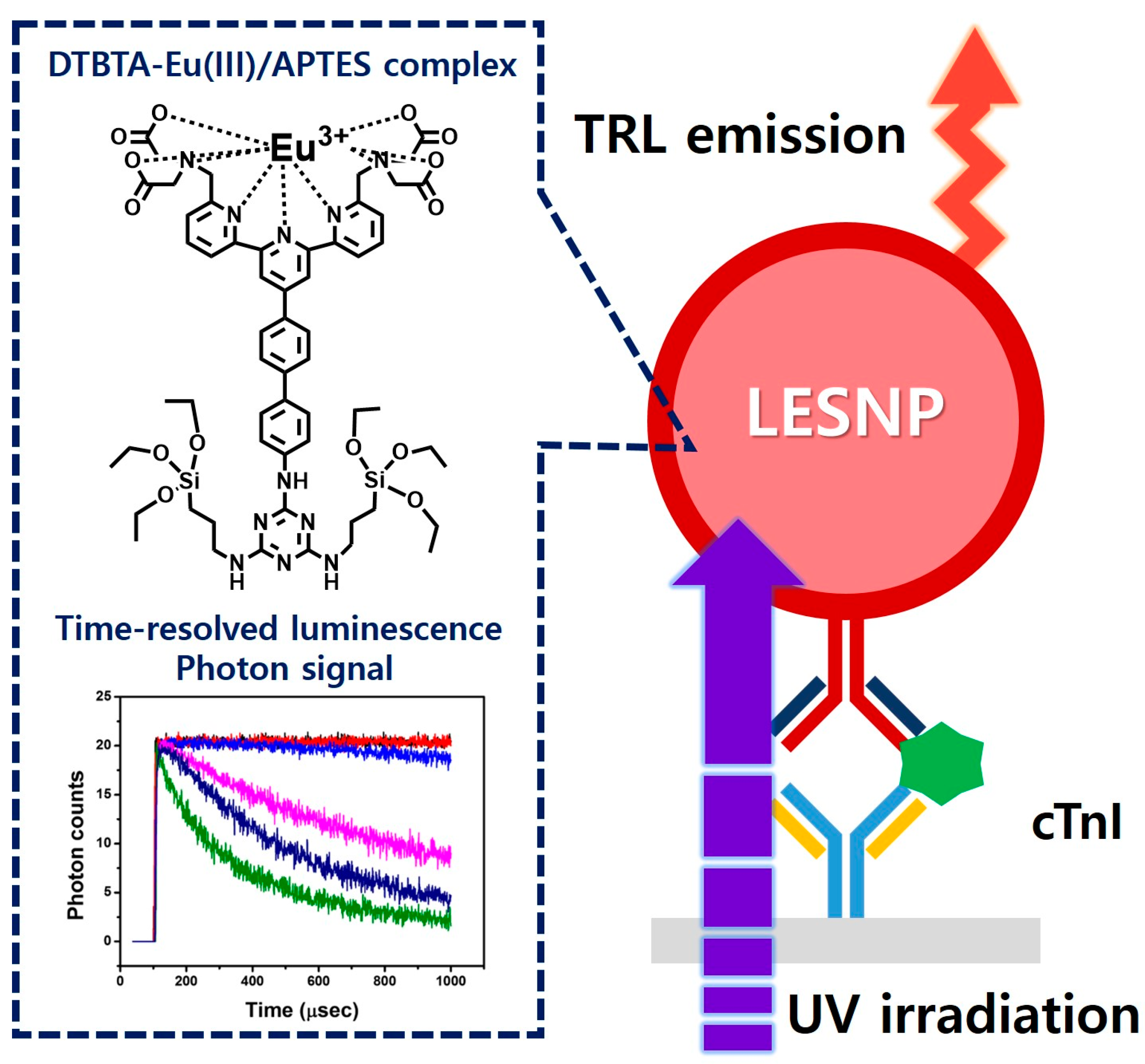
Encapsulation-Stabilized, Europium Containing Nanoparticle as a Probe for Time-Resolved luminescence Detection of Cardiac Troponin I
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent and Apparatus
2.2. Preparation of Europium-Chelates/Aminosilane Complex
2.3. Synthesis of Lanthanide Chelate-Encapsulated Silica Nanoparticles
2.4. Antibody Conjugation on Synthesized Amine Group-Terminated LESNPs
2.5. Conjugation of the Surface Modification of LESNP
2.6. Construction of the cTnI Immunosensing Surface
2.7. Fabrication of the Homemade Time-Resolved Luminescence Analyzer
2.8. TRL Immunosensing of cTnI Using LESNP
3. Results and Discussion
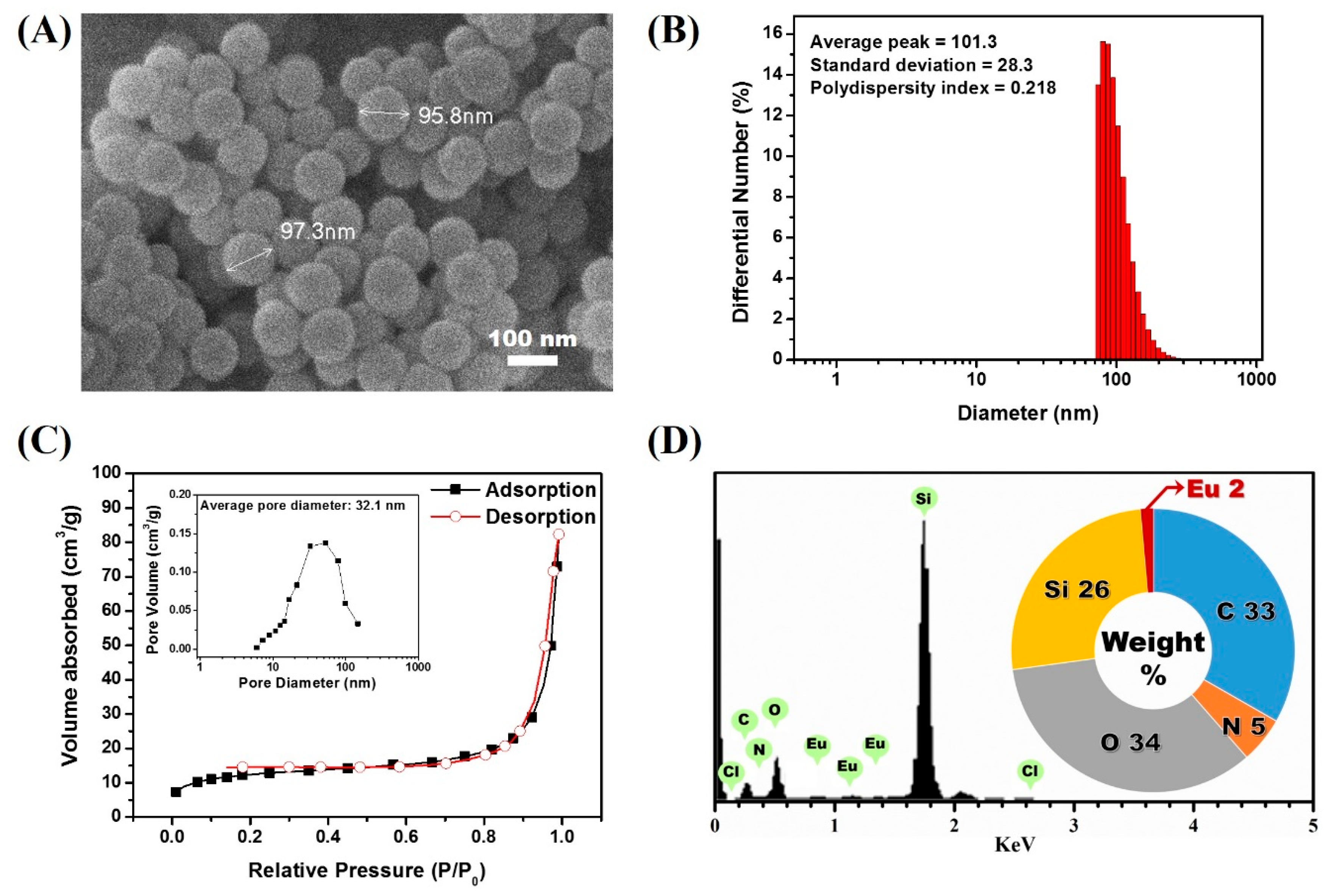
3.1. Synthesis of Lanthanide Chelate-Encapsulated Silica Nanoparticles
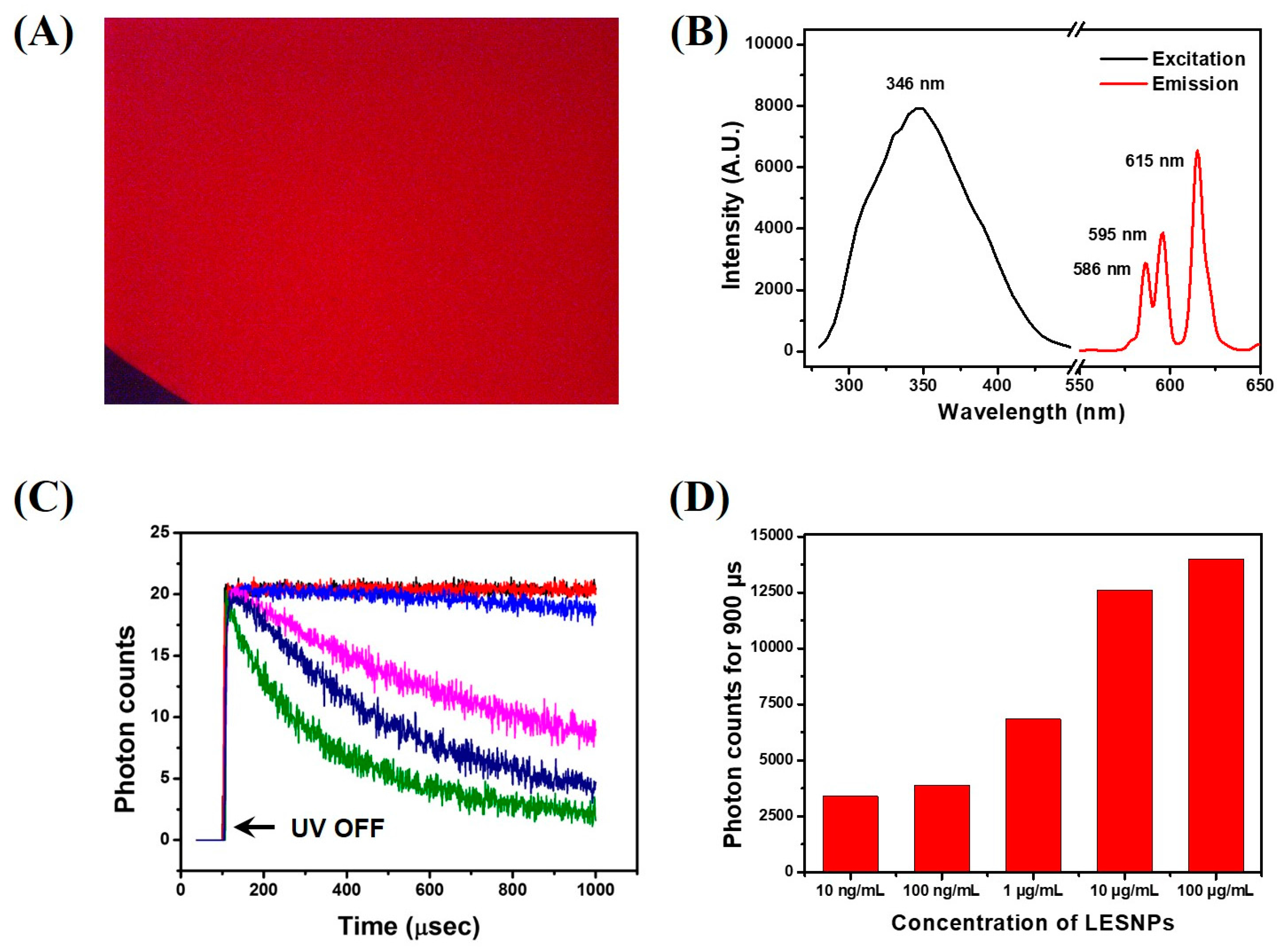
3.2. Optical Property of the Synthesized LESNP
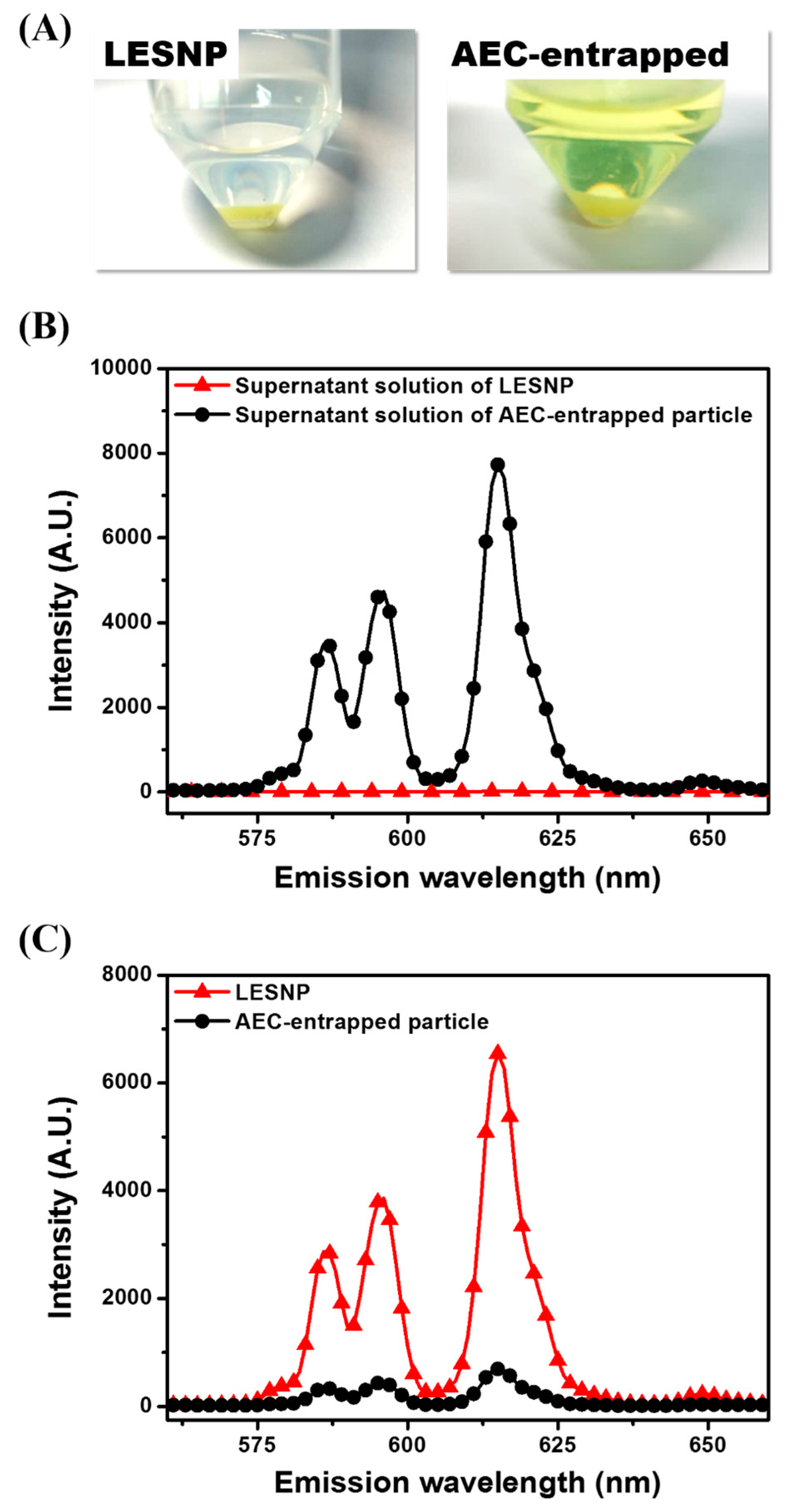
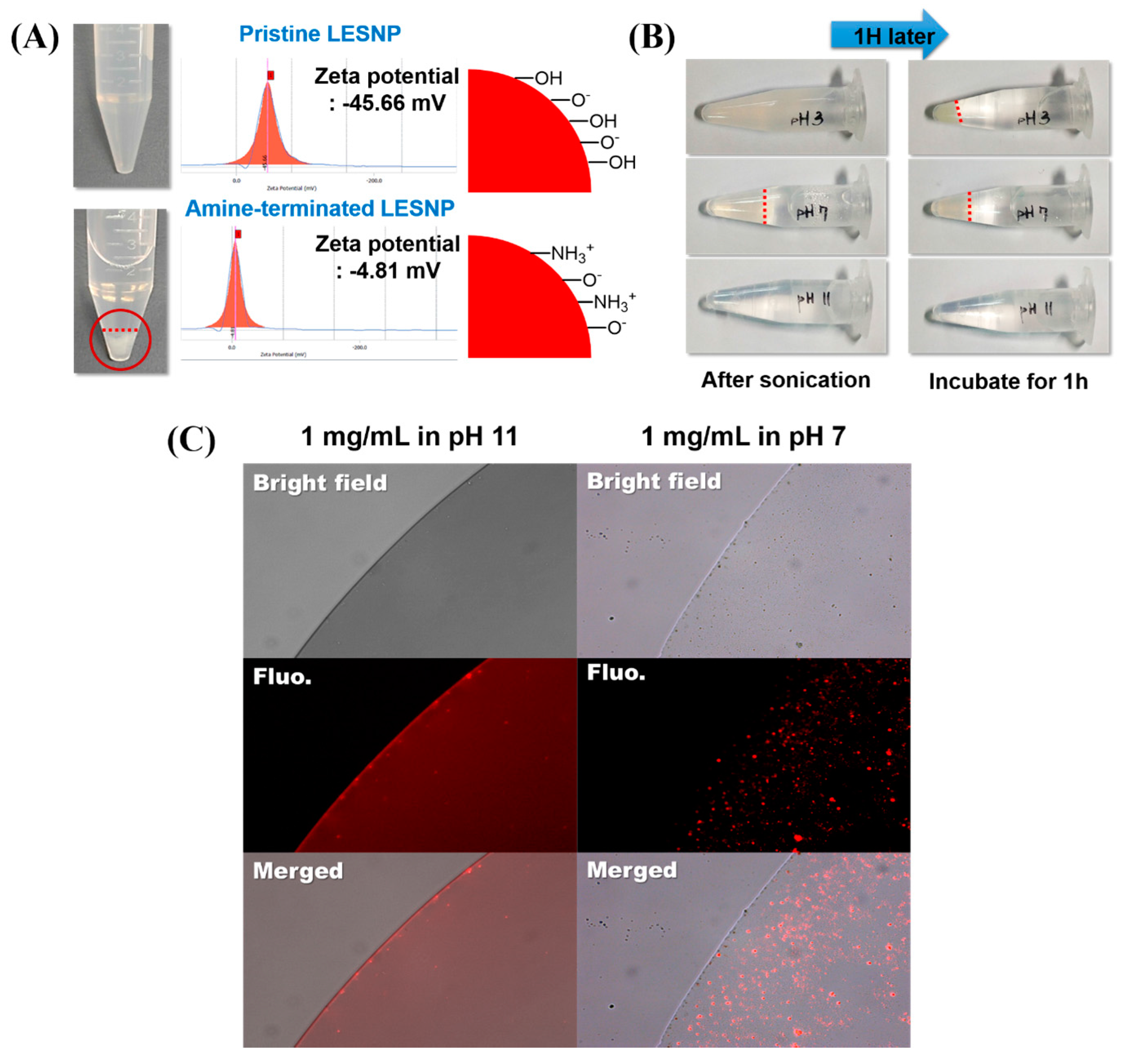
3.3. Confirmation of the Physical Stabtility of LESNPs
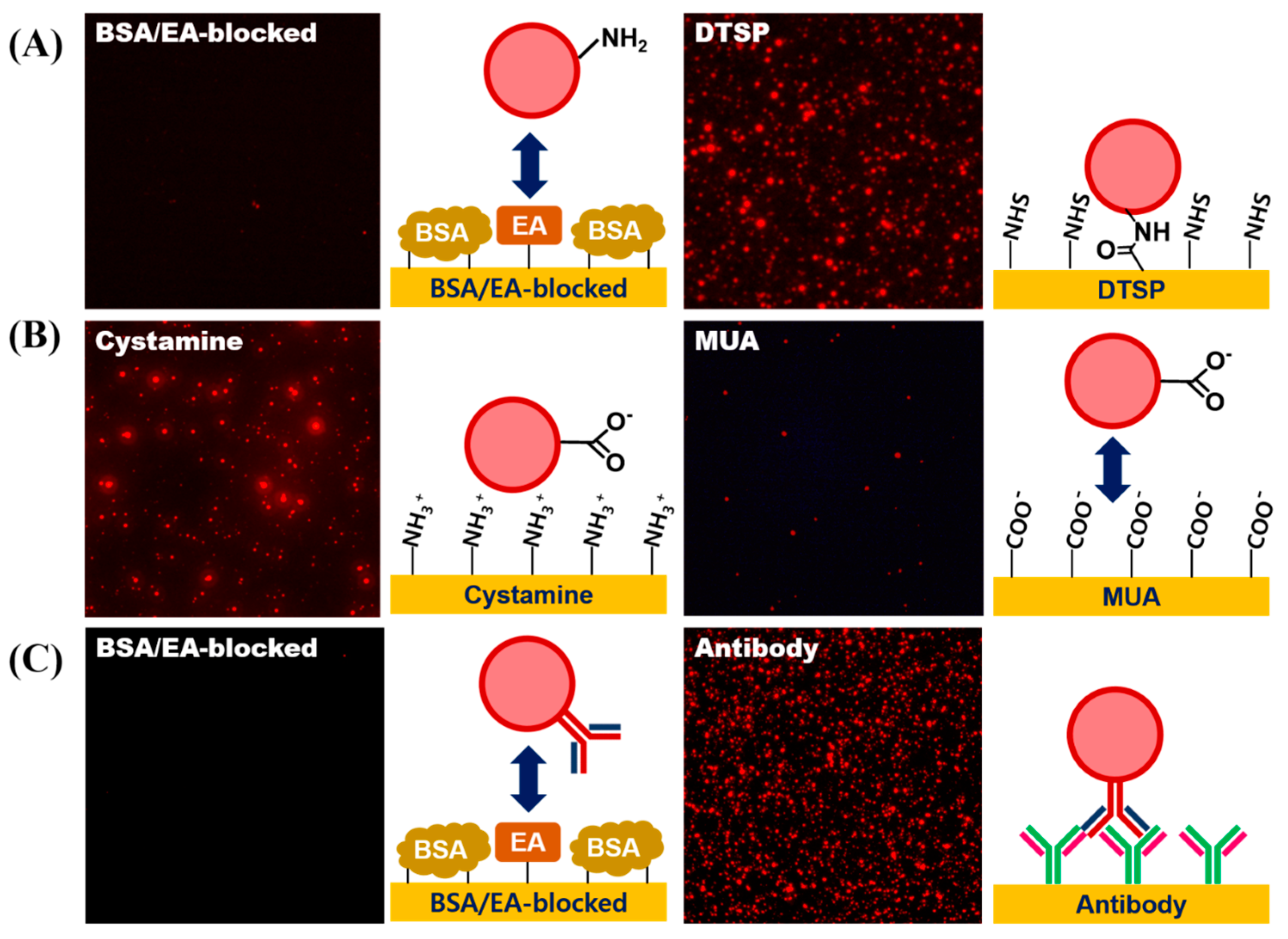
3.4. Surface Modification of LESNPs for Biofunctionalization
3.5. Conjugation of Biomolecules on LESNP
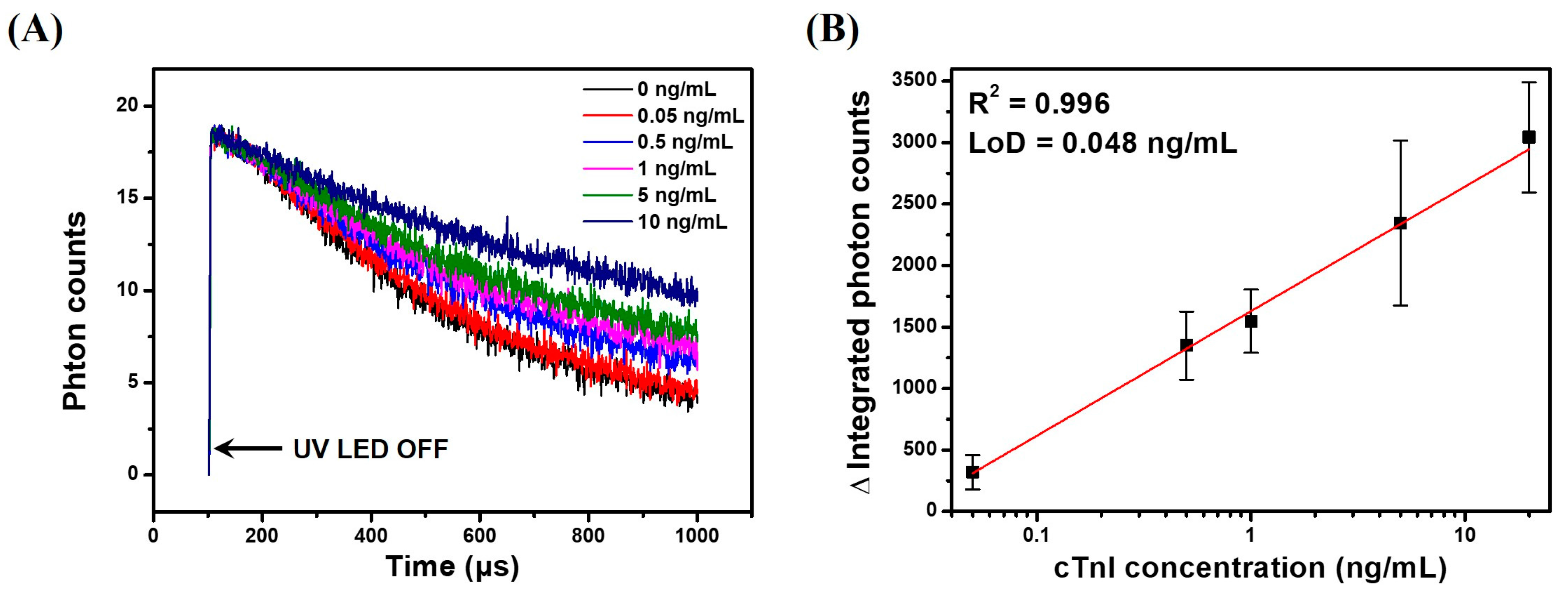
3.6. TRL Immunoassay of cTnI Using Anti-cTnI IgG-Conjugated LESNP
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gubala, V.; Harris, L.; Ricco, A.; Tan, M.; Williams, D. Point of care diagnostics: Status and Future. Anal. Chem. 2012, 84, 487–515. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.; Schneider, E.; Lam, E.; Hrapovic, S.; Luong, J. One-step antibody immobilization-based rapid and highly-sensitive sandwich ELISA procedure for potential in vitro diagnostics. Sci. Rep. 2014, 4, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hwang, S.; Oh, H. Serological tests for the diagnosis of infectious diseases. BioChip J. 2016, 10, 346–353. [Google Scholar] [CrossRef]
- Devi, R.; Doble, M.; Verma, R. Nanomaterials for early detection of cancer biomarker with special emphasis on gold nanoparticles in immunoassay/sensors. Biosens. Bioelectr. 2015, 68, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Reichlin, T.; Hochholzer, W.; Bassetti, S.; Steuer, S.; Stelzig, C.; Hartwiger, S.; Biedert, S.; Schaub, N.; Buerge, C.; Potocki, M.; et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N. Engl. J. Med. 2009, 361, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Colinson, P.; Gaza, D.; Goodacre, S. The clinical and diagnostic performance characteristics of the high sensitivity abbott cardiac troponin I assay. Clin. Biochem. 2015, 48, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Fathil, M.; Arshad, M.; Gopinath, S.; Hashim, U.; Adzhri, R.; Ayub, R.; Ruslinda, A.; Nuzaihan, M.; Azman, A.; Zaki, M.; et al. Diagnostics on acute myocardiac infarction: Cardiac troponin biomarkers. Biosens. Bioelectr. 2015, 70, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yu, Q.; Duan, Y. Fluorescent labels in biosensors for pathogen detection. Crit. Rev. Biotechnol. 2015, 35, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Keum, Y.; Li, Q.; Shelverd, W.; Guo, L. Sensitive immunoassay detection of multiple environmental chemicals on protein microarrays using DNA/dye conjugate as a fluorescent label. J. Environ. Monit. 2012, 14, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Nooney, R.; Rebello, V.; Keegan, G.; O’Connell, C.; Byrne, D.; McDonagh, C. Highly sensitive detection of c-reactive protein using a novel dissolution approach in a dye-doped silica nanoparticle-based fluorescence immunoassay. Anal. Methods 2017, 9, 994–1003. [Google Scholar] [CrossRef]
- Escosura-Muñiz, A.; Parolo, C.; Merkoçi, A. Immunosensing using nanoparticles. Mater. Today 2010, 13, 24–34. [Google Scholar] [CrossRef]
- Chen, H.; Wu, S.; Dong, F.; Cheng, W.; Li, Q.; Ding, S.; Luo, R. A novel chemiluminescence immunoassay for highly sensitive and specific detection of protein using rolling circle amplification and the multiplex binding system. Sens. Actuators B Chem. 2015, 221, 328–333. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Liang, M.; Xu, H.; Tang, S.; Yang, H.; Songa, H. Sensitive fluorescence immunoassay of alpha-fetoprotein through copper ions modulated growth of quantum dots in-situ. Sens. Actuators B Chem. 2017, 247, 408–413. [Google Scholar] [CrossRef]
- Goryacheva, O.; Beloglazova, N.; Vostrikova, A.; Pozharov, M.; Sobolev, A.; Goryacheva, I. Lanthanide-to-quantum dot Förster resonance energy transfer (FRET): Application for immunoassay. Talanta 2017, 164, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhan, S.; Xu, H.; Meng, X.; Xiong, Y.; Chen, X. Ultrasensitive fluorescence immunoassay for detection of ochratoxin A using catalase-mediated fluorescence quenching of CdTe QDs. Nanoscale 2016, 8, 9390–9397. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Rossi, C.; Kijek, T.; Henchal, E.; Ludwig, G. Comparison of dissociation-enhanced lanthanide fluorescent immunoassays to enzyme-linked immunosorbent assays for detection of staphylococcal enterotoxin b, yersinia pestis-specific f1 antigen, and Venezuelan equine encephalitis virus. Clin. Diagn. Lab. Immunol. 2001, 8, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Cho, I.H.; Park, J.N.; Seo, S.M.; Paek, S.H. A high-performance fluorescence immunoassay based on the relaxation of quenching, exemplified by detection of cardiac troponin I. Sensors 2016, 16, 669. [Google Scholar] [CrossRef] [PubMed]
- Giannitsis, E.; Kurz, K.; Hallermayer, K.; Jarausch, J.; Jaffe, A.S.; Katus, H.A. Analytical validation of a high-sensitivity cardiac troponin t assay. Clin. Chem. 2010, 56, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tian, J.; Zhao, Y.; Zhao, S. Ag/Au nanoparticles coated graphene electrochemical sensor for ultrasensitive analysis of carcinoembryonic antigen in clinical immunoassay. Sens. Actuators B Chem. 2015, 206, 570–576. [Google Scholar] [CrossRef]
- Jarolim, P. High sensitivity cardiac troponin assays in the clinical laboratories. Clin. Chem. Lab. Med. 2015, 53, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.Y.; Yan, X.P. Bioconjugated persistent luminescence nanoparticles for Föster resonance energy transfer immunoassay of prostate specific antigen in serum and cell extracts without in situ excitation. Chem. Commun. 2015, 51, 3903–3906. [Google Scholar] [CrossRef] [PubMed]
- Sirkka, N.; Lyytikäinen, A.; Savukoski, T.; Soukka, T. Upconverting nanophosphors as reporters in a highly sensitive heterogeneous immunoassay for cardiac troponin I. Anal. Chim. Acta 2016, 925, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Martí, A.A.; Li, X.; Jockusch, S.; Stevens, N.; Li, Z.; Raveendra, X.; Kalachikov, S.; Morozova, I.; Russo, J.J.; Akins, D.L.; et al. Design and characterization of two-dye and three-dye binary fluorescent probes for mRNA detection. Tetrahedron 2007, 63, 3591–3600. [Google Scholar] [CrossRef] [PubMed]
- Hagan, A.K.; Zuchner, T. Lanthanide-based time-resolved luminescence immunoassays. Anal. Bioanal. Chem. 2011, 400, 2847–2864. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tan, W.B.; Zhang, Y.; Fan, X.; Wang, M. Luminescent nanomaterials for biological labelling. Nanotechnology 2006, 17, R1–R13. [Google Scholar] [CrossRef]
- Gao, M.; Su, H.; Li, S.; Lin, Y.; Ling, X.; Qin, A.; Tang, B.Z. An easily accessible aggregation-induced emission probe for lipid droplet-specific imaging and movement tracking. Chem. Commun. 2017, 53, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Geibler, D.; Qiu, X.; Wegner, K.D.; Hildebrandt, N. A rapid, amplification-free sensitive diagnostic assay for single-step multiplexed fluorescence detection of microRNA. Angew. Chem. Int. Ed. 2015, 54, 10024–10029. [Google Scholar] [CrossRef] [PubMed]
- Seydack, M. Nanoparticle labels in immunosensing using optical detection methods. Biosens. Bioelectron. 2005, 20, 2454–2469. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.; Zheng, W.; Liu, Y.; Zhu, H.; Chen, X. Luminescent biodetection based on lanthanide-doped inorganic nanoprobes. Coord. Chem. Rev. 2014, 273, 13–29. [Google Scholar] [CrossRef]
- Lopez, E.; Chypre, C.; Alpha, B.; Mathis, G. Europium(III) trisbipyridine cryptate label for time-resolved fluorescence detection of polymerase chain reaction products fixed on a solid support. Clin. Chem. 1993, 39, 196–201. [Google Scholar] [PubMed]
- Nishioka, T.; Yuan, J.; Yamamoto, Y.; Sumitomo, K.; Wang, Z.; Hashino, K.; Hosoya, C.; Ikawa, K.; Wang, G.; Matsumoto, K. New luminescent europium(III) chelates for DNA labeling. Inorg. Chem. 2006, 45, 4088–4096. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Jin, D.; Drozdowicz-Tomsia, K.; Yuan, J.; Goldys, E.M. Europium chelate (BHHCT-Eu3+) and its metal nanostructure enhanced luminescence applied to bioassays and time-gated bioimaging. Langmuir 2010, 26, 10036–10043. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K. Time-resolved luminescence microscopy and microarray using europium chelate labels. In Microscopy: Science, Technology, Applications and Education; Méndez-Vilas, A., Díaz, J., Eds.; Formatex: Badajoz, Spain, 2010; Volume 3, pp. 2129–2136. [Google Scholar]
- Amoroso, A.J.; Pope, S.J.A. Using lanthanide ions in molecular bioimaging. Chem. Soc. Rev. 2015, 44, 4723–4742. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Selvin, P.R. Carbostyril derivatives as antenna molecules for luminescent lanthanide chelates. Bioconj. Chem. 2004, 15, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Hemmila, I.; Laitala, V. Progress in lanthanides as luminescent probes. J. Fluoresc. 2005, 15, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, S.; He, D. A novel europium chelate coated nanosphere for time-resolved fluorescence immunoassay. PLoS ONE 2015, 10, e0129689. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Tan, M.; Wang, G.; Yuan, J. Novel fluorescent europium chelate-doped silica nanoparticle: Preparation, characterization and time-resolved fluorometric application. J. Mater. Chem. 2004, 14, 851–856. [Google Scholar] [CrossRef]
- Aikawa, T.; Mizuno, A.; Kohri, M.; Taniguchi, T. Polystyrene latex particles containing europium complexes prepared by miniemulsion polymerization using bovine serum albumin as a surfactant for biochemical diagnosis. Colloids Surf. B Biointerface 2016, 145, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Murcia, M.J.; Naumann, C.A. Biofunctionalization of fluorescent nanoparticles. In Nanotechnologies for the Life Sciences; Challa, S.S., Kumar, R., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2005; Volume 1, pp. 1–40. [Google Scholar]
- Bagwe, R.P.; Hilliard, L.R.; Tan, W. Surface modification of silica nanoparticles to reduce aggregation and non-specific binding. Langmuir 2006, 25, 4357–4362. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.J.; Marand, E. Hybrid inorganic-organic materials based on a 6FDA-6FpDA-DABA polyimide and silica: Physical characterization studies. Polymer 2002, 43, 2385–2400. [Google Scholar] [CrossRef]
- Landfester, K.; Weiss, C.K. Encapsulation by miniemulsion polymerization. In Modern Techniques for Nano-Microreactors/-Reactions; Frank, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–49. [Google Scholar]
- Rahman, I.A.; Padavettan, V. Synthesis of silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—A review. J. Nanomater. Mat. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Han, Y.D.; Kim, K.; Yang, S.S.; Yoon, H.C. A fluoro-microbead guiding chip for simple and quantification immunoassay of cardiac troponin I (cTnI). Biosens. Bioelectron. 2011, 26, 3818–3824. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, F.; Wang, A.; Guo, M.; Zhao, J.; Qi, W.; Yingjie, G.; Gu, J.; Zhu, G. pH dictates the release of hydrophobic drug cocktail from mesoporous nanoarchitecture. ACS Appl. Mater. Interfaces 2013, 5, 11828–11835. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, J. Dye sensitized luminescent europium nanoparticles and its time-resolved fluorometric assay for DNA. Anal. Chim. Acta 2007, 587, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Bruls, D.M.; Evers, T.H.; Kahlman, J.A.H.; Lankvelt, P.J.W.; Ovsyanko, M.; Pelssers, E.G.M.; Schleipen, J.J.H.B.; Theije, F.K.; Verschuren, C.A.; Wijk, T.; et al. Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles. Lab Chip 2009, 9, 3504–3510. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.C.; Kim, H.S. Multilayered assembly of dendrimers with enzymes on gold: Thickness-controlled biosensing interface. Anal. Chem. 2000, 72, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, S.; Paynter, S.; Russell, D.A.; Sapsford, K.E. Self-assembled monolayers: A versatile tool for the formulation of bio-surfaces. Trends Anal. Chem. 2000, 19, 530–540. [Google Scholar] [CrossRef]
- Park, Y.M.; Kim, S.J.; Kim, K.; Han, Y.D.; Yang, S.S.; Yoon, H.C. Lectin-based optical sensing for quantitative analysis of cancer antigen CA15–3 as a breast cancer marker. Sens. Actuators B Chem. 2013, 186, 571–579. [Google Scholar] [CrossRef]
- Jonkheijm, P.; Weinrich, D.; Schrder, H.; Niemeyer, C.M.; Waldmann, H. Chemical strategies for generating protein biochips. Angew. Chem. Int. Ed. 2008, 47, 9618–9647. [Google Scholar] [CrossRef] [PubMed]
- Howarter, J.A.; Youngblood, J.P. Optimization of silica silanization by 3-aminopropyltriethoxysilane. Langmuir 2006, 22, 11142–11147. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, S.W.; Yang, S.S. The optimization of PDMS-PMMA bonding process using silane primer. BioChip J. 2010, 4, 148–154. [Google Scholar] [CrossRef]
- Wu, J.; Ye, Z.; Wang, G.; Jin, D.; Yuan, J.; Guan, Y.; Piper, J. Visible-light-sensitized highly luminescent europium nanoparticles: Preparation and application for time-gated luminescence bioimaging. J. Mater. Chem. 2009, 19, 1258–1264. [Google Scholar] [CrossRef]
- Nakahara, Y.; Tatsumi, Y.; Akimoto, I.; Osaki, S.; Doi, M.; Kimura, K. Fluorescent silica nanoparticles modified chemically with terbium complexes as potential bioimaging probes: Their fluorescence and colloidal properties in water. New J. Chem. 2015, 39, 1452–1458. [Google Scholar] [CrossRef]
- Gotoh, Y.; Tsukada, M.; Minoura, N. Chemical modification of silk fibroin with cyanuric chloride-activated poly(ethylene glycol): Analyses of reaction site by proton NMR spectroscopy and conformation of the conjugates. Bioconj. Chem. 1993, 4, 554–559. [Google Scholar] [CrossRef]
- Klobes, P.; Meyer, K.; Munro, R.G. Porosity and Specific Surface Area Measurements for Solid Materials; Special Publication 960-17; National Institute of Standards and Techenology: Gaithersburg, MD, USA, 2006; pp. 1–79.
- Hohenesche, C.F.; Unger, K.K.; Eberle, T. Agglomerated non-porous silica nanoparticles as model carriers in polyethylene synthesis. J. Mol. Catal. A Chem. 2004, 221, 185–199. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Hashino, K.; Ito, M.; Ikawa, K.; Nishioka, T.; Matsumoto, K. Sodium dodecyl sulfate polyacrylamide slab gel electrophoresis and hydroxyethyl cellulose gel capillary electrophoresis of luminescent lanthanide chelate-labeled proteins with time-resolved detection. Anal. Sci. 2009, 25, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.J.; Monton, H.; O’Kennedy, R.; Williams, D.E.; Nogues, C.; Crean, C.; Gubala, V. Controlling colloidal stability of silica nanoparticles during bioconjugation reactions with proteins and improving their longer-term stability, handling and storage. J. Mater. Chem. B 2015, 3, 2043–2055. [Google Scholar] [CrossRef]
- Park, J.H.; Gu, L.; Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kneuer, C.; Sameti, M.; Haltner, E.G.; Schiestel, T.; Schirra, H.; Schmidt, H.; Lehr, C.-M. Silica nanoparticles modified with aminosilanes as carriers for plasmid DNA. Int. J. Pharm. 2000, 196, 257–261. [Google Scholar] [CrossRef]
- Tholen, D.W.; Linnet, K.; Kondratovich, M.; Armbruster, D.A.; Garrett, P.E.; Jones, R.L.; Kroll, M.H.; Lezuin, R.M.; Pankratz, T.J.; Scassellati, G.A.; et al. Protocols for Determination of Limit of Detection and Limits of Quantification; Approved Guideline EP17-A; NCCLS: Wayne, PA, USA, 2004; ISBN 1-56238-551-8. [Google Scholar]
- Orbulescu, J.; Micic, M.; Ensor, M.; Trajkovic, S.; Daunert, S.; Leblanc, R.M. Human cardiac troponin i: A langmuir monolayer study. Langmuir 2010, 26, 3268–3275. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.R.; Han, Y.D.; Chun, H.J.; Lee, K.W.; Hong, D.-K.; Lee, K.-N.; C. Yoon, H. Encapsulation-Stabilized, Europium Containing Nanoparticle as a Probe for Time-Resolved luminescence Detection of Cardiac Troponin I. Biosensors 2017, 7, 48. https://doi.org/10.3390/bios7040048
Kim KR, Han YD, Chun HJ, Lee KW, Hong D-K, Lee K-N, C. Yoon H. Encapsulation-Stabilized, Europium Containing Nanoparticle as a Probe for Time-Resolved luminescence Detection of Cardiac Troponin I. Biosensors. 2017; 7(4):48. https://doi.org/10.3390/bios7040048
Chicago/Turabian StyleKim, Ka Ram, Yong Duk Han, Hyeong Jin Chun, Kyung Won Lee, Dong-Ki Hong, Kook-Nyung Lee, and Hyun C. Yoon. 2017. "Encapsulation-Stabilized, Europium Containing Nanoparticle as a Probe for Time-Resolved luminescence Detection of Cardiac Troponin I" Biosensors 7, no. 4: 48. https://doi.org/10.3390/bios7040048
APA StyleKim, K. R., Han, Y. D., Chun, H. J., Lee, K. W., Hong, D.-K., Lee, K.-N., & C. Yoon, H. (2017). Encapsulation-Stabilized, Europium Containing Nanoparticle as a Probe for Time-Resolved luminescence Detection of Cardiac Troponin I. Biosensors, 7(4), 48. https://doi.org/10.3390/bios7040048





