Spontaneous Charge Generation in Flowing Albumin Solutions at 35 °C and 38 °C
Abstract
:1. Introduction
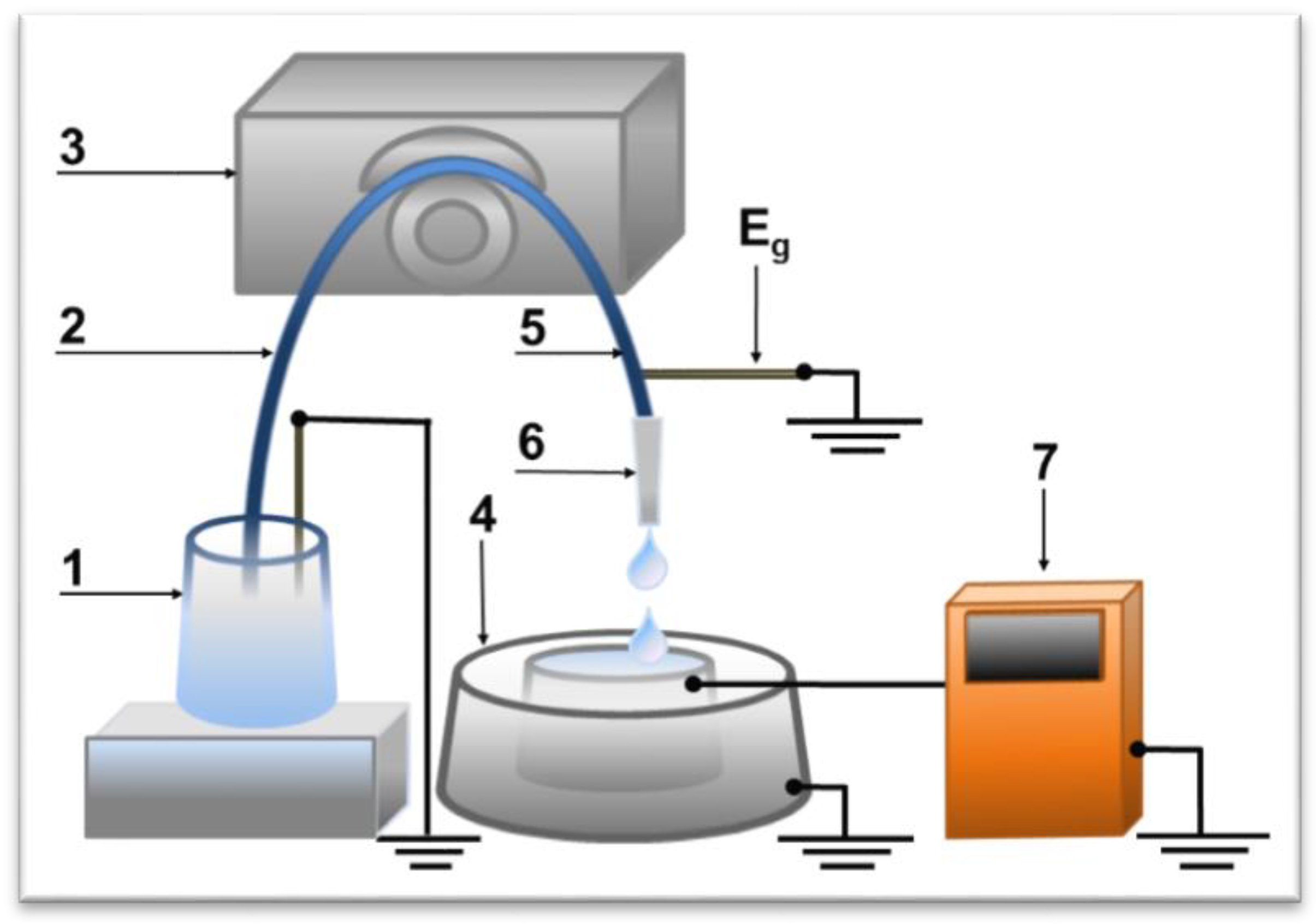
2. Materials and Methods
3. Results
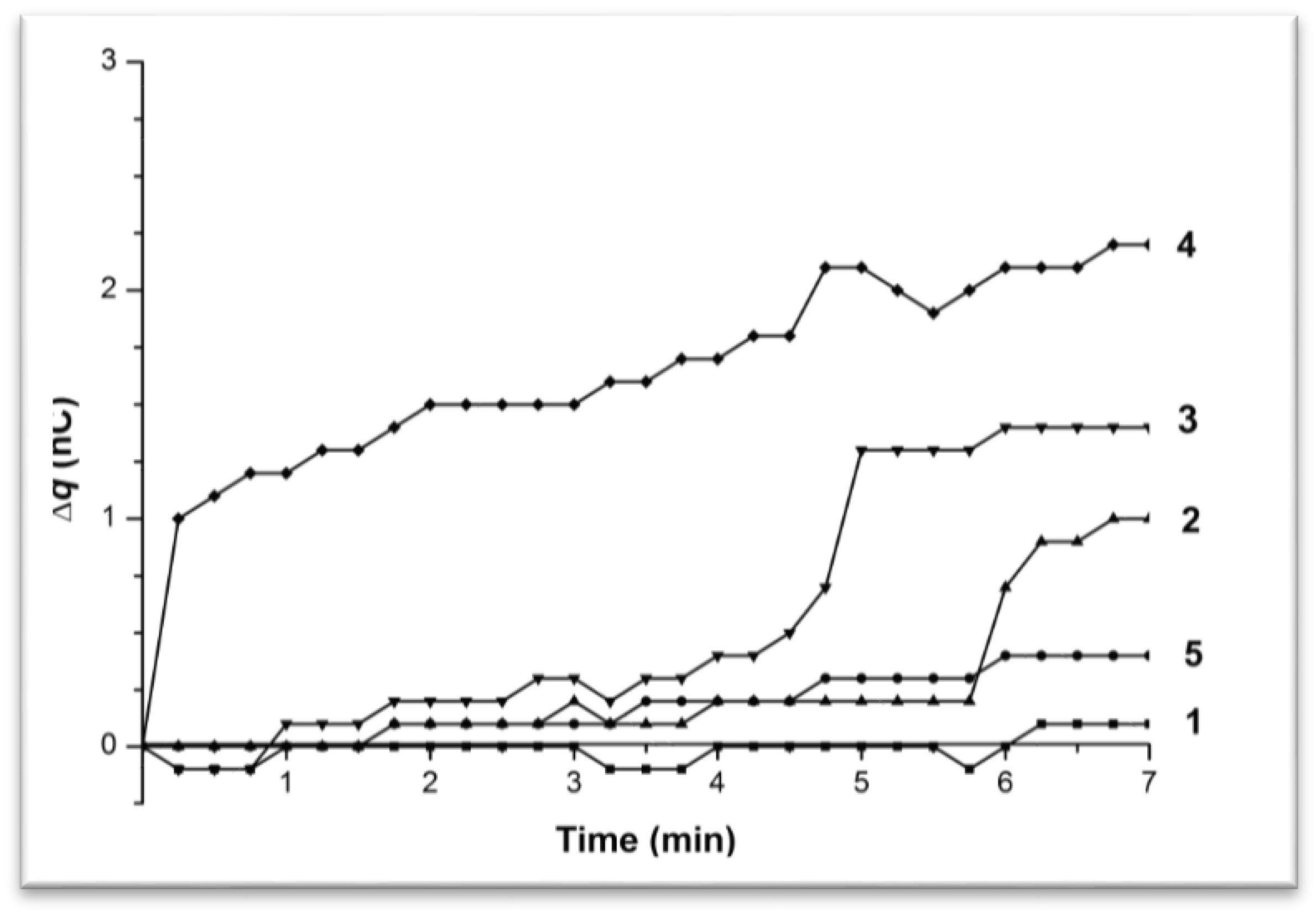
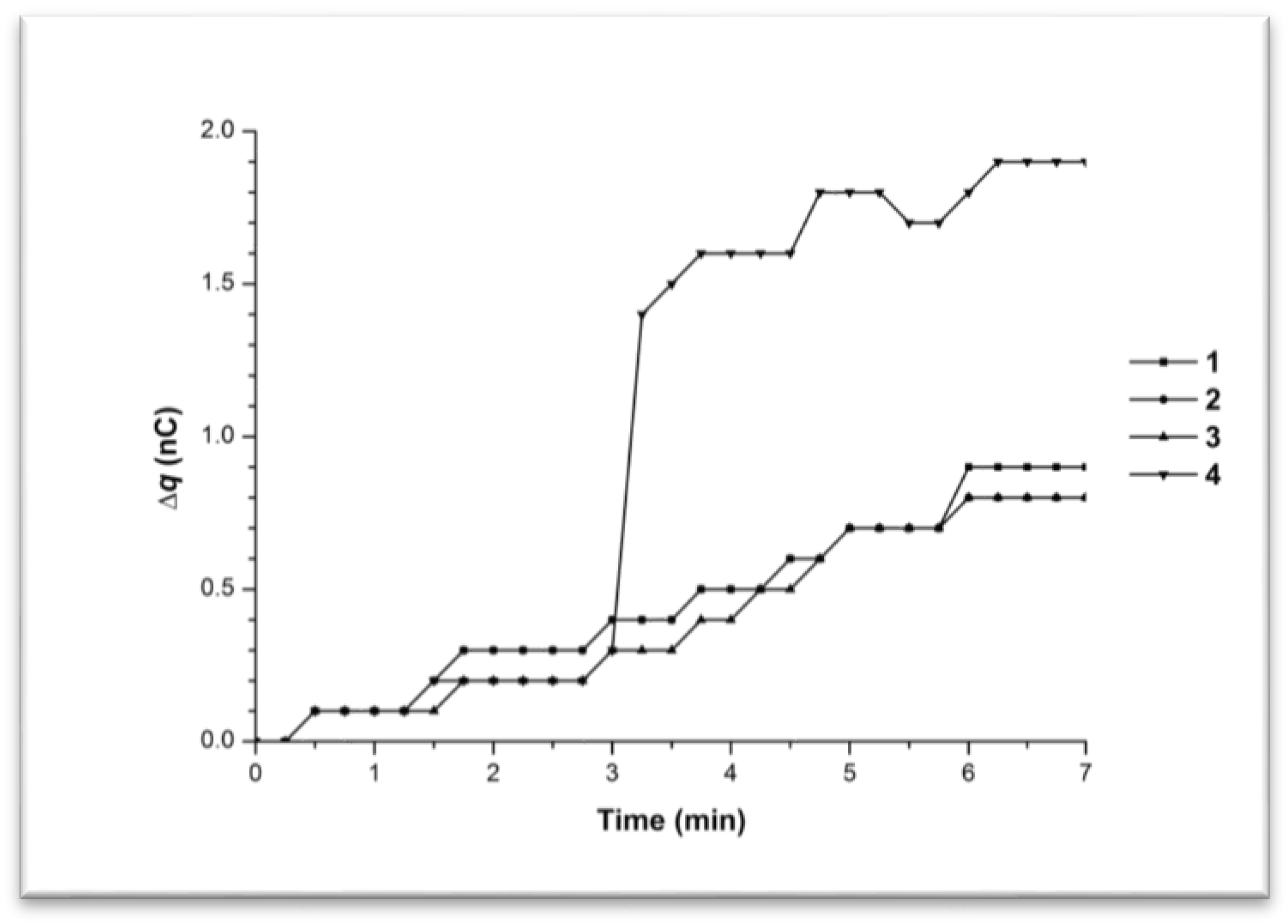
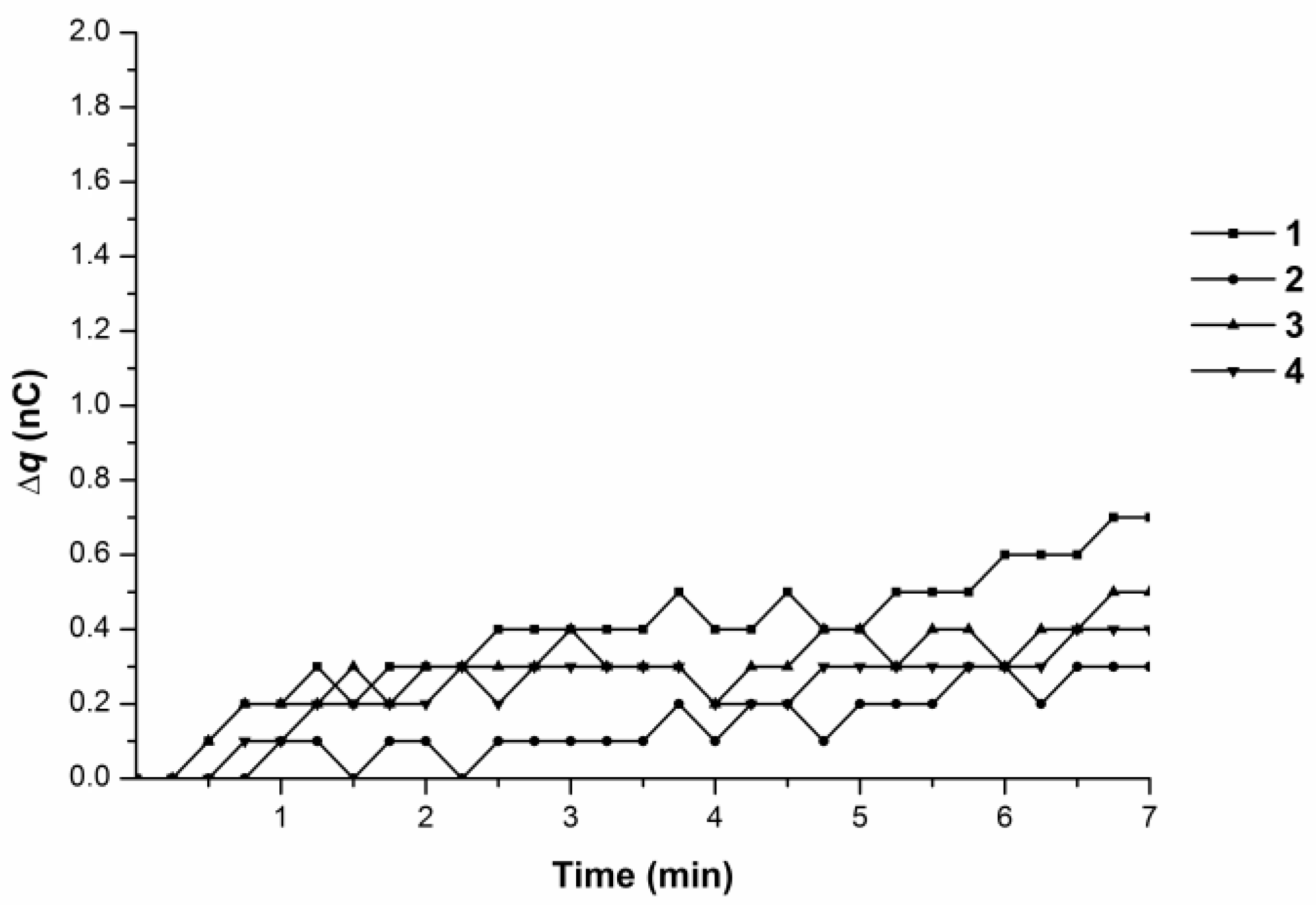
3.1. Monitoring Charge Generation and Accumulation in Water and Protein Solution at 35 °C
3.2. Monitoring Charge Generation and Accumulation in Water and Protein Solution at 38 °C
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tian, R.; Regonda, S.; Gao, J.; Liu, Y.; Hu, W. Ultrasensitive protein detection using lithographically defined Si multi-nanowire field effect transistors. Lab Chip 2011, 11, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.D.; Pleshakova, T.; Malsagova, K.; Kozlov, A.; Kaysheva, A.; Kopylov, A.; Izotov, A.; Andreeva, E.; Kanashenko, S.; Usanov, S.; et al. Highly sensitive protein detection by combination of atomic force microscopy fishing with charge generation and mass spectrometry analysis. FEBS J. 2014, 281, 4705–4717. [Google Scholar] [CrossRef] [PubMed]
- Pleshakova, T.O.; Malsagova, K.A.; Kozlov, A.F.; Kanashenko, S.L.; Ivanova, N.D.; Sadovskaya, T.A.; Archakov, A.I.; Ivanov, Y.D. Highly sensitive AFM-fishing of albumin. Patogenez 2016, 3, 30–36. (In Russian) [Google Scholar]
- Cai, Z.; Zhang, J.-T.; Xue, F.; Hong, Z.; Punihaole, D.; Asher, S.A. 2D photonic crystal protein hydrogel coulometer for sensing serum albumin ligand binding. Anal. Chem. 2014, 86, 4840–4847. [Google Scholar] [CrossRef] [PubMed]
- Archakov, A.I.; Ivanov, Y.D.; Lisitsa, A.V.; Zgoda, V.G. Biospecific irreversible fishing coupled with atomic force microscopy for detection of extremely low-abundant proteins. Proteomics 2009, 9, 1326–1343. [Google Scholar] [CrossRef] [PubMed]
- Pleshakova, T.O.; Shumov, I.D.; Ivanov, Y.D.; Malsagova, K.A.; Kaysheva, A.L.; Archakov, A.I. AFM-Based Technologies as the Way Towards the Reverse Avogadro Number. Biochem. Suppl. Ser. B Biomed. Chem. 2015, 9, 244–257. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Hayden, O.; Lakadamyali, M.; Zhuang, X.; Lieber, C.M. Electrical detection of single viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar] [CrossRef] [PubMed]
- Malsagova, K.A.; Ivanov, Y.D.; Pleshakova, T.O.; Kaysheva, A.L.; Shumov, I.D.; Kozlov, A.F.; Archakov, A.I.; Popov, V.P.; Fomin, B.I.; Latyshev, A.V. A SOI-nanowire biosensor for the multiple detection of D-NFATc1 protein in the serum. Anal. Methods 2015, 7, 8078–8085. [Google Scholar] [CrossRef]
- Malsagova, K.A.; Ivanov, Y.D.; Pleshakova, T.O.; Kozlov, A.F.; Krokhin, N.V.; Kaysheva, A.L.; Shumov, I.D.; Popov, V.P.; Naumova, O.V.; Fomin, B.I.; et al. SOI-nanowire biosensor for detection of D-NFATc1 protein. Biochem. Suppl. Ser. B Biomed. Chem. 2014, 8, 220–225. [Google Scholar] [CrossRef]
- Baldessari, F. Electrokinetics in nanochannels: Part I. Electric double layer overlap and channel-to-well equilibrium. J. Colloid Interface Sci. 2008, 325, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Lee, H.; Im, D.J.; Kang, I.S.; Lim, G.; Kim, D.S.; Kang, K.H. Spontaneous electrical charging of droplets by conventional pipetting. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.D.; Pleshakova, T.O.; Malsagova, K.A.; Kaysheva, A.L.; Kopylov, A.T.; Izotov, A.A.; Tatur, V.Y.; Vesnin, S.G.; Ivanova, N.D.; Ziborov, V.S.; et al. AFM-based protein fishing in the pulsed electric field. Biochem. Suppl. Ser. B Biomed. Chem. 2015, 9, 121–129. [Google Scholar] [CrossRef]
- Pershin, S. Conversion of ortho-para H2O isomers in water and a jump in erythrocyte fluidity through a microcapillary at a temperature of 36.6 ± 0.3 °C. Phys. Wave Phenom. 2009, 17, 241–250. [Google Scholar] [CrossRef]
- McCarty, L.S.; Whitesides, G.M. Electrostatic charging due to separation of ions at interfaces: Contact electrification of ionic electrets. Angew. Chem. Int. Ed. 2008, 47, 2188–2207. [Google Scholar] [CrossRef] [PubMed]
- Kholmanskiy, A.S. Two types of anomalous thermodynamics of water. Apriori. Ser. Iestestvennye I Tekh. Nauk. 2015, 1, 1–17. (In Russian) [Google Scholar]
- Bushuev, E.N. The calculation of temperature dependence of water ion production, specific conductance and extremely diluted electrolytic solution. Vestnik IGAU 2007, 2, 49–52. (In Russian) [Google Scholar]
- Gill, D.S.; Ali, V. Behavior of bovine serum albumin in aqueous solutions of some sodium salts of organic acids, tetraethylammonium bromide and dextrose investigated by ultrasonic velocity, viscosity and density measurements. Indian J. Chem. 1999, 38, 651–655. [Google Scholar]
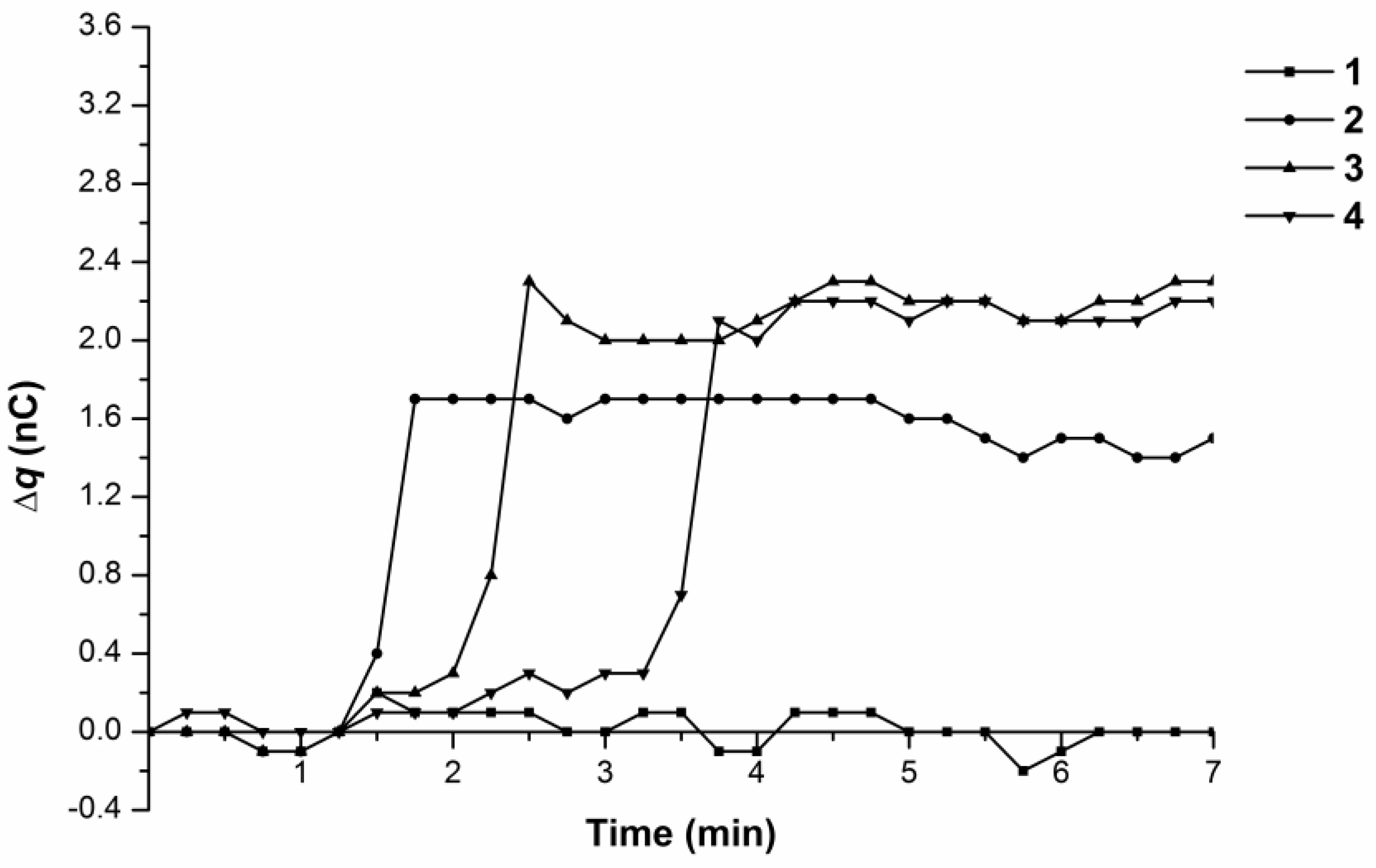

| Temperature | Type of Charge Accumulation | Water | 10−15 M BSA Solution | 10−4 M BSA Solution |
|---|---|---|---|---|
| T = 35 °C | Slow increase | 0–0.3 nC/min | 0.1 nC/min | 0.1 nC/min no abrupt charge accumulation |
| Sharp increase | 1–4 nC/min | 4 nC/min | ||
| T = 38 °C | Slow increase | 0–0.12 nC/min | 0.2 nC/min | no charge accumulation |
| Sharp increase | 3–4 nC/min | 3–8 nC/min |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, Y.D.; Kozlov, A.F.; Galiullin, R.A.; Kolesanova, E.F.; Pleshakova, T.O. Spontaneous Charge Generation in Flowing Albumin Solutions at 35 °C and 38 °C. Biosensors 2017, 7, 60. https://doi.org/10.3390/bios7040060
Ivanov YD, Kozlov AF, Galiullin RA, Kolesanova EF, Pleshakova TO. Spontaneous Charge Generation in Flowing Albumin Solutions at 35 °C and 38 °C. Biosensors. 2017; 7(4):60. https://doi.org/10.3390/bios7040060
Chicago/Turabian StyleIvanov, Yuri D., Andrey F. Kozlov, Rafael A. Galiullin, Ekaterina F. Kolesanova, and Tatyana O. Pleshakova. 2017. "Spontaneous Charge Generation in Flowing Albumin Solutions at 35 °C and 38 °C" Biosensors 7, no. 4: 60. https://doi.org/10.3390/bios7040060
APA StyleIvanov, Y. D., Kozlov, A. F., Galiullin, R. A., Kolesanova, E. F., & Pleshakova, T. O. (2017). Spontaneous Charge Generation in Flowing Albumin Solutions at 35 °C and 38 °C. Biosensors, 7(4), 60. https://doi.org/10.3390/bios7040060





