Surface Plasmon Resonance (SPR) for the Evaluation of Shear-Force-Dependent Bacterial Adhesion
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Formation of Glycans-Modified SPR Interfaces
2.3. Determination of the Amount of Glycans on the SPR Interfaces
2.4. Instrumentation
2.4.1. SPR
2.4.2. UV/Vis Measurements
2.4.3. X-Ray Photoelectron Spectroscopy
2.5. Bacteria
3. Results and Discussion
3.1. Modification of Gold SPR Interfaces with Aminoheptyl α-D-Mannopyranoside (HM)
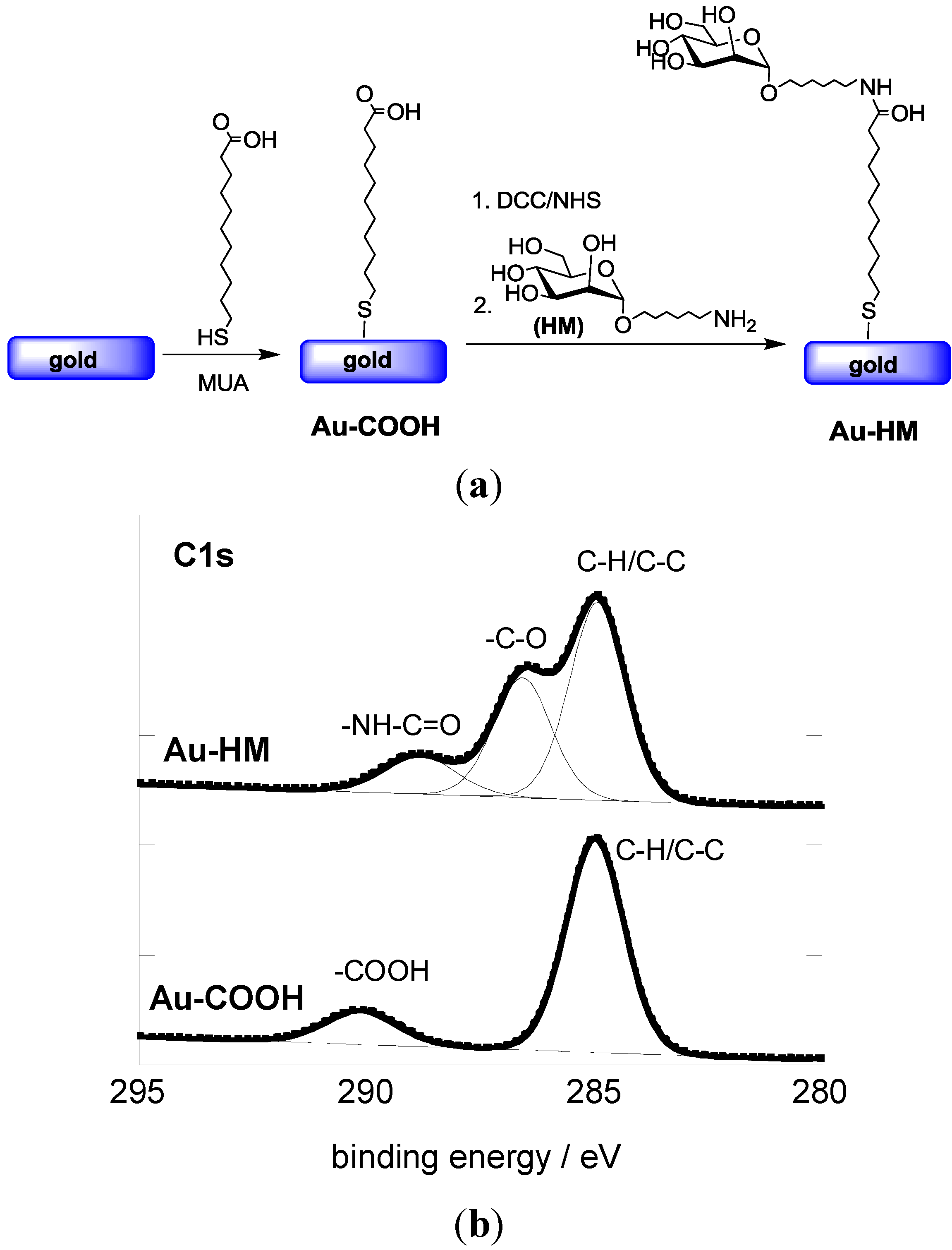
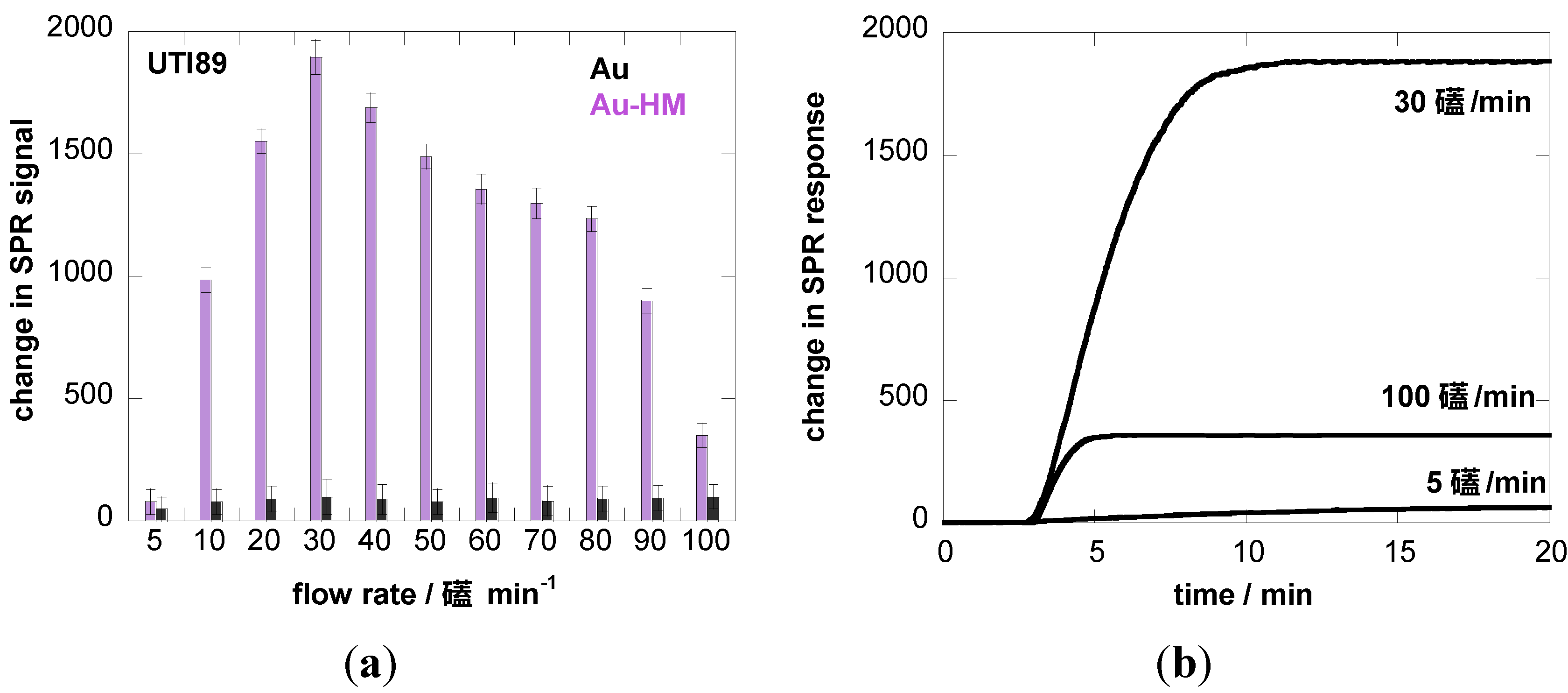
3.2. Adhesion Behavior of E. coli UTI89 to Au-HM under Different Flow Rates

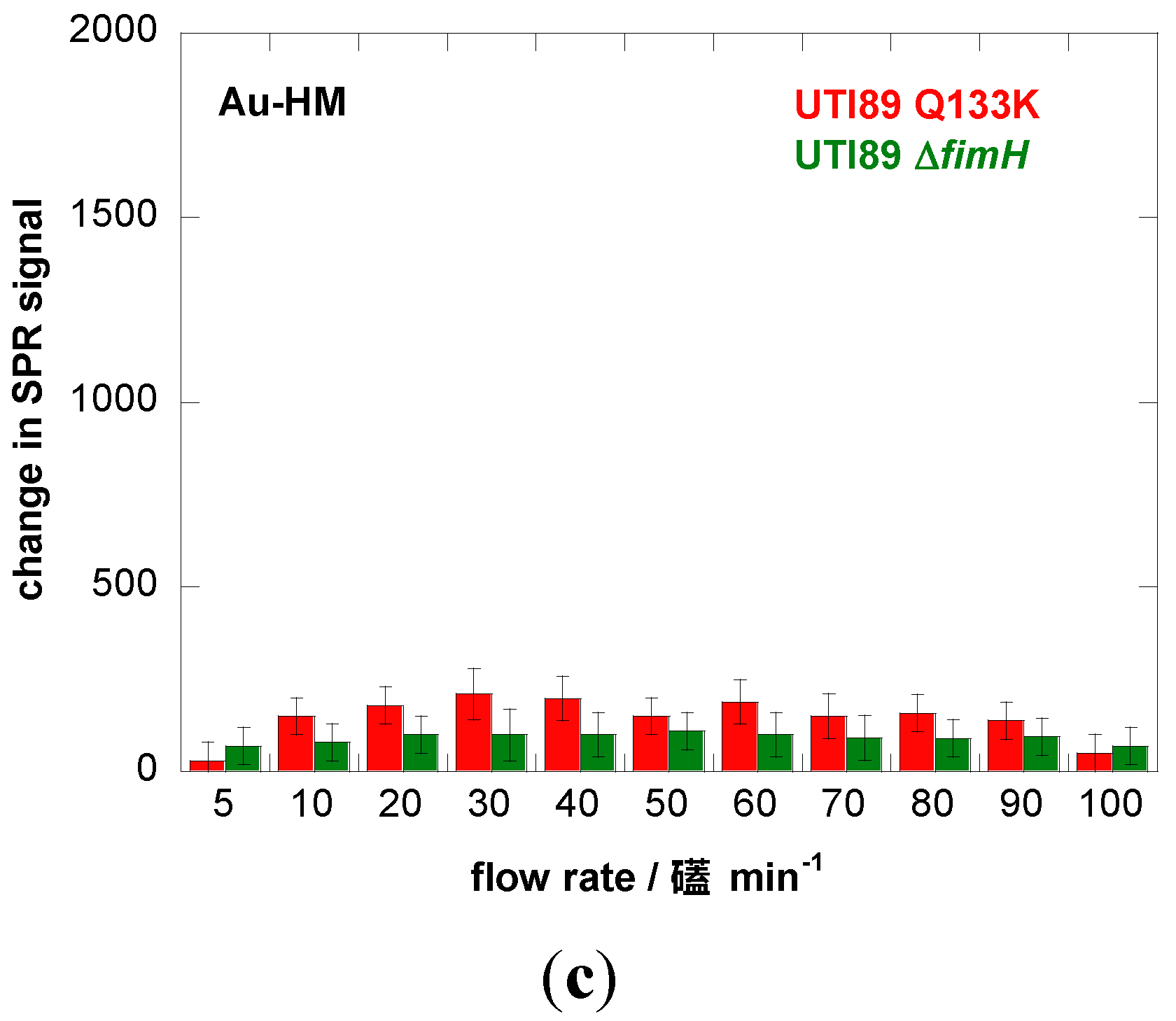
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interests
References
- Scarano, S.; Mascini, M.; Turner, A.P.F.; Minunni, M. Surface plasmon resonance imaging for affinity-based biosensors. Biosens. Bioelectron. 2010, 25, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A. Lable-free screening of bio-molecular interactions. Anal. Bioanal. Chem. 2003, 377, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Lesniewski, A.; Kaminska, I.; Vlandas, A.; Vasilescu, A.; Niedziolka-Jonsson, J.; Pichonat, E.; Happy, H.; Boukherroub, R.; Szunerits, S. Lysozyme detection on aptamer functionalized graphene-coated SPR interfaces. Biosens. Bioelectron. 2013, 50, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Zagorodko, O.; Spadavecchia, J.; Serrano, Y.S.; Larroulet, I.; Pesquera, A.; Zurutuza, A.; Boukherroub, R.; Szunerits, S. Highly sensitive detection of DNA hybridization on commercialized graphene coated surface plasmon resonance interfaces. Anal. Chem. 2014, 86, 11211–11216. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Dostalek, J.; Chen, S.F.; Rasooly, A.; Jiang, S.Y.; Yee, S.S. Spectral surface plasmon resonance biosensor for detection of staphylococcal enterotoxin B in milk. Int. J. Food Microbiol. 2002, 75, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Naimushin, A.N.; Soelberg, S.D.; Nguyen, D.K.; Dunlap, L.; Bartholomew, D.; Elkind, J.; Melendez, J.; Furlong, C.E. Detection of Staphylococcus aureus enterotoxin B at femtomolar levels with a miniature integrated two-channel surface plasmon resonance (SPR) sensor. Biosens. Bioelectron. 2002, 17, 573–584. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, C.R.; Hirama, T.; Lee, K.K.; Altman, E.; Young, N.M. Quantitative analysis of bacterial toxin affinity and specificity for glycolipid receptors by surface plasmon resonance. J. Biol. Chem. 1997, 272, 5533–5538. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.M.; Chen, S.F.; Taylor, A.D.; Homola, J.; Hock, B.; Jiang, S.Y. Detection of low-molecular-weight domoic acid using surface plasmon resonance sensor. Sens. Actuators B 2005, 107, 193–201. [Google Scholar] [CrossRef]
- Kanda, V.; Kitov, P.; Bundle, D.R.; McDermott, M.T. Surface plasmon resonance imaging measurements of the inhibition of Shiga-like toxin by synthetic multivalent inhibitors. Anal. Chem. 2005, 77, 7497–7504. [Google Scholar] [CrossRef] [PubMed]
- Fratamico, P.M.; Strobaugh, T.P.; Medina, M.B.; Gehring, A.G. Detetion of Escherichia coli O157:H7 using a surface plasmon resonance biosensor. Biotechnol. Tech. 1998, 12, 571–576. [Google Scholar] [CrossRef]
- Taylor, A.D.; Ladd, J.; Yu, Q.; Chen, S.; Homola, J.; Jiang, S. Quantitative and simultaneous detection of four foodborne bacterial pathogens with a multi-channel SPR sensor. Biosens. Bioelectron. 2006, 22, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Singh, A.; Naidoo, R.; Wu, P.; McDermott, M.T.; Evoy, S. Chemically immobilized T4-bacteriophage for specific Escherichia coli detection using surface plasmon resonance. Analyst 2011, 136, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.K.; Lee, W.Y.; Chun, B.S.; Bae, Y.M.; Lee, Y.M.; Choi, J.W. The fabricaiton of protein chip based on surface plasmon resonance for detections of pathogens. Biosens. Bioelectron. 2005, 20, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, R.; Fournier-Wirth, C.; Coste, J.; Chebib, H.; Saikali, Y.; Vittori, O.; Errachid, A.; Cloarec, J.P.; Martelet, C.; Jaffrezic-Renault, N. Label-free detection of bacteria by electrochemical impedance spectroscopy: Comparision to surface plasmon resonance. Anal. Chem. 2007, 79, 4879–7886. [Google Scholar] [CrossRef] [PubMed]
- Yazagan, I.; Noah, N.M.; Toure, O.; Zhang, S.; Sadik, O.A. Biosensor for selective detection of E. coli in spinach using the strong affinity of derivatized mannose with fimbrial lectin. Biosens. Bioelectron. 2014, 61, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Barka-Bouaifel, F.; Bouckaert, J.; Yamakawa, N.; Boukerroub, R.; Szunerits, S. Graphene-coated surface plasmon resonance interfaces for studying the interactions between bacteria and surfaces. ACS Appl. Mater. Interfaces 2014, 6, 5422–5431. [Google Scholar] [CrossRef] [PubMed]
- Durka, M.; Buffet, K.; Iehl, J.; Holler, M.; Nierengarten, J.F.; Taganna, J.; Bouckaer, T. J.; Vincent, S.P. The functional valency of dodecamannosylated fullerenes with Escherichia coli FimH-Towards novel bacterial antiadhesives. Chem. Commun. 2011, 47, 1321–1323. [Google Scholar] [CrossRef]
- Le, T.I.; Aprikian, P.; Kidd, B.A.; Forero-Shelton, M.; Tchesnokova, V.; Rajagopal, P.; Rodriguez, V.; Interlandi, G.; Klevit, R.; Vogel, V.; et al. Structural basis for mechanical force regulation of the adhesin FimH via finger trap-like beta sheet twisting. Cell 2010, 141, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Pereverzev, Y.V.; Preshdo, O.V.; Forero, M.; Sokurenko, E.V.; Thomas, W.E. The two-pathway model for the catch-slip transition in biological adhesion. Biophys. J. 2005, 89, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Gibcus, M.J.; van der Mei, H.C.; Busscher, H.J. Influence of fluid shear and microbubbles on bacterial detachment from a surface. Appl. Environ. Microbiol. 2005, 71, 3668–3673. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.; Forero, M.; Yakovenko, O.; Nilsson, L.; Vicini, P.; Sokurenko, E.; Vogel, V. Catch-bond model derived from allostery explains force-activated bacterial adhesion. Biophys. J. 2006, 90, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Sokurenko, E.V.; Vogel, V.; Thomas, W.E. Catch bond mechanism of force-enhanced adhesion: Counter-intuitive, elusive but widespread? Cell Host Microbe 2008, 16, 314–323. [Google Scholar] [CrossRef]
- Yakovenko, O.; Sharma, S.; Forero, M.; Tchesnokova, V.; Aprikian, P.; Kidd, B.; March, A.; Vogel, V.; Sokurenko, E.; Thomas, W. FimH forms catch bonds that are enhanced by mechanical force due to allosteric regulation. J. Biol. Chem. 2008, 283, 11596–11605. [Google Scholar] [CrossRef] [PubMed]
- Klinth, J.E.; Castelain, M.; Uhlin, B.E.; Axner, O. The influence of pH on the specific adhesion of P piliated Escherichia coli. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Bouckaert, J.; Berglund, J.; Schembri, M.; de Genst, E.; Cools, L.; Wuhrer, M.; Hung, C.S.; Pinker, J.; Slattegard, R.; Zavialov, A.; et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol. Microbiol. 2005, 55, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Londardi, E.; Moonens, K.; Buts, L.; de Boer, A.R.; Olsson, J.D.M.; Weiss, M.S.; Fabre, E.; Guérardel, Y.; Deelder, A.M.; Oscarson, S.; et al. Structural sampling of glycan interaction profiles reveals mucosal receptors for fimbrial adhesins of enterotoxigenic Escherichia coli. Biology 2013, 2, 894–917. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Hung, C.S.; Pinkner, J.S.; Walker, J.N.; Cusumano, C.K.; Li, Z.; Bouckaert, J.; Gordone, J.I.; Hultgren, S.J. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc. Natl. Acad. Sci. USA 2009, 106, 22439–22444. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Delgado, M.; Fu, A.; Alcouffe, P.; Gouin, S.G.; Fleury, E.; Katz, J.L.; Ganachaud, F.; Bernard, J. Simple but precise engineering of functional nanocapsules through nanoprecipitation. Angew. Chem. Int. Ed. 2014, 53, 6910–6913. [Google Scholar] [CrossRef]
- Barras, A.; Martin, F.A.; Bande, O.; Baumann, J.S.; Ghigo, J.M.; Boukherroub, R.; Beloin, C.; Siriwardena, A.; Szunerits, S. Glycan-functionalized diamond nanoparticles as potent E. coli anti-adhesives. Nanoscale 2013, 5, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Pinker, J.S.; Roth, R.; Heuser, J.; Nicholes, A.V.; Abraha, S.N.; Hultgren, S.J. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc. Natl. Acad. Sci. USA 1995, 92, 2081–2085. [Google Scholar] [CrossRef] [PubMed]
- Touaibia, M.; Wellens, A.; Shiao, T.C.; Wang, Q.; Sirois, S.; Bouckaert, J.; Roy, R. Mannosylated G(0) dendrimers with nanomolar affinities to Escherichia coli FimH. ChemMedChem 2007, 2, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Maalouli, N.; Barras, A.; Siriwardena, A.; Boukherroub, R.; Szunerits, S. Comparison of photo- and Cu(I)-catalyzed “click” chemistries for the formation of carbohydrate SPR interfaces. Analyst 2013, 138, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Alves, I.; Kurylo, I.; Coffinier, Y.; Siriwardena, A.; Zaitsev, V.; Harté, E.; Boukherroub, R.; Szunerits, S. Plasmon waveguide resonances for the sensing of glycans-lectin interactions. Anal. Chim. Acta 2015, 873, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, J.; Mackenzie, J.; de Paz, J.L.; Chipwaza, B.; Choudhury, D.; Zavialov, A.; Mannerstedt, K.; Anderson, J.; Pierard, D.; Wyns, L.; et al. The affinity of the FimH fimbrial adhesin is receptor-driven and quasi-independent of Escherichia coli pathotypes. Mol. Microbiol. 2006, 61, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Bakker, D.P.; BusScher, H.J.; van der Mei, H.C. Bacterial deposition in a parallel plate and a stagnation point flow chamber: Microbial adhesion mechanisms depend on the mass transport conditions. Microbiology 2002, 148, 597–603. [Google Scholar] [PubMed]
- Boks, N.P.; Norde, W.; van der Mei, H.C.; Busscher, H.J. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces. Microbiology 2008, 154, 3122–3133. [Google Scholar] [CrossRef] [PubMed]
- Tchesnokova, V.; Aprikian, P.; Yakovenko, O.; Larock, C.; Kidd, B.A.; Vogel, V.; Thomas, W.; Sokurenko, E. Integrin-like allosteric properties of the catch bond-forming FimH adhesin of Escherichia coli. J. Biol. Chem. 2008, 283, 7823–7833. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.; Trintchina, E.; Forero, M.; Vogel, V.; Sokurenko, E.V. Bacterial adhesion to target cells enhanced by shear force. Cell 2002, 109, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Lecuyer, S.; Rusconi, R.; Shen, Y.; Forsyth, A.; Vlamakis, H.; Kolter, R.; Stone, H.A. Shear Stress Increases the Residence Time of Adhesion of Pseudomonas aeruginosa. Biophys. J. 2011, 100, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.J.; Seed, P.C.; Hultgren, S.J. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell. Microbiol. 2007, 9, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagorodko, O.; Bouckaert, J.; Dumych, T.; Bilyy, R.; Larroulet, I.; Serrano, A.Y.; Dorta, D.A.; Gouin, S.G.; Dima, S.-O.; Oancea, F.; et al. Surface Plasmon Resonance (SPR) for the Evaluation of Shear-Force-Dependent Bacterial Adhesion. Biosensors 2015, 5, 276-287. https://doi.org/10.3390/bios5020276
Zagorodko O, Bouckaert J, Dumych T, Bilyy R, Larroulet I, Serrano AY, Dorta DA, Gouin SG, Dima S-O, Oancea F, et al. Surface Plasmon Resonance (SPR) for the Evaluation of Shear-Force-Dependent Bacterial Adhesion. Biosensors. 2015; 5(2):276-287. https://doi.org/10.3390/bios5020276
Chicago/Turabian StyleZagorodko, Oleksandr, Julie Bouckaert, Tetiana Dumych, Rostyslav Bilyy, Iban Larroulet, Aritz Yanguas Serrano, Dimitri Alvarez Dorta, Sebastien G. Gouin, Stefan-Ovidiu Dima, Florin Oancea, and et al. 2015. "Surface Plasmon Resonance (SPR) for the Evaluation of Shear-Force-Dependent Bacterial Adhesion" Biosensors 5, no. 2: 276-287. https://doi.org/10.3390/bios5020276
APA StyleZagorodko, O., Bouckaert, J., Dumych, T., Bilyy, R., Larroulet, I., Serrano, A. Y., Dorta, D. A., Gouin, S. G., Dima, S.-O., Oancea, F., Boukherroub, R., & Szunerits, S. (2015). Surface Plasmon Resonance (SPR) for the Evaluation of Shear-Force-Dependent Bacterial Adhesion. Biosensors, 5(2), 276-287. https://doi.org/10.3390/bios5020276









