Advances and Innovations in Conjugated Polymer Fluorescent Sensors for Environmental and Biological Detection
Abstract
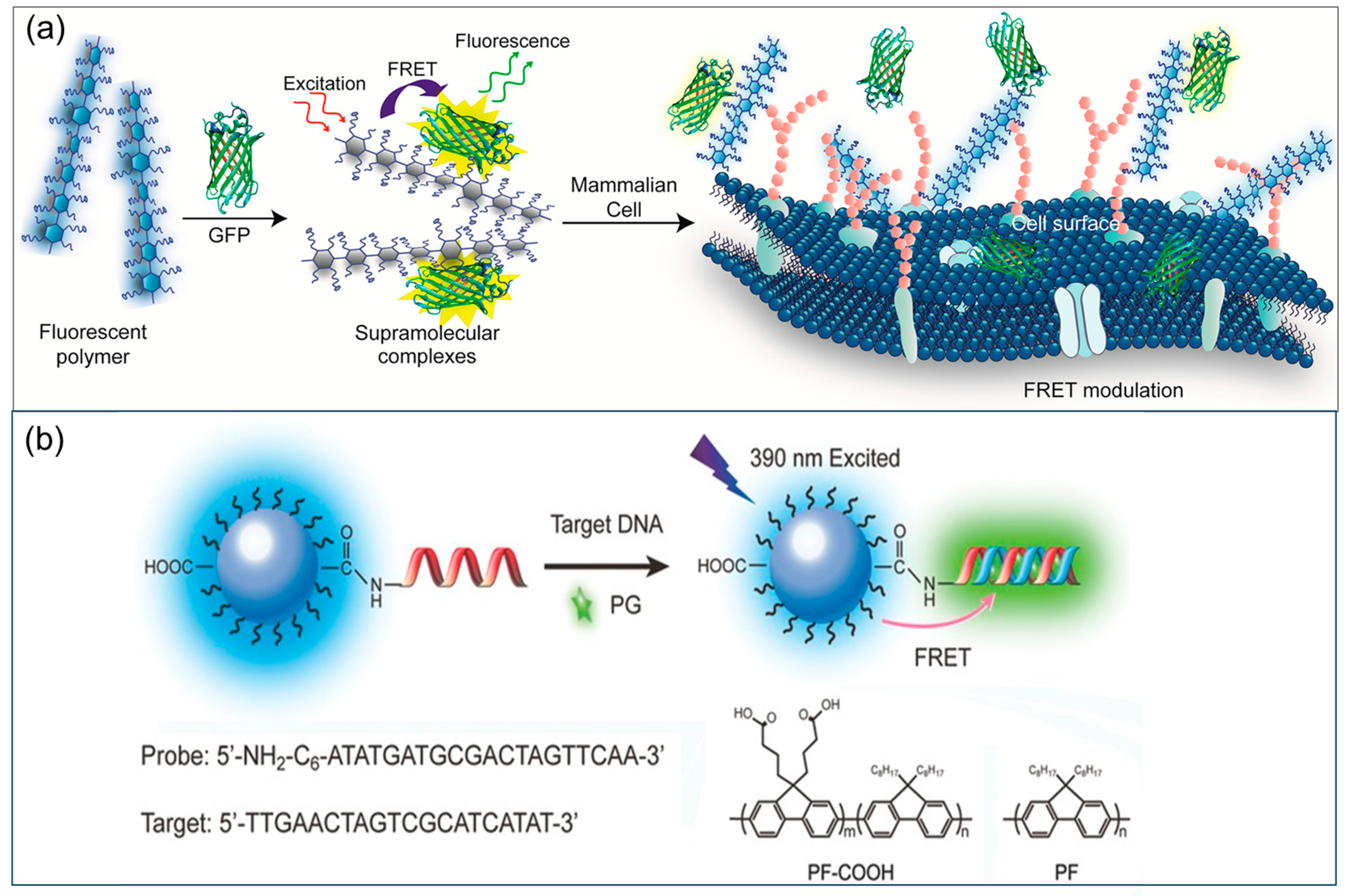
1. Introduction
2. Fluorescent Properties of Conjugated Polymers
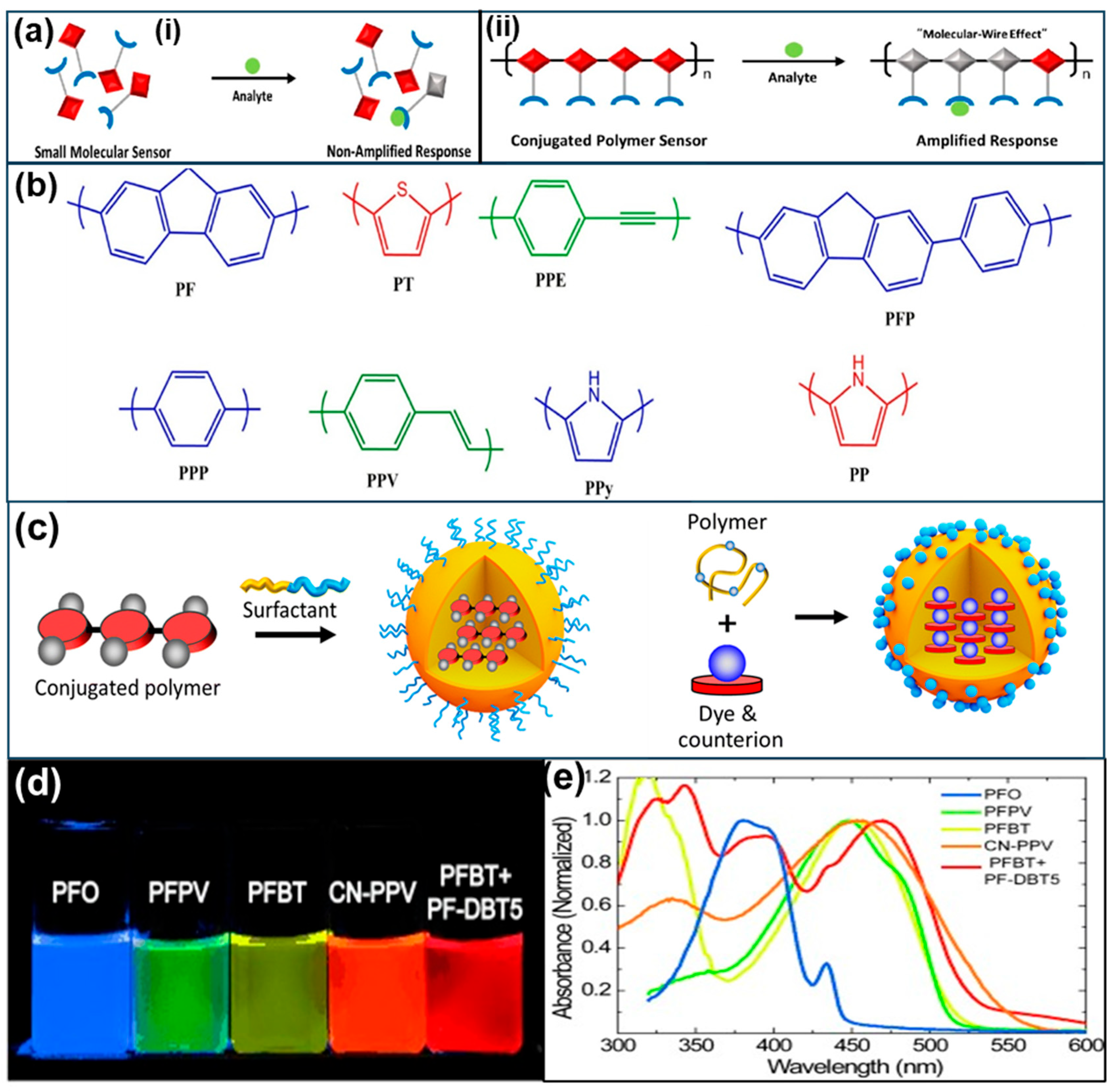
3. Working Mechanism of Conjugated Polymer Fluorescent Sensors
4. Advantages and Disadvantages of Conjugated Polymers in Fluorescent Sensors
5. Recent Advances and Innovations in CP-Based Fluorescent Sensors in Environmental and Biological Applications
5.1. Conjugated Polymer-Based Fluorescent Probes for Detection of Metal Ions
5.2. Conjugated Polymer-Based Fluorescent Sensors for Detection of Explosives

| CPs | Types of Sensors | Explosives | LOD | Refs |
|---|---|---|---|---|
| Poly [2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] | Fluorescence quenching sensor | 1,4-dinitrobenzene | 6.3 ppm | [145] |
| PDY-132 (Super Yellow) * | Fluorescence sensor | 2,4-DNT | 8.17 μM | [134] |
| TBP–BB * | Fluorescence probe | 2,4,6-trinitrophenol | 0.1 mM | [137] |
| Poly(9,9-dioctylfluorene2,7-diyl)-co-bisthiophene | Handheld explosive sensor | Nitrobenzene | 17 μM | [146] |
| Oligo(p-phenylenevinylene) gelator | Fluorescence sensor | Trinitrotoluene (TNT) Dinitrotoluene (DNT) | 5 ppb | [136] |
| Fuorene-based CPs | Colorimetric and fluorometric sensor | 2,4,6-trinitrophenol (TNP) | 3.2 pM | [147] |
| Poly(3,3′-((2-phenyl-9H-fluorene-9,9-diyl)bis(hexane-6,1-diyl))bis(1-methyl-1H-imidazol-3-ium)bromide) | Fluorescence sensor | Nitroexplosive picric acid | 30.9 pM | [140] |
| Poly(aryleneethynylenesiloles) | Fluorescent chemosensors | Picric acid TNT | 10 μM | [148] |
| Folded tetraphenylethene | Fluorescent chemosensors | Picric acid | 0.57 mM | [149] |
| Conjugated microporous polymers | Fluorescent chemosensors | TNT | 5.85 μM | [138] |
| 9,9-dioctyl-2,7-dibromofluorene | Fluorescent chemosensors | TNT | 1.6 μM | [150] |
| Polycarbazole | Fluorescent chemosensors | TNT DNT | 5 ppb 100 ppb | [151] |
| Poly(dichloromethylphenylsilane) | Fluorescent chemosensors | TNT | 1.76 μM | [152] |
| Poly(2,3,5,6-styrylpyrazine) | Fluorescent quenching sensor | TNT | 1.76 μM | [144] |
| Conjugated porous polymer (CPP) | Fluorescent quenching sensor | TNT | 5 ppb | [153] |
| Hyperbranched conjugated polymer nanoparticle | Fluorescent chemosensors | TNT | 3.7 nM | [143] |
| Poly(1,4-divinyl-2,5-dioctyloxybenzene-alt-5,8-dibromo-2,3-diphenylquinoxaline) | Fluorescent quenching sensor | TNT | 5 ppm | [154] |
| Donor–acceptor polymer | Fluorescent quenching sensor | Picric acid TNT | 2 ppb 23 ppb | [155] |
| 2,2’-biimidazole-containing conjugated polymer | Fluorescent quenching sensor | Picric acid | 0.51 μM | [156] |
| Di(naphthalen-2-yl)-1,2-diphenylethene | Fluorescent chemosensors | Picric acid | 0.181 μM | [157] |
| Poly [4,4′-(((2-phenyl-9H-fluorene-9,9-diyl)bis(hexane-6,1-diyl))bis(oxy))dianiline)] | Fluorescent chemosensors | Picric acid | 57.8 nM | [158] |
| PTPA-TPE | Fluorescent quenching sensor | Picric acid | 72 ppb | [159] |
| Polybenzimidazole | Fluorescent quenching sensor | DNT | 3.3 ppb | [160] |
| Polycarbazole | Fluorescent quenching sensor | DNT | - | [161] |
| 2,7-dibromo-9,9-dihexylfluorene | Fluorescent quenching sensor | DNT | 0.4 mM | [162] |
| 1,1,2,2-tetrakis(4-formyl-(1,1′-biphenyl))-ethane | Fluorescent quenching sensor | TNP | 0.09 μM | [163] |
| Poly(triphenylamine-co-benzothiadiazole)s | Fluorescent chemosensors | 1,3,5-trinitrobenzene (TNB) | 19 μM | [164] |
| Polycarbazoles | Fluorescent quenching sensor | TNB | 50 nM | [165] |
5.3. Conjugated Polymer-Based Fluorescent Sensors for Detection of Pathogens
5.4. Conjugated Polymer-Based Fluorescent Sensors for Detection of Other Biomolecules
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Murfin, L.C.; Wu, L.; Lewis, S.E.; James, T.D. Fluorescent small organic probes for biosensing. Chem. Sci. 2021, 12, 3406–3426. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ding, L.; Lv, F. Surfactant assemblies encapsulating fluorescent probes as selective and discriminative sensors for metal ions. Coord. Chem. Rev. 2021, 432, 213696. [Google Scholar] [CrossRef]
- Xu, W.; Ren, C.; Teoh, C.L.; Peng, J.; Gadre, S.H.; Rhee, H.-W.; Lee, C.-L.K.; Chang, Y.-T. An Artificial Tongue Fluorescent Sensor Array for Identification and Quantitation of Various Heavy Metal Ions. Anal. Chem. 2014, 86, 8763–8769. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Bencini, A.; Blake, A.J.; Caltagirone, C.; De Filippo, G.; Devillanova, F.A.; Garau, A.; Gelbrich, T.; Hursthouse, M.B.; et al. Tuning the Selectivity/Specificity of Fluorescent Metal Ion Sensors Based on N2S2 Pyridine-Containing Macrocyclic Ligands by Changing the Fluorogenic Subunit: Spectrofluorimetric and Metal Ion Binding Studies. Inorg. Chem. 2007, 46, 4548–4559. [Google Scholar] [CrossRef]
- Lake, R.J.; Yang, Z.; Zhang, J.; Lu, Y. DNAzymes as Activity-Based Sensors for Metal Ions: Recent Applications, Demonstrated Advantages, Current Challenges, and Future Directions. Acc. Chem. Res. 2019, 52, 3275–3286. [Google Scholar] [CrossRef]
- Reimann, M.J.; Salmon, D.R.; Horton, J.T.; Gier, E.C.; Jefferies, L.R. Water-Soluble Sulfonate Schiff-Base Ligands as Fluorescent Detectors for Metal Ions in Drinking Water and Biological Systems. ACS Omega 2019, 4, 2874–2882. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F. Luminescent metal-organic frameworks as potential sensory materials for various environmental toxic agents. Coord. Chem. Rev. 2019, 401, 213065. [Google Scholar] [CrossRef]
- Shanmugaraju, S.; Mukherjee, P.S. π-Electron rich small molecule sensors for the recognition of nitroaromatics. Chem. Commun. 2015, 51, 16014–16032. [Google Scholar] [CrossRef]
- Peng, J.; Li, J.; Xu, W.; Wang, L.; Su, D.; Teoh, C.L.; Chang, Y.-T. Silica Nanoparticle-Enhanced Fluorescent Sensor Array for Heavy Metal Ions Detection in Colloid Solution. Anal. Chem. 2018, 90, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.V.; Lee, Y.-C. Electrospray technique for preparation of core-shell materials: A mini-review. Part. Aerosol Res. 2018, 14, 49–63. [Google Scholar] [CrossRef]
- Lin, Z.-Y.; Xue, S.-F.; Chen, Z.-H.; Han, X.-Y.; Shi, G.; Zhang, M. Bioinspired Copolymers Based Nose/Tongue-Mimic Chemosensor for Label-Free Fluorescent Pattern Discrimination of Metal Ions in Biofluids. Anal. Chem. 2018, 90, 8248–8253. [Google Scholar] [CrossRef]
- Hussain, S.; Zhu, C.; Yue, Z.; Hao, Y.; Gao, R.; Wei, J. Rational design of signal amplifying fluorescent conjugated polymers for environmental monitoring applications: Recent advances and perspectives. Coord. Chem. Rev. 2024, 499, 215480. [Google Scholar] [CrossRef]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Sai-Anand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles: Preparation, properties, functionalization and biological applications. Chem. Soc. Rev. 2013, 42, 6620–6633. [Google Scholar] [CrossRef]
- Zhou, H.; Chua, M.H.; Tang, B.Z.; Xu, J. Aggregation-induced emission (AIE)-active polymers for explosive detection. Polym. Chem. 2019, 10, 3822–3840. [Google Scholar] [CrossRef]
- Wu, W.; Bazan, G.C.; Liu, B. Conjugated-Polymer-Amplified Sensing, Imaging, and Therapy. Chem 2017, 2, 760–790. [Google Scholar] [CrossRef]
- Wang, B.; Queenan, B.N.; Wang, S.; Nilsson, K.P.R.; Bazan, G.C. Precisely Defined Conjugated Oligoelectrolytes for Biosensing and Therapeutics. Adv. Mater. 2019, 31, 1806701. [Google Scholar] [CrossRef]
- Sakai, R. Conjugated polymers applicable to colorimetric and fluorescent anion detection. Polym. J. 2016, 48, 59–65. [Google Scholar] [CrossRef]
- Rochat, S.; Swager, T.M. Conjugated Amplifying Polymers for Optical Sensing Applications. ACS Appl. Mater. Interfaces 2013, 5, 4488–4502. [Google Scholar] [CrossRef]
- Zeglio, E.; Rutz, A.L.; Winkler, T.E.; Malliaras, G.G.; Herland, A. Conjugated Polymers for Assessing and Controlling Biological Functions. Adv. Mater. 2019, 31, 1806712. [Google Scholar] [CrossRef]
- Van Tran, V.; Phung, V.-D.; Do, H.H. Morphological advances and innovations in conjugated polymer films for high-performance gas sensors. Talanta 2025, 292, 127904. [Google Scholar] [CrossRef] [PubMed]
- Drumm, D.W.; Bilic, A.; Tachibana, Y.; Miller, A.; Russo, S.P. Optical properties of a conjugated-polymer-sensitised solar cell: The effect of interfacial structure. Phys. Chem. Chem. Phys. 2015, 17, 14489–14494. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Eshbaugh, A.A.; Schanze, K.S. Low-Bandgap Donor−Acceptor Conjugated Polymer Sensitizers for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2011, 133, 3063–3069. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamamoto, S.-i.; Koizumi, T. Effects of molecular weight on the optical and electrochemical properties of EDOT-based π-conjugated polymers. Sci. Rep. 2017, 7, 1078. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Albinsson, B. Photoinduced charge and energy transfer in molecular wires. Chem. Soc. Rev. 2015, 44, 845–862. [Google Scholar] [CrossRef]
- Jana, R.; Pathak, T.P.; Sigman, M.S. Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners. Chem. Rev. 2011, 111, 1417–1492. [Google Scholar] [CrossRef]
- Zani, L.; Dessì, A.; Franchi, D.; Calamante, M.; Reginato, G.; Mordini, A. Transition metal-catalyzed cross-coupling methodologies for the engineering of small molecules with applications in organic electronics and photovoltaics. Coord. Chem. Rev. 2019, 392, 177–236. [Google Scholar] [CrossRef]
- Alvarez, A.; Costa-Fernández, J.M.; Pereiro, R.; Sanz-Medel, A.; Salinas-Castillo, A. Fluorescent conjugated polymers for chemical and biochemical sensing. TrAC Trends Anal. Chem. 2011, 30, 1513–1525. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, H.; Schanze, K.S. Polymer Chain Length Dependence of Amplified Fluorescence Quenching in Conjugated Polyelectrolytes. Macromolecules 2008, 41, 3422–3428. [Google Scholar] [CrossRef]
- Rothberg, L.J.; Yan, M.; Papadimitrakopoulos, F.; Galvin, M.E.; Kwock, E.W.; Miller, T.M. Photophysics of phenylenevinylene polymers. Synth. Met. 1996, 80, 41–58. [Google Scholar] [CrossRef]
- Wu, C.; Chiu, D.T. Highly Fluorescent Semiconducting Polymer Dots for Biology and Medicine. Angew. Chem. Int. Ed. 2013, 52, 3086–3109. [Google Scholar] [CrossRef] [PubMed]
- Ashoka, A.H.; Aparin, I.O.; Reisch, A.; Klymchenko, A.S. Brightness of fluorescent organic nanomaterials. Chem. Soc. Rev. 2023, 52, 4525–4548. [Google Scholar] [CrossRef]
- Wu, C.; Szymanski, C.; McNeill, J. Preparation and Encapsulation of Highly Fluorescent Conjugated Polymer Nanoparticles. Langmuir 2006, 22, 2956–2960. [Google Scholar] [CrossRef]
- Wu, C.; Bull, B.; Szymanski, C.; Christensen, K.; McNeill, J. Multicolor Conjugated Polymer Dots for Biological Fluorescence Imaging. ACS Nano 2008, 2, 2415–2423. [Google Scholar] [CrossRef]
- Tian, Z.; Yu, J.; Wu, C.; Szymanski, C.; McNeill, J. Amplified energy transfer in conjugated polymer nanoparticle tags and sensors. Nanoscale 2010, 2, 1999–2011. [Google Scholar] [CrossRef]
- Jiang, Y.; McNeill, J. Light-Harvesting and Amplified Energy Transfer in Conjugated Polymer Nanoparticles. Chem. Rev. 2017, 117, 838–859. [Google Scholar] [CrossRef]
- Xiao, F.; Fang, X.; Li, H.; Xue, H.; Wei, Z.; Zhang, W.; Zhu, Y.; Lin, L.; Zhao, Y.; Wu, C.; et al. Light-Harvesting Fluorescent Spherical Nucleic Acids Self-Assembled from a DNA-Grafted Conjugated Polymer for Amplified Detection of Nucleic Acids. Angew. Chem. Int. Ed. 2022, 61, e202115812. [Google Scholar] [CrossRef]
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional nanoparticles through π-conjugated polymer self-assembly. Nat. Rev. Mater. 2021, 6, 7–26. [Google Scholar] [CrossRef]
- Ashoka, A.H.; Kong, S.-H.; Seeliger, B.; Andreiuk, B.; Soares, R.V.; Barberio, M.; Diana, M.; Klymchenko, A.S. Near-infrared fluorescent coatings of medical devices for image-guided surgery. Biomaterials 2020, 261, 120306. [Google Scholar] [CrossRef]
- Huang, H.; Wang, K.; Tan, W.; An, D.; Yang, X.; Huang, S.; Zhai, Q.; Zhou, L.; Jin, Y. Design of a Modular-Based Fluorescent Conjugated Polymer for Selective Sensing. Angew. Chem. Int. Ed. 2004, 43, 5635–5638. [Google Scholar] [CrossRef]
- Jiang, J.; Li, S.; Yan, Y.; Fang, M.; Chen, M.; Peng, K.; Nie, L.; Feng, Y.; Wang, X. Pendant structure governed the selectivity of Pd2+ using disubstituted polyacetylenes with sulfur functions and the application of thiophanate-methyl detection. Sens. Actuators B Chem. 2017, 247, 36–45. [Google Scholar] [CrossRef]
- Hussain, S.; Malik, A.H.; Iyer, P.K. FRET-assisted selective detection of flavins via cationic conjugated polyelectrolyte under physiological conditions. J. Mater. Chem. B 2016, 4, 4439–4446. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Yuan, H.; Xu, S.; An, H.; Niu, R.; Zhan, Y. Nucleobase-Functionalized Conjugated Polymer for Detection of Copper(II). ACS Appl. Mater. Interfaces 2014, 6, 9601–9607. [Google Scholar] [CrossRef]
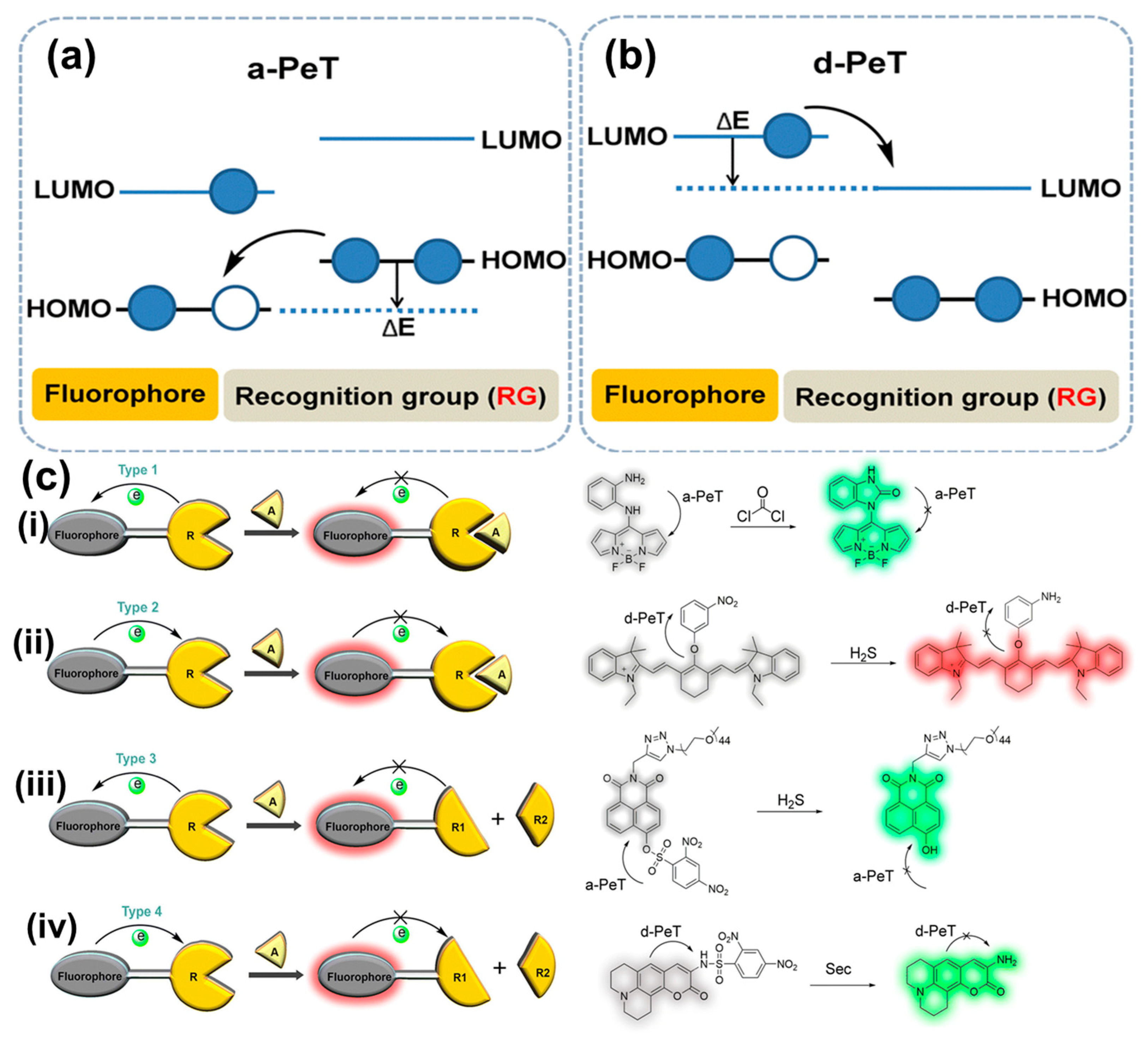
- Daly, B.; Ling, J.; de Silva, A.P. Current developments in fluorescent PET (photoinduced electron transfer) sensors and switches. Chem. Soc. Rev. 2015, 44, 4203–4211. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wei, Y.; Zhu, C.; Cao, J.; Han, D.-M. Ratiometric fluorescent signals-driven smartphone-based portable sensors for onsite visual detection of food contaminants. Coord. Chem. Rev. 2022, 458, 214442. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.-L.; Wang, J.-H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef]
- Ma, X.; Sun, R.; Cheng, J.; Liu, J.; Gou, F.; Xiang, H.; Zhou, X. Fluorescence Aggregation-Caused Quenching versus Aggregation-Induced Emission: A Visual Teaching Technology for Undergraduate Chemistry Students. J. Chem. Educ. 2016, 93, 345–350. [Google Scholar] [CrossRef]
- Escudero, D. Revising Intramolecular Photoinduced Electron Transfer (PET) from First-Principles. Acc. Chem. Res. 2016, 49, 1816–1824. [Google Scholar] [CrossRef]
- Niu, H.; Liu, J.; O’Connor, H.M.; Gunnlaugsson, T.; James, T.D.; Zhang, H. Photoinduced electron transfer (PeT) based fluorescent probes for cellular imaging and disease therapy. Chem. Soc. Rev. 2023, 52, 2322–2357. [Google Scholar] [CrossRef]
- de Silva, A.P.; Moody, T.S.; Wright, G.D. Fluorescent PET (Photoinduced Electron Transfer) sensors as potent analytical tools. Analyst 2009, 134, 2385–2393. [Google Scholar] [CrossRef]
- Xia, H.-C.; Xu, X.-H.; Song, Q.-H. BODIPY-Based Fluorescent Sensor for the Recognization of Phosgene in Solutions and in Gas Phase. Anal. Chem. 2017, 89, 4192–4197. [Google Scholar] [CrossRef]
- Wang, R.; Yu, F.; Chen, L.; Chen, H.; Wang, L.; Zhang, W. A highly selective turn-on near-infrared fluorescent probe for hydrogen sulfide detection and imaging in living cells. Chem. Commun. 2012, 48, 11757–11759. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, J.; Wang, Z.; Tao, F.; Yang, L.; Yuan, G.; Sun, W.; Zhang, X. A color turn-on fluorescent probe for real-time detection of hydrogen sulfide and identification of food spoilage. Chem. Commun. 2021, 57, 5012–5015. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, S.-H.; Wu, L.; Dong, Q.-J.; Yang, W.-C.; Yang, G.-F. Detection of Intracellular Selenol-Containing Molecules Using a Fluorescent Probe with Near-Zero Background Signal. Anal. Chem. 2016, 88, 6084–6091. [Google Scholar] [CrossRef]
- Yang, L.; Niu, J.-Y.; Sun, R.; Xu, Y.-J.; Ge, J.-F. The pH-influenced PET processes between pyronine and different heterocycles. Org. Biomol. Chem. 2017, 15, 8402–8409. [Google Scholar] [CrossRef]
- Yang, X.; Wang, K.; Guo, C. A fluorescent optode for sodium ion based on the inner filter effect. Anal. Chim. Acta 2000, 407, 45–52. [Google Scholar] [CrossRef]
- Neema, P.M.; Tomy, A.M.; Cyriac, J. Chemical sensor platforms based on fluorescence resonance energy transfer (FRET) and 2D materials. TrAC Trends Anal. Chem. 2020, 124, 115797. [Google Scholar] [CrossRef]
- Ganiga, M.; Cyriac, J. FRET based ammonia sensor using carbon dots. Sens. Actuators B Chem. 2016, 225, 522–528. [Google Scholar] [CrossRef]
- Murphy, C.B.; Zhang, Y.; Troxler, T.; Ferry, V.; Martin, J.J.; Jones, W.E. Probing Förster and Dexter Energy-Transfer Mechanisms in Fluorescent Conjugated Polymer Chemosensors. J. Phys. Chem. B 2004, 108, 1537–1543. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, D.; Duan, L. Modulation of Förster and Dexter Interactions in Single-Emissive-Layer All-Fluorescent WOLEDs for Improved Efficiency and Extended Lifetime. Adv. Funct. Mater. 2020, 30, 1907083. [Google Scholar] [CrossRef]
- Chan, C.-Y.; Tanaka, M.; Lee, Y.-T.; Wong, Y.-W.; Nakanotani, H.; Hatakeyama, T.; Adachi, C. Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission. Nat. Photonics 2021, 15, 203–207. [Google Scholar] [CrossRef]
- Jeon, C.Y.; Palanisamy, P.; Lee, H.S.; Lee, H.; Kim, H.U.; Chae, M.Y.; Kwon, J.H. Stable Thermally Activated Delayed Fluorescence-Sensitized Red Fluorescent Devices through Physical Suppression of Dexter Energy Transfer. Adv. Mater. Interfaces 2023, 10, 2300147. [Google Scholar] [CrossRef]
- Speiser, S. Photophysics and Mechanisms of Intramolecular Electronic Energy Transfer in Bichromophoric Molecular Systems: Solution and Supersonic Jet Studies. Chem. Rev. 1996, 96, 1953–1976. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Zhu, L.; Liu, Z.; Lin, W. Through bond energy transfer (TBET)-based fluorescent chemosensors. J. Photochem. Photobiol. C Photochem. Rev. 2020, 44, 100371. [Google Scholar] [CrossRef]
- Qi, J.; Hu, X.; Dong, X.; Lu, Y.; Lu, H.; Zhao, W.; Wu, W. Towards more accurate bioimaging of drug nanocarriers: Turning aggregation-caused quenching into a useful tool. Adv. Drug Deliv. Rev. 2019, 143, 206–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, M.-H. Recent Progress in Fluorescent Vesicles with Aggregation-induced Emission. Chin. J. Polym. Sci. 2019, 37, 352–371. [Google Scholar] [CrossRef]
- Wang, J.; Lv, F.; Liu, L.; Ma, Y.; Wang, S. Strategies to design conjugated polymer based materials for biological sensing and imaging. Coord. Chem. Rev. 2018, 354, 135–154. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Zhang, Y.; Fan, J.; Wang, C.-K.; Lin, L. Theoretical Study of the Mechanism of Aggregation-Caused Quenching in Near-Infrared Thermally Activated Delayed Fluorescence Molecules: Hydrogen-Bond Effect. J. Phys. Chem. C 2019, 123, 24705–24713. [Google Scholar] [CrossRef]
- Cai, Y.; Ji, X.; Zhang, Y.; Liu, C.; Zhang, Z.; Lv, Y.; Dong, X.; He, H.; Qi, J.; Lu, Y.; et al. Near-infrared fluorophores with absolute aggregation-caused quenching and negligible fluorescence re-illumination for in vivo bioimaging of nanocarriers. Aggregate 2023, 4, e277. [Google Scholar] [CrossRef]
- Pak, Y.L.; Wang, Y.; Xu, Q. Conjugated polymer based fluorescent probes for metal ions. Coord. Chem. Rev. 2021, 433, 213745. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal–organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, V.; Park, D.; Lee, Y.-C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Tran, V.V.; Phung, V.-D.; Do, H.H. Advances and innovations in hydrogel particles for sustainable purification of contaminants in aqueous solutions. Chem. Eng. J. 2024, 486, 150324. [Google Scholar] [CrossRef]
- Chen, Q.Y.; DesMarais, T.; Costa, M. Metals and Mechanisms of Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 537–554. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Lee, J.; Pyo, M.; Lee, S.-h.; Kim, J.; Ra, M.; Kim, W.-Y.; Park, B.J.; Lee, C.W.; Kim, J.-M. Hydrochromic conjugated polymers for human sweat pore mapping. Nat. Commun. 2014, 5, 3736. [Google Scholar] [CrossRef]
- Wu, Y.-X.; Li, J.-B.; Liang, L.-H.; Lu, D.-Q.; Zhang, J.; Mao, G.-J.; Zhou, L.-Y.; Zhang, X.-B.; Tan, W.; Shen, G.-L.; et al. A rhodamine-appended water-soluble conjugated polymer: An efficient ratiometric fluorescence sensing platform for intracellular metal-ion probing. Chem. Commun. 2014, 50, 2040–2042. [Google Scholar] [CrossRef]
- Auria-Luna, F.; Foss, F.W.; Molina-Canteras, J.; Velazco-Cabral, I.; Marauri, A.; Larumbe, A.; Aparicio, B.; Vázquez, J.L.; Alberro, N.; Arrastia, I.; et al. Supramolecular chemistry in solution and solid–gas interfaces: Synthesis and photophysical properties of monocolor and bicolor fluorescent sensors for barium tagging in neutrinoless double beta decay. RSC Appl. Interfaces 2025, 2, 185–199. [Google Scholar] [CrossRef]
- Roja, S.S.; Sneha Sunil, P.; Darussalam, M.M.; Manoharan, V.; Prakash, J.; Ranjith Kumar, R. Benzo [4,5]imidazole[2,1-b]quinazoline-1(2H)-one: An efficient fluorescent probe for the selective and sensitive detection of Cu(II) ions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 333, 125853. [Google Scholar] [CrossRef]
- Kim, B.; Jung, I.H.; Kang, M.; Shim, H.-K.; Woo, H.Y. Cationic Conjugated Polyelectrolytes-Triggered Conformational Change of Molecular Beacon Aptamer for Highly Sensitive and Selective Potassium Ion Detection. J. Am. Chem. Soc. 2012, 134, 3133–3138. [Google Scholar] [CrossRef] [PubMed]
- Le, V.S.; Kim, B.; Lee, W.; Jeong, J.-E.; Yang, R.; Woo, H.Y. Ratiometric Fluorescent Ion Detection in Water with High Sensitivity via Aggregation-Mediated Fluorescence Resonance Energy Transfer Using a Conjugated Polyelectrolyte as an Optical Platform. Macromol. Rapid Commun. 2013, 34, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.L.; Jeong, J.-E.; Jung, I.H.; Kim, B.; Le, V.S.; Kim, I.; Kyhm, K.; Woo, H.Y. Conjugated Polyelectrolyte and Aptamer Based Potassium Assay via Single- and Two-Step Fluorescence Energy Transfer with a Tunable Dynamic Detection Range. Adv. Funct. Mater. 2014, 24, 1748–1757. [Google Scholar] [CrossRef]
- Giovannitti, A.; Nielsen, C.B.; Rivnay, J.; Kirkus, M.; Harkin, D.J.; White, A.J.P.; Sirringhaus, H.; Malliaras, G.G.; McCulloch, I. Sodium and Potassium Ion Selective Conjugated Polymers for Optical Ion Detection in Solution and Solid State. Adv. Funct. Mater. 2016, 26, 514–523. [Google Scholar] [CrossRef]
- Senthilkumar, T.; Parekh, N.; Nikam, S.B.; Asha, S.K. Orientation effect induced selective chelation of Fe2+ to a glutamic acid appended conjugated polymer for sensing and live cell imaging. J. Mater. Chem. B 2016, 4, 299–308. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; Saikia, G.; Iyer, P.K. Aqueous polyfluorene probe for the detection and estimation of Fe3+ and inorganic phosphate in blood serum. J. Mater. Chem. 2011, 21, 2502–2507. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, B.; Wen, Y.; Lu, L.; Xu, J. Facile fabrication of a cost-effective, water-soluble, and electrosynthesized poly(9-aminofluorene) fluorescent sensor for the selective and sensitive detection of Fe(III) and inorganic phosphates. Sens. Actuators B Chem. 2012, 171–172, 786–794. [Google Scholar] [CrossRef]
- Li, L.; He, F.; Wang, X.; Ma, N.; Li, L. Reversible pH-Responsive Fluorescence of Water-Soluble Polyfluorenes and Their Application in Metal Ion Detection. ACS Appl. Mater. Interfaces 2012, 4, 4927–4933. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, G.; Xu, J.; Wen, Y.; Lu, B.; Zhang, J.; Ding, W. Novel highly selective fluorescent sensor based on electrosynthesized poly(9-fluorenecarboxylic acid) for efficient and practical detection of iron(III) and its agricultural application. Sens. Actuators B Chem. 2016, 230, 123–129. [Google Scholar] [CrossRef]
- Ding, W.; Xu, J.; Wen, Y.; Zhang, H.; Zhang, J. Facile fabrication of fluorescent poly(5-cyanoindole) thin film sensor via electropolymerization for detection of Fe3+ in aqueous solution. J. Photochem. Photobiol. A Chem. 2016, 314, 22–28. [Google Scholar] [CrossRef]
- Huang, S.; Du, P.; Min, C.; Liao, Y.; Sun, H.; Jiang, Y. Poly(1-amino-5-chloroanthraquinone): Highly Selective and Ultrasensitive Fluorescent Chemosensor For Ferric Ion. J. Fluoresc. 2013, 23, 621–627. [Google Scholar] [CrossRef]
- Wang, H.; He, F.; Yan, R.; Wang, X.; Zhu, X.; Li, L. Citrate-Induced Aggregation of Conjugated Polyelectrolytes for Al3+-Ion-Sensing Assays. ACS Appl. Mater. Interfaces 2013, 5, 8254–8259. [Google Scholar] [CrossRef]
- Dong, W.; Wu, H.; Chen, M.; Shi, Y.; Sun, J.; Qin, A.; Tang, B.Z. Anionic conjugated polytriazole: Direct preparation, aggregation-enhanced emission, and highly efficient Al3+ sensing. Polym. Chem. 2016, 7, 5835–5839. [Google Scholar] [CrossRef]
- Tu, M.-C.; Rajwar, D.; Ammanath, G.; Alagappan, P.; Yildiz, U.H.; Liedberg, B. Visual detection of Al3+ ions using conjugated copolymer-ATP supramolecular complex. Anal. Chim. Acta 2016, 912, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Su, H.-C.; Chen, Y. Synthesis and chemosensory application of water-soluble polyfluorenes containing carboxylated groups. Org. Biomol. Chem. 2014, 12, 5682–5690. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Feng, X.; Tang, H.; Liu, L.; Yang, Q.; Wang, S. Development of Film Sensors Based on Conjugated Polymers for Copper (II) Ion Detection. Adv. Funct. Mater. 2011, 21, 845–850. [Google Scholar] [CrossRef]
- Álvarez-Diaz, A.; Salinas-Castillo, A.; Camprubí-Robles, M.; Costa-Fernández, J.M.; Pereiro, R.; Mallavia, R.; Sanz-Medel, A. Conjugated Polymer Microspheres for “Turn-Off”/“Turn-On” Fluorescence Optosensing of Inorganic Ions in Aqueous Media. Anal. Chem. 2011, 83, 2712–2718. [Google Scholar] [CrossRef]
- Lou, X.; Zhang, Y.; Li, S.; Ou, D.; Wan, Z.; Qin, J.; Li, Z. A new polyfluorene bearing pyridine moieties: A sensitive fluorescent chemosensor for metal ions and cyanide. Polym. Chem. 2012, 3, 1446–1452. [Google Scholar] [CrossRef]
- Chakraborty, C.; Singh, P.; Maji, S.K.; Malik, S. Conjugated Polyfluorene-based Reversible Fluorescent Sensor for Cu(II) and Cyanide Ions in Aqueous Medium. Chem. Lett. 2013, 42, 1355–1357. [Google Scholar] [CrossRef]
- Guan, M.; Xu, C.; Ma, J.; Yang, T.; Liu, J.; Feng, G. A Conjugated Polymer Fluorescent Sensor for Continuous Identification of Copper(II) and Pyrophosphate in Blood Serum and Synovial Fluid. Anal. Sci. 2019, 35, 625–630. [Google Scholar] [CrossRef]
- Qian, J.; Wu, D.; Cai, P.; Xia, J. Nitrogen atom free polythiophene derivative as an efficient chemosensor for highly selective and sensitive Cu2+ and Ag+ detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 218, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, J.; Guo, C.; Pei, M.; Zhang, G. Simple hydrazide-based fluorescent sensors for highly sensitive and selective optical signaling of Cu2+ and Hg2+ in aqueous solution. Sens. Actuators B Chem. 2014, 193, 157–165. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Zhang, Y.; Sun, J.; Cao, L.; Ji, J.; Feng, F. Facile crosslinking of polythiophenes by polyethylenimine via ester aminolysis for selective Cu(II) detection in water. Biosens. Bioelectron. 2018, 109, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, H.; Zhang, J.; Ding, W.; Xu, J.; Wen, Y. Highly selective fluorescent sensor based on electrosynthesized oligo(1-pyreneboronic acid) enables ultra-trace analysis of Cu2+ in environment and agro-product samples. Sens. Actuators B Chem. 2017, 253, 224–230. [Google Scholar] [CrossRef]
- Sil, A.; Islam, S.N.; Patra, S.K. Terpyridyl appended poly(metaphenylene-alt-fluorene) π-conjugated fluorescent polymers: Highly selective and sensitive turn off probes for the detection of Cu2+. Sens. Actuators B Chem. 2018, 254, 618–628. [Google Scholar] [CrossRef]
- Liu, B.; Dai, H.; Bao, Y.; Du, F.; Tian, J.; Bai, R. 2,6-Substituted pyridine derivative-containing conjugated polymers: Synthesis, photoluminescence and ion-sensing properties. Polym. Chem. 2011, 2, 1699–1705. [Google Scholar] [CrossRef]
- Zhang, G.; Wen, Y.; Guo, C.; Xu, J.; Lu, B.; Duan, X.; He, H.; Yang, J. A cost-effective and practical polybenzanthrone-based fluorescent sensor for efficient determination of palladium (II) ion and its application in agricultural crops and environment. Anal. Chim. Acta 2013, 805, 87–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Shi, L.; Zhang, L.; Du, H.; Huang, H.; Xiao, Y.; Zhang, Y.; He, X.; Wang, K. Novel pyrene–pyridine oligomer nanorods for super-sensitive fluorescent detection of Pd2+. Analyst 2020, 145, 5631–5637. [Google Scholar] [CrossRef]
- Yong, X.; Wan, W.; Su, M.; You, W.; Lu, X.; Yan, Y.; Qu, J.; Liu, R.; Masuda, T. Thiourea-functionalized poly(phenyleneethynylene): Fluorescent chemosensors for anions and cations. Polym. Chem. 2013, 4, 4126–4133. [Google Scholar] [CrossRef]
- Cao, S.; Pei, Z.; Xu, Y.; Zhang, R.; Pei, Y. Polytriazole bridged with 2,5-diphenyl-1,3,4-oxadiazole moieties: A highly sensitive and selective fluorescence chemosensor for Ag+. RSC Adv. 2015, 5, 45888–45896. [Google Scholar] [CrossRef]
- Cui, W.; Wang, L.; Xiang, G.; Zhou, L.; An, X.; Cao, D. A colorimetric and fluorescence “turn-off” chemosensor for the detection of silver ion based on a conjugated polymer containing 2,3-di(pyridin-2-yl)quinoxaline. Sens. Actuators B Chem. 2015, 207, 281–290. [Google Scholar] [CrossRef]
- Xiang, G.; Cui, W.; Lin, S.; Wang, L.; Meier, H.; Li, L.; Cao, D. A conjugated polymer with ethyl 2-(2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl) acetate units as a novel fluorescent chemosensor for silver(I) detection. Sens. Actuators B Chem. 2013, 186, 741–749. [Google Scholar] [CrossRef]
- Qin, C.; Wong, W.-Y.; Wang, L. A Water-Soluble Organometallic Conjugated Polyelectrolyte for the Direct Colorimetric Detection of Silver Ion in Aqueous Media with High Selectivity and Sensitivity. Macromolecules 2011, 44, 483–489. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Song, F.; Wei, G.; Cheng, Y.; Zhu, C. A highly selective and sensitive polymer-based OFF-ON fluorescent sensor for Hg2+ detection incorporating salen and perylenyl moieties. J. Mater. Chem. 2012, 22, 478–482. [Google Scholar] [CrossRef]
- Hussain, S.; De, S.; Iyer, P.K. Thiazole-Containing Conjugated Polymer as a Visual and Fluorometric Sensor for Iodide and Mercury. ACS Appl. Mater. Interfaces 2013, 5, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, X.; Wang, X.; Pei, M.; Zhang, G. Highly sensitive detection of Hg2+ in aqueous solution using cationic polythiophene derivatives. New J. Chem. 2013, 37, 4163–4169. [Google Scholar] [CrossRef]
- Giri, D.; Bankura, A.; Patra, S.K. Poly(benzodithieno-imidazole-alt-carbazole) based π-conjugated copolymers: Highly selective and sensitive turn-off fluorescent probes for Hg2+. Polymer 2018, 158, 338–353. [Google Scholar] [CrossRef]
- Lee, K.M.; Chen, X.; Fang, W.; Kim, J.-M.; Yoon, J. A Dual Colorimetric and Fluorometric Sensor for Lead Ion Based on Conjugated Polydiacetylenes. Macromol. Rapid Commun. 2011, 32, 497–500. [Google Scholar] [CrossRef]
- Narkwiboonwong, P.; Tumcharern, G.; Potisatityuenyong, A.; Wacharasindhu, S.; Sukwattanasinitt, M. Aqueous sols of oligo(ethylene glycol) surface decorated polydiacetylene vesicles for colorimetric detection of Pb2+. Talanta 2011, 83, 872–878. [Google Scholar] [CrossRef]
- Wang, M.; Wang, F.; Wang, Y.; Zhang, W.; Chen, X. Polydiacetylene-based sensor for highly sensitive and selective Pb2+ detection. Dyes Pigm. 2015, 120, 307–313. [Google Scholar] [CrossRef]
- Wang, D.-E.; Wang, Y.; Tian, C.; Zhang, L.; Han, X.; Tu, Q.; Yuan, M.; Chen, S.; Wang, J. Polydiacetylene liposome-encapsulated alginate hydrogel beads for Pb2+ detection with enhanced sensitivity. J. Mater. Chem. A 2015, 3, 21690–21698. [Google Scholar] [CrossRef]
- Kang, D.H.; Jung, H.-S.; Ahn, N.; Yang, S.M.; Seo, S.; Suh, K.-Y.; Chang, P.-S.; Jeon, N.L.; Kim, J.; Kim, K. Janus-Compartmental Alginate Microbeads Having Polydiacetylene Liposomes and Magnetic Nanoparticles for Visual Lead(II) Detection. ACS Appl. Mater. Interfaces 2014, 6, 10631–10637. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-Y.; Li, H.-H.; Wu, P.-J.; Chen, C.-P.; Huang, Y.-C.; Chan, Y.-H. Dual Colorimetric and Fluorescent Sensor Based On Semiconducting Polymer Dots for Ratiometric Detection of Lead Ions in Living Cells. Anal. Chem. 2015, 87, 4765–4771. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Wang, G.; Tang, W. A highly sensitive and selective optical sensor for Pb2+ by using conjugated polymers and label-free oligonucleotides. Biosens. Bioelectron. 2013, 39, 231–235. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, C.-Y.; Yang, C.-C.; Young, A.C.; Jang, S.-H.; Chen, W.-C.; Jen, A.K.Y. 2-(2′-Hydroxyphenyl)benzoxazole-Containing Two-Photon-Absorbing Chromophores as Sensors for Zinc and Hydroxide Ions. Chem. Mater. 2008, 20, 1977–1987. [Google Scholar] [CrossRef]
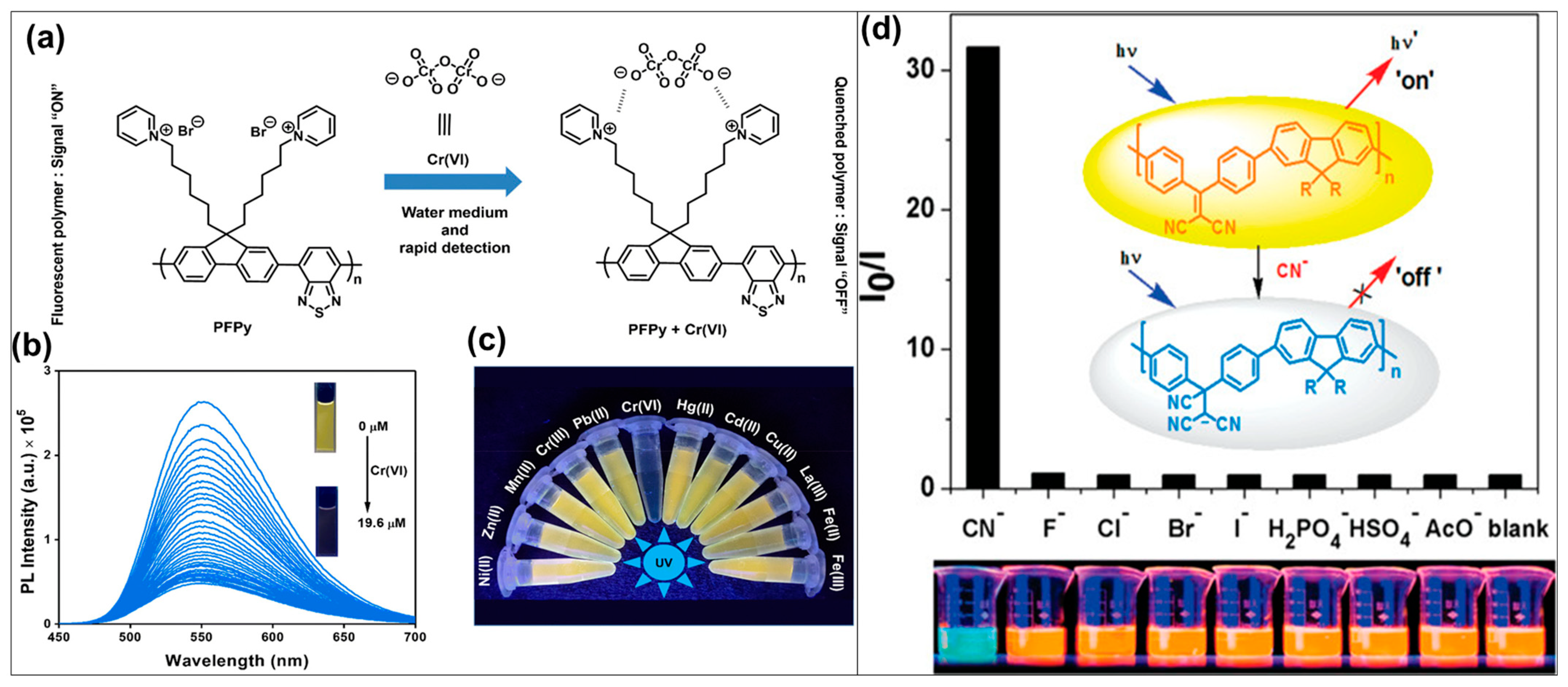
- Mohandoss, S.; Sivakamavalli, J.; Vaseeharan, B.; Stalin, T. Host-guest molecular recognition based fluorescence On-Off-On chemosensor for nanomolar level detection of Cu2+ and Cr2O72− ions: Application in XNOR logic gate and human lung cancer living cell imaging. Sens. Actuators B Chem. 2016, 234, 300–315. [Google Scholar] [CrossRef]
- Ding, B.; Guo, C.; Liu, S.X.; Cheng, Y.; Wu, X.X.; Su, X.M.; Liu, Y.Y.; Li, Y. A unique multi-functional cationic luminescent metal–organic nanotube for highly sensitive detection of dichromate and selective high capacity adsorption of Congo red. RSC Adv. 2016, 6, 33888–33900. [Google Scholar] [CrossRef]
- Tanwar, A.S.; Chanu, M.A.; Parui, R.; Barman, D.; Im, Y.-H.; Krishnan Iyer, P. Dynamic quenching mechanism based optical detection of carcinogenic Cr(vi) in water and on economical paper test strips via a conjugated polymer. RSC Appl. Polym. 2024, 2, 196–204. [Google Scholar] [CrossRef]
- Wu, X.; Xu, B.; Tong, H.; Wang, L. Highly Selective and Sensitive Detection of Cyanide by a Reaction-Based Conjugated Polymer Chemosensor. Macromolecules 2011, 44, 4241–4248. [Google Scholar] [CrossRef]
- Qu, Y.; Wu, Y.; Gao, Y.; Qu, S.; Yang, L.; Hua, J. Diketopyrrolopyrrole-based fluorescent conjugated polymer for application of sensing fluoride ion and bioimaging. Sens. Actuators B Chem. 2014, 197, 13–19. [Google Scholar] [CrossRef]
- Salinas, Y.; Martínez-Máñez, R.; Marcos, M.D.; Sancenón, F.; Costero, A.M.; Parra, M.; Gil, S. Optical chemosensors and reagents to detect explosives. Chem. Soc. Rev. 2012, 41, 1261–1296. [Google Scholar] [CrossRef]
- Bolse, N.; Eckstein, R.; Schend, M.; Habermehl, A.; Eschenbaum, C.; Hernandez-Sosa, G.; Lemmer, U. A digitally printed optoelectronic nose for the selective trace detection of nitroaromatic explosive vapours using fluorescence quenching. Flex. Print. Electron. 2017, 2, 024001. [Google Scholar] [CrossRef]
- Gillanders, R.N.; Campbell, I.A.; Glackin, J.M.E.; Samuel, I.D.W.; Turnbull, G.A. Ormosil-coated conjugated polymers for the detection of explosives in aqueous environments. Talanta 2018, 179, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Gross, D.E.; Huang, H.; Yang, D.; Yang, X.; Discekici, E.; Xue, Z.; Zhao, H.; Moore, J.S.; Zang, L. Diffusion-Controlled Detection of Trinitrotoluene: Interior Nanoporous Structure and Low Highest Occupied Molecular Orbital Level of Building Blocks Enhance Selectivity and Sensitivity. J. Am. Chem. Soc. 2012, 134, 4978–4982. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Xu, Y.; Wang, X.; Li, H.; Wu, X.; Tong, H.; Wang, L. Porous films based on a conjugated polymer gelator for fluorescent detection of explosive vapors. Polym. Chem. 2013, 4, 5056–5059. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Wang, J.; Wei, X.; Lu, W. Conjugated Microporous Polymer-Based Fluorescent Probe for Selective Detection of Nitro-explosives and Metal Nitrates. ACS Appl. Mater. Interfaces 2025, 17, 4033–4043. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Z.; Tian, M.; Schroeder, B.C.; Aliev, A.E.; Faul, C.F.J. Luminescent and Swellable Conjugated Microporous Polymers for Detecting Nitroaromatic Explosives and Removing Harmful Organic Vapors. ACS Appl. Mater. Interfaces 2019, 11, 48352–48362. [Google Scholar] [CrossRef]
- Li, K.; Liu, B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem. Soc. Rev. 2014, 43, 6570–6597. [Google Scholar] [CrossRef]
- Malik, A.H.; Hussain, S.; Kalita, A.; Iyer, P.K. Conjugated Polymer Nanoparticles for the Amplified Detection of Nitro-explosive Picric Acid on Multiple Platforms. ACS Appl. Mater. Interfaces 2015, 7, 26968–26976. [Google Scholar] [CrossRef]
- Alizadeh, N.; Akbarinejad, A.; Ghoorchian, A. Photophysical Diversity of Water-Soluble Fluorescent Conjugated Polymers Induced by Surfactant Stabilizers for Rapid and Highly Selective Determination of 2,4,6-Trinitrotoluene Traces. ACS Appl. Mater. Interfaces 2016, 8, 24901–24908. [Google Scholar] [CrossRef]
- Huang, J.; Gu, J.; Meng, Z.; Jia, X.; Xi, K. Signal enhancement of sensing nitroaromatics based on highly sensitive polymer dots. Nanoscale 2015, 7, 15413–15420. [Google Scholar] [CrossRef]
- Wu, X.; Hang, H.; Li, H.; Chen, Y.; Tong, H.; Wang, L. Water-dispersible hyperbranched conjugated polymer nanoparticles with sulfonate terminal groups for amplified fluorescence sensing of trace TNT in aqueous solution. Mater. Chem. Front. 2017, 1, 1875–1880. [Google Scholar] [CrossRef]
- Ma, X.-S.; Cui, Y.-Z.; Ding, Y.-Q.; Tao, F.-R.; Zheng, B.; Yu, R.-H.; Huang, W. 2D hyperbranched conjugated polymer for detecting TNT with excellent exciton migration. Sens. Actuators B Chem. 2017, 238, 48–57. [Google Scholar] [CrossRef]
- Chu, F.; Tsiminis, G.; Spooner, N.A.; Monro, T.M. Explosives detection by fluorescence quenching of conjugated polymers in suspended core optical fibers. Sens. Actuators B Chem. 2014, 199, 22–26. [Google Scholar] [CrossRef]
- Neves, T.; Marques, L.; Martelo, L.; Burrows, H.D. Conjugated polymer-based explosives sensor: Progresses in the design of a handheld device. In Proceedings of the SENSORS, 2014 IEEE, Valencia, Spain, 2–5 November 2014; pp. 1415–1418. [Google Scholar]
- Batool, R.; Riaz, N.; Junaid, H.M.; Waseem, M.T.; Khan, Z.A.; Nawazish, S.; Farooq, U.; Yu, C.; Shahzad, S.A. Fluorene-Based Fluorometric and Colorimetric Conjugated Polymers for Sensitive Detection of 2,4,6-Trinitrophenol Explosive in Aqueous Medium. ACS Omega 2022, 7, 1057–1070. [Google Scholar] [CrossRef]
- Shu, W.; Guan, C.; Guo, W.; Wang, C.; Shen, Y. Conjugated poly(aryleneethynylenesiloles) and their application in detecting explosives. J. Mater. Chem. 2012, 22, 3075–3081. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, P.; He, B.; Luo, W.; Zhao, Z.; Tang, B.Z. New fluorescent through-space conjugated polymers: Synthesis, optical properties and explosive detection. Polym. Chem. 2018, 9, 558–564. [Google Scholar] [CrossRef]
- Liu, W.; Chen, H.; Zhang, S.; Li, P.; Wu, W. Synthesis of Novel Fluorescent Conjugated Polymers and Their Rapid Gas-Phase Detection of Trace Nitro-Explosives. Macromol. Chem. Phys. 2024, 225, 2300325. [Google Scholar] [CrossRef]
- Nie, H.; Zhao, Y.; Zhang, M.; Ma, Y.; Baumgarten, M.; Müllen, K. Detection of TNT explosives with a new fluorescent conjugated polycarbazole polymer. Chem. Commun. 2011, 47, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tao, F.; Zhang, Y.; Li, T.; Raymo, F.M.; Cui, Y. Detection of nitroaromatic explosives by a 3D hyperbranched σ–π conjugated polymer based on a POSS scaffold. J. Mater. Chem. A 2017, 5, 14343–14354. [Google Scholar] [CrossRef]
- Novotney, J.L.; Dichtel, W.R. Conjugated Porous Polymers For TNT Vapor Detection. ACS Macro Lett. 2013, 2, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Q.; Zhang, J.; Gu, J.; Zhang, L. Synthesis of two conjugated polymers as TNT chemosensor materials. Sens. Actuators B Chem. 2010, 149, 155–160. [Google Scholar] [CrossRef]
- Xu, B.; Wu, X.; Li, H.; Tong, H.; Wang, L. Selective Detection of TNT and Picric Acid by Conjugated Polymer Film Sensors with Donor–Acceptor Architecture. Macromolecules 2011, 44, 5089–5092. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, N.; Bai, R.; Bao, Y. Aggregation-enhanced FRET-active conjugated polymer nanoparticles for picric acid sensing in aqueous solution. J. Mater. Chem. C 2018, 6, 266–270. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yao, J.; Zhuang, Z.; Ni, C.; Yao, H.; Su, D.; Zhou, J.; Zhao, Z. AEE-active conjugated polymers based on di(naphthalen-2-yl)-1,2-diphenylethene for sensitive fluorescence detection of picric acid. Dyes Pigm. 2020, 174, 108041. [Google Scholar] [CrossRef]
- Tanwar, A.S.; Hussain, S.; Malik, A.H.; Afroz, M.A.; Iyer, P.K. Inner Filter Effect Based Selective Detection of Nitroexplosive-Picric Acid in Aqueous Solution and Solid Support Using Conjugated Polymer. ACS Sens. 2016, 1, 1070–1077. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, Q.; Guo, X.; Ouyang, T.; Dong, W.; Duan, Q. Highly sensitive, selective and reliable detection of picric acid in aqueous media based on conjugated porous polymer nanoparticles. Microchem. J. 2022, 183, 108022. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, M. A multifunctional fluorescence sensor for DNT vapor and Fe3+ based on a conjugated polymer. Dyes Pigm. 2024, 229, 112306. [Google Scholar] [CrossRef]
- Liu, G.; Abdurahman, A.; Zhang, Z.; Feng, Y.; Li, F.; Zhang, M. New three-component conjugated polymers and their application as super rapid-response fluorescent probe to DNT vapor. Sens. Actuators B Chem. 2019, 296, 126592. [Google Scholar] [CrossRef]
- Long, Y.; Chen, H.; Yang, Y.; Wang, H.; Yang, Y.; Li, N.; Li, K.; Pei, J.; Liu, F. Electrospun Nanofibrous Film Doped with a Conjugated Polymer for DNT Fluorescence Sensor. Macromolecules 2009, 42, 6501–6509. [Google Scholar] [CrossRef]
- Jiang, S.; Meng, L.; Ma, W.; Qi, Q.; Zhang, W.; Xu, B.; Liu, L.; Tian, W. Morphology controllable conjugated network polymers based on AIE-active building block for TNP detection. Chin. Chem. Lett. 2021, 32, 1037–1040. [Google Scholar] [CrossRef]
- Dong, W.; Ma, Z.; Duan, Q.; Fei, T. Crosslinked fluorescent conjugated polymer nanoparticles for high performance explosive sensing in aqueous media. Dyes Pigm. 2018, 159, 128–134. [Google Scholar] [CrossRef]
- Dong, W.; Fei, T.; Palma-Cando, A.; Scherf, U. Aggregation induced emission and amplified explosive detection of tetraphenylethylene-substituted polycarbazoles. Polym. Chem. 2014, 5, 4048–4053. [Google Scholar] [CrossRef]
- Wolfe, N.D.; Dunavan, C.P.; Diamond, J. Origins of major human infectious diseases. Nature 2007, 447, 279–283. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-Soluble Conjugated Polymers for Imaging, Diagnosis, and Therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef]
- Wang, F.; Ma, M.; Cao, H.; Chai, X.; Huang, M.; Liu, L. Conjugated polymer materials for detection and discrimination of pathogenic microorganisms: Guarantee of biosafety. Biosaf. Health 2022, 4, 79–86. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, Q.; Liu, L.; Wang, S. Visual optical discrimination and detection of microbial pathogens based on diverse interactions of conjugated polyelectrolytes with cells. J. Mater. Chem. 2011, 21, 7905–7912. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Q.; Yao, C.; Li, S.; Zhang, P.; Sun, H.; Lv, F.; Liu, L.; Li, L.; Wang, S. Conjugated Polyelectrolyte–Silver Nanostructure Pair for Detection and Killing of Bacteria. Adv. Mater. Technol. 2017, 2, 1700033. [Google Scholar] [CrossRef]
- Hai, W.; Goda, T.; Takeuchi, H.; Yamaoka, S.; Horiguchi, Y.; Matsumoto, A.; Miyahara, Y. Specific Recognition of Human Influenza Virus with PEDOT Bearing Sialic Acid-Terminated Trisaccharides. ACS Appl. Mater. Interfaces 2017, 9, 14162–14170. [Google Scholar] [CrossRef]
- Bai, H.; Lu, H.; Fu, X.; Zhang, E.; Lv, F.; Liu, L.; Wang, S. Supramolecular Strategy Based on Conjugated Polymers for Discrimination of Virus and Pathogens. Biomacromolecules 2018, 19, 2117–2122. [Google Scholar] [CrossRef]
- Yan, W.; Yuan, H.; Li, R.; Fan, Y.; Zhan, Y.; Qi, J.; An, H.; Niu, R.; Li, G.; Xing, C. Conjugated Polythiophene/Porphyrin Complex for Rapid and Simple Detection of Bacteria in Drinking Water. Macromol. Chem. Phys. 2015, 216, 1603–1608. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Han, H.-H.; Tang, X.-Y.; Zhou, D.-M.; Wu, C.; Chen, G.-R.; He, X.-P.; Tian, H. Sialylglycan-Assembled Supra-Dots for Ratiometric Probing and Blocking of Human-Infecting Influenza Viruses. ACS Appl. Mater. Interfaces 2017, 9, 25164–25170. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Chen, H.; Hu, R.; Li, M.; Lv, F.; Liu, L.; Wang, S. Supramolecular Conjugated Polymer Materials for in Situ Pathogen Detection. ACS Appl. Mater. Interfaces 2016, 8, 31550–31557. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, Z.; Liu, L.; Lv, F.; Wang, Y.; Wang, S. Cationic Conjugated Polymers for Discrimination of Microbial Pathogens. Adv. Mater. 2014, 26, 4333–4338. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, X.; Yang, Y.; Bai, H.; Cui, Q.; Sun, H.; Li, L.; Wang, S. Conjugated Polymer with Aggregation-Directed Intramolecular Förster Resonance Energy Transfer Enabling Efficient Discrimination and Killing of Microbial Pathogens. Chem. Mater. 2018, 30, 3244–3253. [Google Scholar] [CrossRef]
- He, P.; Lv, F.; Liu, L.; Wang, S. Synthesis of amphiphilic poly(fluorene) derivatives for selective imaging of Staphylococcus aureus. Sci. Bull. 2018, 63, 900–906. [Google Scholar] [CrossRef]
- Gui Ning, L.; Wang, S.; Feng Hu, X.; Ming Li, C.; Qun Xu, L. Vancomycin-conjugated polythiophene for the detection and imaging of Gram-positive bacteria. J. Mater. Chem. B 2017, 5, 8814–8820. [Google Scholar] [CrossRef]
- Hussain, S.; Lv, F.; Qi, R.; Senthilkumar, T.; Zhao, H.; Chen, Y.; Liu, L.; Wang, S. Förster Resonance Energy Transfer Mediated Rapid and Synergistic Discrimination of Bacteria over Fungi Using a Cationic Conjugated Glycopolymer. ACS Appl. Bio Mater. 2020, 3, 20–28. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, Y.; You, L.; Dong, P.-T.; Mei, J.; Cheng, J.-X. Polymer Electrochromism Driven by Metabolic Activity Facilitates Rapid and Facile Bacterial Detection and Susceptibility Evaluation. Adv. Funct. Mater. 2020, 30, 2005192. [Google Scholar] [CrossRef]
- Wu, C.; Hansen, S.J.; Hou, Q.; Yu, J.; Zeigler, M.; Jin, Y.; Burnham, D.R.; McNeill, J.D.; Olson, J.M.; Chiu, D.T. Design of Highly Emissive Polymer Dot Bioconjugates for In Vivo Tumor Targeting. Angew. Chem. Int. Ed. 2011, 50, 3430–3434. [Google Scholar] [CrossRef]
- Liu, J.; Feng, G.; Ding, D.; Liu, B. Bright far-red/near-infrared fluorescent conjugated polymer nanoparticles for targeted imaging of HER2-positive cancer cells. Polym. Chem. 2013, 4, 4326–4334. [Google Scholar] [CrossRef]
- Rana, S.; Elci, S.G.; Mout, R.; Singla, A.K.; Yazdani, M.; Bender, M.; Bajaj, A.; Saha, K.; Bunz, U.H.F.; Jirik, F.R.; et al. Ratiometric Array of Conjugated Polymers–Fluorescent Protein Provides a Robust Mammalian Cell Sensor. J. Am. Chem. Soc. 2016, 138, 4522–4529. [Google Scholar] [CrossRef] [PubMed]
- Howes, P.; Green, M.; Levitt, J.; Suhling, K.; Hughes, M. Phospholipid Encapsulated Semiconducting Polymer Nanoparticles: Their Use in Cell Imaging and Protein Attachment. J. Am. Chem. Soc. 2010, 132, 3989–3996. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Li, K.; Pu, K.-Y.; Ding, D.; Liu, B. Conjugated Polymer and Gold Nanoparticle Co-loaded PLGA Nanocomposites with Eccentric Internal Nanostructure for Dual-modal Targeted Cellular Imaging. Small 2012, 8, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ma, M.; Zai, H.; Zhang, L.; Fu, N.; Huang, W.; Wang, L. Conjugated Polymer Nanoparticles for Label-Free and Bioconjugate-Recognized DNA Sensing in Serum. Adv. Sci. 2015, 2, 1400009. [Google Scholar] [CrossRef]
- Feng, X.; Liu, L.; Wang, S.; Zhu, D. Water-soluble fluorescent conjugated polymers and their interactions with biomacromolecules for sensitive biosensors. Chem. Soc. Rev. 2010, 39, 2411–2419. [Google Scholar] [CrossRef]
- Gaylord, B.S.; Heeger, A.J.; Bazan, G.C. DNA detection using water-soluble conjugated polymers and peptide nucleic acid probes. Proc. Natl. Acad. Sci. USA 2002, 99, 10954–10957. [Google Scholar] [CrossRef]
- He, F.; Tang, Y.; Yu, M.; Feng, F.; An, L.; Sun, H.; Wang, S.; Li, Y.; Zhu, D.; Bazan, G.C. Quadruplex-to-Duplex Transition of G-Rich Oligonucleotides Probed by Cationic Water-Soluble Conjugated Polyelectrolytes. J. Am. Chem. Soc. 2006, 128, 6764–6765. [Google Scholar] [CrossRef]
- Feng, F.; Liu, L.; Wang, S. Fluorescent conjugated polymer-based FRET technique for detection of DNA methylation of cancer cells. Nat. Protoc. 2010, 5, 1255–1264. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, C.; Niu, C.; Wang, X.; Li, Z.; Liu, J. Binding Studies of Cationic Conjugated Polymers and DNA for Label-Free Fluorescent Biosensors. ACS Appl. Polym. Mater. 2022, 4, 6211–6218. [Google Scholar] [CrossRef]


| Properties | Conjugated Polymer | Organic Dyes | Quantum Dots | Metal nanoclusters |
|---|---|---|---|---|
| Amplification efficiency | High (efficient exciton migration, molecular wire effect) | Low (single-fluorophore excitation) | Moderate (can be enhanced with shell coating) | Low |
| Emission tunability | Highly tunable via backbone engineering or copolymerization | Moderate (limited by chemical structure) | Highly tunable by size and composition | Limited (tuned by core size and ligand environment) |
| Molar absorptivity | High (broad absorption bands) | Moderate | Very high (broad, strong absorption) | Low to moderate |
| Quantum yield | High, especially in optimized formulations | High | Moderate to high (improves with passivation) | Moderate |
| Photostability | High (resistant to photobleaching) | Low (prone to photobleaching) | Very high (excellent photostability) | Moderate to high |
| Water solubility/dispersibility | Low, often requires encapsulation or surfactant | High | Moderate (requires surface modification) | High |
| Biocompatibility | Moderate (dependent on functionalization and size) | High | Moderate to high (depends on surface chemistry) | High (depending on ligands used) |
| Structural precision | Low (polymer chains are polydisperse) | High (defined molecular structure) | High (well-controlled nanocrystal structure) | High (atomically defined clusters) |
| Reproducibility | Moderate (affected by synthesis variability) | High (synthetically reproducible) | High | High |
| Aggregation behavior | Prone to ACQ | Stable in dilute solution; aggregation at high concentration | Aggregation risk in salt/bio media | Minimal aggregation; stabilized by ligands |
| Reaction yield of the synthetic processes | 60–90% (highly dependent on monomer solubility and catalyst efficiency) High-molecular-weight formation | 70–95% high yield due to well-optimized reactions | 40–80% (isolated yield) Yield sensitive to precursor ratios | 20–60% (isolated yield) Low yield due to competing nucleation into nanoparticles |
| Environmental stability | Moderate (prone to photobleaching and degradation under oxygen, moisture, and pH variations) | Poor (susceptible to photobleaching and chemical degradation) | High long-term stability | Low (sensitive to aggregation, surface oxidation, and ligand exchange) |
| CPs | Types of Sensors | Metal Ions | LOD | Selectivity | Refs |
|---|---|---|---|---|---|
| PPFP-Br * | Bioassay in an aqueous medium | K+ | 1.5 nM | Na+, Ca2+, Li+, Mg2+, NH4+, Cu2+ | [82] |
| Poly(fluorene-co-phenylene) | Förster resonance energy transfer (FRET) | K+ | 0.7 nM | Na+, Ca2+, Hg2+, Mg2+, Cu2+, Fe2+ | [83] |
| Cationic polyfluorene | Förster resonance energy transfer (FRET) | K+ | 3 nM | Ag+, Ca2+, Li+, Al3+, Cu2+, Zn2+ | [84] |
| Benzo [1,2-b:4,5-b′]dithiophene | Optical solid state sensor | Na+ | 1 mM | K+ | [85] |
| Water-soluble polyfluorene | Aqueous polyfluorene probe | Fe2+ | 46 nM | Cu+, Cu2+, Co2+, Fe3+, Na+, K+, Zn2+, Cr3+, Ag+, Hg2+, Cd2+, Ni2+, Pb2+ | [86] |
| Anionic polyfluorene | Aqueous polyfluorene probe | Fe3+ | 0.33 µM | Mg2+, Ca2+, Mn2+, Mn3+, Fe2+, Al3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+ | [87] |
| Water-soluble poly(9-aminofluorene) | Fluorescent chemosensor | Fe3+ | 3.7 pM | Mg2+, Ca2+, Mn2+, Fe2+, Al3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+, Pb2+ | [88] |
| Water-soluble polyfluorenes | Aqueous polyfluorene probe | Fe3+ | 0.8 µM | Mg2+, Ca2+, Mn2+, Fe2+, Al3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+, Pb2+ | [89] |
| Poly(9-fluorenecarboxylic acid) | Aqueous polyfluorene probe | Fe3+ | 0.611 nM | Pd2+, Cu2+, Hg2+, Ni2+, Co2+, Zn2+, Ba2+, Ca2+, Mg2+, Sn2+, Mn2+, K+, Cr3+, Cd2+, Al3+, Sr2+, Pb2+ | [90] |
| Poly(5-cyanoindole) | Fluorescent chemosensor | Fe3+ | 16 nM | Cu2+, Hg2+, Ni2+, Co2+, Zn2+, Ba2+, Ca2+, Mg2+, Sn2+, Mn2+, K+, Cr3+, Cd2+, Al3+, Sr2+, Pb2+ | [91] |
| Poly(1-amino-5-chloroanthraquinone) | Fluorescent chemosensor | Fe3+ | 0.02 nM | Na+, Cu2+, Hg2+, Ni2+, Co2+, Zn2+, Ba2+, Ca2+, Mg2+, Sn2+, Mn2+, K+, Cr3+, Cd2+,Al3+,Ce2+, Pb2+ | [92] |
| Poly[p(phenylene ethynylene)-alt-(thienylene ethyn-ylene)] | Förster resonance energy transfer (FRET) | Fe3+ | 0.3 µM | Ag+, Cu2+, Hg2+, Ni2+, Co2+, Zn2+, Ba2+, Ca2+, Mg2+, Yb3+, Mn2+, Cr3+, Cd2+, Al3+, Ce2+, Pb2+ | [79] |
| Poly(p-phenyleneethynylene) | Ion-sensing assay | Al3+ | 0.37 μM | Ag+, Cu2+, Hg2+, Co2+, Zn2+, Ga3+, Mg2+, Mn2+, Cr3+, Fe2+, Pb2+, Fe3+ | [93] |
| Anionic polytriazole | Fluorescent chemosensor | Al3+ | 1.1 μM | Na+, K+, Ag+, Cu2+, Ni2+, Co2+, Zn2+, Ca2+, Mg2+, Mn2+, Cr3+, Fe3+, Pb2+ | [94] |
| Polythiophene | A colorimetric and fluorescent sensor | Al3+ | 4 μM | Na+, K+, Li+, Cu2+, Hg2+, Ni2+, Co2+, Zn2+, Ca2+, Mg2+, Mn2+, Cr3+, Cd2+, Fe2+, Pb2+, Fe3+ | [95] |
| Water-soluble polyfluorenes | Fluorescent chemosensor | Cu+ Cu2+ | 0.25 μM 0.25 μM | Na+, K+, Li+, Ag+, Ni2+, Co2+, Zn2+, Ca2+, Ba2+, Cd2+ | [96] |
| Amine-functionalized polyfluorene | Fluorescent film sensor | Cu2+ | 5 μM | Na+, Cu2+, Hg2+, Ni2+, Co2+, Zn2+, Ca2+, Mg2+, Mn2+, Ba2+, Pb2+, Fe3+ | [97] |
| Double bond functionalized polyfluorene | Fluorescent optosensor | Cu2+ | 1 nM | Na+, K+, Hg2+, Co2+, Zn2+, Ca2+, Mg2+, Mn2+, Pb2+, Fe2+ | [98] |
| Polyfluorene | Fluorescent chemosensor | Cu2+ | 0.5 μM | Na+, K+, Li+, Hg2+, Ni2+, Zn2+, Ca2+, Mg2+, Mn2+, Cr3+, Cd2+, Fe2+, Pb2+, Al3+, Ba2+, Fe3+, Ag+ | [99] |
| Sulfate-functionalized polyfluorene | Fluorescent chemosensor | Cu2+ | 2.5 µM | Hg2+, Ni2+, Zn2+, Sn2+, Mg2+, Fe2+, Pb2+, Al3+, Fe3+, Ag+, Au3+, Co2+ | [100] |
| Poly(phenylene ethylene) | Fluorescent chemosensor | Cu2+ | 24 nM | Na+, K+, Li+, Hg2+, Ni2+, Zn2+, Ca2+, Mg2+, Mn2+, Cr6+, Cd2+, Fe3+, Pb2+, Al3+, Ba2+, Fe3+, Ag+, Co2+, Pt2+, Be2+, Cs+, As2+ | [101] |
| Water-soluble polythiophene | Fluorescent chemosensor | Cu2+ | 10 nM | K+, Li+, Hg2+, Ni2+, Zn2+, Ca2+, Mg2+, Mn2+, Fe2+, Pb2+, Ba2+, Ag+, Co2+ | [44] |
| Poly3-[2-(2-methoxy-ethoxy)-ethoxy]-thiophene | Fluorescent chemosensor | Cu2+ | 0.45 μM | Na+, Ni2+, Zn2+, Mg2+, Mn2+, Cr3+, Cd2+, Fe3+, Sn4+, Al3+, Ag+, Co2+ | [102] |
| poly 3-[1-(2-hydrazino-2-oxoethyl)piperidin-4-ylidene]methylthiophene | Fluorescent chemosensor | Cu2+ Hg2+ | 20 nM 2 nM | Ni2+, Sr3+, Cr3+, Fe3+, Pb2+, Al3+, Ag+, Li+, Co2+, Cd2+, Mn2+ | [103] |
| Crosslinked polythiophenes | Fluorescent nano probe | Cu2+ | 10 nM | Na+, K+, Hg2+, Zn2+, Ca2+, Cd2+, Fe2+, Pb2+, Al3+, Fe3+, Ag+, Co2+ | [104] |
| Oligo(1-pyreneboronic acid) | Fluorescent sensor | Cu2+ | 23 pM | Na+, K+, Hg2+, Ni2+, Zn2+, Ca2+, Mg2+, Mn2+, Cr3+, Cd2+, Sr2+, Pb2+, Al3+, Ba2+, Fe3+ | [105] |
| Terpyridyl appended poly(metaphenylene-alt-fluorene) | Turn-off fluorescent probe | Cu2+ | 13–14 μM | Na+, K+, Hg2+, Ni2+, Zn2+, Ca2+, Mg2+, Co2+, Cr3+, Cd2+, Sr2+, Pb2+, Ag+, Ba2+, Fe3+ | [106] |
| 2,6-bis(4-bromophenyl)-4-phenylpyridine | Turn-off fluorescent probe | Pd2+ | 1 μM | Pt4+, Hg2+, Ni2+, Zn2+, Ca2+, Co2+, Cd2+, Ag+, Fe3+, Cu2+ | [107] |
| Polybenzanthrone | Fluorescent chemosensor | Pd2+ | 0.27 nM | K+, Hg2+, Ni2+, Zn2+, Ca2+, Mg2+, Mn2+, Cr3+, Cd2+, Sr2+, Pb2+, Al3+, Ba2+, Fe2+, Fe3+, Co2+ | [108] |
| Pyrene-pyridine oligomer nanorods | Fluorescent nanosensors | Pd2+ | 70 nM | Na+, Hg2+, Ni2+, Zn2+, Ca2+, Mg2+, Mn2+, Cr3+, Cd2+, Pb2+, Al3+, Ba2+, Fe2+, Fe3+, Co2+ | [109] |
| Thiourea-functionalized poly(phenyleneethynylene) | Fluorescent chemosensors | Ag+ | 47.6 μM | Na+, K+, Zn2+, Mn2+, Pb2+, Co2+, Cu2+ | [110] |
| Polytriazoles | Fluorescent chemosensors | Ag+ | 0.42 μM | K+, Na+, Hg2+, Ni2+, Zn2+, Ca2+, Mg2+, Mn2+, Cr3+, Cd2+, Cu2+, Pb2+, Ba2+, Co2+ | [111] |
| 2,3-di(pyridin-2-yl)quinoxaline | Colorimetric and fluorescence “turn-off” chemosensor | Ag+ | 0.5 μM | Na+, Li+, Hg2+, Ni2+, Zn2+, Ca2+, Mg2+, Cu2+, Cr3+, Cd2+, Sr2+, Al3+, Ba2+, Zr4+, Fe3+, Co2+, La3+ | [112] |
| Ethyl 2-(2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl)acetate | Fluorescent chemosensor | Ag+ | 0.05 μM | Na+, K+, Hg2+, Ni2+, Zn2+, Ca2+, Mg2+, Cu2+, Cr3+, Cd2+, Al3+, Ba2+, Fe2+, Fe3+, Co2+ | [113] |
| Water-soluble organometallic conjugated polyelectrolyte | Colorimetric and fluorescent sensor | Ag+ | 0.5 μM | Li+, Cs+, Hg2+, Ni2+, Zn2+, Ca2+, Mg2+, Cu2+, Cd2+, Fe3+, Co2+, Pb2+ | [114] |
| 1,7-bis((3-formyl-4-hydroxyphenyl) ethynyl)perylene | Off–on fluorescent sensor | Hg2+ | 0.7 μM | Na+, K+, Ni2+, Zn2+, Ca2+, Ag+, Cr3+, Cd2+, Al3+, Fe3+, Co2+, Pb2+ | [115] |
| Poly-p-phenylene | Fluorescence “turn-off/turn-on” and colorimetric sensor | Hg2+ | 2.1 nM | Ni2+, Zn2+, Mn2+, Cu2+, Cr3+, Cd2+, Fe2+, Co2+ | [116] |
| Cationic polythiophene | Turn-off fluorescent probes | Hg2+ | 1 nM | Li+, Ag+, Ni2+, Zn2+, Ca2+, Cu2+, Cr3+, Cd2+, Al3+, Ba2+, Sr2+, Pb2+, Co2+, Mn2+ | [117] |
| Poly(benzodithieno-imidazole-alt-carbazole) | Turn-off fluorescent probes | Hg2+ | 0.2 μM | Na+, K+, Pb2+, Ni2+, Zn2+, Ca2+, Mg2+, Cu2+, Cr3+, Cd2+, Al3+, Ba2+, Sr2+, Fe3+, Co2+, Ag+ | [118] |
| Polydiacetylene | Dual colorimetric and fluorometric sensor | Pb2+ | 3.8 μM | Li+, K+, Ni2+, Zn2+, Ca2+, Mg2+, Cs+, Cr3+, Cd2+, Al3+, Mn2+, Co2+ | [119] |
| Polydiacetylene vesicles | Colorimetric and fluorescent sensor | Pb2+ | 5 μM | Na+, K+, Li+, Ni2+, Zn2+, Ca2+, Mg2+, Cu2+, Hg2+, Fe2+, Co2+, Cd2+ | [120] |
| Polydiacetylene | Fluorescent and colorimetric sensors | Pb2+ | 1 μM | Na+, Li+, Ni2+, Zn2+, Mg2+, Cu2+, Cr3+, Cd2+, Al3+, Fe3+, Co2+, Ag+, Hg2+, Mn2+ | [121] |
| Polydiacetylene liposome | Fluorescent and colorimetric sensors | Pb2+ | 3 μM | Na+, K+, Mn2+, Ca2+, Mg2+, Cu2+, Cd2+, Ba2+, Fe3+, Co2+, Ag+, Hg2+ | [122] |
| Polydiacetylene liposome | Colorimetric and fluorescent sensor | Pb2+ | 0.1 mM | Ni2+, Fe2+, Ca2+, Mg2+, Cu2+, Cd2+, Co2+, Hg2+ | [123] |
| Polydiacetylene dots | Dual colorimetric and fluorescent sensor | Pb2+ | 0.5 μM | Ni2+, Fe2+, Ca2+, As3+, Mn2+, Cd2+, Co2+, Cu2+ | [124] |
| Water-soluble cationic polythiophene | Fluorescent sensor | Pb2+ | 6 nM | Na+, K+, Li+, Ca2+, Mg2+, Cu2+, Cd2+, Ba2+, Fe2+, Co2+, Zn2+, Hg2+, Ni2+ | [125] |
| Benzo[a]imidazo [5,1,2-cd] fluorophores | Bicolor fluorescent sensor | Ba2+ | 0.059 μM | Na+, K+, Ca2+, Mg2+, Sr2+ | [80] |
| Benzo [4,5]imidazo [2,1,b] quinones | Bicolor fluorescent sensor | Cu2+ | 74.4 nM | Na+, K+, Sr2+, Cr3+, Cd2+, Ba2+, Fe3+, Co2+, Zn2+, Al3+, Ni2+ | [81] |
| CPs | Types of Sensors | Pathogen | LOD | Refs |
|---|---|---|---|---|
| PEDOT | Quartz crystal microbalance | H1N1 | 0.12 HAU | [172] |
| Poly(l-lysine) (PLL)/poly(acrylic acid) (PAA) | Metal-enhanced fluorescence | E. coli | 5 × 105 CFU | [171] |
| Cationic polythiophene derivative | Fluorescence biosensor | S. aureus E. coli C. albicans | 1.2 × 108 CFU/mL 1.0 × 108 CFU/mL 4 × 107 CFU/mL | [173] |
| Cationic porphyrin | Förster resonance energy transfer | E. coli | 0.1 × 106 CFU/mL | [174] |
| 2,6-Sialyllactose-DCM | Förster resonance energy transfer | H3N2 H7N9 H10N8 | 256 HAU | [175] |
| Cationic poly(fluorene-co-phenylene) | Fluorescence biosensor | B. subtilis E. faecalis S. aureus E. coli P. aeruginosa C. albicans S. cerecisiae | - | [176] |
| Cationic poly(phenylene vinylene) derivative | Fluorescence biosensor | B. subtilis E. coli C. albicans | OD 600 = 1.0 OD 600 = 1.0 OD 600 = 2.0 | [177] |
| PFDBT-BIMEG * | Förster resonance energy transfer | S. aureus E. coli C. albicans | OD600 = 0.5 | [178] |
| Poly(fluorene-co-phenylene) | Fluorescence biosensor | S. aureus | OD600 = 2.0 | [179] |
| Polythiophene | Fluorescence biosensor | S. aureus S. epidermidis | OD600 = 1.0 | [180] |
| Cationic conjugated glycopolymer | Förster resonance energy transfer | E. coli | OD600 = 0.025 | [181] |
| Poly(3,4- propylenedioxythiophen-alt-3,4-ethylenedioxythiophene) copolymer (PPE) | Colorimetric and fluorescence biosensor | S. aureus E. coli | OD600 = 0.25 | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phung, V.-D.; Tran, V.V. Advances and Innovations in Conjugated Polymer Fluorescent Sensors for Environmental and Biological Detection. Biosensors 2025, 15, 580. https://doi.org/10.3390/bios15090580
Phung V-D, Tran VV. Advances and Innovations in Conjugated Polymer Fluorescent Sensors for Environmental and Biological Detection. Biosensors. 2025; 15(9):580. https://doi.org/10.3390/bios15090580
Chicago/Turabian StylePhung, Viet-Duc, and Vinh Van Tran. 2025. "Advances and Innovations in Conjugated Polymer Fluorescent Sensors for Environmental and Biological Detection" Biosensors 15, no. 9: 580. https://doi.org/10.3390/bios15090580
APA StylePhung, V.-D., & Tran, V. V. (2025). Advances and Innovations in Conjugated Polymer Fluorescent Sensors for Environmental and Biological Detection. Biosensors, 15(9), 580. https://doi.org/10.3390/bios15090580