Foreign Body Reaction to Neural Implants: A Comparative Study of Polymer Toxicity and Tissue Response
Abstract
1. Introduction
2. Results
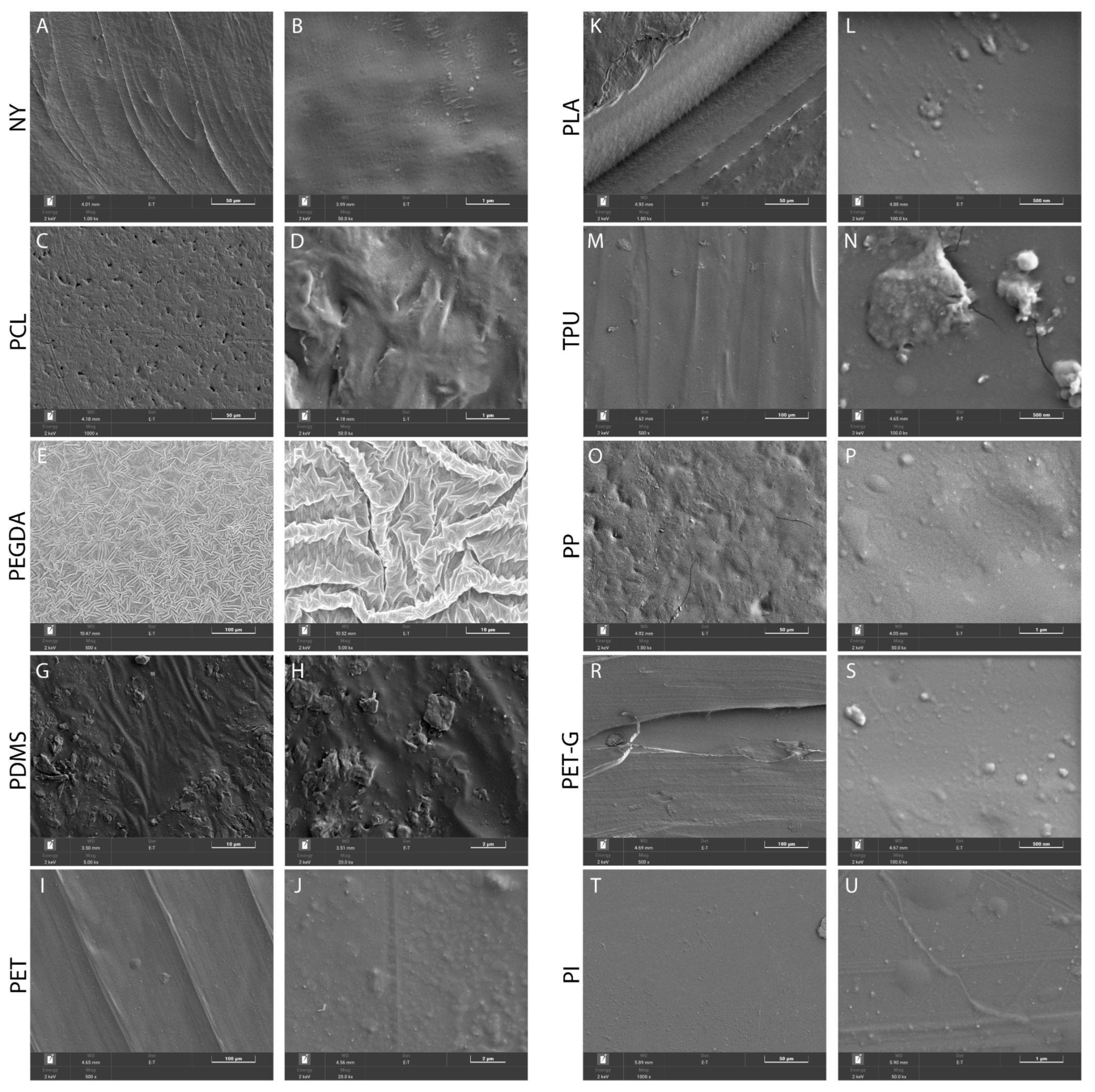
2.1. Surface Properties of the Scaffolds
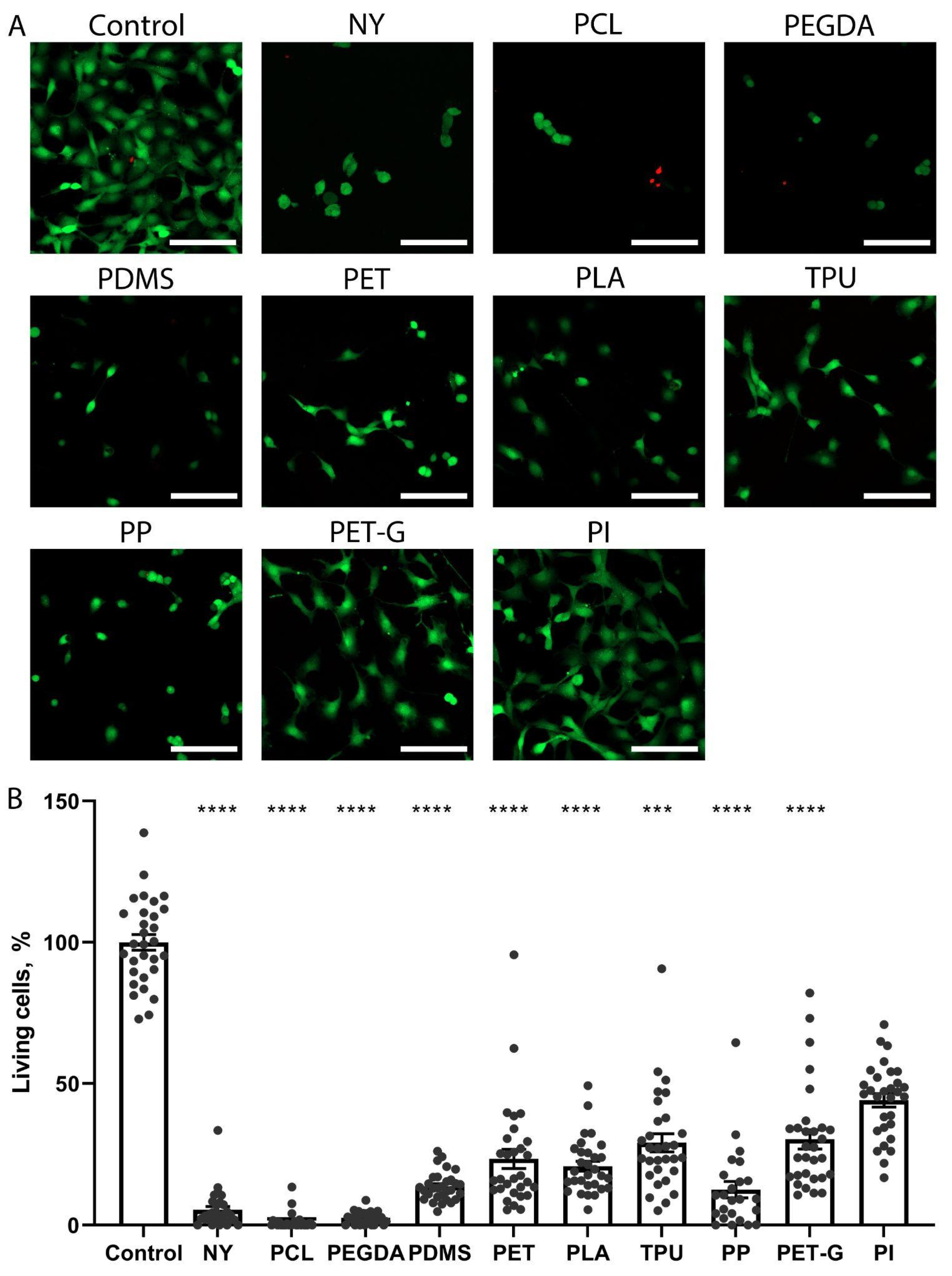
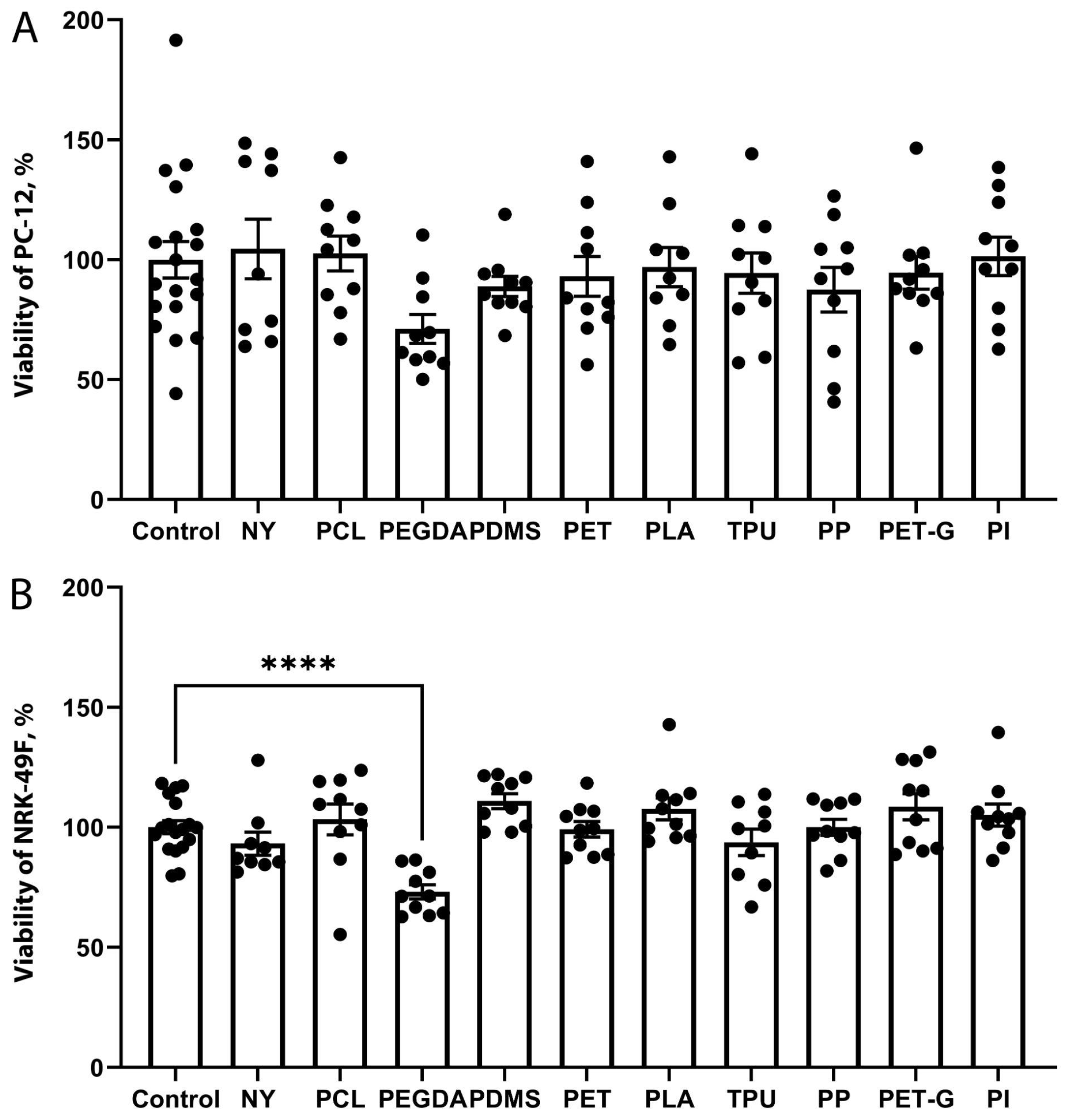
2.2. The Growth of Neuronal PC-12 Cells on the Scaffolds
2.3. The Growth of Fibroblast NRK-49F Cells on the Scaffolds
2.4. Comparison of the Toxicity of Substrate Materials
2.5. Morphological Changes in the Brain During Implant Placement
3. Discussion
4. Materials and Methods
4.1. The Preparation of Polymer Scaffolds
4.2. Surface Characterization Using SEM
4.3. Cell Cultures and Biocompatibility Assessment
4.4. In Vitro Toxicity Assessment of Scaffolds
4.5. Animal Experiments
4.6. Histological Evaluation
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMSO | Dimethyl sulfoxide |
| FBR | Foreign body reaction |
| FBS | Fetal bovine serum |
| NBF | Neutral buffered formaldehyde |
| NY | Nylon 618 |
| PCL | Polycaprolactone |
| PDMS | Polydimethylsiloxane |
| PEGDA | Polyethylene glycol diacrylate |
| PET | Polyethylene terephthalate |
| PET-G | Polyethylene terephthalate glycol |
| PI | Polyimide |
| PLA | Polylactide |
| PP | Polypropylene |
| SEM | Scanning electron microscopy |
| TPU | Thermoplastic polyurethane |
References
- Shen, K.; Chen, O.; Edmunds, J.L.; Piech, D.K.; Maharbiz, M.M. Translational Opportunities and Challenges of Invasive Electrodes for Neural Interfaces. Nat. Biomed. Eng. 2023, 7, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Giraldo, C.; Genta, M.; Goding, J.; Green, R. Biomimetic Approaches towards Device-Tissue Integration. In Handbook of Neuroengineering; Springer: Singapore, 2021; pp. 261–286. ISBN 9789811528484. [Google Scholar]
- Valeriani, D.; Santoro, F.; Ienca, M. The Present and Future of Neural Interfaces. Front. Neurorobotics 2022, 16, 953968. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.E.; Meng, E. A Review for the Peripheral Nerve Interface Designer. J. Neurosci. Methods 2020, 332, 108523. [Google Scholar] [CrossRef]
- Gori, M.; Vadalà, G.; Giannitelli, S.M.; Denaro, V.; Di Pino, G. Biomedical and Tissue Engineering Strategies to Control Foreign Body Reaction to Invasive Neural Electrodes. Front. Bioeng. Biotechnol. 2021, 9, 659033. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the Mechanisms of Biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Budday, S.; Nay, R.; de Rooij, R.; Steinmann, P.; Wyrobek, T.; Ovaert, T.C.; Kuhl, E. Mechanical Properties of Gray and White Matter Brain Tissue by Indentation. J. Mech. Behav. Biomed. Mater. 2015, 46, 318–330. [Google Scholar] [CrossRef]
- Weltman, A.; Yoo, J.; Meng, E. Flexible, Penetrating Brain Probes Enabled by Advances in Polymer Microfabrication. Micromachines 2016, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Carnicer-Lombarte, A.; Chen, S.-T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 622524. [Google Scholar] [CrossRef]
- Lotti, F.; Ranieri, F.; Vadalà, G.; Zollo, L.; Di Pino, G. Invasive Intraneural Interfaces: Foreign Body Reaction Issues. Front. Neurosci. 2017, 11, 497. [Google Scholar] [CrossRef]
- Gou, S.; Yang, S.; Cheng, Y.; Yang, S.; Liu, H.; Li, P.; Du, Z. Applications of 2D Nanomaterials in Neural Interface. Int. J. Mol. Sci. 2024, 25, 8615. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Jung, F. The Pathology of the Foreign Body Reaction against Biomaterials. J. Biomed. Mater. Res. Part A 2017, 105, 927–940. [Google Scholar] [CrossRef]
- Gulino, M.; Kim, D.; Pané, S.; Santos, S.D.; Pêgo, A.P. Tissue Response to Neural Implants: The Use of Model Systems toward New Design Solutions of Implantable Microelectrodes. Front. Neurosci. 2019, 13, 689. [Google Scholar] [CrossRef]
- Prodanov, D.; Delbeke, J. Mechanical and Biological Interactions of Implants with the Brain and Their Impact on Implant Design. Front. Neurosci. 2016, 10, 11. [Google Scholar] [CrossRef]
- Hatsopoulos, N.G.; Donoghue, J.P. The Science of Neural Interface Systems. Annu. Rev. Neurosci. 2009, 32, 249–266. [Google Scholar] [CrossRef]
- Sung, C.; Jeon, W.; Nam, K.S.; Kim, Y.; Butt, H.; Park, S. Multimaterial and Multifunctional Neural Interfaces: From Surface-Type and Implantable Electrodes to Fiber-Based Devices. J. Mater. Chem. B 2020, 8, 6624–6666. [Google Scholar] [CrossRef]
- Ferguson, M.; Sharma, D.; Ross, D.; Zhao, F. A Critical Review of Microelectrode Arrays and Strategies for Improving Neural Interfaces. Adv. Healthc. Mater. 2019, 8, e1900558. [Google Scholar] [CrossRef]
- Wunderlich, H.; Kozielski, K.L. Next Generation Material Interfaces for Neural Engineering. Curr. Opin. Biotechnol. 2021, 72, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for Neural Implants. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Green, R.A.; Lovell, N.H.; Wallace, G.G.; Poole-Warren, L.A. Conducting Polymers for Neural Interfaces: Challenges in Developing an Effective Long-Term Implant. Biomaterials 2008, 29, 3393–3399. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, A.; Descamps, E.; Bergaud, C. A Review on Mechanical Considerations for Chronically-Implanted Neural Probes. J. Neural Eng. 2018, 15, 031001. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of Cell Adhesion, Proliferation and Differentiation on Materials Designed for Body Implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In Vitro Cytotoxicity Testing of Polycations: Influence of Polymer Structure on Cell Viability and Hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Galindo, J.M.; San-Millán, M.I.; Castillo-Sarmiento, C.A.; Ballesteros-Yáñez, I.; Vázquez, E.; Merino, S.; Herrero, M.A. Optimization of 3D Synthetic Scaffolds for Neuronal Tissue Engineering Applications. Chemistry 2024, 30, e202302481. [Google Scholar] [CrossRef] [PubMed]
- Aye, S.-S.S.; Li, R.; Boyd-Moss, M.; Long, B.; Pavuluri, S.; Bruggeman, K.; Wang, Y.; Barrow, C.R.; Nisbet, D.R.; Williams, R.J. Scaffolds Formed via the Non-Equilibrium Supramolecular Assembly of the Synergistic ECM Peptides RGD and PHSRN Demonstrate Improved Cell Attachment in 3D. Polymers 2018, 10, 690. [Google Scholar] [CrossRef]
- Myllymaa, S.; Myllymaa, K.; Korhonen, H.; Djupsund, K.; Tanila, H.; Lappalainen, R. Development of Flexible Thin Film Microelectrode Arrays for Neural Recordings. In 14th Nordic-Baltic Conference on Biomedical Engineering and Medical Physics, Riga, Latvia, 16–20 June 2008; IFMBE Proceedings; Springer: Berlin/Heidelberg, Germany, 2008; pp. 286–289. ISBN 9783540693666. [Google Scholar]
- Kumar, K.; Deshpande, K.; Kalur, N.; Chauhan, G.; Chugh, D.; Ganesh, S.; Ramakrishnan, A. Polyimide-Based Flexible Multi-Electrode Arrays: Synthesis, Microfabrication, and In-Vivo Validation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Deroanne, C.F.; Lapiere, C.M.; Nusgens, B.V. In Vitro Tubulogenesis of Endothelial Cells by Relaxation of the Coupling Extracellular Matrix-Cytoskeleton. Cardiovasc. Res. 2001, 49, 647–658. [Google Scholar] [CrossRef]
- Engler, A.; Bacakova, L.; Newman, C.; Hategan, A.; Griffin, M.; Discher, D. Substrate Compliance versus Ligand Density in Cell on Gel Responses. Biophys. J. 2004, 86, 617–628. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Shahriari, D.; Loke, G.; Tafel, I.; Park, S.; Chiang, P.-H.; Fink, Y.; Anikeeva, P. Scalable Fabrication of Porous Microchannel Nerve Guidance Scaffolds with Complex Geometries. Adv. Mater. 2019, 31, e1902021. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.; Baranes, K.; Park, M.; Choi, I.S.; Kang, K.; Shefi, O. Interactions of Neurons with Physical Environments. Adv. Healthc. Mater. 2017, 6, 1700267. [Google Scholar] [CrossRef] [PubMed]
- Boulingre, M.; Portillo-Lara, R.; Green, R.A. Biohybrid Neural Interfaces: Improving the Biological Integration of Neural Implants. Chem. Commun. 2023, 59, 14745–14758. [Google Scholar] [CrossRef]
- Kumosa, L.S. Commonly Overlooked Factors in Biocompatibility Studies of Neural Implants. Adv. Sci. 2023, 10, e2205095. [Google Scholar] [CrossRef]
- Wurth, S.; Capogrosso, M.; Raspopovic, S.; Gandar, J.; Federici, G.; Kinany, N.; Cutrone, A.; Piersigilli, A.; Pavlova, N.; Guiet, R.; et al. Long-Term Usability and Bio-Integration of Polyimide-Based Intra-Neural Stimulating Electrodes. Biomaterials 2017, 122, 114–129. [Google Scholar] [CrossRef]
- Dorrier, C.E.; Jones, H.E.; Pintarić, L.; Siegenthaler, J.A.; Daneman, R. Emerging Roles for CNS Fibroblasts in Health, Injury and Disease. Nat. Rev. Neurosci. 2022, 23, 23–34. [Google Scholar] [CrossRef]
- Scheid, S.; Goebel, U.; Ulbrich, F. Neuroprotection Is in the Air—Inhaled Gases on Their Way to the Neurons. Cells 2023, 12, 2480. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.P.; McCann, S.K.; Walker, A.M.; Premji, Z.A.; Rogan, K.J.; Hutton, M.J.H.; Gray, L.J. Neuroprotection by Anaesthetics in Rodent Models of Traumatic Brain Injury: A Systematic Review and Network Meta-Analysis. Br. J. Anaesth. 2018, 121, 1272–1281. [Google Scholar] [CrossRef]
- Silachev, D.N.; Usatikova, E.A.; Pevzner, I.B.; Zorova, L.D.; Babenko, V.A.; Gulyaev, M.V.; Pirogov, Y.A.; Plotnikov, E.Y.; Zorov, D.B. Effect of Anesthetics on Efficiency of Remote Ischemic Preconditioning. Biochemistry 2017, 82, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Feng, D.; Zhang, Y.-F.; Shang, Y.; Wu, Y.; Li, X.-F.; Pei, L. Chloral Hydrate Preconditioning Protects against Ischemic Stroke via Upregulating Annexin A1. CNS Neurosci. Ther. 2015, 21, 718–726. [Google Scholar] [CrossRef]
- Bocharnikov, A.D.; Boeva, E.A.; Milovanova, M.A.; Antonova, V.V.; Yakupova, E.I.; Grechko, A.V. Nueroprotection by Anesthetics in Brain Injury Models. Gen. Reanimatol. 2024, 20, 65–69. [Google Scholar] [CrossRef]
- Du, Z.J.; Kolarcik, C.L.; Kozai, T.D.Y.; Luebben, S.D.; Sapp, S.A.; Zheng, X.S.; Nabity, J.A.; Cui, X.T. Ultrasoft Microwire Neural Electrodes Improve Chronic Tissue Integration. Acta Biomater. 2017, 53, 46–58. [Google Scholar] [CrossRef]
- Azemi, E.; Gobbel, G.T.; Cui, X.T. Seeding Neural Progenitor Cells on Silicon-Based Neural Probes. J. Neurosurg. 2010, 113, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Adewole, D.O.; Struzyna, L.A.; Burrell, J.C.; Harris, J.P.; Nemes, A.D.; Petrov, D.; Kraft, R.H.; Chen, H.I.; Serruya, M.D.; Wolf, J.A.; et al. Development of Optically Controlled “Living Electrodes” with Long-Projecting Axon Tracts for a Synaptic Brain-Machine Interface. Sci. Adv. 2021, 7, eaay5347. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, A.D.; Woolley, A.J.; Poole-Warren, L.A.; Thomson, C.E.; Green, R.A. A Critical Review of Cell Culture Strategies for Modelling Intracortical Brain Implant Material Reactions. Biomaterials 2016, 91, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, E.S.; Pevzner, I.B.; Zorova, L.D.; Silachev, D.N.; Babenko, V.A.; Manskikh, V.N.; Gulyaev, M.V.; Pirogov, Y.A.; Plotnikov, E.Y.; Zorov, D.B. Changes in the Number of Neurons, Astrocytes and Microglia in the Brain After Ischemic Stroke Assessed by Immunohistochemistry and Immunoblotting. Tsitologiia 2016, 58, 534–542. [Google Scholar] [CrossRef]


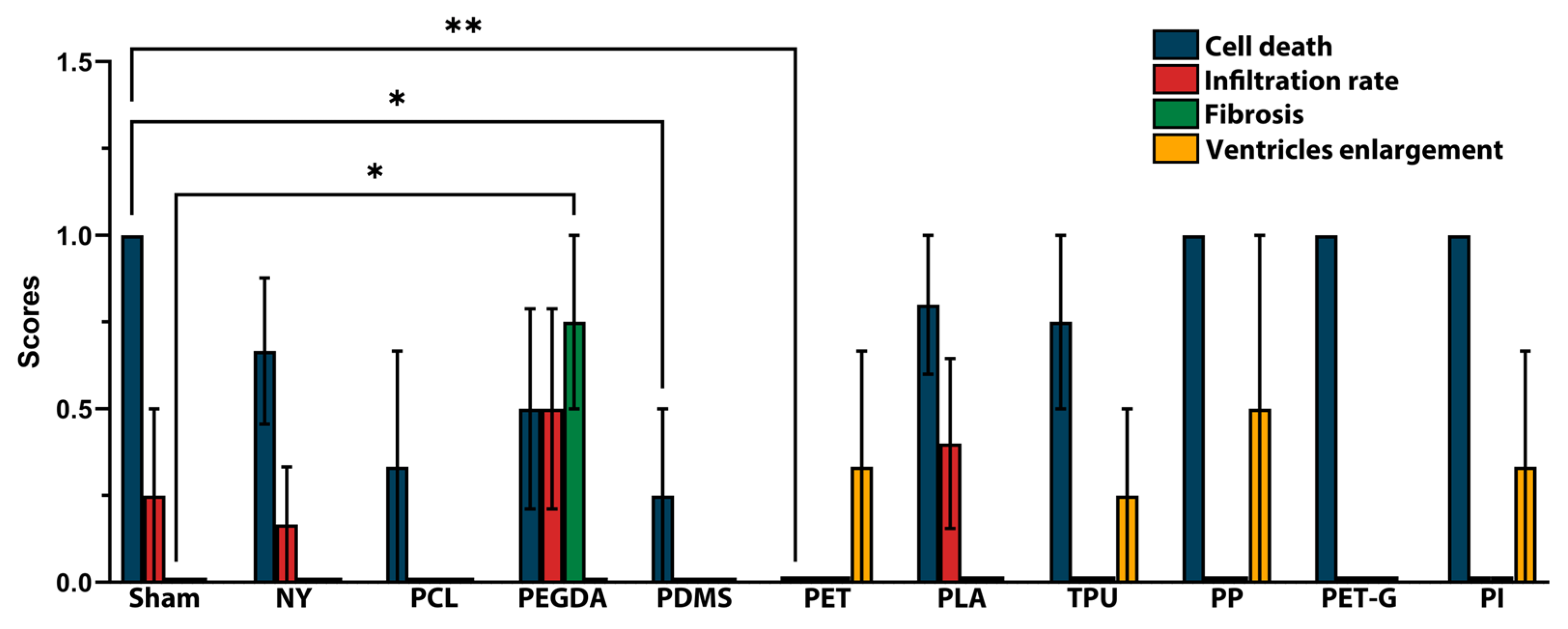

| Feature | Score | Description |
|---|---|---|
| Cell death | 0 | None, or only in the area of injection in the cortex |
| 1 | Degraded neurons in the peri-implant area | |
| 2 | Massive degeneration of neurons at the peri-implant area with edema of neuropil | |
| 3 | Extensive degeneration of neurons and neuropil deterioration with active immune response | |
| Infiltration rate | 0 | None |
| 1 | Mild: presence of activated glial cells and rare macrophages | |
| 2 | Moderate: Focal dense immune infiltrate containing glial cells, macrophages, and lymphocytes | |
| 3 | Severe: Granuloma formation with multinucleated giant cells | |
| Fibrosis | 0 | None |
| 1 | Sparse collagen fibers are non-uniformly spread around the implant | |
| 2 | Uniform layer of collagen fibers all around the implant | |
| 3 | Extensive fibrotic scar | |
| Ventricles enlargement | 0 | None |
| 1 | Unilateral ventricle enlargement | |
| 2 | Bilateral ventricle enlargement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makievskaya, C.; Brezgunova, A.; Andrianova, N.; Kelm, E.; Solovyova, M.; Naumova, G.; Zeinalova, A.; Gancharova, O.; Bushkova, T.; Kozlov, D.; et al. Foreign Body Reaction to Neural Implants: A Comparative Study of Polymer Toxicity and Tissue Response. Biosensors 2025, 15, 599. https://doi.org/10.3390/bios15090599
Makievskaya C, Brezgunova A, Andrianova N, Kelm E, Solovyova M, Naumova G, Zeinalova A, Gancharova O, Bushkova T, Kozlov D, et al. Foreign Body Reaction to Neural Implants: A Comparative Study of Polymer Toxicity and Tissue Response. Biosensors. 2025; 15(9):599. https://doi.org/10.3390/bios15090599
Chicago/Turabian StyleMakievskaya, Ciara, Anna Brezgunova, Nadezda Andrianova, Evgeny Kelm, Maria Solovyova, Gelena Naumova, Alina Zeinalova, Olga Gancharova, Tatiana Bushkova, Daniil Kozlov, and et al. 2025. "Foreign Body Reaction to Neural Implants: A Comparative Study of Polymer Toxicity and Tissue Response" Biosensors 15, no. 9: 599. https://doi.org/10.3390/bios15090599
APA StyleMakievskaya, C., Brezgunova, A., Andrianova, N., Kelm, E., Solovyova, M., Naumova, G., Zeinalova, A., Gancharova, O., Bushkova, T., Kozlov, D., Putlayev, V., Evdokimov, P., Petrov, A., Lebedev, M., Plotnikov, E., & Popkov, V. (2025). Foreign Body Reaction to Neural Implants: A Comparative Study of Polymer Toxicity and Tissue Response. Biosensors, 15(9), 599. https://doi.org/10.3390/bios15090599






