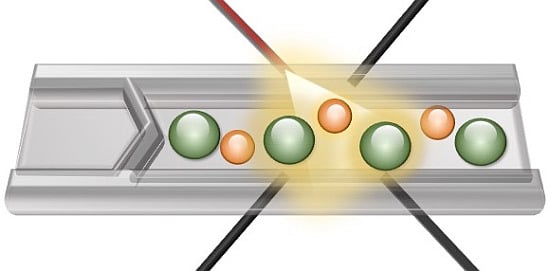
Multi-Pixel Photon Counters for Optofluidic Characterization of Particles and Microalgae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microfluidics
2.2. Optics and Electronics
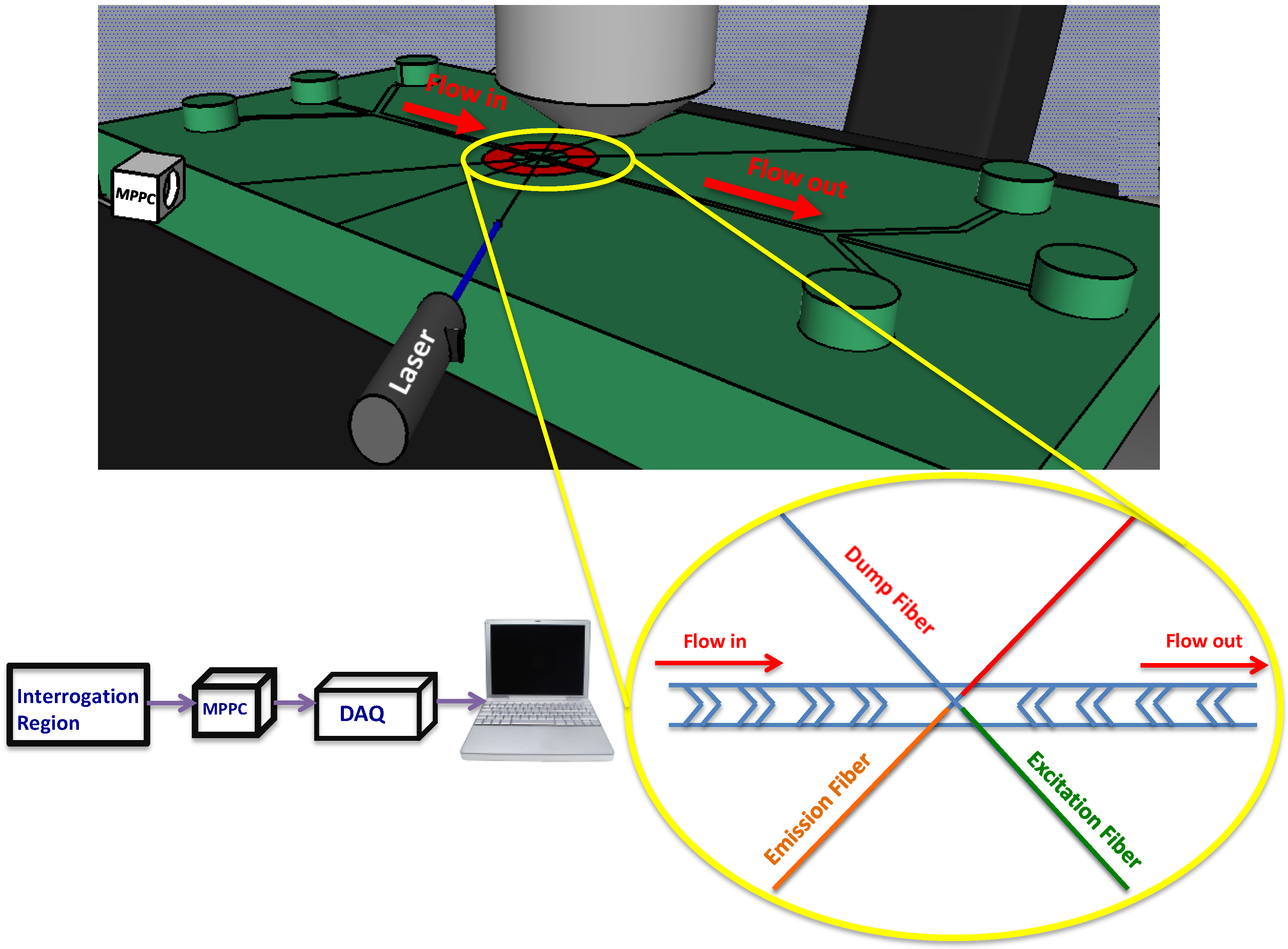
3. Results and Discussion
3.1. COMSOL Modeling
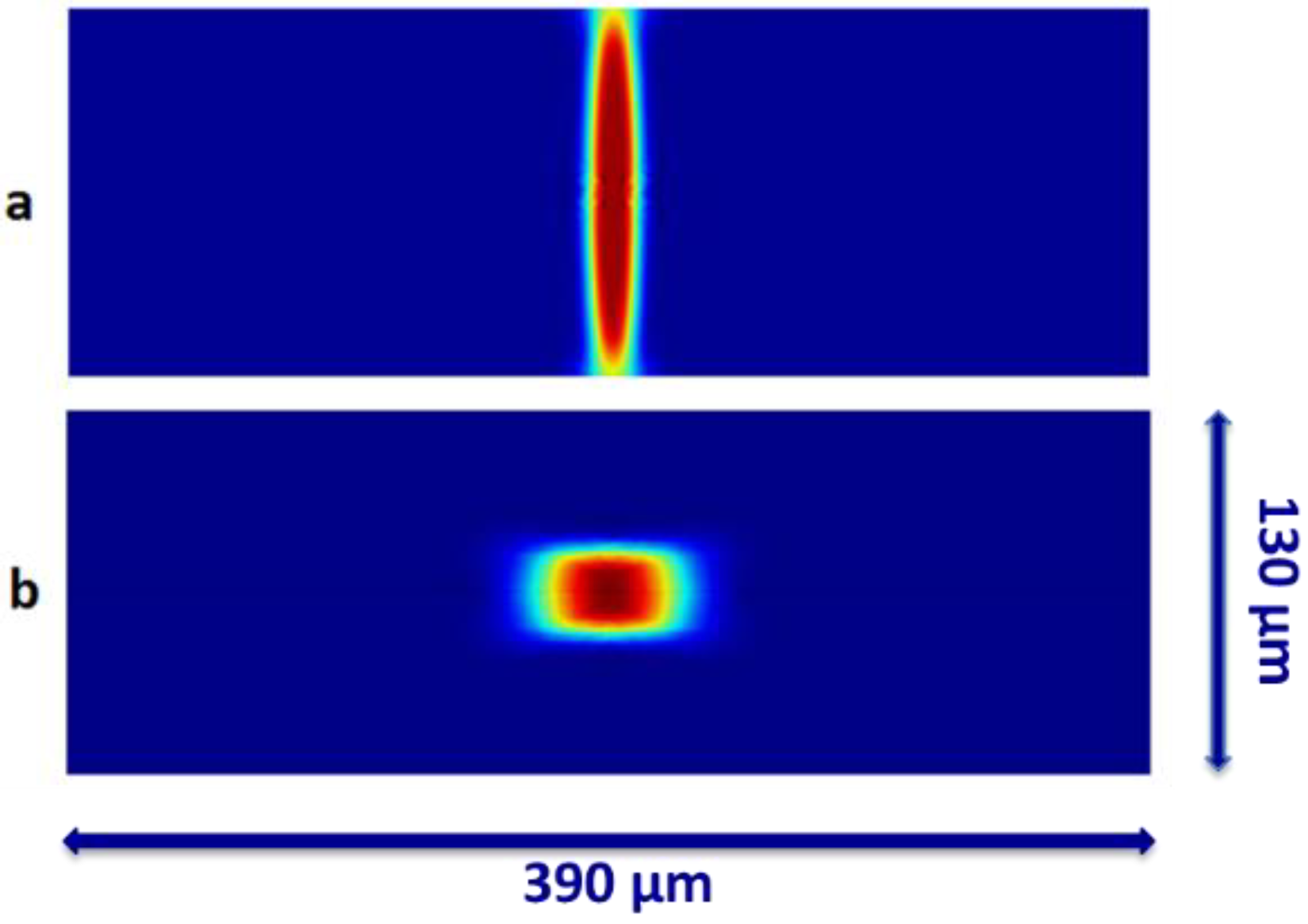
3.2. Experimental Results
3.3. Characterization of Microbeads
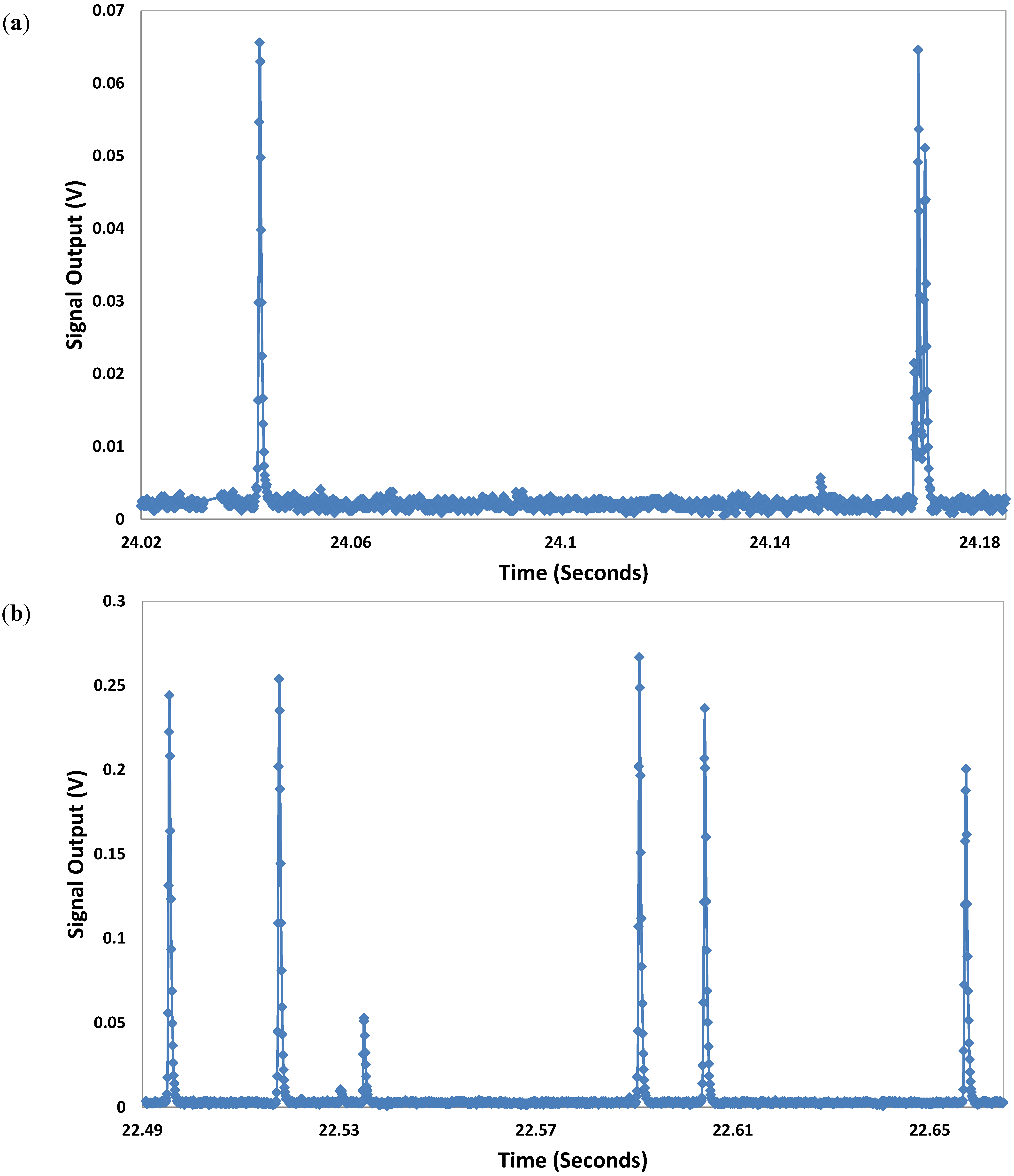
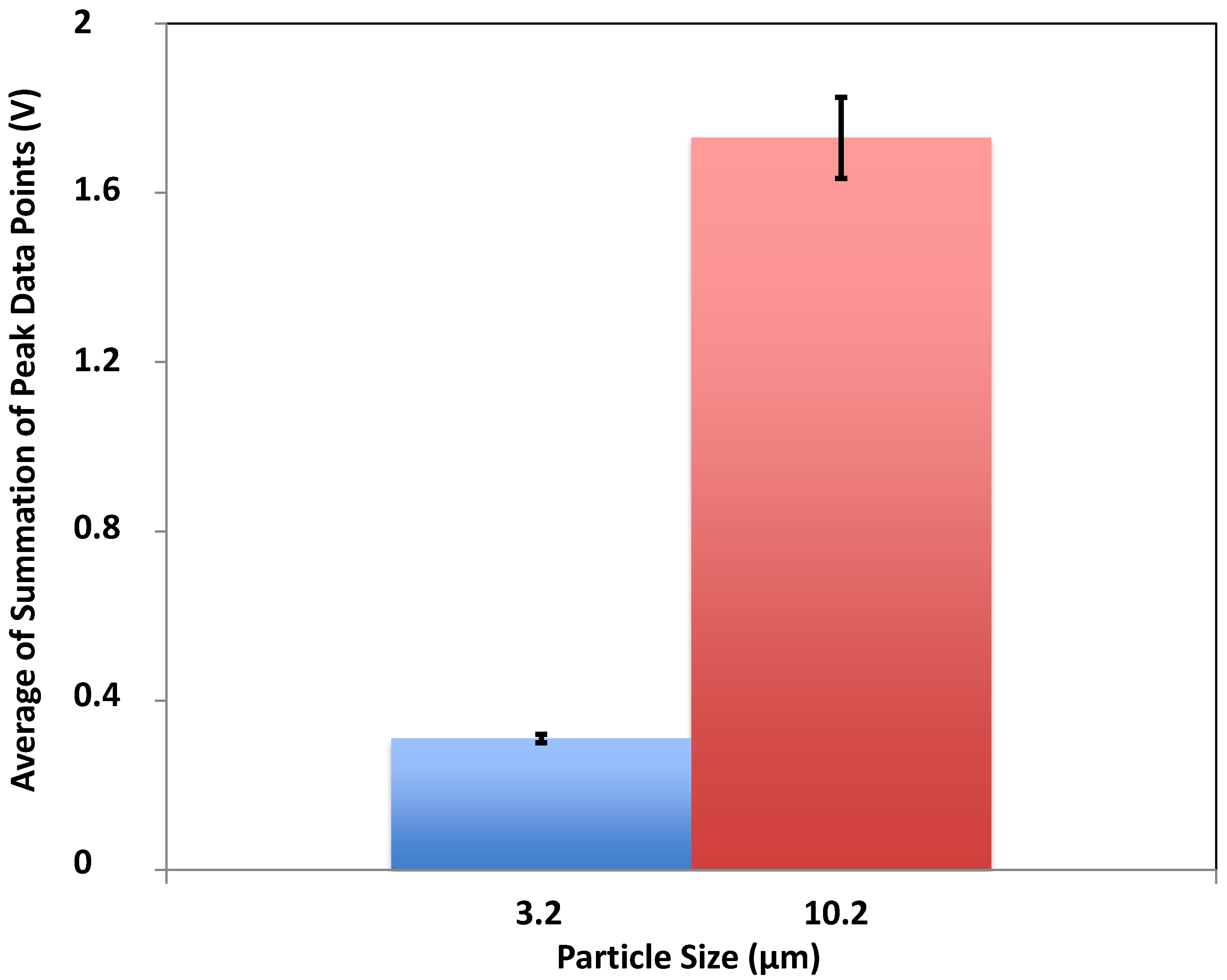
3.4. Characterization of Microalgae
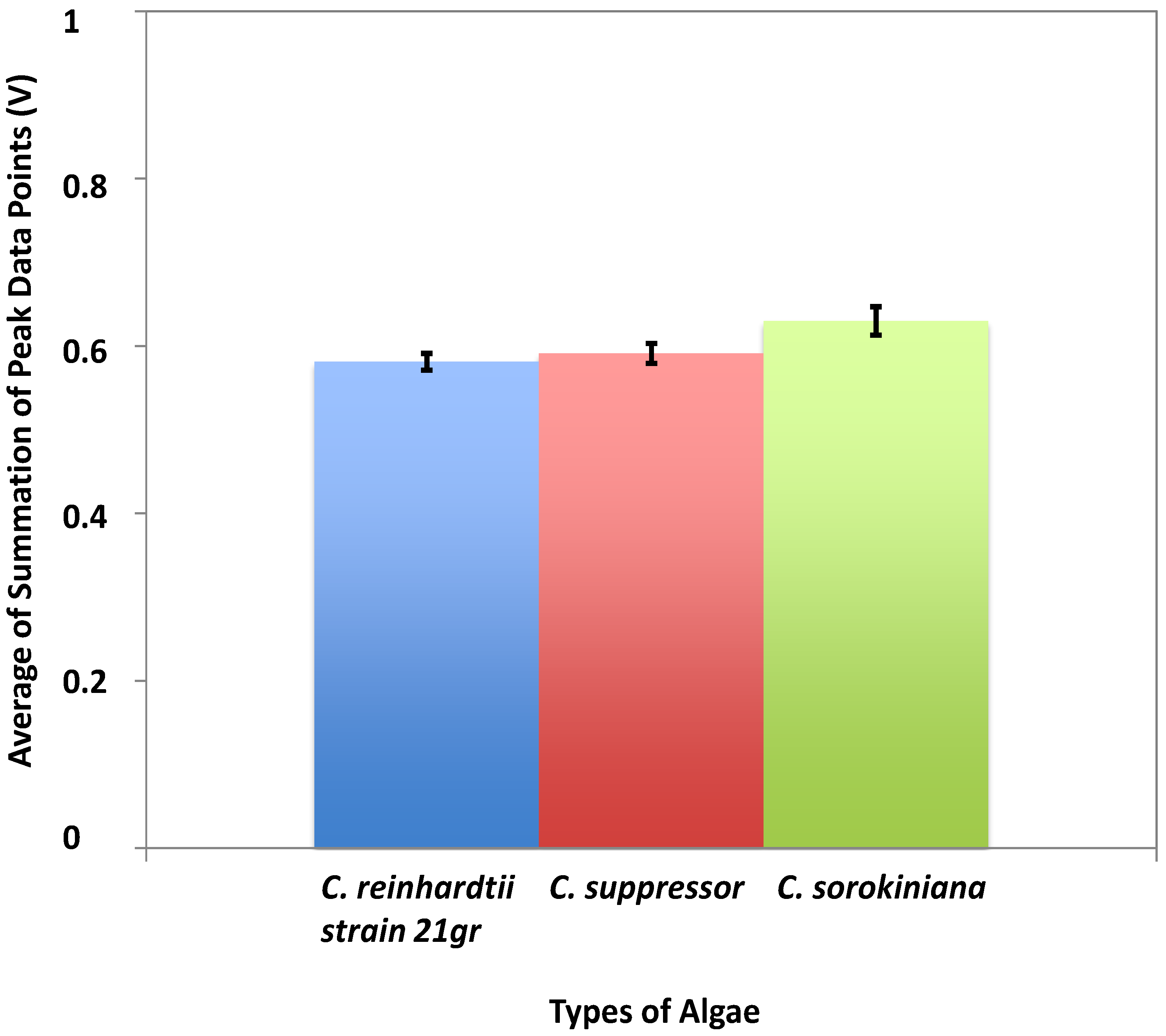
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Hashemi, N.; Howell, P.B.; Erickson, J.S.; Golden, J.P.; Ligler, F.S. Dynamic reversibility of hydrodynamic focusing for recycling sheath fluid. Lab Chip 2010, 10, 1952–1959. [Google Scholar]
- Thyssen, M.; Denis, M. Temporal and spatial high-frequency monitoring of phytoplankton by automated flow cytometry and pulse-shape analysis. In Global Change: Mankind-Marine Environment Interactions; Ceccaldi, H.-J., Dekeyser, I., Girault, M., Stora, G., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 293–298. [Google Scholar]
- Hu, Z.; Glidle, A.; Ironside, C.; Cooper, J.M.; Yin, H. An integrated microspectrometer for localised multiplexing measurements. Lab Chip 2015, 15, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Amini, H.; Lee, W.; di Carlo, D. Inertial microfluidic physics. Lab Chip 2014, 14, 2739–2761. [Google Scholar] [CrossRef] [PubMed]
- Dierssen, H.M. Perspectives on empirical approaches for ocean color remote sensing of chlorophyll in a changing climate. Proc. Natl. Acad. Sci. USA 2010, 107, 17073–17078. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Nawaz, A.A.; Lin, S.-C.S.; Lapsley, M.I.; Zhao, Y.; McCoy, J.P.; El-Deiry, W.S.; Huang, T.J. An integrated, multiparametric flow cytometry chip using “microfluidic drifting” based three-dimensional hydrodynamic focusing. Biomicrofluidics 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.P.; Kim, J.S.; Erickson, J.S.; Hilliard, L.R.; Howell, P.B.; Anderson, G.P.; Nasir, M.; Ligler, F.S. Multi-wavelength microflow cytometer using groove-generated sheath flow. Lab Chip 2009, 9, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, N.; Erickson, J.S.; Golden, J.P.; Ligler, F.S. Optofluidic characterization of marine algae using a microflow cytometer. Biomicrofluidics 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, N.; Erickson, J.S.; Golden, J.P.; Jackson, K.M.; Ligler, F.S. Microflow Cytometer for optical analysis of phytoplankton. Biosens. Bioelectron. 2011, 26, 4263–4269. [Google Scholar] [CrossRef] [PubMed]
- Tseng, D.; Mudanyali, O.; Oztoprak, C.; Isikman, S.O.; Sencan, I.; Yaglidere, O.; Ozcan, A. Lensfree microscopy on a cellphone. Lab Chip 2010, 10, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Kotz, K.T.; Petrofsky, A.C.; Haghgooie, R.; Granier, R.; Toner, M.; Tompkins, R.G. Inertial focusing cytometer with integrated optics for particle characterization. Technology 2013, 1, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kiesel, P.; Martini, J.; Recht, M.; Bern, M.; Johnson, N.; Huck, M. Flow cytometry on a chip. In Point-of-Care Diagnostics on a Chip; Issadore, D., Westervelt, R.M., Eds.; Springer: Berlin, Germany, 2013; pp. 47–69. [Google Scholar]
- Ateya, D.; Erickson, J.; Howell, P., Jr.; Hilliard, L.; Golden, J.; Ligler, F. The good, the bad, and the tiny: A review of microflow cytometry. Anal. Bioanal. Chem. 2008, 391, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Nobuhara, T.; Taguchi, M.; Nakaya, T.; Murakami, T.; Nakadaira, T.; Tamura, Y.; Yoshimura, K.; Kawagoe, K.; Iijima, T.; et al. Development of Multi-Pixel Photon Counters. Available online: http://www.slac.stanford.edu/econf/C0604032/papers/0126.PDF (accessed on 2 February 2015).
- Gomi, S.; Hano, H.; Iijima, T.; Itoh, S.; Kawagoe, K.; Kim, S.H.; Kubota, T.; Matsumura, T.; Maeda, T.; Mazuka, Y.; et al. Development and Study of the Multi Pixel Photon Counter. Available online: https://indico.cern.ch/event/3062/session/9/contribution/15/material/slides/3.pdf (accessed on 2 February 2015).
- Vacheret, A.; Barker, G.J.; Dziewiecki, M.; Guzowski, P.; Haigh, M.D.; Hartfiel, B.; Izmaylovd, A.; Johnstonb, W.; Khabibullind, M.; Khotjantsev, A.; et al. Characterization and Simulation of the Response of Multi-Pixel Photon Counters to Low Light Levels. Available online: http://arxiv.org/pdf/1101.1996.pdf (accessed on 2 February 2015).
- Soto, O.; Rojas, R.; Kuleshov, S.; Hakobyan, H.; Toro, A.; Brooks, W.K.; Rios, R. Novel Hamamatsu Multi-Pixel Photon Counter (MPPC) Array Studies for the GlueX Experiment: New Results. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2014, 739, 89–97. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, C.; Zhu, C.; Liu, L. Feasibility study of Multi-Pixel Photon Counter serving as the detector in digital optical communications. Opt. Int. J. Light Electron Opt. 2013, 124, 5781–5786. [Google Scholar] [CrossRef]
- Bai, Z.; Mendoza Reyes, J.M.; Montazami, R.; Hashemi, N. On-chip development of hydrogel microfibers from round to square/ribbon shape. J. Mater. Chem. A. 2014, 2, 4878–4884. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asrar, P.; Sucur, M.; Hashemi, N. Multi-Pixel Photon Counters for Optofluidic Characterization of Particles and Microalgae. Biosensors 2015, 5, 308-318. https://doi.org/10.3390/bios5020308
Asrar P, Sucur M, Hashemi N. Multi-Pixel Photon Counters for Optofluidic Characterization of Particles and Microalgae. Biosensors. 2015; 5(2):308-318. https://doi.org/10.3390/bios5020308
Chicago/Turabian StyleAsrar, Pouya, Marta Sucur, and Nastaran Hashemi. 2015. "Multi-Pixel Photon Counters for Optofluidic Characterization of Particles and Microalgae" Biosensors 5, no. 2: 308-318. https://doi.org/10.3390/bios5020308
APA StyleAsrar, P., Sucur, M., & Hashemi, N. (2015). Multi-Pixel Photon Counters for Optofluidic Characterization of Particles and Microalgae. Biosensors, 5(2), 308-318. https://doi.org/10.3390/bios5020308