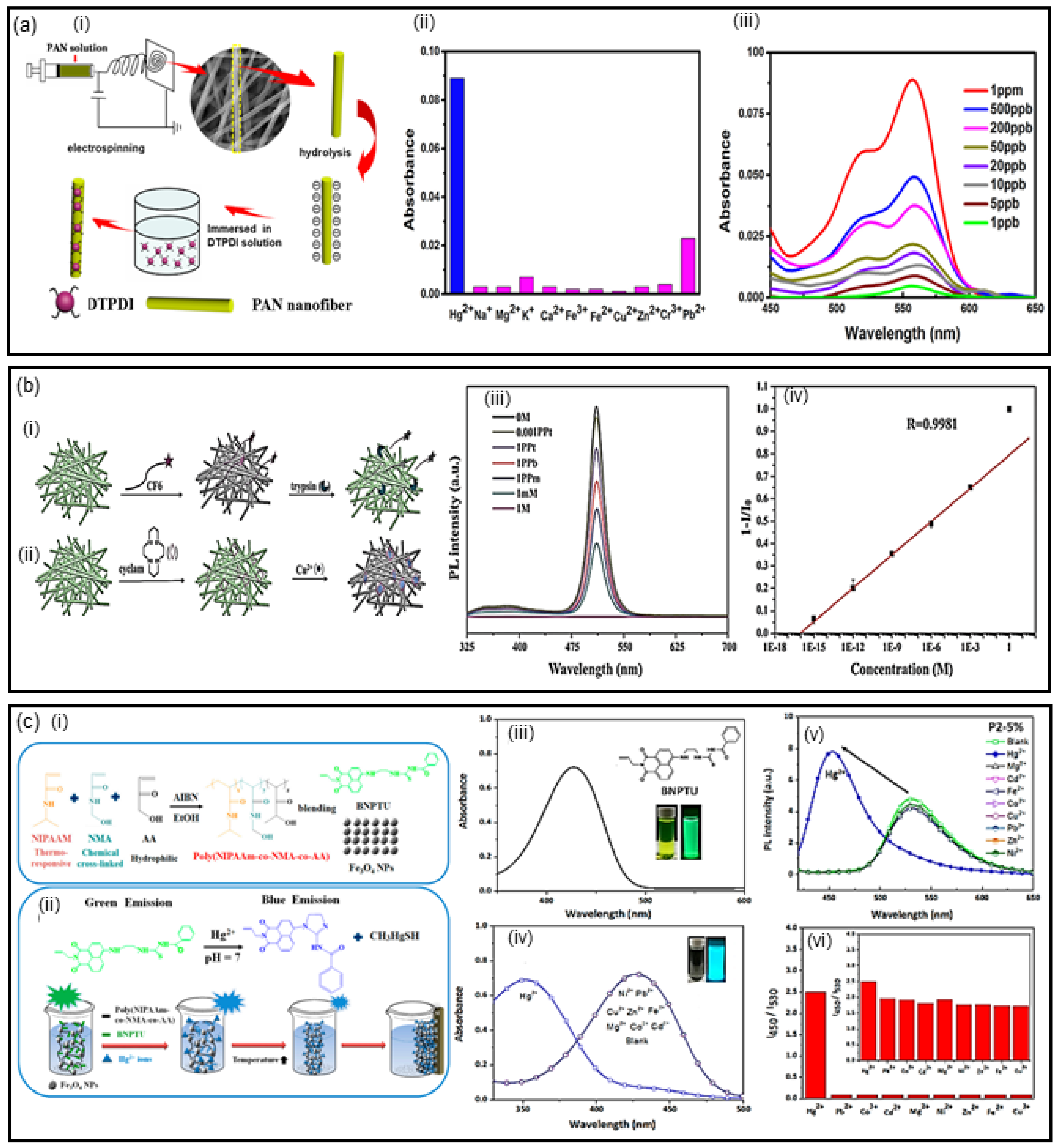
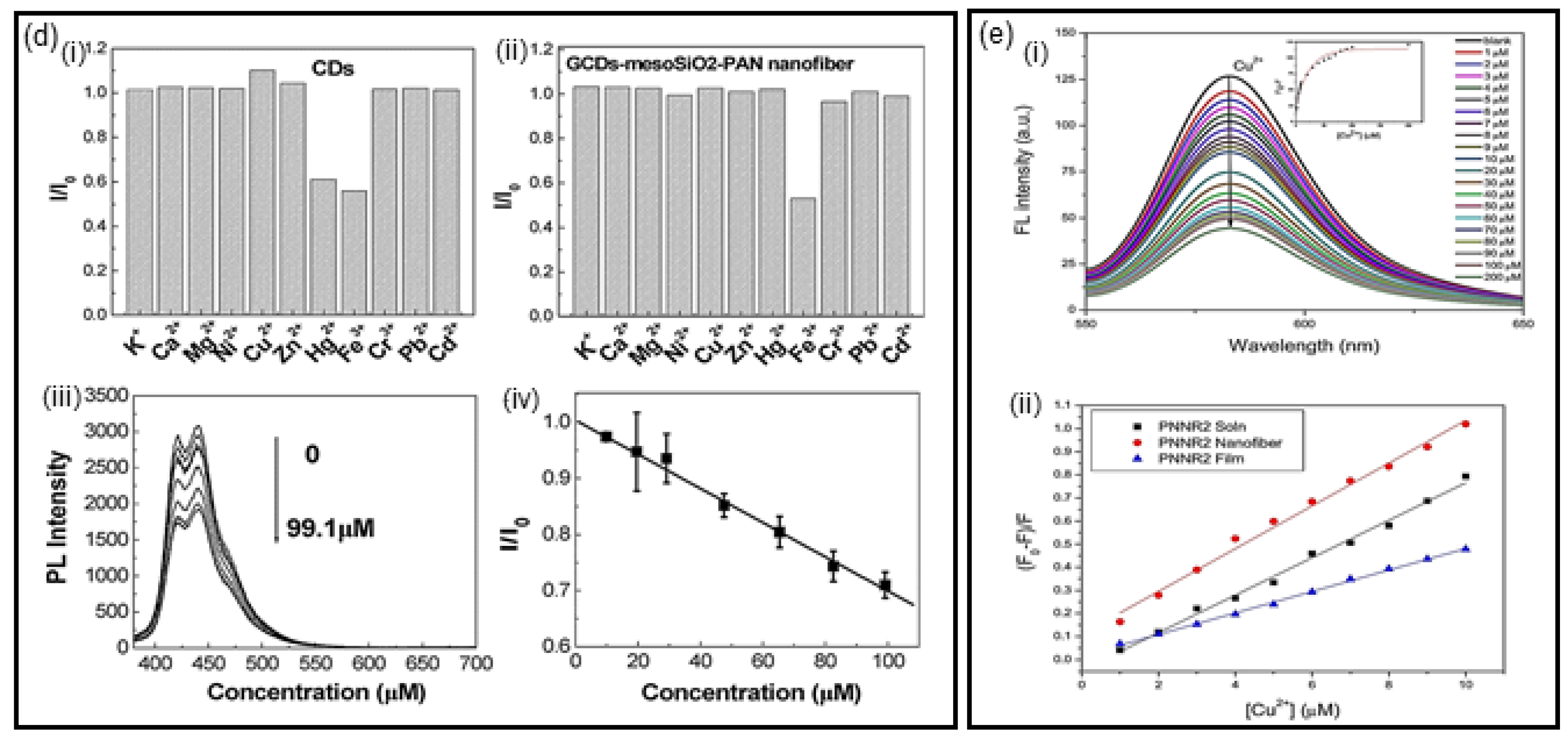
2.1. Optical Detection Using Fluorescence
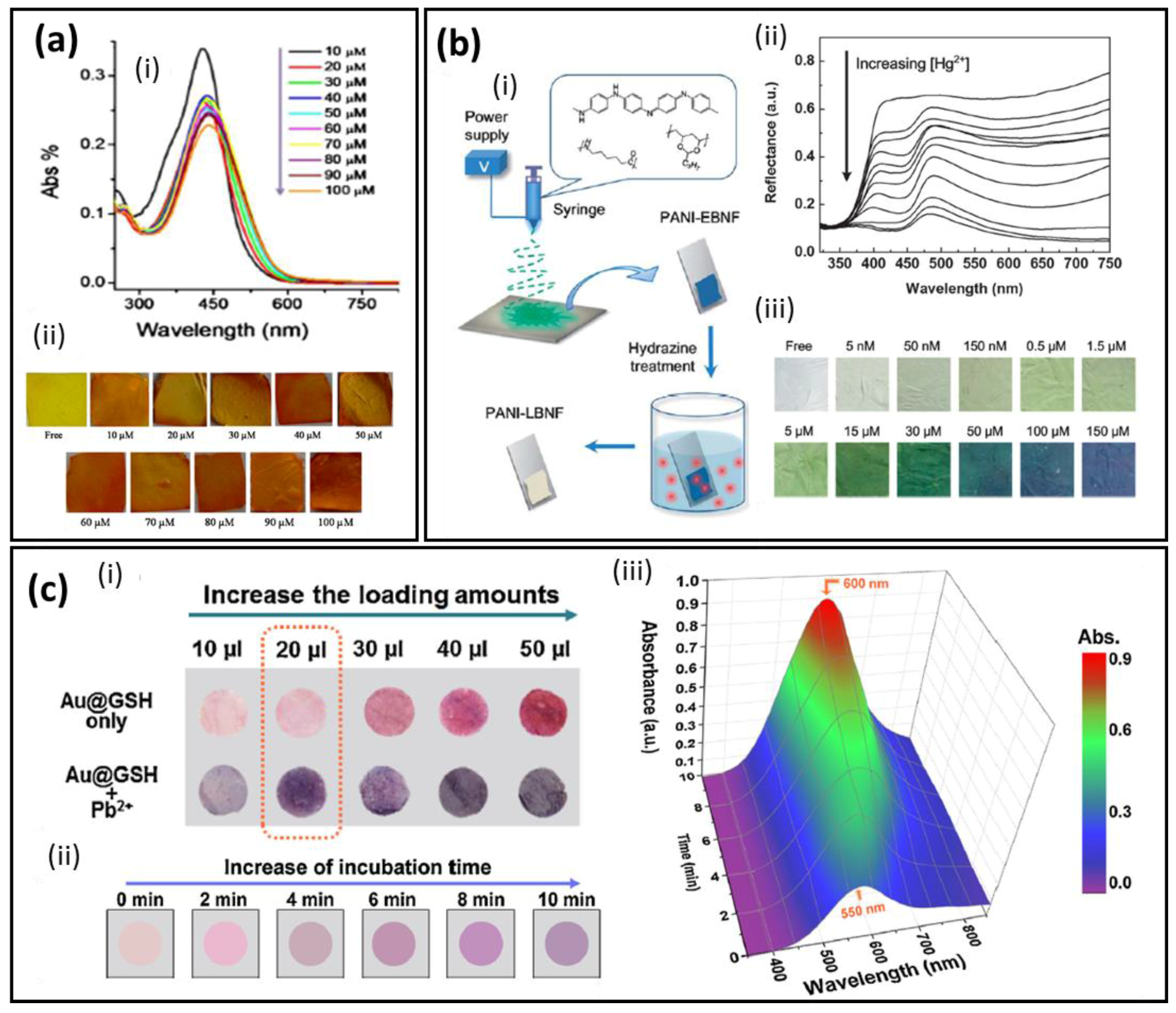
2.3. Optical Detection Using Colorimetry
3. Conclusions and Future Outlook
Acknowledgments
Conflicts of Interest
References
- Ying, Y.; Ying, W.; Li, Q.; Meng, D.; Ren, G.; Yan, R.; Peng, X. Recent advances of nanomaterial-based membrane for water purification. Appl. Mater. Today 2017, 7, 144–158. [Google Scholar] [CrossRef]
- March, G.; Nguyen, T.D.; Piro, B. Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kim, K.-H.; Bansal, V.; Lazarides, T.; Kumar, N. Progress in the sensing techniques for heavy metal ions using nanomaterials. J. Ind. Eng. Chem. 2017, 54, 30–43. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, L.; Zeng, G.; Zhang, C.; Zhang, Y.; Xie, X. Current progress in biosensors for heavy metal ions based on DNAzymes/DNA molecules functionalized nanostructures: A review. Sens. Actuators B Chem. 2016, 223, 280–294. [Google Scholar] [CrossRef]
- Pandey, S.K.; Singh, P.; Singh, J.; Sachan, S.; Srivastava, S.; Singh, S.K. Nanocarbon-based Electrochemical Detection of Heavy Metals. Electroanalysis 2016, 28, 2472–2488. [Google Scholar] [CrossRef]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent Trends in Macro-, Micro-, and Nanomaterial-Based Tools and Strategies for Heavy-Metal Detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Bhardwaj, S.K.; Bhardwaj, N.; Paul, A.K.; Kumar, P.; Kim, K.H.; Deep, A. Progress in the biosensing techniques for trace-level heavy metals. Biotechnol. Adv. 2016, 34, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gou, H.; Al-Ogaidi, I.; Wu, N. Nanostructured Sensors for Detection of Heavy Metals: A Review. ACS Sustain. Chem. Eng. 2013, 1, 713–723. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent Progress in Nanomaterial-Based Optical Aptamer Assay for the Detection of Food Chemical Contaminants. ACS Appl. Mater. Interfaces 2017, 9, 23287–23301. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Zhao, N.; Wang, Y.; Ma, M.; Fang, L.; Gu, Y.; Jia, Y.; Liu, J. On-line/on-site analysis of heavy metals in water and soils by laser induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2017, 137, 39–45. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Han, J.; Kwon, S.; Kang, S.; Jang, A. Development of a rotary disc voltammetric sensor system for semi-continuous and on-site measurements of Pb(II). Chemosphere 2016, 143, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, M.; Yang, C. Fluorescent hydrogel waveguide for on-site detection of heavy metal ions. Sci. Rep. 2017, 7, 7902. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Persano, L.; Camposeo, A.; Jang, J.S.; Koo, W.T.; Kim, S.J.; Cho, H.J.; Kim, I.D.; Pisignano, D. Electrospun Nanostructures for High Performance Chemiresistive and Optical Sensors. Macromol. Mater. Eng. 2017, 302, 1–37. [Google Scholar] [CrossRef]
- Zhang, N.; Qiao, R.; Su, J.; Yan, J.; Xie, Z.; Qiao, Y.; Wang, X.; Zhong, J. Recent Advances of Electrospun Nanofibrous Membranes in the Development of Chemosensors for Heavy Metal Detection. Small 2017, 13, 1604293. [Google Scholar] [CrossRef] [PubMed]
- Schoolaert, E.; Hoogenboom, R.; De Clerck, K. Colorimetric Nanofibers as Optical Sensors. Adv. Funct. Mater. 2017. [Google Scholar] [CrossRef]
- Piriya, V.S.A.; Joseph, P.; Daniel, S.C.G.K.; Lakshmanan, S.; Kinoshita, T.; Muthusamy, S. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Mcdonagh, C.; Burke, C.S.; Maccraith, B.D. Optical Chemical Sensors. Chem. Rev. 2008, 353, 400–422. [Google Scholar] [CrossRef] [PubMed]
- Spricigo, P.C.; Foschini, M.M.; Ribeiro, C.; Corrêa, D.S.; Ferreira, M.D. Nanoscaled Platforms Based on SiO2 and Al2O3 Impregnated with Potassium Permanganate Use Color Changes to Indicate Ethylene Removal. Food Bioprocess Technol. 2017, 10, 1622–1630. [Google Scholar] [CrossRef]
- Mercante, L.A.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Electrospinning-based (bio)sensors for food and agricultural applications: A review. Trends Anal. Chem. 2017, 91, 2017. [Google Scholar] [CrossRef]
- Roque, A.P.; Mercante, L.A.; Scagion, V.P.; Oliveira, J.E.; Mattoso, L.H.C.; De Boni, L.; Mendonca, C.R.; Correa, D.S. Fluorescent PMMA/MEH-PPV electrospun nanofibers: Investigation of morphology, solvent, and surfactant effect. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1388–1394. [Google Scholar] [CrossRef]
- Terra, I.A.A.; Sanfelice, R.C.; Valente, G.T.; Correa, D.S. Optical sensor based on fluorescent PMMA/PFO electrospun nanofibers for monitoring volatile organic compounds. J. Appl. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Korent Urek, Š.; Frančič, N.; Turel, M.; Lobnik, A. Sensing heavy metals using mesoporous-based optical chemical sensors. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Celebioglu, A.; Uyar, T. Real-time selective visual monitoring of Hg2+ detection at ppt level: An approach to lighting electrospun nanofibers using gold nanoclusters. Sci. Rep. 2015, 5, 10403. [Google Scholar] [CrossRef] [PubMed]
- Kacmaz, S.; Ertekin, K.; Suslu, A.; Ergun, Y.; Celik, E.; Cocen, U. Sub-nanomolar sensing of ionic mercury with polymeric electrospun nanofibers. Mater. Chem. Phys. 2012, 133, 547–552. [Google Scholar] [CrossRef]
- Liang, F.-C.; Kuo, C.-C.; Chen, B.-Y.; Cho, C.-J.; Hung, C.-C.; Chen, W.-C.; Borsali, R. RGB-Switchable Porous Electrospun Nanofiber Chemoprobe-Filter Prepared from Multifunctional Copolymers for Versatile Sensing of pH and Heavy Metals. ACS Appl. Mater. Interfaces 2017, 9, 16381–16396. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, K.; Yin, M.; Chang, J.; Geng, Y.; Pan, K. Fluorescent nanofibrous membrane (FNFM) for the detection of mercuric ion (II) with high sensitivity and selectivity. Sens. Actuators B Chem. 2017, 238, 120–127. [Google Scholar] [CrossRef]
- Liang, F.-C.; Luo, Y.-L.; Kuo, C.-C.; Chen, B.-Y.; Cho, C.-J.; Lin, F.-J.; Yu, Y.-Y.; Borsali, R. Novel Magnet and Thermoresponsive Chemosensory Electrospinning Fluorescent Nanofibers and Their Sensing Capability for Metal Ions. Polymers 2017, 9, 136. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.; Xu, H.; Xiao, L.; Wang, Y.; Shen, H.; Wang, H.; Yuan, Q. Luminescent properties and sensing performance of a carbon quantum dot encapsulated mesoporous silica/polyacrylonitrile electrospun nanofibrous membrane. J. Mater. Sci. 2016, 51, 6801–6811. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Kuo, C.-C.; Huang, Y.-S.; Lu, S.-T.; Liang, F.-C.; Jiang, D.-H. Novel Highly Selective and Reversible Chemosensors Based on Dual-Ratiometric Fluorescent Electrospun Nanofibers with pH- and Fe3+-Modulated Multicolor Fluorescence Emission. ACS Appl. Mater. Interfaces 2015, 7, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, S.; Ondigo, D.A.; Zugle, R.; Tshentu, Z.; Nyokong, T.; Torto, N. A highly selective and sensitive pyridylazo-2-naphthol-poly(acrylic acid) functionalized electrospun nanofiber fluorescence “turn-off” chemosensory system for Ni2+. Anal. Methods 2012, 4, 1729. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Huang, J.; Cai, J.; Zhu, J.; Yang, X.; Shen, J.; Li, C. Perovskite quantum dots encapsulated in electrospun fiber membranes as multifunctional supersensitive sensors for biomolecules, metal ions and pH. Nanoscale Horiz. 2017, 2, 225–232. [Google Scholar] [CrossRef]
- Wu, W.C.; Lai, H.J. Preparation of thermo-responsive electrospun nanofibers containing rhodamine-based fluorescent sensor for Cu2+ detection. J. Polym. Res. 2016, 23. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Celebioglu, A.; Balusamy, B.; Uyar, T. Immobilization of gold nanoclusters inside porous electrospun fibers for selective detection of Cu(II): A strategic approach to shielding pristine performance. Sci. Rep. 2015, 5, 15608. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Yang, Q.; Fei, X.; Sun, M.; Song, Y. A reusable nanofibrous film chemosensor for highly selective and sensitive optical signaling of Cu2+ in aqueous media. Chem. Commun. 2013, 49, 4833. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-J.; Chen, C.-Y. Thermo-responsive electrospun nanofibers doped with 1,10-phenanthroline-based fluorescent sensor for metal ion detection. J. Mater. Sci. 2016, 51, 1620–1631. [Google Scholar] [CrossRef]
- Kim, C.; Hwang, J.Y.; Ku, K.S.; Angupillai, S.; Son, Y.A. A renovation of non-aqueous Al3+ sensor to aqueous media sensor by simple recyclable immobilize electrospun nano-fibers and its uses for live sample analysis. Sens. Actuators B Chem. 2016, 228, 259–269. [Google Scholar] [CrossRef]
- Wang, M.; Meng, G.; Huang, Q.; Qian, Y. Electrospun 1,4-DHAQ-doped cellulose nanofiber films for reusable fluorescence detection of trace Cu2+ and further for Cr3+. Environ. Sci. Technol. 2012, 46, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Anzenbacher, P.; Li, F.; Palacios, M.A. Toward wearable sensors: Fluorescent attoreactor mats as optically encoded cross-reactive sensor arrays. Angew. Chem. Int. Ed. 2012, 51, 2345–2348. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, H.; Zhang, J.; Deng, L.; Wu, S.; He, J.; Dong, A. Poly(vinyl alcohol) electrospun nanofibrous membrane modified with spirolactam–rhodamine derivatives for visible detection and removal of metal ions. RSC Adv. 2014, 4, 51381–51388. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, M.; Wu, W.; Xu, H.; Cheng, S.; Fan, L.-J. Polyacrylonitrile/noble metal/SiO2 nanofibers as substrates for the amplified detection of picomolar amounts of metal ions through plasmon-enhanced fluorescence. Nanoscale 2015, 7, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, Y.; Wang, L.; He, J.; Al-Deyab, S.S.; El-Newehy, M.; Yu, J.; Ding, B. Simultaneous visual detection and removal of lead(II) ions with pyromellitic dianhydride-grafted cellulose nanofibrous membranes. J. Mater. Chem. A 2015, 3, 18180–18189. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Yin, X.; Ding, B.; Sun, G.; Ke, T.; Chen, J.; Yu, J. Colorimetric strips for visual lead ion recognition utilizing polydiacetylene embedded nanofibers. J. Mater. Chem. A 2014, 2, 18304–18312. [Google Scholar] [CrossRef]
- Li, Y.; Ding, B.; Sun, G.; Ke, T.; Chen, J.; Al-Deyab, S.S.; Yu, J. Solid-phase pink-to-purple chromatic strips utilizing gold probes and nanofibrous membranes combined system for lead (II) assaying. Sens. Actuators B Chem. 2014, 204, 673–681. [Google Scholar] [CrossRef]
- Raj, S.; Shankaran, D.R. Curcumin based biocompatible nanofibers for lead ion detection. Sens. Actuators B Chem. 2016, 226, 318–325. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Celebioglu, A.; Uyar, T. Flexible and highly stable electrospun nanofibrous membrane incorporating gold nanoclusters as an efficient probe for visual colorimetric detection of Hg(II). J. Mater. Chem. A 2014, 2, 12717–12723. [Google Scholar] [CrossRef]
- Si, Y.; Wang, X.; Li, Y.; Chen, K.; Wang, J.; Yu, J.; Wang, H.; Ding, B. Optimized colorimetric sensor strip for mercury(II) assay using hierarchical nanostructured conjugated polymers. J. Mater. Chem. A 2014, 2, 645–652. [Google Scholar] [CrossRef]
- Najarzadekan, H.; Sereshti, H. Development of a colorimetric sensor for nickel ion based on transparent electrospun composite nanofibers of polycaprolactam-dimethylglyoxime/polyvinyl alcohol. J. Mater. Sci. 2016, 51, 8645–8654. [Google Scholar] [CrossRef]
- Saithongdee, A.; Praphairaksit, N.; Imyim, A. Electrospun curcumin-loaded zein membrane for iron(III) ions sensing. Sens. Actuators B Chem. 2014, 202, 935–940. [Google Scholar] [CrossRef]
- Ondigo, D.A.; Tshentu, Z.R.; Torto, N. Electrospun nanofiber based colorimetric probe for rapid detection of Fe2+ in water. Anal. Chim. Acta 2013, 804, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer International Publishing: Baltimore, MD, USA, 2006. [Google Scholar]
- Zhang, J.; Cheng, F.; Li, J.; Zhu, J.-J.; Lu, Y. Fluorescent nanoprobes for sensing and imaging of metal ions: Recent advances and future perspectives. Nano Today 2016, 11, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Becerro, A.I.; Carrillo-Carrión, C.; Núñez, N.O.; Zyuzin, M.V.; Laguna, M.; González-Mancebo, D.; Ocaña, M.; Parak, W.J. Rare earth based nanostructured materials: Synthesis, functionalization, properties and bioimaging and biosensing applications. Nanophotonics 2017, 6. [Google Scholar] [CrossRef]
- Lim, S.H.; Kemling, J.W.; Feng, L.; Suslick, K.S. A colorimetric sensor array of porous pigments. Analyst 2009, 134, 2453–2457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Yoon, J. Recent Advances in Development of Chiral Fluorescent and Colorimetric Sensors. Chem. Rev. 2014, 114, 4918–4959. [Google Scholar] [CrossRef] [PubMed]
- Hadar, H.A.; Bulatov, V.; Dolgin, B.; Schechter, I. Detection of heavy metals in water using dye nano-complexants and a polymeric film. J. Hazard. Mater. 2013, 260, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kasai, H.; Nakanishi, H.; Suzuki, T.M. Test Strips for Heavy-Metal Ions Fabricated from Nanosized Dye Compounds. Angew. Chem. Int. Ed. 2006, 45, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, E.; Pradhan, N. Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: A review. Sens. Actuators B Chem. 2017, 238, 888–902. [Google Scholar] [CrossRef]
- Alex, S.A.; Chandrasekaran, N.; Mukherjee, A. State-of-the-art strategies for the colorimetric detection of heavy metals using gold nanorods based on aspect ratio reduction. Anal. Methods 2016, 8, 2131–2137. [Google Scholar] [CrossRef]
- Guo, L.; Xu, Y.; Ferhan, A.R.; Chen, G.; Kim, D.-H. Oriented Gold Nanoparticle Aggregation for Colorimetric Sensors with Surprisingly High Analytical Figures of Merit. J. Am. Chem. Soc. 2013, 135, 12338–12345. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemicasl creativity behind the assay. A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Uzun, L.; Denizli, A. Colorimetric Sensor Array Based on Gold Nanoparticles and Amino Acids for Identification of Toxic Metal Ions in Water. ACS Appl. Mater. Interfaces 2014, 6, 18395–18400. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Shi, X.; Gu, W.; Zhang, Y.; Xian, Y. A colorimetric sensor based on catechol-terminated mixed self-assembled monolayers modified gold nanoparticles for ultrasensitive detections of copper ions. Analyst 2012, 137, 3365–3371. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Chang, H.-T. Parameters for selective colorimetric sensing of mercury(II) in aqueous solutions using mercaptopropionic acid-modified gold nanoparticles. Chem. Commun. 2007, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
| Analyte | Polymeric matrix | Recognition material | LOD (mg·L−1) | Reference |
|---|---|---|---|---|
| Single detection | ||||
| Hg(II) | PCL | AuNC | 5.0 × 10−8 | [24] |
| EC | EMIMBF4 | 1.4 × 10−5 | [25] | |
| poly(MMA-co-BNPTU-co-RhBAM) | BNPTU | 4.0 × 10−3 | [26] | |
| PAN | DTPDI | 1.0 × 10−3 | [27] | |
| poly(NIPAAm-co-NMA-co-AA) | BNPTU | 2.0 × 10−2 | [28] | |
| Fe(III) | PAN | CDs | 0.2 | [29] |
| poly(HEMA-co-NMA-co-NBD) | SRhBOH | 5.6 | [30] | |
| Ni(II) | PAN-PAA | PAN-PAA | 7.0 × 10−3 | [31] |
| Cu(II) | PMMA | CsPbBr3 QDs | 6.0 × 10−11 | [32] |
| PNNR | PNNR | 6.4 × 10−3 | [33] | |
| CA | DTT.AuNC | 5.0 × 10−2 | [34] | |
| poly(MMA-co-AHPA) | RhB-hydrazine | 9.5 × 10−2 | [35] | |
| poly(NIPAAm-co-NMA) | F-phen | - | [36] | |
| Al(III) | PU | R2PP | 2.0 × 10−4 | [37] |
| Multiple detection | ||||
| Cu(II)/Cr(III) | CA | 1,4-DHAQ | 2.0 × 10−4 for Cu(II) and Cr(III) | [38] |
| Co(II)/Zn(II) | PU | DS-5N, FL-5N and NBD-5N | 2.0 for Co(II) and 3.0 for Zn(II) | [39] |
| Fe(III)/Cr(III)/Hg(II) | PVA | SRD and SSRD | 5.6 × 10−2 for Fe(III), 5.2 × 10−2 for Cr(III) and 0.1 for Hg(II) | [40] |
| Pb(II)/Hg(II)/Fe(III)/Mn(II)/Ni(II)/Cd(II) | PAN | CPEs | - | [41] |
| Analyte | Polymeric Matrix | Recognition Material | LOD (mg·L−1) | Reference |
|---|---|---|---|---|
| Pb(II) | CA | PMDA | 1.0 × 10−2 | [42] |
| PAN | PCDA and PCDA-5EG | 1.0 × 10−1 | [43] | |
| PA6/PVDF | Au@GSH NPs | 1.0 × 10−1 | [44] | |
| CA | curcumin | 4.1 | [45] | |
| Hg(II) | PVA | AuNC | 1.0 × 10−3 | [46] |
| PA6/PVB | PANI | 1.0 × 10−3 | [47] | |
| Ni(II) | N6 | DMG | 2.0 × 10−3 | [48] |
| Fe(III) | Zein | curcumin | 4.0 × 10−1 | [49] |
| Fe(II) | PVBC | PIMH | 1.0 × 10−4 (solution) and 2.0 × 10−3 (solid state) | [50] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terra, I.A.A.; Mercante, L.A.; Andre, R.S.; Correa, D.S. Fluorescent and Colorimetric Electrospun Nanofibers for Heavy-Metal Sensing. Biosensors 2017, 7, 61. https://doi.org/10.3390/bios7040061
Terra IAA, Mercante LA, Andre RS, Correa DS. Fluorescent and Colorimetric Electrospun Nanofibers for Heavy-Metal Sensing. Biosensors. 2017; 7(4):61. https://doi.org/10.3390/bios7040061
Chicago/Turabian StyleTerra, Idelma A. A., Luiza A. Mercante, Rafaela S. Andre, and Daniel S. Correa. 2017. "Fluorescent and Colorimetric Electrospun Nanofibers for Heavy-Metal Sensing" Biosensors 7, no. 4: 61. https://doi.org/10.3390/bios7040061
APA StyleTerra, I. A. A., Mercante, L. A., Andre, R. S., & Correa, D. S. (2017). Fluorescent and Colorimetric Electrospun Nanofibers for Heavy-Metal Sensing. Biosensors, 7(4), 61. https://doi.org/10.3390/bios7040061






