In Vivo Study on the Safe Use of a Novel Intraoperative Sensing Tool for Tissue Stiffness Assessment in Endoscopic Surgery
Abstract
1. Introduction
2. Materials and Methods
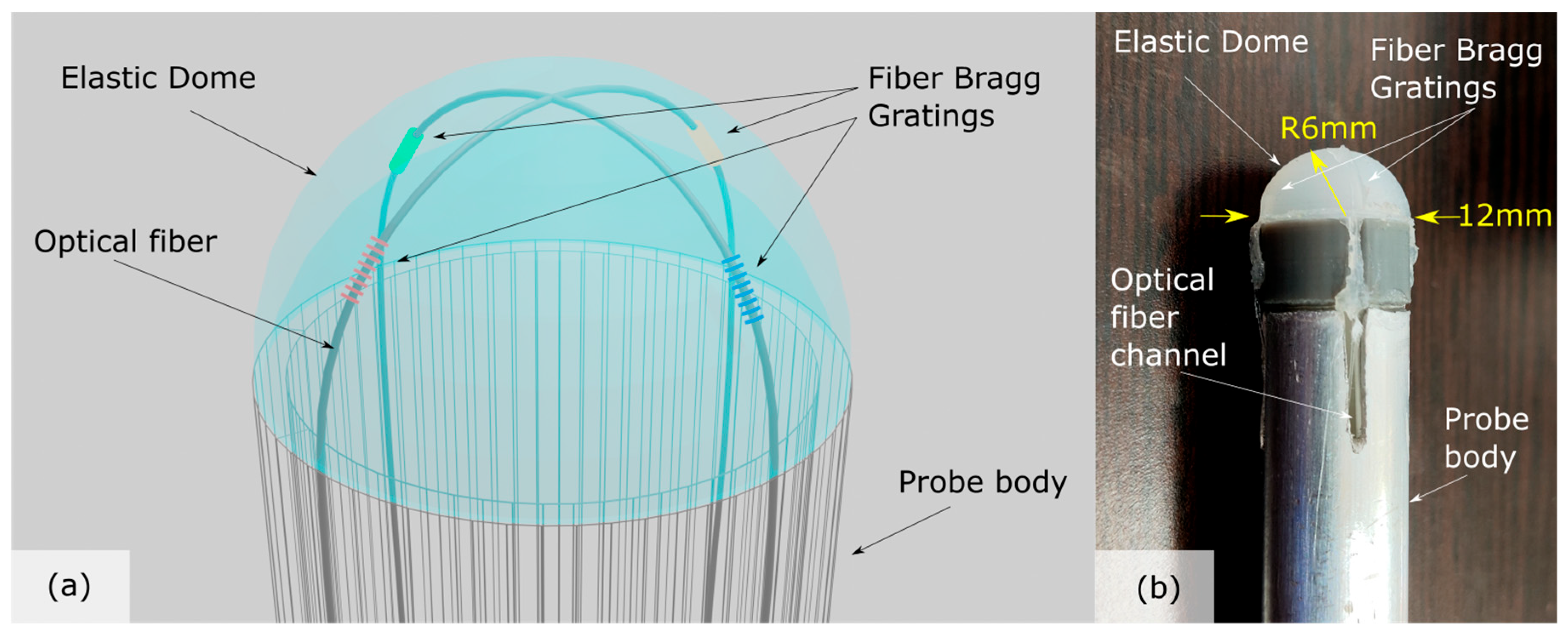
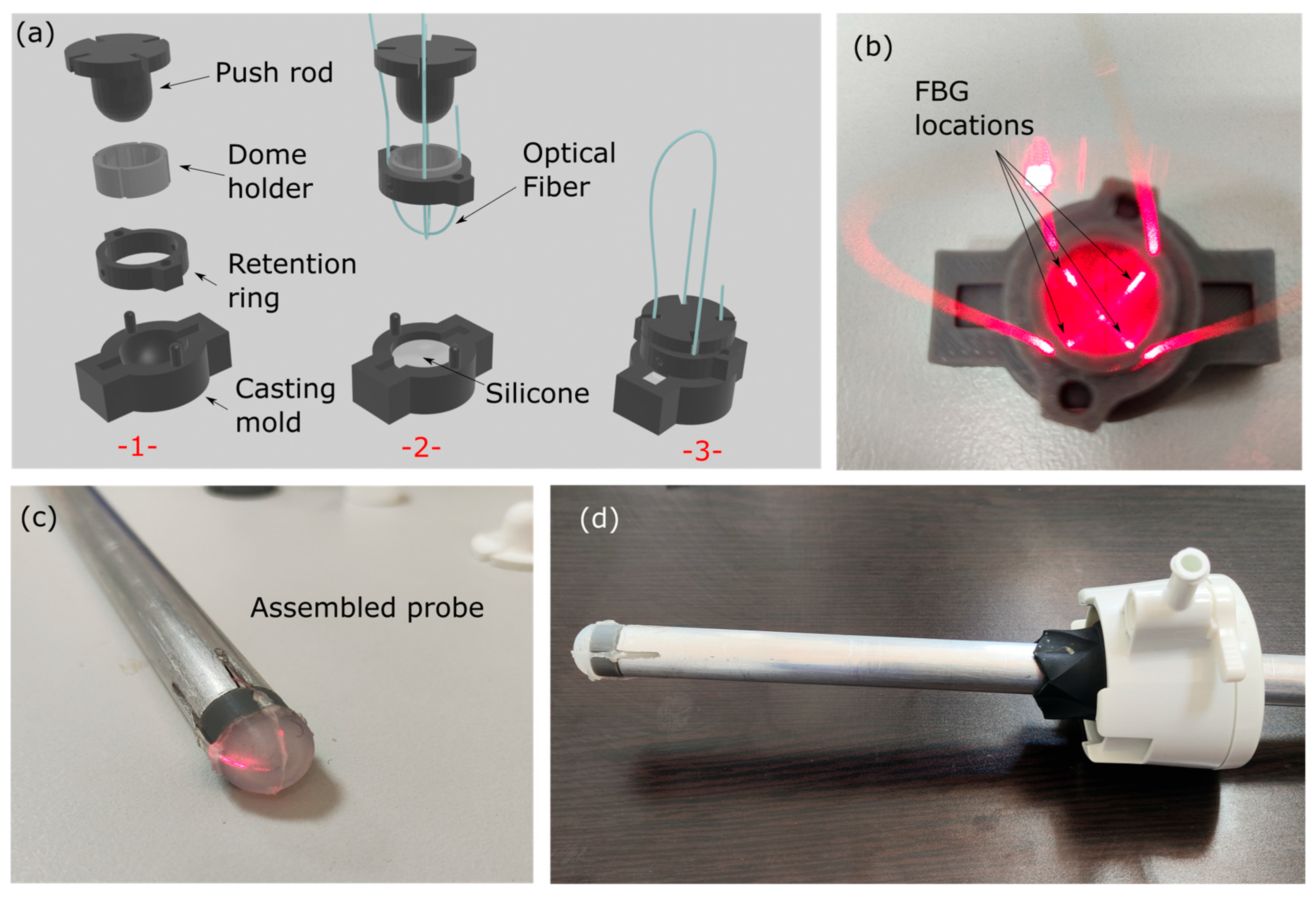
2.1. Palpation Probe Design, Manufacturing, Monitoring and Materials
2.2. Anesthesia and Surgical Technique
2.3. EPT Physiological Effects—Safety Assessment
- Cardiopulmonary function: To assess safety and the potential effect of intraoperative EPT application to the cardiopulmonary function, basic physiologic parameters including blood pressure, pulse rate and SpO2 were recorded before the initial and after the final application of the EPT.
- Inflammatory response, liver and renal function: Blood samples were drawn before the initial and after the final application of the EPT during laparoscopy to assess safety and its potential effects on inflammatory response and liver and renal function. White blood cell count, neutrophils, and C-reactive protein were used for the assessment of the inflammatory response, aspartic (AST) and alanine (ALT) aminotransferase for liver function, and urea and creatinine levels for renal function evaluation.
- Histopathological assessment: After the completion of laparoscopy, a midline laparotomy was performed. All areas of EPT—tissue contact (left side of the acrylic clip) were retrieved along with the neighboring tissue on the right side of the clip, the latter serving as normal control tissue. After initial formalin fixation all samples were evaluated in hematoxylin—eosin-stained sections for the following parameters: inflammation, necrosis, and stromal changes (hyalinization and changes in vessel wall).
2.4. EPT Performance in the Assessement of Solid Objects Underneath Normal Liver Parenchyma
3. Results
3.1. EPT Effects and Safety Profile
3.1.1. Cardiopulmonary Function
3.1.2. Inflammatory Response, Liver and Renal Function
3.1.3. Effects on Tissues Histology
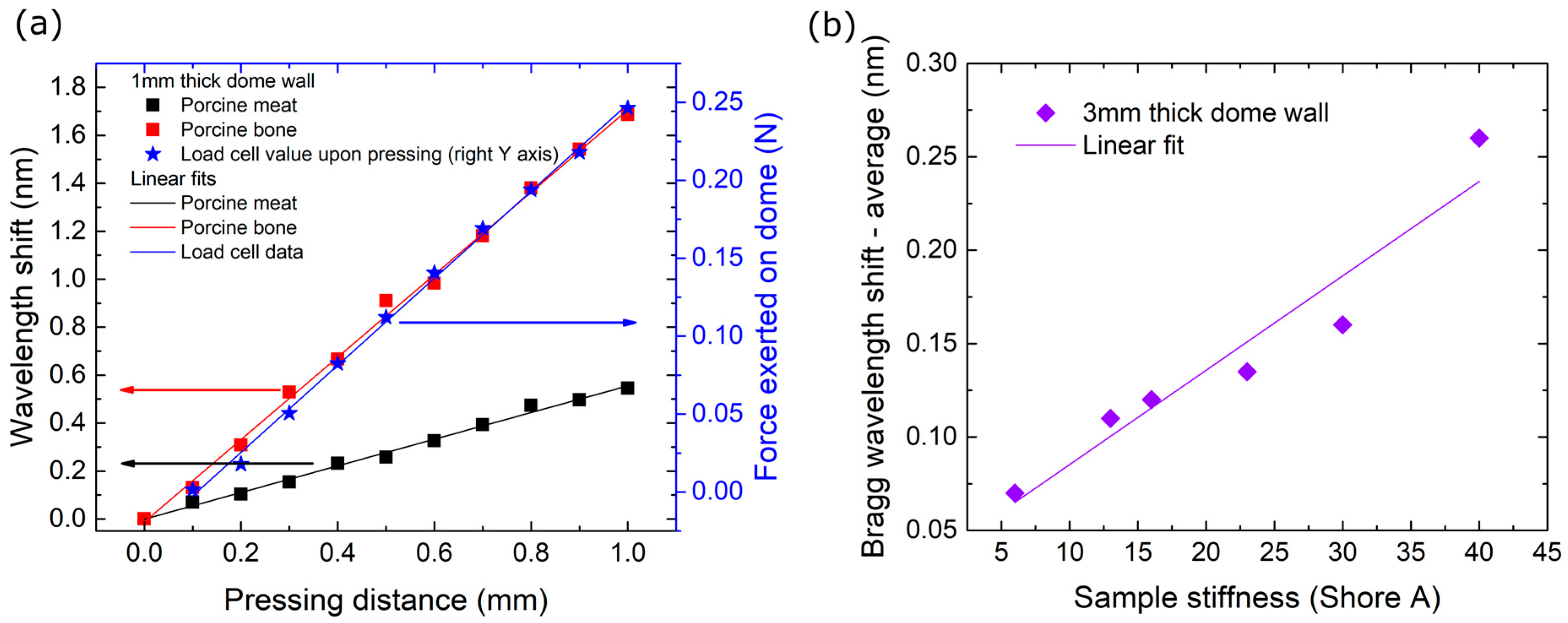
3.2. Stiffness Mapping Results
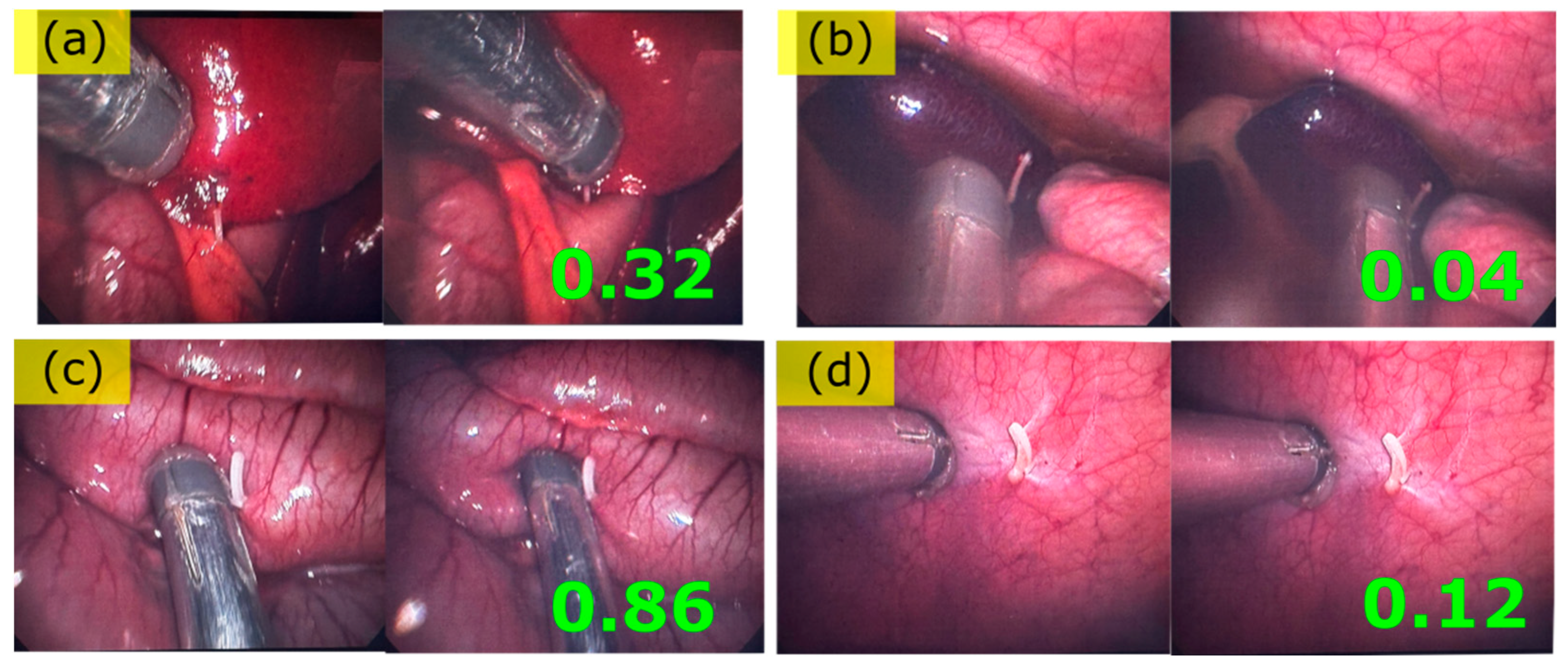
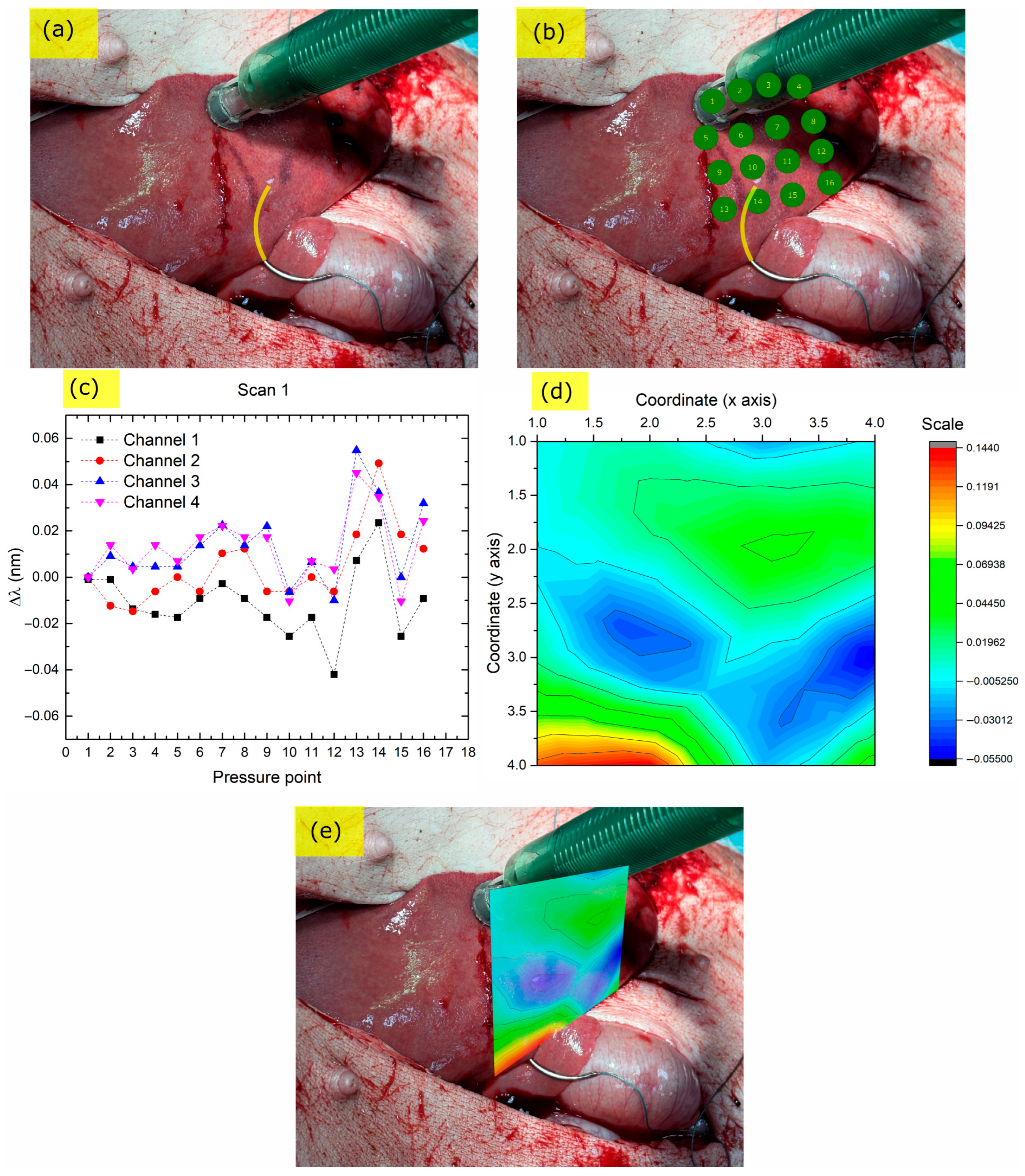
3.2.1. Endoscopic Palpation Scans
3.2.2. Liver, Ex-Vivo, Scan 1
3.2.3. Liver, Ex Vivo, Scan 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPT | Endoscopic palpation tool |
| MIS | Minimally invasive surgery |
| FBG | Fiber Bragg grating |
| FP | Fabry-Pérot |
| VCSEL | Vertical-cavity surface-emitting laser |
References
- Colan, J.; Davila, A.; Hasegawa, Y. Tactile Feedback in Robot-Assisted Minimally Invasive Surgery: A Systematic Review. Int. J. Med. Robot. 2024, 20, e70019. [Google Scholar] [CrossRef]
- Selim, M.; Dresscher, D.; Abayazid, M. A Comprehensive Review of Haptic Feedback in Minimally Invasive Robotic Liver Surgery: Advancements and Challenges. Int. J. Med. Robot. 2024, 20, e2605. [Google Scholar] [CrossRef] [PubMed]
- Puangmali, P.; Althoefer, K.; Seneviratne, L.D.; Murphy, D.; Dasgupta, P. State-of-the-Art in Force and Tactile Sensing for Minimally Invasive Surgery. IEEE Sens. J. 2008, 8, 371–381. [Google Scholar] [CrossRef]
- Othman, W.; Lai, Z.-H.A.; Abril, C.; Barajas-Gamboa, J.S.; Corcelles, R.; Kroh, M.; Qasaimeh, M.A. Tactile Sensing for Minimally Invasive Surgery: Conventional Methods and Potential Emerging Tactile Technologies. Front. Robot. AI 2022, 8, 705662. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Shi, X.; Yu, D.; Guo, X. Vision-Based High-Resolution Tactile Sensor by Using Visual Light Ring. IEEE Sens. Lett. 2022, 6, 1–4. [Google Scholar] [CrossRef]
- Di, J.; Dugonjic, Z.; Fu, W.; Wu, T.; Mercado, R.; Sawyer, K.; Most, V.R.; Kammerer, G.; Speidel, S.; Fan, R.E.; et al. Using Fiber Optic Bundles to Miniaturize Vision-Based Tactile Sensors. IEEE Trans. Robot. 2024, 41, 62–81. [Google Scholar] [CrossRef]
- Takashima, K.; Yoshinaka, K.; Okazaki, T.; Ikeuchi, K. An Endoscopic Tactile Sensor for Low Invasive Surgery. Sens. Actuators Phys. 2005, 119, 372–383. [Google Scholar] [CrossRef]
- Takahashi, T.; Suzuki, M.; Iwamoto, S.; Aoyagi, S. Capacitive Tactile Sensor Based on Dielectric Oil Displacement out of a Parylene Dome into Surrounding Channels. Micromachines 2012, 3, 270–278. [Google Scholar] [CrossRef]
- Charalambides, A.; Bergbreiter, S. A Novel All-Elastomer MEMS Tactile Sensor for High Dynamic Range Shear and Normal Force Sensing. J. Micromechanics Microengineering 2015, 25, 095009. [Google Scholar] [CrossRef]
- Kim, U.; Kim, Y.B.; Seok, D.-Y.; So, J.; Choi, H.R. A Surgical Palpation Probe With 6-Axis Force/Torque Sensing Capability for Minimally Invasive Surgery. IEEE Trans. Ind. Electron. 2018, 65, 2755–2765. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Li, T.-H.; Chou, I.-C.; Teng, Y.-J. Piezoelectric Tactile Sensor for Submucosal Tumor Detection in Endoscopy. Sens. Actuators Phys. 2016, 244, 299–309. [Google Scholar] [CrossRef]
- Chi, C.; Sun, X.; Xue, N.; Li, T.; Liu, C. Recent Progress in Technologies for Tactile Sensors. Sensors 2018, 18, 948. [Google Scholar] [CrossRef] [PubMed]
- Man, J.; Jin, Z.; Chen, J. Magnetic Tactile Sensor with Bionic Hair Array for Sliding Sensing and Object Recognition. Adv. Sci. 2024, 11, 2306832. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Liu, P.; Xu, C.; Lai, J.; Yuan, W.; Ren, H. ElastoSight: Miniature Tactile Sensors for Multimodal Force and Geometry Estimation in Robotic Intraluminal Palpation. In Proceedings of the 2025 IEEE 8th International Conference on Soft Robotics (RoboSoft), Lausanne, Switzerland, 22–26 April 2025; IEEE: New York, NY, USA, 2025; pp. 1–7. [Google Scholar]
- Raitt, D.G.; Huseynov, M.; Homer-Vanniasinkam, S.; Wurdemann, H.A.; Abad, S.-A. Soft-Tipped Sensor with Compliance Control for Elasticity Sensing and Palpation. IEEE Trans. Robot. 2024, 40, 2430–2441. [Google Scholar] [CrossRef]
- Faragasso, A.; Stilli, A.; Bimbo, J.; Wurdemann, H.A.; Althoefer, K. Multi-Axis Stiffness Sensing Device for Medical Palpation. In Proceedings of the 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Hamburg, Germany, 28 September–2 October 2015; IEEE: New York, NY, USA, 2015; pp. 2711–2716. [Google Scholar]
- Prathap, P.B.; Saara, K. Quantifying Efficacy of the Fiber Bragg Grating Sensors in Medical Applications: A Survey. J. Opt. 2024, 53, 4180–4201. [Google Scholar] [CrossRef]
- Manavi Roodsari, S.; Freund, S.; Angelmahr, M.; Seppi, C.; Rauter, G.; Schade, W.; Cattin, P.C. Deep Learning-Based Approach for High Spatial Resolution Fibre Shape Sensing. Commun. Eng. 2024, 3, 1–10. [Google Scholar] [CrossRef]
- Machaca, S.; Cao, E.; Chi, A.; Adrales, G.; Kuchenbecker, K.J.; Brown, J.D. Wrist-Squeezing Force Feedback Improves Ac-curacy and Speed in Robotic Surgery Training. In Proceedings of the 2022 9th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), Seoul, Republic of Korea, 21 August 2022; IEEE: New York, NY, USA, 2022; pp. 1–8. [Google Scholar]
- Nair, B.R.; Aravinthkumar, A.; Vinod, B. Advancing Robotic Surgery: Affordable Kinesthetic and Tactile Feedback Solutions for Endotrainers. In Proceedings of the Fifth International Conference on Control, Robotics, and Intelligent System, Macau, China, 28 October 2024. [Google Scholar]
- Dong, S.; Xiong, X.; Lou, Y.; Luo, D.; Wu, J.; Yang, T.; Liu, H.; Dong, Y. Development of FBG Miniature Force Sensors for Tactile Sensing in Robot-Assisted Minimally Invasive Surgery. In Proceedings of the Fourth International Computational Imaging Conference (CITA 2024), Xiamen, China, 20–22 September 2024; SPIE: Bellingham, WA, USA, 2025; Volume 13542, pp. 289–294. [Google Scholar]
- Lv, C.; Wang, S.; Shi, C. A High-Precision and Miniature Fiber Bragg Grating-Based Force Sensor for Tissue Palpation during Minimally Invasive Surgery. Ann. Biomed. Eng. 2020, 48, 669–681. [Google Scholar] [CrossRef]
- Ping, Z.; Zhang, T.; Gong, L.; Zhang, C.; Zuo, S. Miniature Flexible Instrument with Fibre Bragg Grating-Based Triaxial Force Sensing for Intraoperative Gastric Endomicroscopy. Ann. Biomed. Eng. 2021, 49, 2323–2336. [Google Scholar] [CrossRef]
- Othman, W.; Vandyck, K.E.; Abril, C.; Barajas-Gamboa, J.S.; Pantoja, J.P.; Kroh, M.; Qasaimeh, M.A. Stiffness Assessment and Lump Detection in Minimally Invasive Surgery Using In-House Developed Smart Laparoscopic Forceps. IEEE J. Transl. Eng. Health Med. 2022, 10, 1–10. [Google Scholar] [CrossRef]
- Ly, H.H.; Tanaka, Y.; Fujiwara, M. A Tactile Sensor Using the Acoustic Reflection Principle for Assessing the Contact Force Component in Laparoscopic Tumor Localization. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 289–299. [Google Scholar] [CrossRef]
- Gan, L.; Duan, W.; Akinyemi, T.O.; Du, W.; Omisore, O.M.; Wang, L. Development of a Fiber Bragg Grating-Based Force Sensor for Minimally Invasive Surgery–Case Study of Ex-Vivo Tissue Palpation. IEEE Trans. Instrum. Meas. 2021, 72, 1–12. [Google Scholar] [CrossRef]
- Qu, W.; Ma, C.; Chen, Y.; Peng, D.; Liu, S.; Luo, L. A High-Sensitivity Force Sensor for Minimally Invasive Surgery Palpation Based on Fabry-P4erot Cavity and Rectangular Slot Hollow Structure. IEEE Trans. Instrum. Meas. 2025, 74, 1–10. [Google Scholar]
- Leong, C.Y.; Cheng, X.; Cui, J.; Gunawardena, D.S.; Tam, H.-Y. Artificial Skin Based on Polymer Optical Fiber Bragg Grating Arrays for Robotic Tactile Perception. J. Light. Technol. 2023, 42, 3022–3029. [Google Scholar] [CrossRef]
- Waltermann, C.; Bethmann, K.; Doering, A.; Jiang, Y.; Baumann, A.L.; Angelmahr, M.; Schade, W. Multiple Off-Axis Fiber Bragg Gratings for 3D Shape Sensing. Appl. Opt. 2018, 57, 8125–8133. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Q.; Zhao, M.; Chen, C.; Pan, S.; Yuan, M. Tactile Perception Technologies and Their Applications in Minimally Invasive Surgery: A Review. Front. Physiol. 2020, 11, 611596. [Google Scholar] [CrossRef]
- Eklund, A.; Bergh, A.; Lindahl, O. A Catheter Tactile Sensor for Measuring Hardness of Soft Tissue: Measurement in a Silicone Model and in an in Vitro Human Prostate Model. Med. Biol. Eng. Comput. 1999, 37, 618–624. [Google Scholar] [CrossRef]
- Alleblas, C.C.J.; Vleugels, M.P.H.; Stommel, M.W.J.; Nieboer, T.E. Performance of a Haptic Feedback Grasper in Laparoscopic Surgery: A Randomized Pilot Comparison with Conventional Graspers in a Porcine Model. Surg. Innov. 2019, 26, 573–580. [Google Scholar] [CrossRef]
- Trejos, A.L.; Jayender, J.; Perri, M.T.; Naish, M.D.; Patel, R.V.; Malthaner, R.A. Robot-Assisted Tactile Sensing for Minimally Invasive Tumor Localization. Int. J. Robot. Res. 2009, 28, 1118–1133. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Dong, L.; Han, X.; Du, W.; Zhai, J.; Pan, C.; Wang, Z.L. Self-Powered High-Resolution and Pressure-Sensitive Triboelectric Sensor Matrix for Real-Time Tactile Mapping. Adv. Mater. 2016, 28, 2896–2903. [Google Scholar] [CrossRef]
- Li, T.; Shi, C.; Ren, H. A High-Sensitivity Tactile Sensor Array Based on Fiber Bragg Grating Sensing for Tissue Palpation in Minimally Invasive Surgery. IEEE/ASME Trans. Mechatron. 2018, 23, 2306–2315. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, S.; Liu, Z.; Huang, Y.; Huang, J.; Huang, X.; Chen, J.; Fang, B.; Peng, D. Smart Laparoscopic Grasper In-tegrated with Fiber Bragg Grating Based Tactile Sensor for Real-time Force Feedback. J. Biophotonics 2022, 15, e202100331. [Google Scholar] [CrossRef]
- Murakami, Y.; Tsuchiya, H. Bending Losses of Coated Single-Mode Optical Fibers. IEEE J. Quantum Electron. 1978, 14, 495–501. [Google Scholar] [CrossRef]


| Swine 1 | Swine 2 | |||
|---|---|---|---|---|
| Vitals | Before | After | Before | After |
| HR (bpm) | 110 | 130 | 120 | 129 |
| Systolic (mmHg) | 117 | 110 | 125 | 110 |
| Diastolic (mmHg) | 90 | 93 | 95 | 85 |
| SpO2 (%) | 96 | 95 | 97 | 95 |
| T (°C) | 110 | 130 | 120 | 129 |
| Laboratory | ||||
| WBC (×103/μL) | 5900 | 9600 | 6200 | 6400 |
| Neut (%) | 36% | 52% | 41% | 47% |
| Hb (g/dL) | 10.9 | 11.2 | 12.6 | 12.5 |
| PLT (×103/μL) | 204 | 207 | 175 | 196 |
| Ur (mg/dL) | 32.2 | 34.1 | 34.2 | 31 |
| Cr (mg/dL) | 1.4 | 1.4 | 2.2 | 2.4 |
| AST (IU/L) | 46 | 64 | 159 | 148 |
| ALT (IU/L) | 58 | 61 | 67 | 60 |
| CRP (mg/dL) | 0.06 | 0.06 | 0.06 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Violakis, G.; Antonakis, P.; Kritsotakis, E.; Kozonis, T.; Chardalias, L.; Papalois, A.; Agrogiannis, G.; Kampouroglou, E.; Vardakis, N.; Kostakis, S.; et al. In Vivo Study on the Safe Use of a Novel Intraoperative Sensing Tool for Tissue Stiffness Assessment in Endoscopic Surgery. Biosensors 2025, 15, 581. https://doi.org/10.3390/bios15090581
Violakis G, Antonakis P, Kritsotakis E, Kozonis T, Chardalias L, Papalois A, Agrogiannis G, Kampouroglou E, Vardakis N, Kostakis S, et al. In Vivo Study on the Safe Use of a Novel Intraoperative Sensing Tool for Tissue Stiffness Assessment in Endoscopic Surgery. Biosensors. 2025; 15(9):581. https://doi.org/10.3390/bios15090581
Chicago/Turabian StyleViolakis, Georgios, Pantelis Antonakis, Emmanouil Kritsotakis, Theodoros Kozonis, Leonidas Chardalias, Apostolos Papalois, Georgios Agrogiannis, Effrosyni Kampouroglou, Nikolaos Vardakis, Stylianos Kostakis, and et al. 2025. "In Vivo Study on the Safe Use of a Novel Intraoperative Sensing Tool for Tissue Stiffness Assessment in Endoscopic Surgery" Biosensors 15, no. 9: 581. https://doi.org/10.3390/bios15090581
APA StyleViolakis, G., Antonakis, P., Kritsotakis, E., Kozonis, T., Chardalias, L., Papalois, A., Agrogiannis, G., Kampouroglou, E., Vardakis, N., Kostakis, S., Athanasaki, E., Zhang, Z., Angelmahr, M., Konstadoulakis, M., & Polygerinos, P. (2025). In Vivo Study on the Safe Use of a Novel Intraoperative Sensing Tool for Tissue Stiffness Assessment in Endoscopic Surgery. Biosensors, 15(9), 581. https://doi.org/10.3390/bios15090581








