Abstract
Polyquinone derivatives are widely recognized in the literature for their remarkable properties, their biocompatibility, simple synthesis, and easy bio-functionalization. We have shown that polyquinones present very stable electroactivity in neutral aqueous medium within the cathodic potential domain avoiding side oxidation of interfering species. Besides, they can act as immobilized redox transducers for probing biomolecular interactions in sensors. Our group has been working on devices based on such modified electrodes with a view to applications for proteins, antibodies and organic pollutants using a reagentless label-free electrochemical immunosensor format. Herein, these developments are briefly reviewed and put into perspective.
1. General Introduction
1.1. Generalities
An antigen (Ag) is a nanometer-sized molecule with molecular weight higher than 1,500 Da to which the immune system of animals responds by synthesizing antibodies (Ab) able to selectively bind the antigen. There are five primary classes of antibodies, namely IgG, IgM, IgA, IgD and IgE, but only IgG antibodies are used in immunosensors. Ab-Ag binding is based on non-covalent interactions and is characterized by an association constant, Ka, in the range 105–1013 M. Polyclonal Ab are directed to different binding sites (epitopes) on Ag, with different affinities. Monoclonal Ab, specific to only one epitope of an Ag, are preferred. These Ab generally present higher affinity and specificity.
1.2. Immunoassays
Analytical tools using Ab-Ag couples are called immunoassays. They are capable of direct and specific detection in complex samples, due to highly specific interactions between Ab and Ag. Commercially available immunoassay kits are inexpensive, simple, adapted to field use and constitute a rapid way of determining contaminants in environmental samples. Generally, the detection scheme relies on labeling with radioisotopes (radioimmunoassay, RIA, now scarcely used), chemiluminescent compounds (chemiluminescence immunoassay, CLIA) [1,2] or enzymes (enzyme immunoassay, EIA, and enzyme-linked immunosorbent assay, ELISA) [3,4,5,6]. Usually, the Ab or the Ag is immobilized on a substrate. In the most basic format, an immunocomplex (Ab-Ag) is formed upon contact with a solution containing the labeled analyte (generally the Ag). The analyte concentration in solution is then derived from that of bound analyte. In a more efficient sandwich format, Ab are immobilized on a substrate which is put in contact with the sample containing the Ag, then a labeled secondary antibody is added (see Figure 1(a)). Competitive formats also exist, in which competition takes place between non-labeled and labeled Ag for an immobilized Ab (Figure 1(b)).
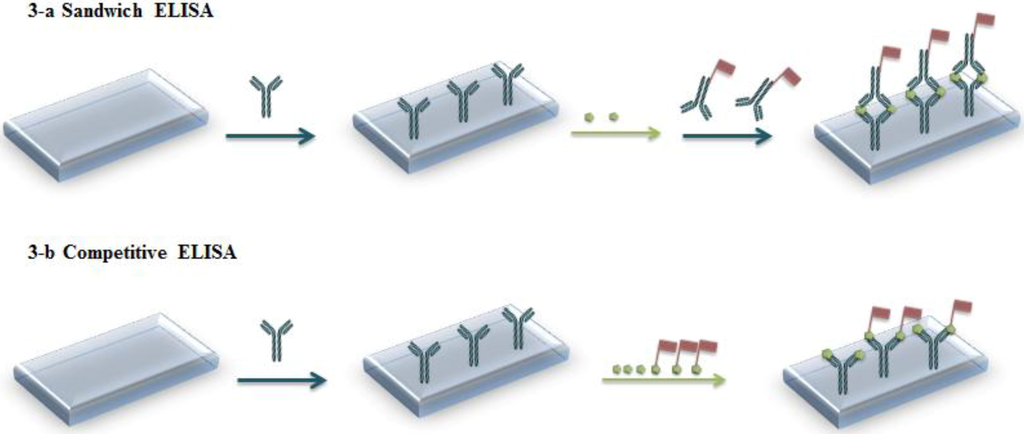
Figure 1.
Principle of enzyme-linked immunosorbent assay (ELISA). (a) Sandwich format (b) Competitive format.
1.3. Immunosensors
Today, most immunoassays are performed using ELISA. They are generally performed in microtiter plates, which demand time and skilled operators. Moreover, ELISA tests are not efficient for simultaneous multiple analysis (multiplexing), continuous detection and transduction into an electronically usable signal. In the near future, biosensors using antibody-antigen complexes (called immunosensors) may provide faster and easier methods. An immunosensor consists of a sensing element (an immobilized Ab or Ag) and a transducer which translates the biomolecular recognition event into an electrical signal. This can be performed indirectly through SPR [7,8]. However, SPR is expensive, requires specialized technicians and is rarely employed for on-site measurement. In addition, optic-based detections in general are fragile. Transduction can also be performed through piezoelectric techniques [9] or near field microscopies (AFM, SECM) [10], with few of the same inconveniences. Field-effect transistors are competitive [11,12], but we focus in this paper on electrochemical sensors which are much more widely reported in the literature.
1.4. Electrochemical Immunosensors
Electrochemical biosensors have attracted considerable attention due to the advantages of robustness, low cost, low power consumption, simplicity, high sensitivity, compatibility with mass manufacturing using existing micro-fabrication technologies, and portability; therefore they are excellent candidates for easy-to-use immunosensors. The most popular format uses a redox reaction from a labeled Ab (or Ag) which provides a current proportional to the analyte concentration. For example, Ab or Ag may be labeled with enzymes producing an electroactive product from a reagent added in the analytical solution [13]. Detection is generally performed by cyclic voltammetry, chronoamperometry or most frequently, impedimetry [14]. However, it would be more interesting to perform electrochemical transduction without labeling the biomolecules by a redox tag or adding redox reagent to the solution. This can be performed with conducting polymers (ECPs).
1.5. Electrochemical Immunosensors Based on Conducting Polymers
ECPs are smart materials because they present at the same time, grafting, coupling and transduction abilities [15,16,17,18] without help of a supplementary redox label put on the Ab or in solution. As Ab-Ag complexes are heavy and hindering, it is expected that their presence in the vicinity of the polymer/solution interface strongly influences ion diffusion from the solution to the polymer/solution interface and mixed charge transport inside the film. This typically leads to a measurable decrease in the electroactivity of the underlying conducting polymer.
1.5.1. Polypyrrole
The most popular ECP for biosensing applications is polypyrrole (PPy) [19,20,21]. In 1991, John et al. [22] described a method for incorporation of antibodies (anti-HSA) in PPy. It was shown by cyclic voltammetry that HSA (human serum albumin) interacted with the electrode surface while no response was obtained with pure PPy. Sadik and Wallace [23] optimized this method with detection limits in the order of nM [24]. Later, based on the same principle, an electrochemical immunosensor for direct detection of small organic antigenic pollutants like pesticides or antibiotics was developed. For example, polychlorinated biphenyls (PCBs) have been detected in water with a detection limit of 0.05 μg·L−1 [25,26,27,28].
1.5.2. Other ECPs
Other conducting polymers were also used for detection of pollutants. For example, Shim et al. [29] fabricated a label free impedimetric immunosensor to detect bisphenol A (endocrine disrupting compounds released into the environment from many kinds of polycarbonate plastics, epoxy resins of food cans, etc.) using polythiophene, with a detection limit of 0.3 ± 0.07 ng·mL−1. Khan et al. [30] performed an impedimetric immunosensor based on a chitosan/polyaniline hybrid to detect ochratoxin-A (a mycotoxin found in food products, human blood, breast milk, tissues and organs of animals). Other polymers were also used, and more particularly polyquinones, which present particular redox properties. For example, a series of aminonaphthoquinones and aminoanthraquinones were originally developed in the 80’s and 90’s for electrocatalytic purposes or energy storage [31,32,33,34,35,36,37]. More recently, other polyquinone films were developed to be used as transducers in biosensors [38,39]. Indeed, even if polyquinone derivatives have been much less investigated than other ECPs, they present good biocompatibility, easy bio-functionalization and remarkably stable electroactivity in neutral aqueous medium [40]. These properties can be used to probe biomolecular interactions [41,42,43,44] due to the high sensitivity of the quinone group to changes in its local physico-chemical environment [45,46,47].
2. Recent Advances on Polyquinone-Modified Electrodes for Immunosensing
2.1. General Approach
2.1.1. Principles
The major bottleneck is how to achieve direct electrochemical transduction when there is no intrinsic charge transfer reaction following molecular recognition. The most original and innovative idea is to directly immobilize the redox transducer on the sensor surface so that its electroactivity can be influenced by steric hindrance of heavy molecules (Ab or proteins) in its neighborhood. The detection of the target is performed simply by recording the redox current before and after recognition. This approach allows the development of easy-to-use, reagentless and label-free electrochemical devices. Several sensing architectures could be designed for such an approach, schematized and summarized in Figure 2 below.
Cases a–c, Figure 2, describe the most common approaches, which use relatively bulky probes. In order to reduce the size of the grafted probe, it is also possible to use peptides, as shown for cases d,e. Finally, small organic molecules may be used (f) instead of proteins or peptides [48].
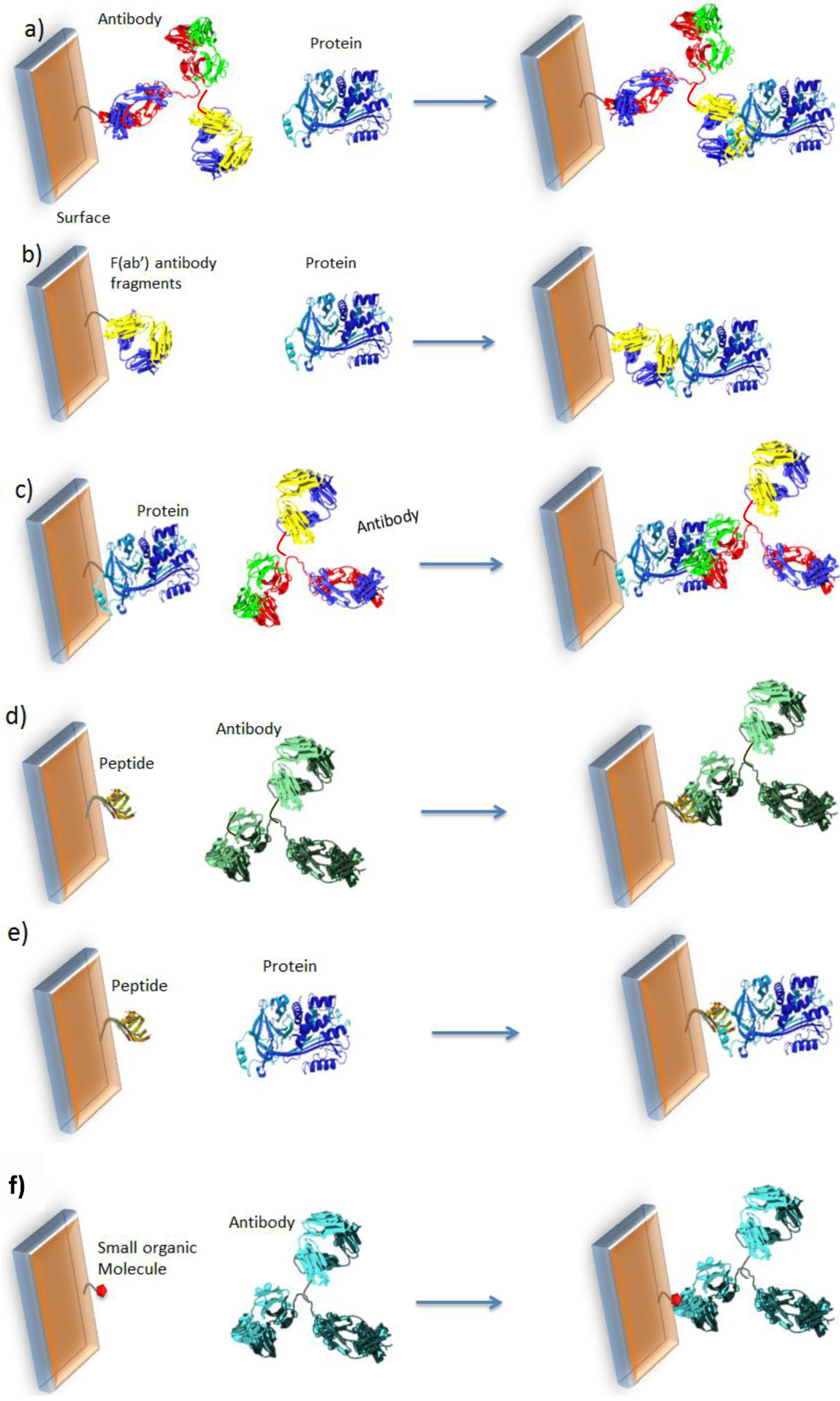
Figure 2.
Classical formats used for detections of proteins or antibodies. (a) Grafted antibodies (Ab) to detect proteins; (b) Grafted antibody fragment F(ab’) to detect proteins; (c) Grafted protein to detect Ab. Use of peptides to detect (d) antibodies or (e) proteins. (f) Use of small organic molecules.
2.1.2. Design
Because the sensor’s architecture must be adaptable to any format from among those schematized in Figure 2, it has to be engineered from “elemental bricks” joined together to form the whole electrochemical sensor, namely the grafting group, the redox transducer and the probe able to selectively complex the target molecule. This construction is schematized in Figure 3 below.
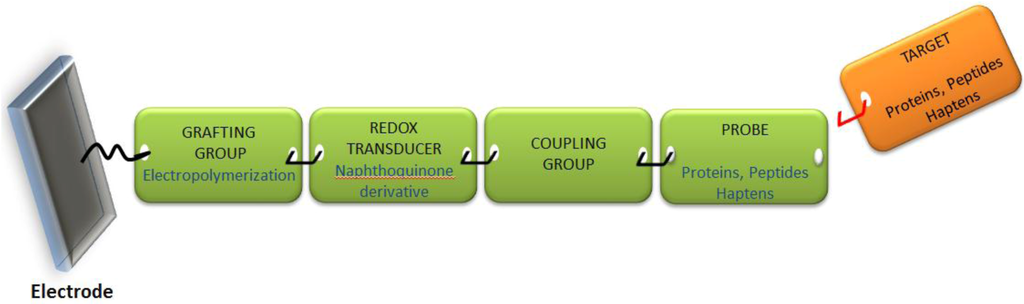
Figure 3.
Schematic view of the “elemental bricks” needed to construct a versatile reagentless and label-free electrochemical sensor.
We selected electropolymerization as the strategy to graft the sensing material, using hydroxynaphthoquinone monomers, which polymerize by electrooxidation of the hydroxyl group. The quinone plays the role of redox transducer, and we developed a coupling strategy to directly graft a spacer (e.g., an alkyl chain bearing a functional group) on the α-carbon of the quinone. This spacer is then used to couple the probe (antibody, protein, peptide or modified hapten).
We first synthesized 5-hydroxy-2-thioacetic acid-1,4-naphthoquinone.(HSNQA) (Figure 4(a)). The reaction of thiols with various hydroxynaphthoquinone derivatives leads, in one step, to substituted quinone rings, under mild conditions [49]. Another spacer was also designed by straightforward carbon-carbon coupling, leading to 5-hydroxy-1,4-naphthoquinone-3-propionic acid (HNQA) (Figure 4(b)) [42].
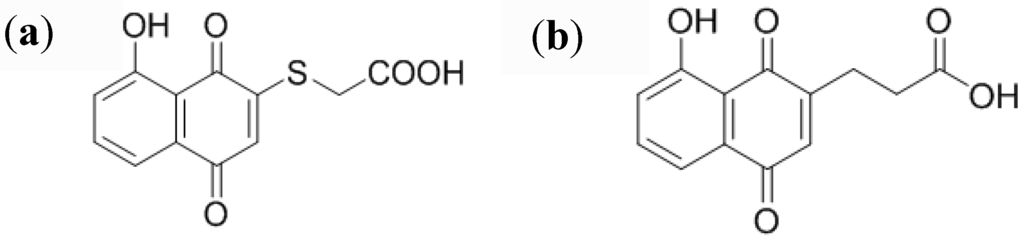
Figure 4.
(a) Structure of 5-hydroxy-2-thioacetic acid-1,4-naphthoquinone) (HSNQA) and (b) 5-hydroxy-1,4-naphthoquinone-3-propionic acid (HNQA).
We obtained a multifunctional conjugated copolymer poly(5-hydroxy-1,4-naphthoquinone-co-(5-hydroxy-2-thioacetic acid-1,4-naphthoquinone), poly(HNQ-co-HSNQA) and used it as the immobilizing and transducing element for a label-free electrochemical immunosensor [44]. Biomolecules can be coupled through peptide links between the –COOH group and the terminal –NH2 group on the bioprobe. The quinone group was used for its redox properties. It is well known that the quinone/hydroquinone system presents an electroactivity which is sensitive to its local environment, particularly cations, protons or sodium ions in aqueous solution. The redox reaction in the film involves the reaction Q + 2H+ + 2e− ⇌ QH2 or Q + 2Na+ + 2e− ⇌ Q2−, 2Na+. Presence of heavy biomolecules in the vicinity of the quinone group leads to a local modification of the proton and sodium apparent diffusion coefficient; therefore quinone electroactivity changes [38].
2.2. Applications
2.2.1. Proteins as Probes
Proteins are macromolecules made of α-amino acids linked together by peptide bonds. The molecular weight is a fundamental characteristic of each protein; it is generally greater than 10,000 Da (10 kDa). The typical model used in immunosensors for the study of protein/antibody interactions is the ovalbumin/anti-ovalbumin (OVA/αOVA) system. We used this system as a model in our quinone-based biosensor. Poly(5-hydroxy-1,4-naphthoquinone-co-5-hydroxy-2-thioacetic acid-1,4-naphthoquinone) (poly(HNQ-co-HSNQA)), was used as both the immobilization and transduction element. OVA was grafted on this polymer and used as probe to detect αOVA present in solution. The immune complex was detected by recording the electrochemical signal using square wave voltammetry (SWV, Figure 5). In aqueous solution, quinones transfer two electrons and two protons in a concerted process. We assume that the presence of two pairs of peaks in PBS is due to two different types of quinones on the electrode surface: in the bulk of the polymer and at the polymer/electrolyte interface. The current decreased upon addition of αOVA, whereas no signal was observed upon addition of non-specific anti-KLH or diluted (1/50) normal serum.
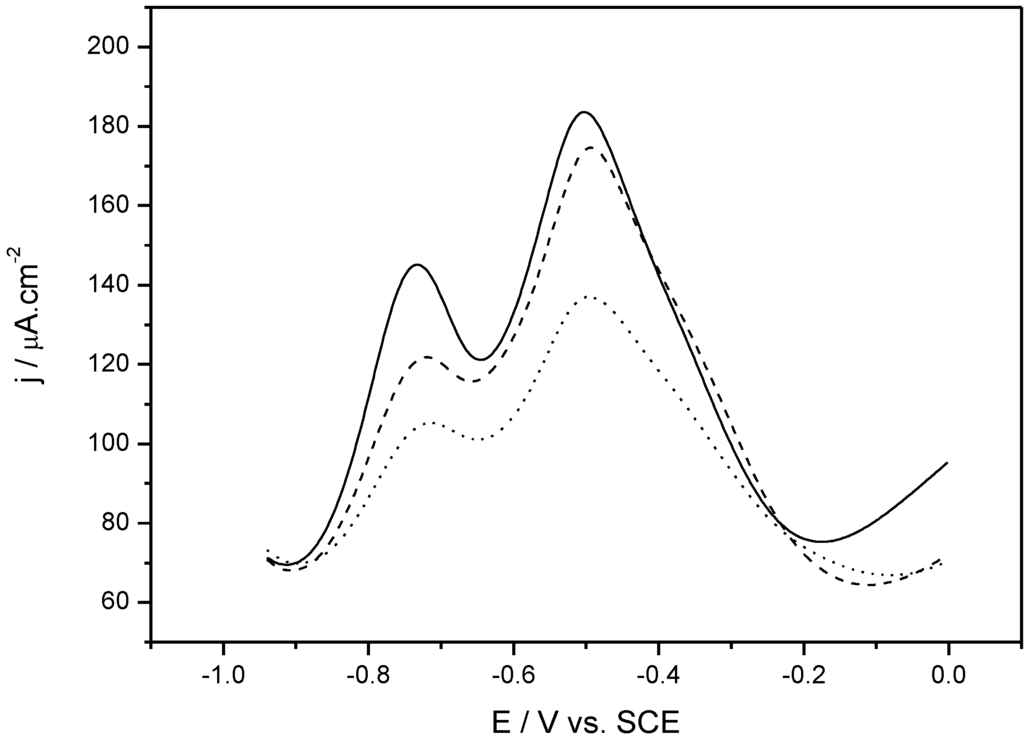
Figure 5.
Square wave voltammetry (SWV) obtained for poly(HNQ-co-HSNQA)-modified electrodes before ovalbumin (OVA) grafting (solid line), after OVA grafting (dashed line) and after anti-ovalbumin (αOVA) complexation (dotted line). Serum dilution: 1/50 [44].
It was proposed (Figure 6(a)) that the lowering of the redox activity of the quinone groups embedded in the polymer film is due to the steric hindrance of αOVA (150 kDa) which is barely four times bigger than OVA (45 kDa) [44].
In the configuration of case a, the sensitivity of the sensor is limited by the fact that the probe (the protein) is relatively big. In terms of mean area occupied by proteins on the electrode surface, we can take the power 2/3 of the molecular weight ratio to determine the relative projected area between probe and target on the electrode surface. It gives (150/45)2/3 = 2.2, which means that αOVA is only ca. two times more hindering than OVA; This is low and not optimal. One way to solve the problem is to reduce the probe size, e.g., using peptides instead of whole proteins (Figure 6(b)).
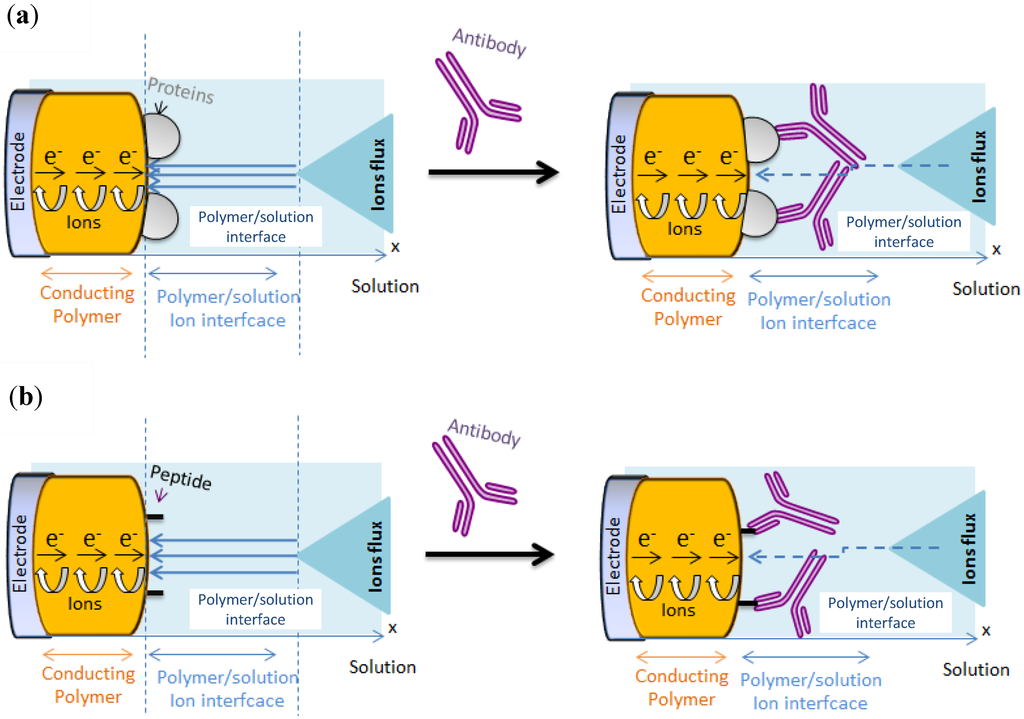
Figure 6.
Transduction principle for poly(HNQ-co-HSNQA)/OVA/αOVA sensor (a) case of protein/antibody complex ; (b) peptide/antibody complex.
2.2.2. Peptides for Biomolecule Grafting
α-Amino acids are the building blocks of proteins and peptides. They carry a primary amine group bound to the α-carbon of the carboxylic acid group, which gives them the generic structure H2N-CHR-COOH wherein R represents the side chain (a functional group) which identifies the α-amino acid. Their general structure is shown in Figure 7.
The α-Amino acids can bind to each other through an amide bond between the α-carboxyl group of one and the α-amine group of another, to form peptides. Compared to proteins, peptides are smaller, structurally less complex and more stable. Their synthesis can be automated, which is a considerable advantage compared to proteins and allows new biological and biomedical applications, as well as for diagnostics. Among other peptides of interest, glutathione (γ-L-glutamyl-L-cysteinyl-glycine) is potentially an efficient linker for biomolecule immobilization on the surface. Indeed, it is a tripeptide, bearing one primary amine, two amide groups, two carboxylic groups and one thiol group, all these groups being chemically reactive (see Figure 8).
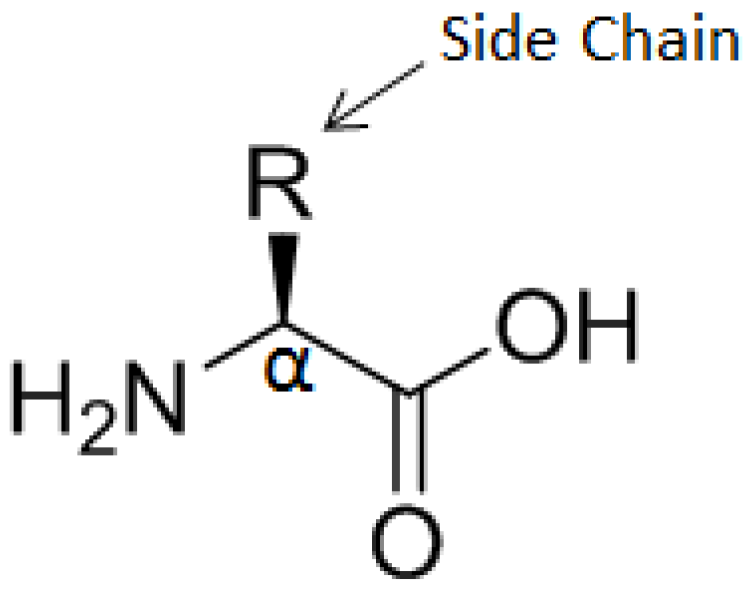
Figure 7.
General structure of α-amino acids.
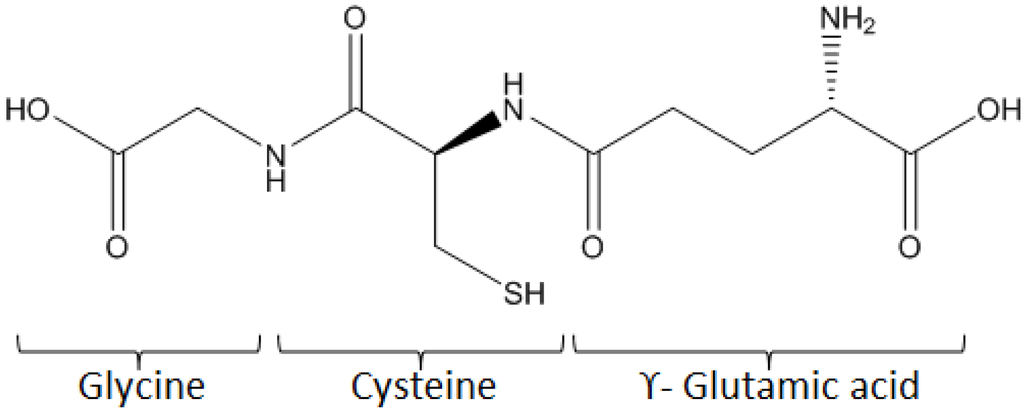
Figure 8.
Structure of glutathione.
We recently developed direct grafting of glutathione (using the thiol group present on the side chain of cysteine) onto 5-hydroxy-1,4-naphthoquinone (HNQ) [41] to obtain 5-hydroxy-3-γ-L-glutamyl-L-cysteinyl-glycine-1,4-naphthoquinone (HNQ-Glu, Figure 9(a)) which was electropolymerized to give the polymer structure shown in Figure 9(b).
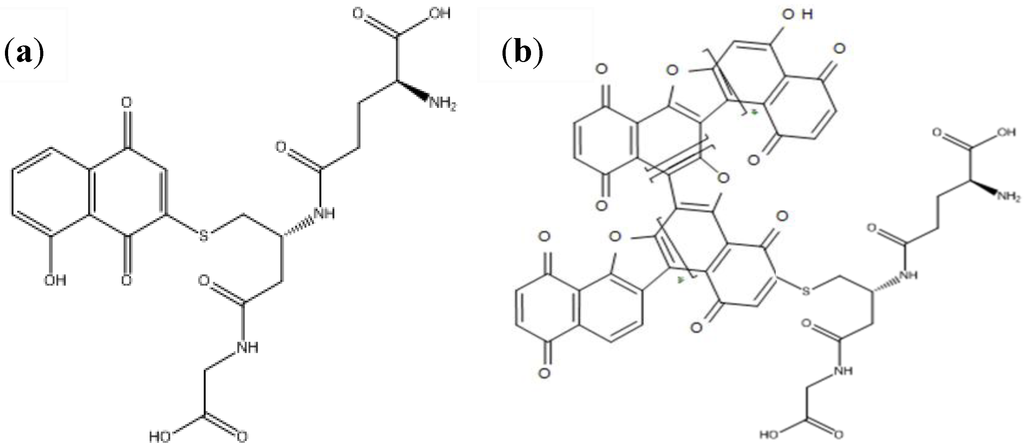
Figure 9.
(a) Structure of the juglone-gluthathione conjugate (HNQ-Glu). (b) Structure of the corresponding conducting polymer poly(HNQ-Glu).
The glutathione moiety was used as the anchoring group for oligonucleotide immobilization in a DNA sensor [41].
2.2.3. Peptides as Probes
Protein-protein or antigen-antibody interactions involve limited areas of both partners. Activities and biological functions of proteins can be analyzed by studying the interactions between shorter synthetic peptide fragments constituting the active site of a protein and their ligands, e.g., for the study of interactions between specific peptide epitopes of a protein and its specific antibody. Following this idea, we developed a strategy for the detection of Human papillomaviruses (HPVs).
It is known that genotypes 16 and 18 of Human papillomavirus (HPV-16 and HPV-18) are implicated in cervical cancer [50,51]. During the natural course of HPV infection, the presence of antibodies is usually observed [52]. Until now, most serological analyses have relied on enzyme-linked immunosorbent assays (ELISAs) [53] or antibody displacement assays [54]. Electrochemical biosensors for HPV detection are scarce. The most common approach consists in using immobilized antibodies which target the virus. A smarter approach consists in immobilizing a peptide present on the virus capside which can target antibodies in the infected serum [55,56]. Electrochemical detection of antibodies using peptide probes have been proposed, e.g., for the Nile virus [57]. For HPV detection, we proposed to target antibodies using HPV-16-L1 peptide (311–325 sequence, Asn-Leu-Ala-Ser-Ser-Asn-Tyr-Phe-Pro-Thr-Pro-Ser-Gly-Ser-Met), allowing HPV detection without labeling or adding reagent into solution. HPV-16-L1 (1,8 kDa) was grafted on the electrode to probe the HPV-16 antibody (much bigger, ca. 150 kDa) [42]. In this format, the big difference in size makes transduction of the molecular recognition much more sensitive than for classical ELISA-like architectures, the relative projected area between probe and target being (150/1.8)2/3 = 19 >>2.2 (see end of Section 2.2.1).
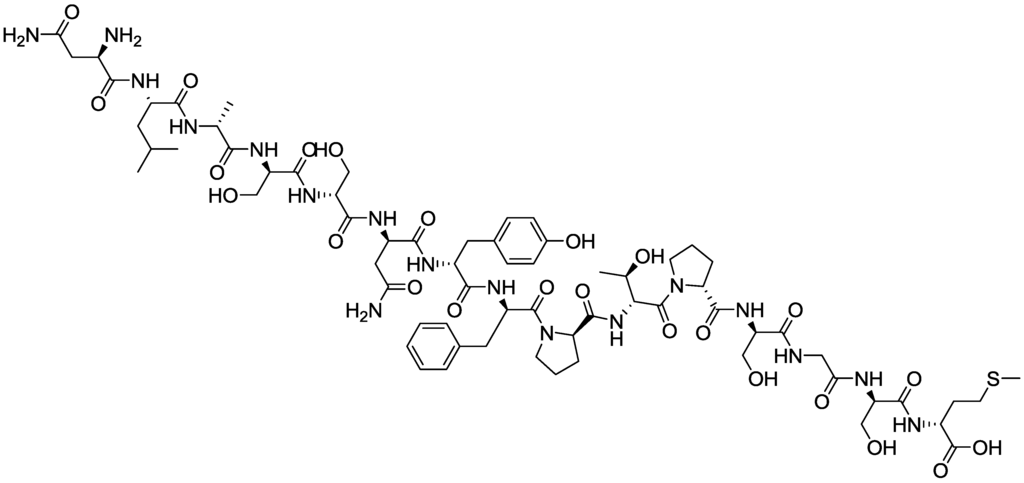
Figure 10.
Structure of Human papillomavirus (HPV-16-L1).
SWV was used to characterize the presence of the antibody. As expected, formation of the HPV-16-L1/anti-HPV complex induces a strong decrease in the polymer electroactivity (Figure 11(a)). If the same experiment is performed with a non-target antibody (e.g., anti-OVA), the current drop is lower than for anti-HPV (Figure 11(b)), which demonstrates the specificity of the molecular recognition.
These results were confirmed by traditional ELISA. It is important to note that due to the chemical nature of the quinone group and its redox process (the film being neutral or negatively charged, but never positively charged), poly(HNQ-co-HSNQA) enables the avoidance of non-specific adsorption of proteins on its surface (Figure 12) and therefore avoids false positives.
This work demonstrates the interest in replacing the heavy Ag probe (in this case, HPV virus) by much smaller fragments such as an oligopeptide constituted of 10 to 20 amino acids, which presents a much smaller size than a bulky (and dangerous to handle) virus.
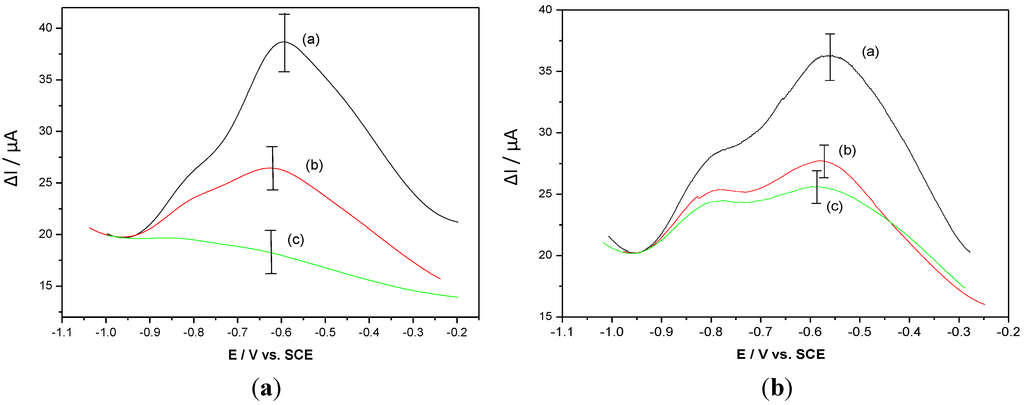
Figure 11.
(a) SWV of a poly(HNQ-co-HSNQA) recorded in PBS before grafting (curve a), after grafting of 5 × 10−8 M HPV-16-L1 (curve b) and after complexation with 5 × 10−8 M α-HPV (curve c). (b) SWV of a poly(HNQ-co-HSNQA) recorded in PBS before grafting (curve a), after grafting of 5 × 10−8 M HPV-16-L1 (curve b) and after complexation with 5 × 10−8 M α-OVA (curve c) [42].
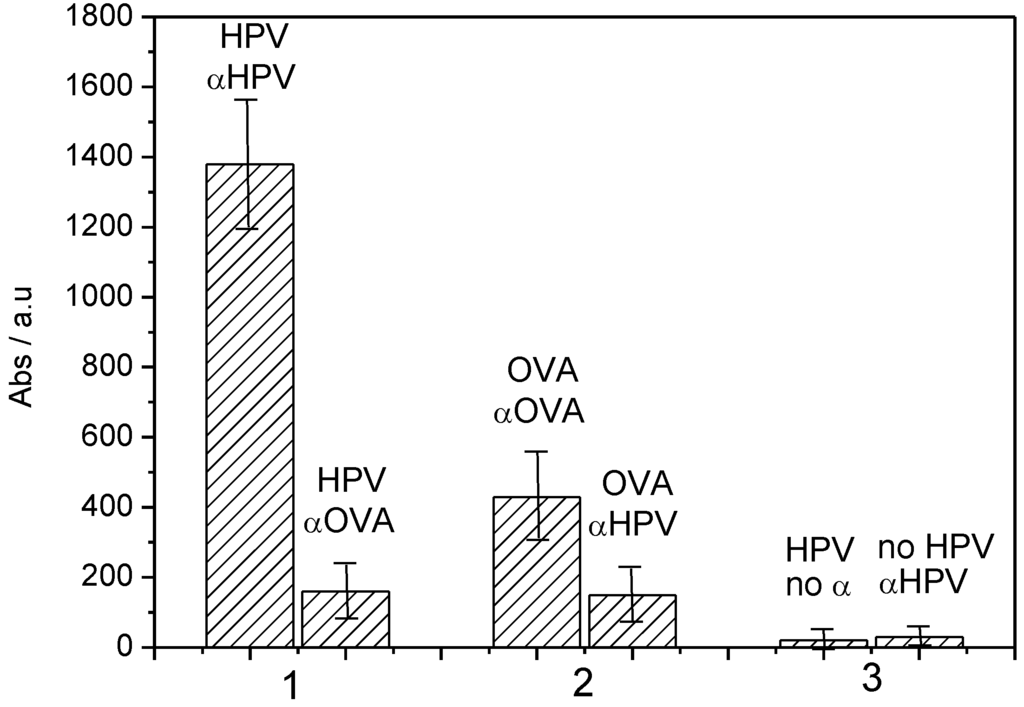
Figure 12.
Absorbance obtained for spectrophotometric ELISA assays corresponding to: (1), HPV-16-L1 grafted, + αHPV (left bar) or + αOVA (right bar). (2) OVA grafted, + αOVA (left bar) or + αHPV (right bar). (3) HPV grafted + no antibody (pure PBS) (left bar), or nothing grafted + αHPV (right bar) [42].
2.2.4. Small Organic Molecules as Probes
Analysis of potable water is correlated with public health and its regulation is under development. More than 10,000 molecules are liable to be regulated within the forthcoming years. For metrology of micro-pollutants in water (for trace quantities of the order of micrograms per liter), analyses are currently mainly achieved through chromatographic techniques coupled to mass spectrometry. However, to gain time and cost we must avoid the separation step. To keep specificity without separation, antibodies are particularly efficient and have already been used, e.g., to detect pesticides [58,59]. For this application, most of the label-free immunosensors described in the literature rely on a transduction format based on impedance [60,61] or amperometry [62] using a redox label. However, it has been shown that the introduction of appropriate functionalities through chemical modification of the sensor surface can provide good sensing abilities without the help of a label [18].
Atrazine (6-chloro-N-ethyl-N-[1-methylethyl]-1,3,5-triazine-2,4-diamine; ATZ) is a widely used pesticide that constitutes an excellent model for small organic pollutants for which antibodies exist. One of the most recent and pertinent examples has been reported by Cosnier et al. [63] who have shown that it is possible to detect 10 pg·mL−1 atrazine by Electrochemical Impedance Spectroscopy (EIS) using an immunosensor based on polypyrrole film N-substituted by nitrilotriacetic acid electrogenerated on gold electrodes. An excellent overview of detection systems for atrazine is given in [64].
It is noteworthy to recall the ability of an antibody to react with more than one antigen. This is known as cross-reactivity and arises because the cross-reacting antigen shares a structure similar to that of the immunizing antigen used to generate antibodies. We used this ability to develop a novel approach and applied this to atrazine (ATZ) detection. It is based on the competitive complexation between the anti-atrazine (αATZ) antibody on one side and atrazine present in the analyzed sample or hydroxyatrazine (HATZ) immobilized on the sensor surface on the other side, in a true direct and label-free format (no redox probe added in solution). In this particular case, the antibody αATZ generated from ATZ can also bind to HATZ (the hydroxylated atrazine), but with lower affinity [65,66,67].
We recently developed [47,68] an electrogenerated polyquinone film functionalized by a hydroxyatrazine moiety, N-(6-(4-hydroxy-6-isopropylamino-1,3,5-triazin-2-ylamino)hexyl)-5-hydroxy-1,4-naphthoquinone-3-propionamide (JUG-HATZ), which contains three functional groups: the hydroxyl group for subsequent electropolymerization, the quinone group to be used as redox transducer and hydroxyatrazine as bioreceptor element (Figure 13).
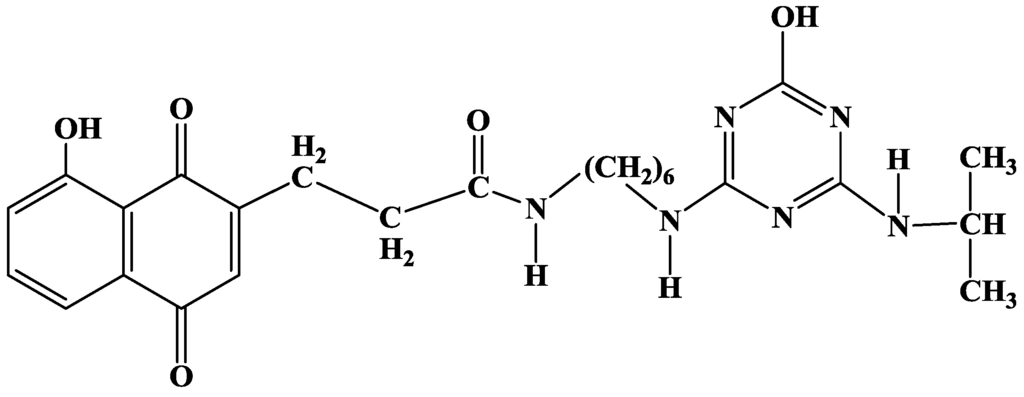
Figure 13.
JUG-HATZ structure.
After formation of the HATZ/αATZ complex, the faradic current of the quinone group decreases (Figure 14, step 2). The electrode modified by poly(JUG-HATZ) where αATZ is complexed is then utilized to detect ATZ in solution. Indeed, αATZ preferentially binds to ATZ, so that a displacement equilibrium occurs (Figure 14, step 3) between ATZ in solution and HATZ incorporated in the polymer. Addition of free atrazine removes the complexed antibodies from the electrode surface leading to an increase in electroactivity. It is proposed that the removal of the αATZ enhances the ionic flux through the interface and leads to this current increase. Here, the relative projected area between probe and target is (150/0.3)2/3 = 63, even higher than for peptide probes (see Section 2.2.3), which may explain the high sensitivity of this architecture.
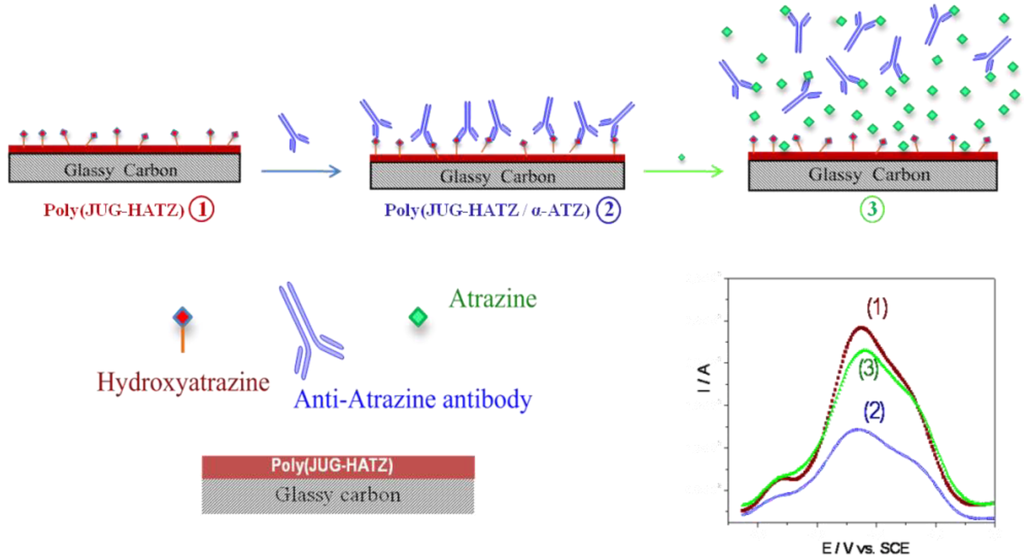
Figure 14.
Immunosensor principle based on the cross-reactivity of an antibody. Example of atrazine (ATZ) detection [47].
This signal-on system allows detection of one of the lowest atrazine concentrations (1 pM) reported in the literature for this kind of reagentless and label-free electrochemical immunosensor (Figure 15).
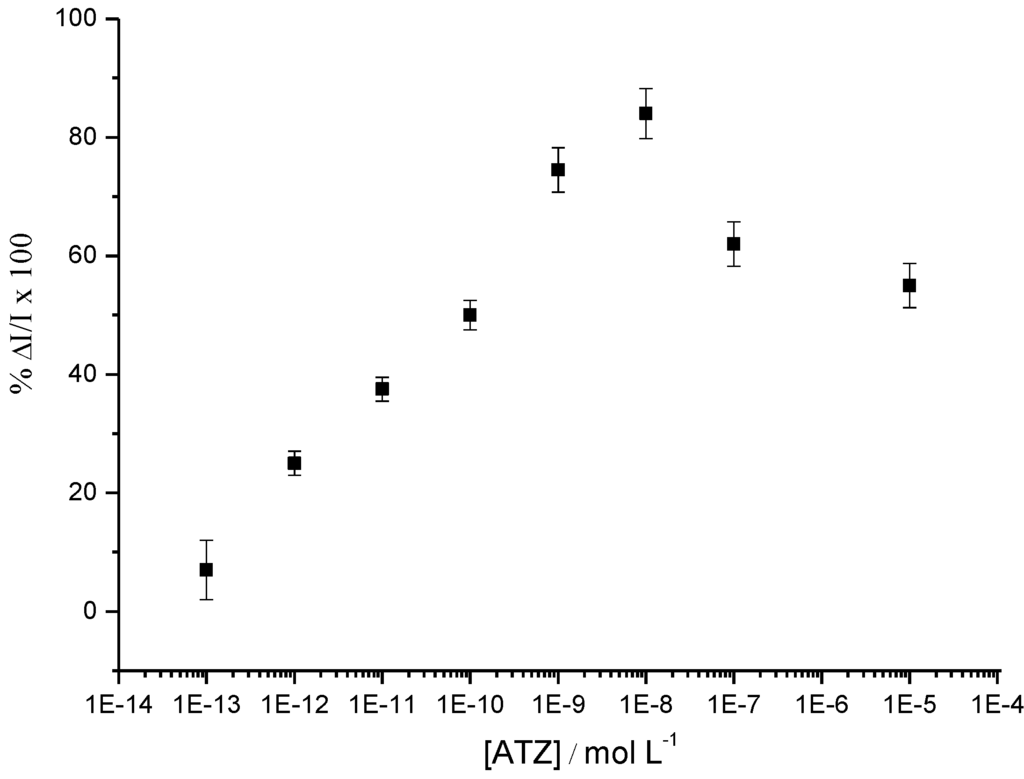
Figure 15.
Percentage of ΔI/I measured by SWV at −0.45/SCE, after addition of ATZ for concentrations from 1 pM up to 10 µM. ΔI = Ipoly(JUG-HATZ) – Ipoly(JUG-HATZ/α-ATZ) and I = Ipoly(JUG-HATZ/α-ATZ) [47].
Similar experiments were performed using electrochemical impedance spectroscopy (EIS). The process allows to detect atrazine with low detection limit (0.2 ng·L−1) in a true label-free format (no redox probe added in solution) by following changes in the electrochemical impedance of the sensor. αATZ has a molecular weight of ca. 150 kDa and an hydrodynamic volume of about 25 nm3, which makes a projected surface of 252/3 = 8.5 nm2. These dimensions are able to significantly decrease the electrolyte-film interface capacitance. On the contrary, when the antibody is removed, CPEef increases, as illustrated by the results shown on Figure 16.
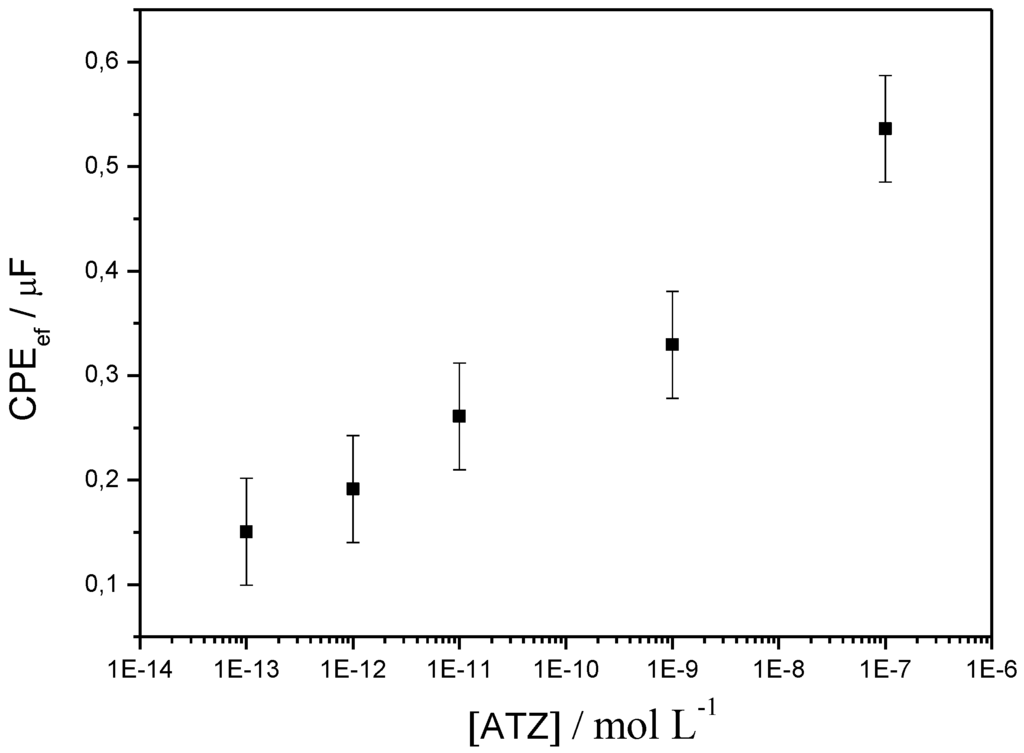
Figure 16.
CPEef as a function of ATZ concentration, from electrochemical impedance spectroscopy (EIS) experiments, on a poly(JUG-HATZ/α-ATZ)-modified electrode. Results obtained for an offset potential of −0.5 V vs. SCE [68].
The limit of detection can be estimated at 10−12 M, ca. 0.2 ng·L−1. Variations are relatively linear (on a log scale) up to 10−7 M (ca. 20 mg·L−1), which covers concentrations found in real ground water samples.
This approach is versatile: it can be extended to numerous other targets (pesticides, organic pollutants or toxins) providing they are antigenic. Work is in progress with such molecules, e.g., bisphenol A, ochratoxin A, isoproturon and glyphosates.
3. Conclusions
The possibility to detect very small amounts of proteins, peptides or pollutants using a cheap technique offers numerous opportunities for point-of-care diagnostics and on-site monitoring. Electrochemical devices transform the analyte capture into an electrical signal and benefit from the expertise developed in the context of microelectronics. This allows consideration of mass production and miniaturization at low cost. The vast majority of existing systems are based on indirect detection by amperometry and need either to label targets by a redox molecule or an enzyme, or to add a redox marker in solution. The need to add a reagent is a drawback which was recently bypassed by the development of direct, reagentless and label-free methods, either using amperometry or impedance. The use of electroactive layers immobilized on electrodes, e.g., ECPs, is promising. As these direct methods are mainly based on steric effects, special attention must be paid to the probe and target dimensions. Typical ELISA-based methods, for which the probe is bigger than the target, are not ideal. It is therefore crucial to decrease the probe size, using Fab’2 antibodies, light proteins or even peptides and small haptens.
Acknowledgments
The authors thank University Paris Diderot—Sorbonne Paris Cité and CNRS for financial support.
References
- Sparreboom, A.; Rongen, H.A.H.; van Bennekom, W.P. Assays for interferons and interleukins in biological matrices. Anal. Chim. Acta 1994, 295, 1–26. [Google Scholar] [CrossRef]
- Rongen, H.A.H.; Hoetelmans, R.M.W.; Bult, A.; van Bennekom, W.P. Chemiluminescence and Immunoassays. J. Pharm. Biomed. Appl. 1994, 12, 433–462. [Google Scholar] [CrossRef]
- Gosling, J.P. Enzyme immunoassay. Immunoassay 1996, 287–308. [Google Scholar] [CrossRef]
- Gosling, J.P. Enzyme immunoassay: With and without separation. In Principles and Practice of Immunoassay, 2nd ed.; Price, C.P., Newman, D.J., Eds.; Stockton Press: London, UK, 1997; pp. 349, 351–351. [Google Scholar]
- Porstmann, T.; Kiessig, S.T. Enzyme immunoassay techniques. J. Immunol. Methods 1992, 150, 5–21. [Google Scholar] [CrossRef]
- Tijssen, P. Principles of immunoassays. Enzymes. Methods Immunol. Anal. 1993, 1, 283–297. [Google Scholar]
- Wegner, G.J.; Lee, H.J.; Corn, R.M. Characterization and optimization of peptide arrays for the study of epitope-antibody interactions using surface plasmon resonance imaging. Anal. Chem. 2002, 74, 5161–5168. [Google Scholar] [CrossRef]
- Kanda, V.; Kariuki, J.K.; Harrison, D.J.; McDermott, M.T. Label-free reading of microarray-based immunoassays with surface plasmon resonance imaging. Anal. Chem. 2004, 76, 7257–7262. [Google Scholar]
- Bizet, K.; Gabrielli, C.; Perrot, H.; Therasse, J. Validation of antibody-based recognition by piezoelectric transducers through electroacoustic admittance analysis. Biosens. Bioelectron. 1998, 13, 259–269. [Google Scholar] [CrossRef]
- Jones, V.W.; Kenseth, J.R.; Porter, M.D.; Mosher, C.L.; Henderson, E. Microminiaturized immunoassays using atomic force microscopy and compositionally patterned antigen arrays. Anal. Chem. 1998, 70, 1233–1241. [Google Scholar]
- Jaffrezic-Renault, N.; Martelet, C. Preparation of well-engineered thin molecular layers on semiconductor-based transducers. Sens. Actuator. A 1992, 32, 307–312. [Google Scholar] [CrossRef]
- Lin, P.; Yan, F. Organic thin-film transistors for chemical and biological sensing. Adv. Mater. 2012, 24, 34–51. [Google Scholar] [CrossRef]
- Skládal, P. Advances in electrochemical immunosensors. Electroanalysis 1997, 9, 737–745. [Google Scholar] [CrossRef]
- Prodromidis, M.I. Impedimetric immunosensors—A review. Electrochim. Acta 2010, 55, 4227–4233. [Google Scholar] [CrossRef]
- Sargent, A.; Sadik, O.A. Monitoring antibody-antigen reactions at conducting polymer-based immunosensors using impedance spectroscopy. Electrochim. Acta 1999, 44, 4667–4675. [Google Scholar] [CrossRef]
- Cosnier, S. Biomolecule immobilization on electrode surfaces by entrapment or attachment to electrochemically polymerized films. A review. Biosens. Bioelectron. 1999, 14, 443–456. [Google Scholar] [CrossRef]
- Gerard, M.; Chaubey, A.; Malhotra, B.D. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Cosnier, S. Biosensors based on electropolymerized films: New trends. Anal. Bioanal. Chem. 2003, 377, 507–520. [Google Scholar] [CrossRef]
- Cosnier, S. Affinity biosensors based on electropolymerized films. Electroanalysis 2005, 17, 1701–1715. [Google Scholar] [CrossRef]
- Ionescu, R.E.; Gondran, C.; Bouffier, L.; Jaffresic-Renault, N.; Martelet, C.; Cosnier, S. Label-free impedimetric immunosensor for sensitive detection of atrazine. Electrochim. Acta 2010, 55, 6228–6232. [Google Scholar] [CrossRef]
- Ramanavičius, A.; Ramanavičienė, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer—Polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar]
- John, R.; Spencer, M.; Wallace, G.G.; Smyth, M.R. Development of a polypyrrole-based human serum albumin sensor. Anal. Chim. Acta 1991, 249, 381–385. [Google Scholar] [CrossRef]
- Sadik, O.A.; Wallace, G.G. Pulsed amperometric detection of proteins using antibody containing conducting polymers. Anal. Chim. Acta 1993, 279, 209–212. [Google Scholar] [CrossRef]
- Lu, W.; Zhao, H.; Wallace, G.G. Pulsed electrochemical detection of proteins using conducting polymer based sensors. Anal. Chim. Acta 1995, 315, 27–33. [Google Scholar] [CrossRef]
- Sadik, O.A.; van Emon, J.M. Designing immunosensors for environmental monitoring. Chemtech 1997, 27, 38–46. [Google Scholar]
- Brender, S.; Sadik, O.A.; van Emon, J.M. Direct electrochemical immunosensor for polychlorinated biphenyls. Environ. Sci. Technol. 1998, 32, 788–797. [Google Scholar] [CrossRef]
- Sadik, O.A. Bioaffinity sensors based on conducting polymers: A short review. Electroanalysis 1999, 11, 839–844. [Google Scholar] [CrossRef]
- Xu, H.; Masila, M.; Yan, F.; Sadik, O.A. Multiarray sensors for pesticides and toxic metals. Proc. SPIE 1999, 3534, 437–445. [Google Scholar]
- Rahman, M.A.; Shiddiky, M.J.A.; Park, J.-S.; Shim, Y.-B. An impedimetric immunosensor for the label-free detection of bisphenol A. Biosens. Bioelectron. 2007, 22, 2464–2470. [Google Scholar] [CrossRef]
- Khan, R.; Dhayal, M. Chitosan/polyaniline hybrid conducting biopolymer base impedimetric immunosensor to detect Ochratoxin-A. Biosens. Bioelectron. 2009, 24, 1700–1705. [Google Scholar] [CrossRef]
- Lacaze, P.C.; Pham, M.C.; Delamar, M.; Dubois, J.E. Extension of the voltammetric theory to inert, permeable thin-film coated rotating-disk electrodes. Behavior of ferrocene-ferricinium ion and quinone-hydroquinone systems on polymer-coated electrodes. J. Electroanal. Chem. 1980, 108, 9–16. [Google Scholar] [CrossRef]
- Leidner, C.R.; Gater, V.K. Ion and electron-transport within amino-quinone polymer-films. J. Electrochem. Soc. 1987, 134, C502. [Google Scholar] [CrossRef]
- Gater, V.K.; Love, M.D.; Liu, M.D.; Leidner, C.R. Quinone molecular films derived from 1,5-diaminoanthraquinone. J. Electronanal. Chem. 1987, 235, 381–385. [Google Scholar] [CrossRef]
- Gater, V.K.; Liu, M.D.; Love, M.D.; Leidner, C.R. Quinone molecular films derived from aminoquinones. J. Electroanal. Chem. 1988, 257, 133–146. [Google Scholar] [CrossRef]
- Miller, L.L.; Zinger, B.; Degrand, C. The effects of cross-linking and anodic surface roughening on quinone polymer carbon electrodes. J. Electroanal. Chem. 1984, 178, 87–99. [Google Scholar] [CrossRef]
- Pham, M.C.; Piro, B.; Bazzaoui, E.A.; Hedayatullah, M.; Lacroix, J.C.; Novak, P.; Haas, O. Anodic oxidation of 5-amino-1,4-naphthoquinone (ANQ) and synthesis of a conducting polymer (PANQ). Synth. Met. 1998, 92, 197–205. [Google Scholar] [CrossRef]
- Haringer, D.; Novak, P.; Haas, O.; Piro, B.; Pham, M.C. Poly(5-amino-1,4-naphthoquinone), a novel lithium-inserting electroactive polymer with high specific charge. J. Electrochem. Soc. 1999, 146, 2393–2396. [Google Scholar] [CrossRef]
- Reisberg, S.; Piro, B.; Noel, V.; Pham, M.C. Selectivity and sensitivity of a reagentless electrochemical DNA sensor studied by square wave voltammetry and fluorescence. Bioelectrochemistry 2006, 69, 172–179. [Google Scholar] [CrossRef]
- Piro, B.; Reisberg, S.; Noel, V.; Pham, M.C. Investigations of the steric effect on electrochemical transduction in a quinone-based DNA sensor. Biosens. Bioelectron. 2007, 22, 3126–3131. [Google Scholar] [CrossRef]
- Piro, B.; Haccoun, J.; Pham, M.C.; Tran, L.D.; Rubin, A.; Perrot, H.; Gabrielli, C. Study of the DNA hybridization transduction behavior of a quinone-containing electroactive polymer by cyclic voltammetry and electrochemical impedance spectroscopy. J. Electroanal. Chem. 2005, 577, 155–165. [Google Scholar] [CrossRef]
- Reisberg, S.; Acevedo, D.F.; Korovitch, A.; Piro, B.; Noel, V.; Buchet, I.; Tran, L.D.; Barbero, C.A.; Pham, M.C. Design of a new electrogenerated polyquinone film substituted with glutathione. Towards direct electrochemical biosensors. Talanta 2010, 80, 1318–1325. [Google Scholar] [CrossRef]
- Piro, B.; Kapella, A.; Le, V.H.; Anquetin, G.; Zhang, Q.D.; Reisberg, S.; Noel, V.; Tran, L.D.; Duc, H.T.; Pham, M.C. Towards the detection of human papillomavirus infection by a reagentless electrochemical peptide biosensor. Electrochim. Acta 2011, 56, 10688–10693. [Google Scholar]
- Zhang, Q.D.; March, G.; Noel, V.; Piro, B.; Reisberg, S.; Tran, L.D.; Hai, L.V.; Abadia, E.; Nielsen, P.E.; Sola, C.; Pham, M.C. Label-free and reagentless electrochemical detection of PCR fragments using self-assembled quinone derivative monolayer: Application to Mycobacterium tuberculosis. Biosens. Bioelectron. 2012, 32, 163–168. [Google Scholar] [CrossRef]
- Piro, B.; Zhang, Q.D.; Reisberg, S.; Noel, V.; Dang, L.A.; Duc, H.T.; Pham, M.C. Direct and rapid electrochemical immunosensing system based on a conducting polymer. Talanta 2010, 82, 608–612. [Google Scholar] [CrossRef]
- Piro, B.; Reisberg, S.; Noel, V.; Pham, M.C. Investigations of the steric effect on electrochemical transduction in a quinone-based DNA sensor. Biosens. Bioelectron. 2007, 12, 3126–3131. [Google Scholar]
- Reisberg, S.; Piro, B.; Noel, V.; Nguyen, T.D.; Nielsen, P.E.; Pham, M.C. Investigation of the charge effect on the electrochemical transduction in a quinone-based DNA sensor. Electrochim. Acta 2008, 54, 346–351. [Google Scholar] [CrossRef]
- Tran, H.V.; Yougnia, R.; Reisberg, S.; Piro, B.; Serradji, N.; Nguyen, T.D.; Tran, L.D.; Dong, C.Z.; Pham, M.C. A label-free electrochemical immunosensor for direct, signal-on and sensitive pesticide detection. Biosens. Bioelectron. 2012, 31, 62–68. [Google Scholar] [CrossRef]
- Sassolas, A.; Prieto-Simón, B.; Marty, J. Biosensors for pesticide detection: New trends. Am. J. Anal. Chem. 2012, 3, 210–232. [Google Scholar] [CrossRef]
- Thomson, R.H. Studies in the juglone series. 3. Addition reactions. J. Org. Chem. 1951, 16, 1082–1090. [Google Scholar] [CrossRef]
- Bosch, F.X.; Manos, M.M.; Munoz, N.; Sherman, M.; Jansen, A.M.; Peto, J.; Schiffman, M.H.; Moreno, V.; Kurman, R.; Shan, K.V. Prevalence of human papillomavirus in cervical-cancer—A worldwide perspective. J. Nat. Cancer. Inst. 1995, 87, 796–802. [Google Scholar] [CrossRef]
- Munoz, N.; Bosch, F.X.; De Sanjose, S.; Herrero, R.; Castellsague, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic classification of human papillomavirus types associated with cervical cancer. New Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef]
- Tyring, S.K. Human papillomavirus infections: Epidemiology, pathogenesis, and host immune response. J. Am. Acad. Dermatol. 2000, 43, S18–S26. [Google Scholar] [CrossRef]
- Dillner, J. The serological response to papillomaviruses. Semin. Cancer Biol. 1999, 9, 423–430. [Google Scholar]
- Yeager, M.D.; Aste-Amezaga, M.; Brown, D.R.; Martin, M.M.; Shah, M.J.; Cook, J.C.; Christensen, N.D.; Ackerson, C.; Lowe, R.S.; Smith, J.F.; Keller, P.; Jansen, K.U. Neutralization of human papillomavirus (HPV) pseudovirions: A novel and efficient approach to detect and characterize HPV neutralizing antibodies. Virology 2000, 278, 570–577. [Google Scholar] [CrossRef]
- Caygill, R.L.; Blair, G.E.; Millner, P.A. A review on viral biosensors to detect human pathogens. Anal. Chim. Acta 2010, 681, 8–15. [Google Scholar] [CrossRef]
- Caygill, R.L.; Hodges, C.S.; Holmes, J.L.; Higson, S.P.J.; Blair, G.E.; Millner, P.A. Novel impedimetric immunosensor for the detection and quantitation of Adenovirus using reduced antibody fragments immobilized onto a conducting copolymer surface. Biosens. Bioelectron. 2012, 32, 104–110. [Google Scholar] [CrossRef]
- Ionescu, R.E.; Cosnier, S.; Herrmann, S.; Marks, R.S. Amperometric immunosensor for the detection of anti-West Nile virus IgG. Anal. Chem. 2007, 79, 8662–8668. [Google Scholar]
- Marty, J.L.; Garcia, D.; Rouillon, R. Biosensors—Potential in pesticide detection. Trends Anal. Chem. 1995, 14, 329–333. [Google Scholar]
- Dennison, M.J.; Turner, A.P.F. Biosensors for environmental monitoring. Biotechnol. Adv. 1995, 13, 1–12. [Google Scholar] [CrossRef]
- Hleli, S.; Martelet, C.; Abdelghani, A.; Burais, N.; Jaffrezic-Renault, N. Atrazine analysis using an impedimetric immunosensor based on mixed biotinylated self-assembled monolayer. Sens. Actuator. B 2006, 113, 711–717. [Google Scholar] [CrossRef]
- Valera, E.; Ramón-Azcón, J.; Barranco, A.; Alfaro, B.; Sánchez-Baeza, F.; Marco, M.-P.; Rodríguez, Á. Determination of atrazine residues in red wine samples. A conductimetric solution. Food Chem. 2010, 122, 888–894. [Google Scholar] [CrossRef]
- Besombes, J.L.; Cosnier, S.; Labbé, P.; Reverdy, G. A biosensor as warning device for the detection of cyanide, chlorophenols, atrazine and carbamate pesticides. Anal. Chim. Acta 1995, 311, 255–263. [Google Scholar] [CrossRef]
- Ionescu, R.E.; Gondran, C.; Bouffier, L.; Jaffrezic-Renault, N.; Martelet, C.; Cosnier, S. Label-free impedimetric immunosensor for sensitive detection of atrazine. Electrochim. Acta 2010, 55, 6228–6232. [Google Scholar] [CrossRef]
- Ballesteros-Gomez, A.; Rubio, S. Recent advances in environmental analysis. Anal. Chem. 2011, 83, 4579–4613. [Google Scholar] [CrossRef]
- Schlaeppi, J.M.; Foery, W.; Ramsteiner, K. Hydroxyatrazine and atrazine determination in soil and water by enzyme-linked immunosorbent-assay using specific monoclonal-antibodies. J. Agric. Food Chem. 1989, 37, 1532–1538. [Google Scholar] [CrossRef]
- Wortberg, M.; Jones, G.B.; Kreissig, S.; Rocke, D.M.; Gee, S.J.; Hammock, B.D. An approach to the construction of an immunoarray for differentiating and quantitating cross reacting analytes. Anal. Chim. Acta 1996, 319, 291–303. [Google Scholar] [CrossRef]
- Charlton, K.; Harris, W.J.; Porter, A.J. The isolation of super-sensitive anti-hapten antibodies from combinatorial antibody libraries derived from sheep. Biosens. Bioelectron. 2001, 16, 639–646. [Google Scholar] [CrossRef]
- Tran, H.V.; Reisberg, S.; Piro, B.; Pham, M.C. Label-free electrochemical immunoaffinity sensor based on impedimetric method for pesticide detection. Electroanalysis 2013, in press. [Google Scholar]
Appendx
Experimentals
Phosphate buffer saline (PBS) had a pH of 7.4 and was provided by Sigma. Aqueous solutions were made with ultrapure (18 MΩ cm) water. Glassy carbon electrodes (GC, 3 mm in diameter, S = 0.07 cm2) were purchased from BASInc. 5-hydroxy-1,4-naphthoquinone (JUG), 1-naphthol (1-NAP), lithium perchlorate (LiClO4), acetonitrile (ACN) and ethanol (EtOH), practical grade, were from Sigma-Aldrich. Atrazine (ATZ) and atrazine-desethyl-2-hydroxy (ATD) were purchased from Supelco (USA). Anti-atrazine (α-ATZ) antibody (monoclonal) was purchased from Thermo Scientific, USA. A. OVA (egg albumin, 2× crystallized) was purchased from Calbiochem, La Jolla, CA, USA. HPV-16-L1 peptide (311–325 sequence, i.e., Asn-Leu-Ala-Ser-Ser-Asn-Tyr-Phe-Pro-Thr-Pro-Ser-Gly-Ser-Met) was purchased from Genscript, USA. Secondary Goat F(ab’)2 anti-Mouse Ig conjugated to horseradish peroxidase was purchased from Tebu (Le Perray-en-Yvelines, France). Antibodies against HPV (αHPV, mouse anti-papillomavirus 16 L1 late protein, monoclonal antibody) and against OVA (αOVA) were obtained from AbD serotec (Morphosys, UK). The electrochemical synthesis of the polymer films was carried out by electro-oxidation of 5 × 10−3 M JUG-HATZ + 10−3 M 1-NAP + 0.1 M LiClO4 in ACN on GC electrodes, under Ar, at a constant potential of 1.25 V (vs. SCE) during 600 s. Square wave voltammetry (SWV) was used to characterize α-ATZ complexation and ATZ detection. The following parameters were used: pulse height 50 mV, pulse width 50 ms, scan increment 2 mV, frequency 12.5 Hz. EIS was performed using an Autolab PGSTAT30 equipped with the FRA module. Impedance spectra were recorded in PBS buffer at room temperature at a given offset potential within a frequency range from 10 kHz to 100 mHz with a perturbation amplitude of 10 mV. As a pretreatment before each experiment, a constant potential corresponding to the one used for EIS was imposed, for 120 s. Solutions were systematically deaerated with Ar before and during experiments.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).