Self-Adaptive Polymer Fabry–Pérot Thermometer for High-Sensitivity and Wide-Linear-Range Sensing
Abstract
1. Introduction
2. Materials and Methods
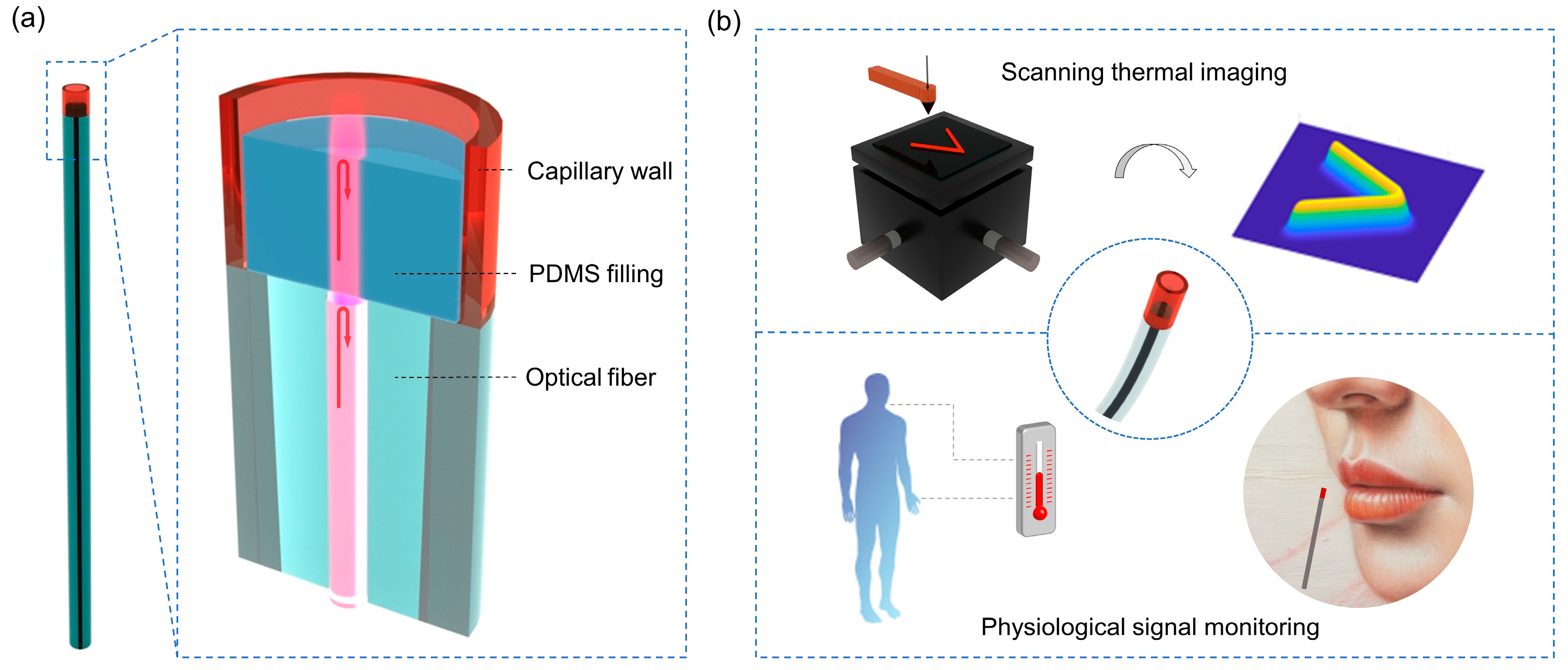
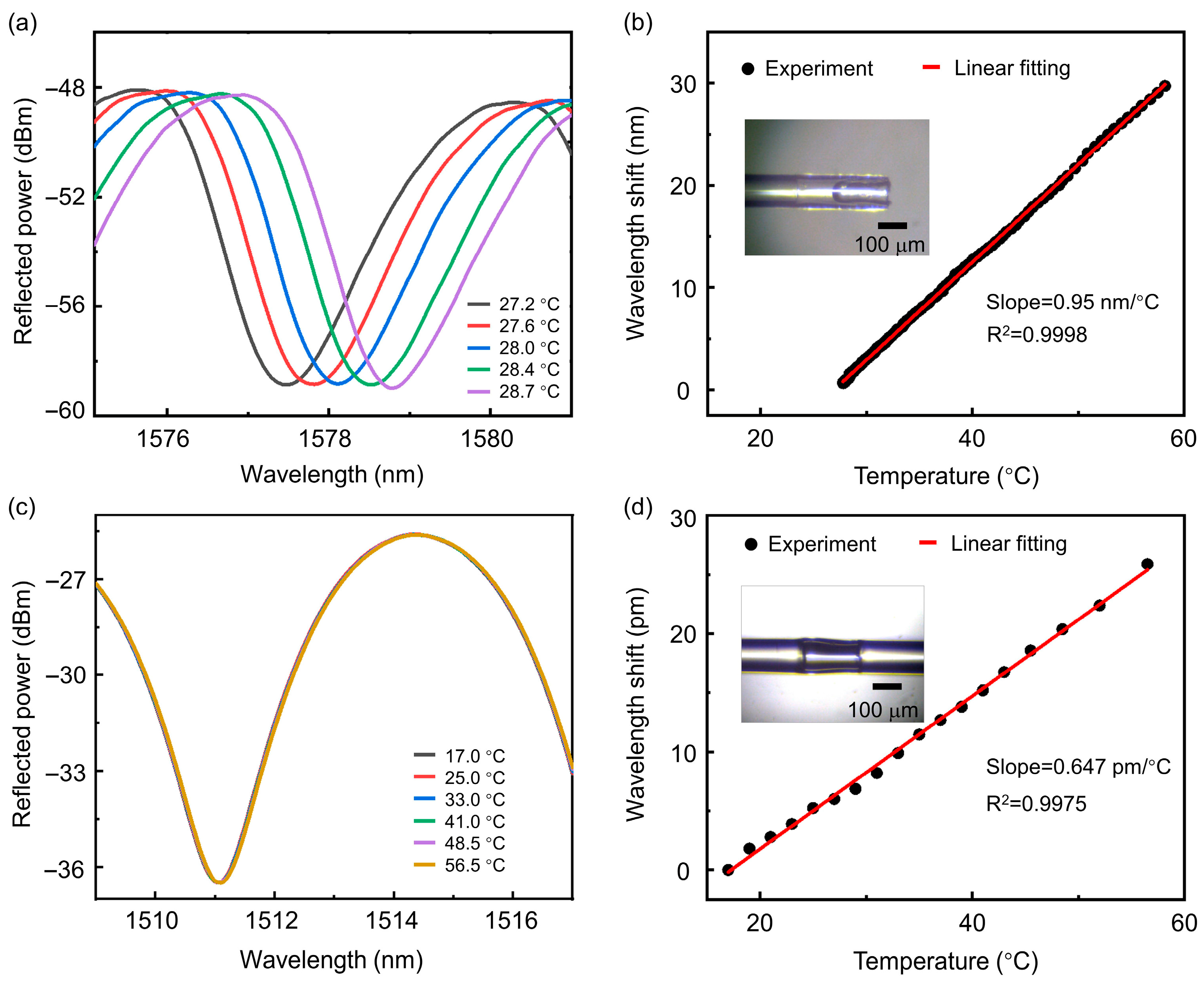
2.1. Preparation and Characterization of PFPI Sensors
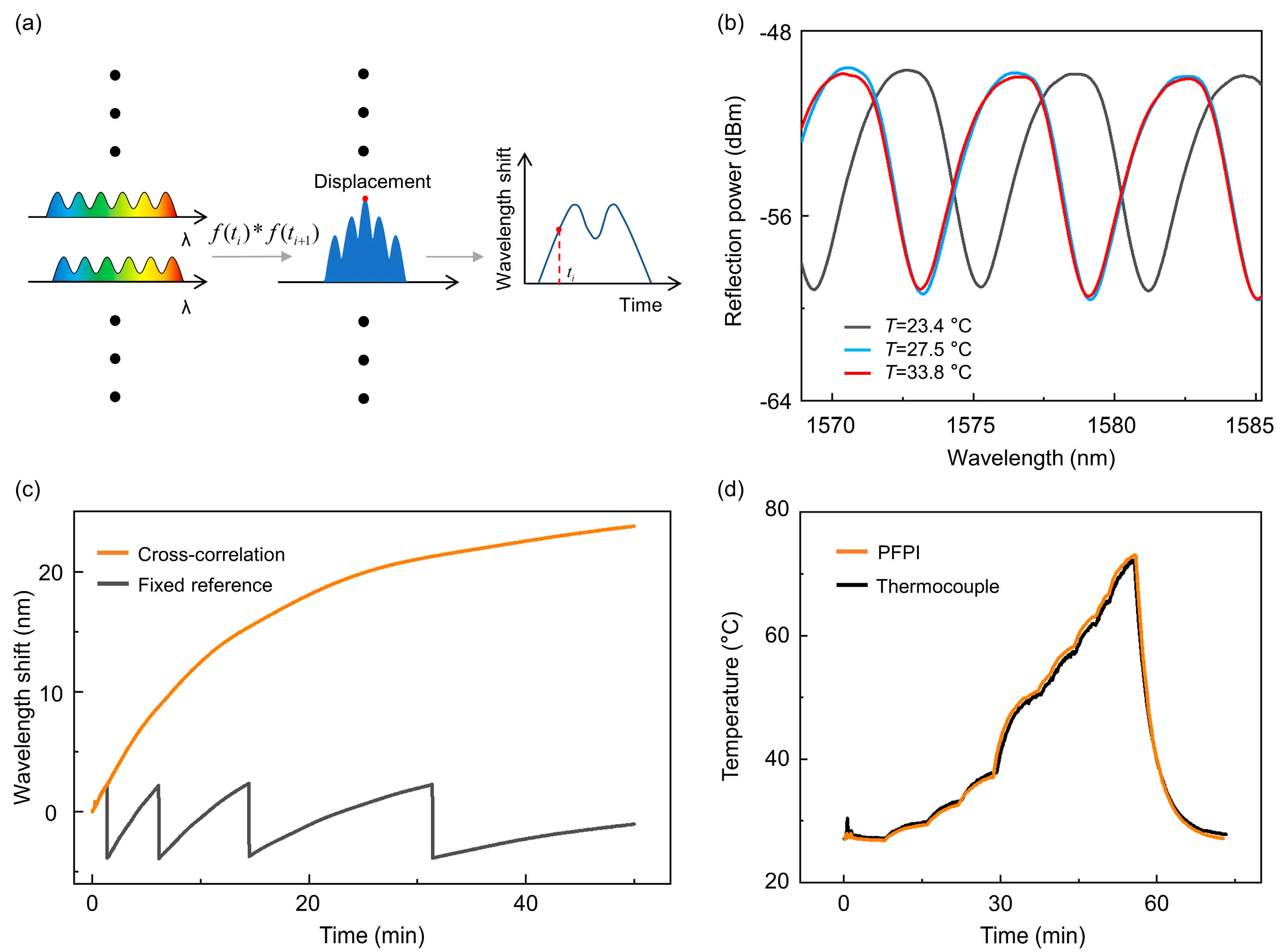
2.2. Cross-Correlation Algorithm for Wide-Range Spectral Shift Tracing
3. Results
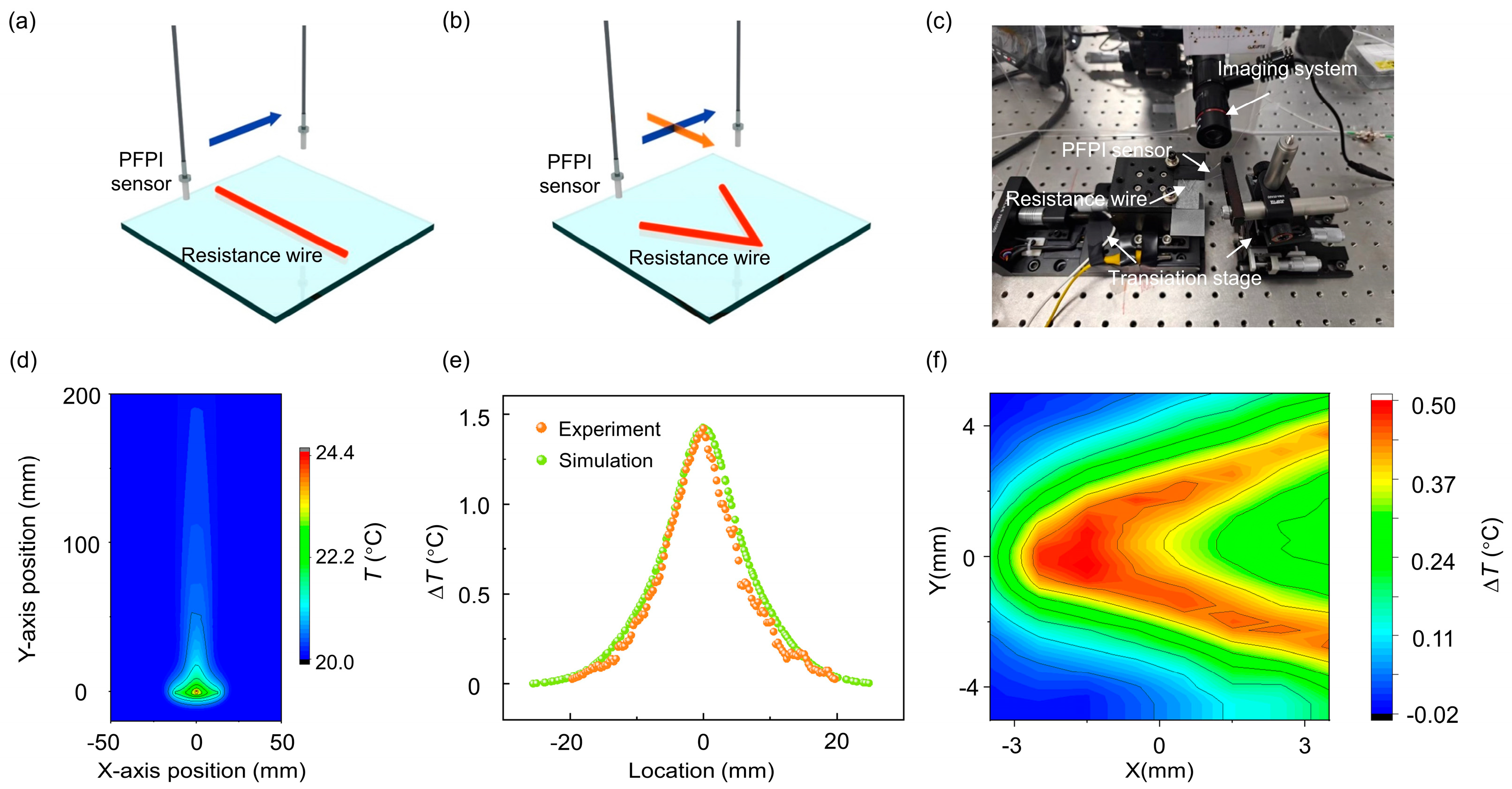
3.1. Scanning Thermal Field Imaging
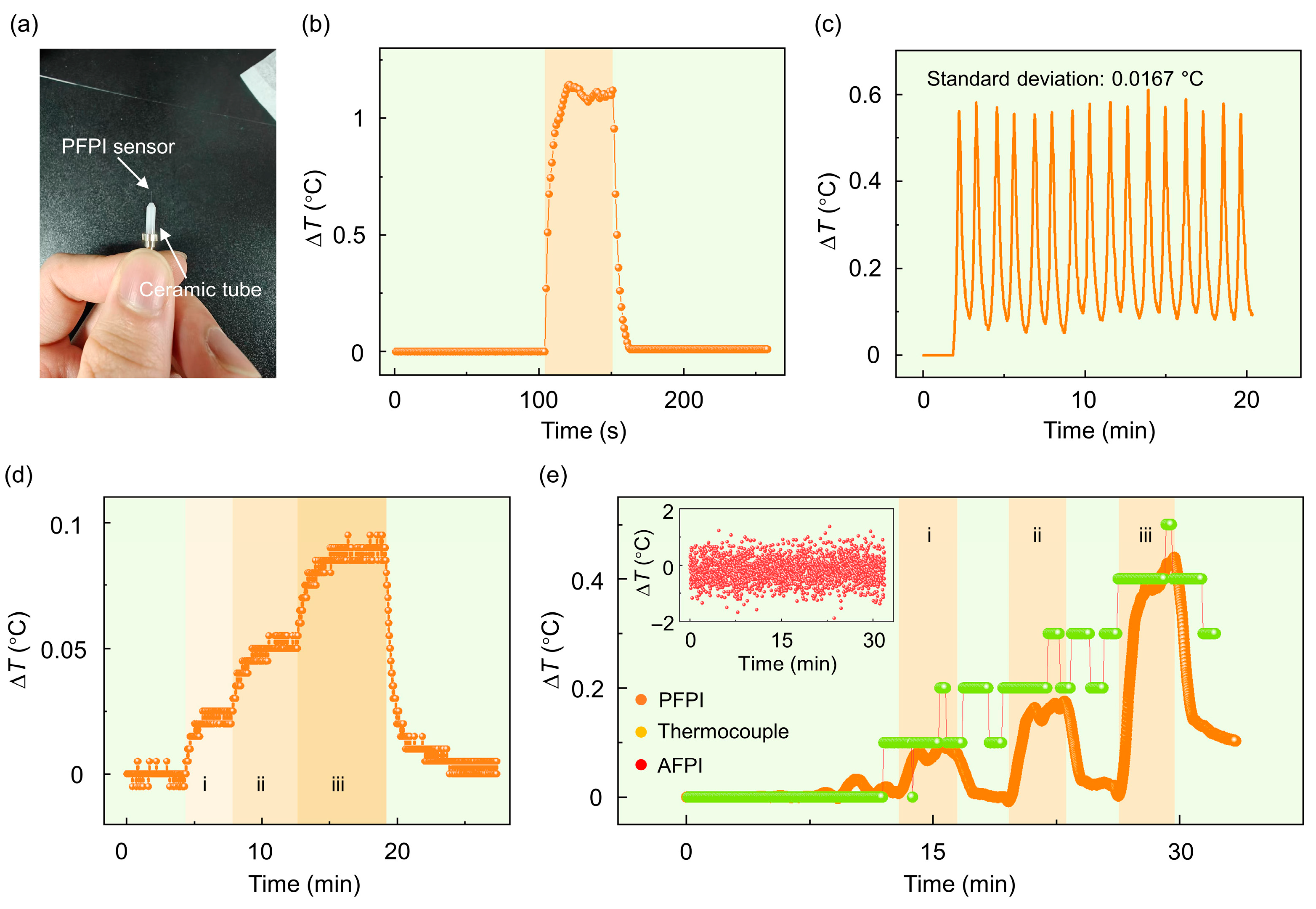
3.2. Body Temperature and Respiratory Signals Monitoring
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FBG | fiber Bragg grating |
| PDMS | polydimethylsiloxane |
| FPI | Fabry–Pérot interferometer |
| APFI | air-filled Fabry–Pérot interferometer |
| FSR | free spectral range |
References
- Sang, A.K.; Froggatt, M.E.; Gifford, D.K.; Kreger, S.T.; Dickerson, B.D. One Centimeter Spatial Resolution Temperature Measurements in a Nuclear Reactor Using Rayleigh Scatter in Optical Fiber. IEEE Sens. J. 2008, 8, 1375–1380. [Google Scholar] [CrossRef]
- Song, K.; Wang, H.; Kamm, G.B.; Pohle, J.; Reis, F.D.C.; Heppenstall, P.; Wende, H.; Siemens, J. The TRPM2 Channel Is a Hypothalamic Heat Sensor That Limits Fever and Can Drive Hypothermia. Science 2016, 353, 1393–1398. [Google Scholar] [CrossRef]
- Shin, J.; Jeong, B.; Kim, J.; Nam, V.B.; Yoon, Y.; Jung, J.; Hong, S.; Lee, H.; Eom, H.; Yeo, J.; et al. Sensitive Wearable Temperature Sensor with Seamless Monolithic Integration. Adv. Mater. 2020, 32, 1905527. [Google Scholar] [CrossRef]
- Trung, T.Q.; Le, H.S.; Dang, T.M.L.; Ju, S.; Park, S.Y.; Lee, N. Freestanding, Fiber-Based, Wearable Temperature Sensor with Tunable Thermal Index for Healthcare Monitoring. Adv. Healthc. Mater. 2018, 7, 1800074. [Google Scholar] [CrossRef]
- Song, E.; Chen, M.; Chen, Z.; Zhou, Y.; Zhou, W.; Sun, H.-T.; Yang, X.; Gan, J.; Ye, S.; Zhang, Q. Mn2+-Activated Dual-Wavelength Emitting Materials toward Wearable Optical Fibre Temperature Sensor. Nat. Commun. 2022, 13, 2166. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A Graphene-Based Electrochemical Device with Thermoresponsive Microneedles for Diabetes Monitoring and Therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Takahashi, A.; Takahashi, Y.; Okubo, K.; Takagi, K.; Fujino, T.; Kusa, S.; Takigawa, M.; Watari, Y.; Yamao, K.; et al. Incidences of Esophageal Injury during Esophageal Temperature Monitoring: A Comparative Study of a Multi-Thermocouple Temperature Probe and a Deflectable Temperature Probe in Atrial Fibrillation Ablation. J. Interv. Card. Electrophysiol. 2014, 39, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chan, P.K.L.; Lu, J.; Huang, B.; Leung, D.C.W. High Dynamic Range Organic Temperature Sensor. Adv. Mater. 2013, 25, 1290. [Google Scholar] [CrossRef]
- Ohashi, M.; Takahashi, Y.; Terakado, N.; Onoue, N.; Shinozaki, T.; Fujiwara, T. Temperature Dependence of Afterglow in Zirconia and Its Optically-Stimulated Luminescence by Bone-through Irradiation for Biological Temperature Probe. Sci. Rep. 2020, 10, 2242. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Z.; Li, L.; Guo, J.; Jin, L.; Lu, J.; Huang, P.; Zhang, S.; Jiao, L. Highly Efficient Red-Emitting Carbon Dots as a “Turn-on” Temperature Probe in Living Cells. Spectrochim. Acta A 2022, 280, 121538. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Hu, R.; Xu, J.; Wang, S.; Li, S.; Li, Y.; Yang, G. Intracellular Fluorescent Temperature Probe Based on Triarylboron Substituted Poly N -Isopropylacrylamide and Energy Transfer. Anal. Chem. 2015, 87, 3694–3698. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Yang, Q.-F.; Chang, L.; Shen, B.; Wang, H.; Leal, M.A.; Wu, L.; Gao, M.; Feshali, A.; Paniccia, M.; et al. Hertz-Linewidth Semiconductor Lasers Using CMOS-Ready Ultra-High-Q Microresonators. Nat. Photon 2021, 15, 346–353. [Google Scholar] [CrossRef]
- Cai, X.; Sushkov, A.B.; Suess, R.J.; Jadidi, M.M.; Jenkins, G.S.; Nyakiti, L.O.; Myers-Ward, R.L.; Li, S.; Yan, J.; Gaskill, D.K.; et al. Sensitive Room-Temperature Terahertz Detection via the Photothermoelectric Effect in Graphene. Nat. Nanotechnol. 2014, 9, 814–819. [Google Scholar] [CrossRef]
- Vicarelli, L.; Vitiello, M.S.; Coquillat, D.; Lombardo, A.; Ferrari, A.C.; Knap, W.; Polini, M.; Pellegrini, V.; Tredicucci, A. Graphene Field-Effect Transistors as Room-Temperature Terahertz Detectors. Nat. Mater. 2012, 11, 865–871. [Google Scholar] [CrossRef]
- Datye, I.M.; Rojo, M.M.; Yalon, E.; Deshmukh, S.; Mleczko, M.J.; Pop, E. Localized Heating and Switching in MoTe2 -Based Resistive Memory Devices. Nano Lett. 2020, 20, 1461–1467. [Google Scholar] [CrossRef]
- Feng, S.; Yan, Y.; Li, H.; Zhang, L.; Yang, S. Thermal Management of 3D Chip with Non-Uniform Hotspots by Integrated Gradient Distribution Annular-Cavity Micro-Pin Fins. Appl. Therm. Eng. 2021, 182, 116132. [Google Scholar] [CrossRef]
- Shoemaker, D.; Malakoutian, M.; Chatterjee, B.; Song, Y.; Kim, S.; Foley, B.M.; Graham, S.; Nordquist, C.D.; Chowdhury, S.; Choi, S. Diamond-Incorporated Flip-Chip Integration for Thermal Management of GaN and Ultra-Wide Bandgap RF Power Amplifiers. IEEE Trans. Compon., Packag. Manufact. Technol. 2021, 11, 1177–1186. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, F.; Tao, Z.; Chang, L.; Shu, H.; Jin, M.; Zhou, Y.; Ge, Z.; Wang, X. Fully On-Chip Microwave Photonic Instantaneous Frequency Measurement System. Laser Photonics Rev. 2022, 16, 2200158. [Google Scholar] [CrossRef]
- Zhang, D.; Fu, Y.; Zhan, H.; Zhao, C.; Gao, X.; Qin, C.; Wang, L. Suppressing Thermal Quenching via Defect Passivation for Efficient Quasi-2D Perovskite Light-Emitting Diodes. Light. Sci. Appl. 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Li, J.; Kim, Y.; Van Os, J.M.C.; Brounts, S.H.; Choi, C.Y. Using Implantable Biosensors and Wearable Scanners to Monitor Dairy Cattle’s Core Body Temperature in Real-Time. Comput. Electron. Agric. 2020, 174, 105453. [Google Scholar] [CrossRef]
- Madonna, V.; Giangrande, P.; Lusuardi, L.; Cavallini, A.; Gerada, C.; Galea, M. Thermal Overload and Insulation Aging of Short Duty Cycle, Aerospace Motors. IEEE Trans. Ind. Electron. 2020, 67, 2618–2629. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, N.; Ma, C. Steady-State Error Estimation and Calibration of a Shielded Total Temperature Probe for Turbomachinery. Int. J. Therm. Sci. 2023, 190, 108298. [Google Scholar] [CrossRef]
- Xiao, A.; Zheng, J.; Wu, X.; Cui, W.; Chen, P.; Liang, J.; Zhong, J.; Huang, Y.; Huang, Y.; Guan, B. Ultrasensitive Detection and Cellular Photothermal Therapy via a Self-Photothermal Modulation Biosensor. Adv. Opt. Mater. 2023, 11, 2202711. [Google Scholar] [CrossRef]
- Li, B.-B.; Wang, Q.-Y.; Xiao, Y.-F.; Jiang, X.-F.; Li, Y.; Xiao, L.; Gong, Q. On Chip, High-Sensitivity Thermal Sensor Based on High-Q Polydimethylsiloxane-Coated Microresonator. Appl. Phys. Lett. 2010, 96, 251109. [Google Scholar] [CrossRef]
- Yang, D.-Q.; Chen, J.; Cao, Q.-T.; Duan, B.; Chen, H.-J.; Yu, X.-C.; Xiao, Y.-F. Operando Monitoring Transition Dynamics of Responsive Polymer Using Optofluidic Microcavities. Light Sci. Appl. 2021, 10, 128. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Z.; Zhao, R.; Feng, L. Exceptional Point Engineered Glass Slide for Microscopic Thermal Mapping. Nat. Commun. 2018, 9, 1764. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Yan, S.-C.; Ruan, Y.-P.; Xu, F.; Lu, Y.-Q. Fiber-Optic Point-Based Sensor Using Specklegram Measurement. Sensors 2017, 17, 2429. [Google Scholar] [CrossRef]
- Luo, H.; Sun, Q.; Xu, Z.; Liu, D.; Zhang, L. Simultaneous Measurement of Refractive Index and Temperature Using Multimode Microfiber-Based Dual Mach–Zehnder Interferometer. Opt. Lett. 2014, 39, 4049. [Google Scholar] [CrossRef]
- Wagih, M.; Shi, J.; Li, M.; Komolafe, A.; Whittaker, T.; Schneider, J.; Kumar, S.; Whittow, W.; Beeby, S. Wide-Range Soft Anisotropic Thermistor with a Direct Wireless Radio Frequency Interface. Nat. Commun. 2024, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Katerinopoulou, D.; Zalar, P.; Sweelssen, J.; Kiriakidis, G.; Rentrop, C.; Groen, P.; Gelinck, G.H.; Van Den Brand, J.; Smits, E.C.P. Large-Area All-Printed Temperature Sensing Surfaces Using Novel Composite Thermistor Materials. Adv. Elect. Mater. 2019, 5, 1800605. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, H.; Li, Z.; Cao, Z.; Zhang, H.; Xu, P.; Li, X. Thermocouple-Integrated Resonant Microcantilever for on-Chip Thermogravimetric (TG) and Differential Thermal Analysis (DTA) Dual Characterization Applications. Microsyst. Nanoeng. 2025, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Öztabak, E.; Gökkaya, O.; Ahn, H. A Novel Method of Measuring Microtube Wall Temperatures by Thermocouples to Investigate sCO2 Flows. Appl. Therm. Eng. 2022, 209, 118299. [Google Scholar] [CrossRef]
- Bai, H.; Kim, Y.S.; Shepherd, R.F. Autonomous Self-Healing Optical Sensors for Damage Intelligent Soft-Bodied Systems. Sci. Adv. 2022, 8, eabq2104. [Google Scholar] [CrossRef]
- Zhao, H.; O’Brien, K.; Li, S.; Shepherd, R.F. Optoelectronically Innervated Soft Prosthetic Hand via Stretchable Optical Waveguides. Sci. Robot. 2016, 1, eaai7529. [Google Scholar] [CrossRef]
- Hossain, R.-E.-N.; Lewis, J.; Moore, A.L. In Situ Infrared Temperature Sensing for Real-Time Defect Detection in Additive Manufacturing. Addit. Manuf. 2021, 47, 102328. [Google Scholar] [CrossRef]
- Chen, J.-H.; Li, D.-R.; Xu, F. Optical Microfiber Sensors: Sensing Mechanisms, and Recent Advances. J. Light. Technol. 2019, 37, 2577–2589. [Google Scholar] [CrossRef]
- Grattan, K.T.V.; Sun, T. Fiber Optic Sensor Technology: An Overview. Sens. Actuat. A-Phys. 2000, 82, 40–61. [Google Scholar] [CrossRef]
- Bao, X.; Chen, L. Recent Progress in Distributed Fiber Optic Sensors. Sensors 2012, 12, 8601–8639. [Google Scholar] [CrossRef]
- Leal-Junior, A.; Frizera, A.; Díaz, C.; Marques, C.; Ribeiro, M.; Pontes, M.J. Material Features Based Compensation Technique for the Temperature Effects in a Polymer Diaphragm-Based FBG Pressure Sensor. Opt. Express 2018, 26, 20590. [Google Scholar] [CrossRef]
- Wu, Q.; Cheng, Y.; Huang, X.; Wei, Q.; Chen, J. Operando Strain and Temperature Measurement of Sodium-Ion Batteries via Optical Fiber Sensors. Opt. Commun. 2025, 577, 131428. [Google Scholar] [CrossRef]
- Kou, J.-L.; Ding, M.; Feng, J.; Lu, Y.-Q.; Xu, F.; Brambilla, G. Microfiber-Based Bragg Gratings for Sensing Applications: A Review. Sensors 2012, 12, 8861–8876. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Fu, H.Y.; Qureshi, K.K.; Guan, B.-O.; Tam, H.Y. High-Pressure and High-Temperature Characteristics of a Fabry–Perot Interferometer Based on Photonic Crystal Fiber. Opt. Lett. 2011, 36, 412. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, L.; Lan, X.; Kaur, A.; Huang, J.; Xiao, H. High-Temperature Fiber-Optic Fabry–Perot Interferometric Pressure Sensor Fabricated by Femtosecond Laser. Opt. Lett. 2013, 38, 4609. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.-Y.; Luo, Y.; Zhang, Z.; Zou, X.; Luo, B.; Pan, W.; Yan, L. Sensitivity-Enhanced Temperature Sensor with Cascaded Fiber Optic Sagnac Interferometers Based on Vernier-Effect. Opt. Commun. 2015, 336, 73–76. [Google Scholar] [CrossRef]
- Hou, L.; Zhao, C.; Xu, B.; Mao, B.; Shen, C.; Wang, D.N. Highly Sensitive PDMS-Filled Fabry–Perot Interferometer Temperature Sensor Based on the Vernier Effect. Appl. Opt. 2019, 58, 4858. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Liang, Y.; Li, L.; Masson, J.-F.; Peng, W. Liquid Crystal Filled Surface Plasmon Resonance Thermometer. Opt. Express 2016, 24, 10904. [Google Scholar] [CrossRef]
- Yang, W.; Li, L.; Zhang, S.; Tian, K. Sensitivity-Enhanced Temperature Sensor Based on PDMS-coated Mach–Zehnder Interferometer. Sensors 2025, 25, 1191. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, D.; Li, D.; Chen, X.; Geng, Y.; Li, X. High-Performance Temperature Sensor via Mach-Zehnder Interferometer Based on Exposed-Core Microstructured Fiber Combined with PDMS. Opt. Fiber Technol. 2025, 93, 104284. [Google Scholar] [CrossRef]
- Yang, R.; Tian, J.; Chen, Z.; Yang, X.; Hou, C.; Meng, Y. High Sensitivity Optical Fiber Temperature Sensor Based upon a Polydimethylsiloxane Filled Fabry-Perot Interferometer. Instrum. Sci. Technol. 2024, 53, 255. [Google Scholar] [CrossRef]
- Zhao, C.; Hou, L.; Kang, J.; Mao, B.; Shen, C.; Jin, S. High-Sensitivity Hydraulic Pressure Sensor Based on Fabry-Perot Interferometer Filled with Polydimethylsiloxane Film. Rev. Sci. Instrum. 2019, 90, 095002. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Cheng, Y.; Xiao, Y.; Liu, H.; Yuan, L. A New Preparation Method for an FPI Sensor with a PDMS Film. In Proceedings of the International Conference on Optical and Photonic Engineering (icOPEN 2024), Foshan, China, 15–18 November 2024; Di, J., Qian, K., Feng, S., Zhou, J., Zou, X., Wang, H., Zuo, C., Eds.; SPIE: Foshan, China, 2025; p. 9. [Google Scholar]
- Müller, A.; Wapler, M.C.; Wallrabe, U. A Quick and Accurate Method to Determine the Poisson’s Ratio and the Coefficient of Thermal Expansion of PDMS. Soft Matter 2019, 15, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, L.; Liu, Z.; Zhang, Y.; Zhang, Y. Surface-Plasmon-Resonance-Based Optical-Fiber Temperature Sensor with High Sensitivity and High Figure of Merit. Opt. Lett. 2017, 42, 2948. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Yu, M.; Liu, J.; Tan, Y.; Chen, J. Self-Adaptive Polymer Fabry–Pérot Thermometer for High-Sensitivity and Wide-Linear-Range Sensing. Biosensors 2025, 15, 602. https://doi.org/10.3390/bios15090602
Cheng Y, Yu M, Liu J, Tan Y, Chen J. Self-Adaptive Polymer Fabry–Pérot Thermometer for High-Sensitivity and Wide-Linear-Range Sensing. Biosensors. 2025; 15(9):602. https://doi.org/10.3390/bios15090602
Chicago/Turabian StyleCheng, Yifan, Maolin Yu, Junjie Liu, Yingling Tan, and Jinhui Chen. 2025. "Self-Adaptive Polymer Fabry–Pérot Thermometer for High-Sensitivity and Wide-Linear-Range Sensing" Biosensors 15, no. 9: 602. https://doi.org/10.3390/bios15090602
APA StyleCheng, Y., Yu, M., Liu, J., Tan, Y., & Chen, J. (2025). Self-Adaptive Polymer Fabry–Pérot Thermometer for High-Sensitivity and Wide-Linear-Range Sensing. Biosensors, 15(9), 602. https://doi.org/10.3390/bios15090602





