Three-Dimensional SERS Substrates: Architectures, Hot Spot Engineering, and Biosensing Applications
Abstract
1. Introduction
2. Comparison Between 2D and 3D SERS Substrates
2.1. Structural Design and Hot Spot Distribution
2.2. Enhancement of Performance and Reproducibility
2.3. Analyte Accessibility and Compatibility with Complex Matrices
2.4. Fabrication and Functional Integration
2.5. Summary of Comparison
2.6. Comparison of Biosensing Applications Between 2D and 3D Substrates
3. SERS Enhancement Principles in 3D Architectures
3.1. Electromagnetic Enhancement
3.2. Chemical Enhancement
3.3. Multiple Scattering and Light Trapping
4. Types of 3D SERS Substrates
4.1. Vertically Aligned Nanowire and Nanorod Arrays
4.2. Dendritic Nanostructures
4.3. Porous Frameworks and Aerogel-Based Scaffolds
4.4. Core–Shell and Hollow Nanosphere Assemblies
4.5. Hierarchical Hybrid Architectures
5. Fabrication Strategies
5.1. Template-Assisted Growth
5.2. Electrochemical and Galvanic Formation of Dendritic Structures
5.3. Dealloying and Freeze-Drying Strategies for Porous and Aerogel Frameworks
5.4. Self-Assembly of Core–Shell and Hollow Nanostructures
5.5. Hybrid Integration Strategies for Hierarchical Architectures
5.6. Comparative Evaluation of Fabrication Methods
6. Biosensing Applications in Biological Sciences
6.1. The Biocompatibility of SERS Substrate Materials
6.2. Glucose Sensing
6.3. Tumor Sensing
6.4. Drug Delivery
6.5. Advantages in 3D SERS in Detection of Low-Abundance Biomarkers
7. Conclusions and Outlook
- (1)
- Stability in Complex Biological Matrices: Biological fluids such as blood, saliva, or tissue homogenates are chemically complex and prone to non-specific adsorption of proteins and macromolecules onto nanostructured surfaces. This can lead to degradation of the signal intensity or aggregation of the nanostructures, particularly in silver-based substrates. Core–shell designs (e.g., Au@Ag) and surface passivation strategies (e.g., PEGylation) are promising, but more universal stabilization methods are needed for long-term operation in variable physiological conditions.
- (2)
- Signal Attenuation Under Extreme or Variable Conditions: Environmental stressors such as high ionic strength, fluctuating pH, temperature changes, and oxidative conditions can impair signal reproducibility. Substrates embedded in hydrogels or flexible matrices may undergo deformation or swelling, disrupting the hot spot architecture. Therefore, further work is required to engineer mechanically and chemically resilient substrates that can retain signal fidelity under such stresses.
- (3)
- Lack of Standardization and Reproducibility: Many 3D SERS fabrication strategies, especially those based on electrochemical growth or dendritic self-assembly, still suffer from high batch-to-batch variability. This hinders quantitative biosensing applications and large-scale deployment. Introducing scalable, template-guided synthesis and machine learning-guided optimization may help overcome this limitation.
- (4)
- Optical Interference and Quantification in Turbid Media: Signal attenuation due to scattering, absorption, or matrix interferences in turbid samples remains a major issue for in vivo and real-time applications. Integration of optical trapping designs, internal standards, and ratiometric SERS techniques can enhance spectral reliability, but requires further refinement for clinical use.
Author Contributions
Funding
Conflicts of Interest
References
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Qian, X.; Nie, S. Single-Molecule and Single-Nanoparticle SERS: From Fundamental Mechanisms to Biomedical Applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Surface-Enhanced Raman Scattering and Biophysics. J. Phys. Condens. Matter 2002, 14, R597–R624. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.J.; Wei, T.; Ma, X.; Zheng, X.S.; Ren, B. Surface-enhanced Raman spectroscopy for bioanalysis: Reliability and challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Jebakumari, K.E.; Murugasenapathi, N.K.; Palanisamy, T. Engineered two-dimensional nanostructures as SERS substrates for biomolecule sensing: A review. Biosensors 2023, 13, 102. [Google Scholar] [CrossRef]
- Liang, X.; Li, N.; Zhang, R.; Yin, P.; Zhang, C.; Yang, N.; Liang, K.; Kong, B. Carbon-based SERS biosensor: From substrate design to sensing and bioapplication. NPG Asia Mater. 2021, 13, 8. [Google Scholar] [CrossRef]
- Zheng, H.; Ding, Q.; Li, C.; Chen, W.; Chen, X.; Lin, Q.; Wang, D.; Weng, Y.; Lin, D. Recent progress in surface-enhanced Raman spectroscopy-based biosensors for the detection of extracellular vesicles. Anal. Methods 2022, 14, 4161–4173. [Google Scholar] [CrossRef]
- Hu, W.; Xia, L.; Hu, Y.; Li, G. Recent progress on three-dimensional substrates for surface-enhanced Raman spectroscopic analysis. Microchem. J. 2022, 172, 106908. [Google Scholar] [CrossRef]
- Mukherjee, A.; Wackenhut, F.; Dohare, A.; Horneber, A.; Lorenz, A.; Müchler, H.; Meixner, A.J.; Mayer, H.A.; Brecht, M. Three-dimensional (3D) surface-enhanced Raman spectroscopy (SERS) substrates: Fabrication and SERS applications. J. Phys. Chem. C 2023, 127, 13689–13698. [Google Scholar] [CrossRef]
- Geng, X.; Wu, C.; Liu, S.; Han, Y.; Song, L.; Zhang, Y. Fabrication optimization and application of 3D hybrid SERS substrates. RSC Adv. 2021, 11, 31400–31407. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Ju, W.; Yang, H.Y.; Li, Z. Dimensional design for surface-enhanced Raman spectroscopy. ACS Mater. Au 2022, 2, 552–575. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhou, X.; Liu, W.; Liu, Y.; Wang, X. Flexible DNA Hydrogel SERS Active Biofilms for Conformal Ultrasensitive Detection of Uranyl Ions from Aquatic Products. Langmuir ACS J. Surf. Colloids 2020, 36, 2930–2936. [Google Scholar] [CrossRef]
- Guan, P.C.; Qi, Q.J.; Wang, Y.Q.; Lin, J.S.; Zhang, Y.J.; Li, J.F. Development of a 3D hydrogel SERS chip for noninvasive, real-time pH and glucose monitoring in sweat. ACS Appl. Mater. Interfaces 2024, 16, 48139–48146. [Google Scholar] [CrossRef]
- Quinn, A.; You, Y.H.; McShane, M.J. Hydrogel Microdomain Encapsulation of Stable Functionalized Silver Nanoparticles for SERS pH and Urea Sensing. Sensors 2019, 19, 3521. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, D.; Yan, M.; Xu, G.; Zhai, H.; Luo, J.; Wang, G.; Jiang, D.; Yuan, Y. Three-dimensional surface-enhanced Raman scattering substrates constructed by integrating template-assisted electrodeposition and post-growth of silver nanoparticles. J. Colloid Interface Sci. 2022, 608, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Q.; Yin, Y. Colloidal self-assembly approaches to smart nanostructured materials. Chem. Rev. 2021, 122, 4976–5067. [Google Scholar] [CrossRef]
- Ali, M.A.; Hu, C.; Yttri, E.A.; Panat, R. Recent advances in 3D printing of biomedical sensing devices. Adv. Funct. Mater. 2022, 32, 2107671. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.; Xu, C.; Liu, D. SERS tags for biomedical detection and bioimaging. Theranostics 2022, 12, 1870. [Google Scholar] [CrossRef]
- Kneipp, K.; Moskovits, M.; Kneipp, H. Surface-enhanced Raman scattering. In Phys. Today; 2007; Volume 60, pp. 40–46. [Google Scholar] [CrossRef]
- Tame, M.S.; McEnery, K.R.; Özdemir, Ş.K.; Lee, J.; Maier, S.A.; Kim, M.S. Quantum Plasmonics. Nat. Phys. 2013, 9, 329–340. [Google Scholar] [CrossRef]
- Mcoyi, M.P.; Mpofu, K.T.; Sekhwama, M.; Mthunzi-Kufa, P. Developments in Localized Surface Plasmon Resonance. Plasmonics 2025, 20, 5481–5520. [Google Scholar] [CrossRef]
- Yi, J.; You, E.-M.; Hu, R.; Wu, D.-Y.; Liu, G.-K.; Yang, Z.-L.; Zhang, H.; Gu, Y.; Wang, Y.-H.; Wang, X.; et al. Surface-enhanced Raman spectroscopy: A half-century historical perspective. Chem. Soc. Rev. 2025, 54, 1453–1551. [Google Scholar] [CrossRef]
- Pandey, P.; Seo, M.-K.; Shin, K.H.; Sohn, J.I. Hierarchically Assembled Plasmonic Metal-Dielectric-Metal Hybrid Nano-Architectures for High-Sensitivity SERS Detection. Nanomaterials 2022, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lindquist, N.C.; Klemme, D.J.; Nagpal, P.; Norris, D.J.; Oh, S.H. Split-wedge antennas with sub-5 nm gaps for plasmonic nanofocusing. Nano Lett. 2016, 16, 7849–7856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, T.; Ai, B.; Gu, P.; Guan, Y.; Wang, Y.; Zhao, Z.; Zhang, G. Multiple plasmonic hot spots platform: Nanogap coupled gold nanoparticles. Appl. Surf. Sci. 2022, 593, 153388. [Google Scholar] [CrossRef]
- Lu, Z.; Ji, J.; Ye, H.; Zhang, H.; Zhang, S.; Xu, H. Quantifying the ultimate limit of plasmonic near-field enhancement. Nat. Commun. 2025, 15, 8803. [Google Scholar] [CrossRef]
- Koya, A.N.; Zhu, X.; Ohannesian, N.; Yanik, A.A.; Alabastri, A.; Zaccaria, R.P.; Krahne, R.; Shih, W.-C.; Garoli, D. Nanoporous metals: From plasmonic properties to applications in enhanced spectroscopy and photocatalysis. ACS Nano 2021, 15, 6038–6060. [Google Scholar] [CrossRef]
- Bhattarai, J.K.; Neupane, D.; Nepal, B.; Mikhaylov, V.; Demchenko, A.V.; Stine, K.J. Preparation, modification, characterization, and biosensing application of nanoporous gold using electrochemical techniques. Nanomaterials 2018, 8, 171. [Google Scholar] [CrossRef]
- Bandarenka, H.V.; Khinevich, N.V.; Burko, A.A.; Redko, S.V.; Zavatski, S.A.; Shapel, U.A.; Mamatkulov, K.Z.; Vorobyeva, M.Y.; Arzumanyan, G.M. 3D silver dendrites for single-molecule imaging by surface-enhanced Raman spectroscopy. ChemNanoMat 2021, 7, 141–149. [Google Scholar] [CrossRef]
- Křápek, V.; Řepa, R.; Foltýn, M.; Šikola, T.; Horák, M. Plasmonic lightning-rod effect. arXiv 2024, arXiv:2407.09454. [Google Scholar] [CrossRef]
- Chakraborti, S.; Basu, R.N.; Panda, S.K. Vertically aligned silicon nanowire array decorated by Ag or Au nanoparticles as SERS substrate for bio-molecular detection. Plasmonics 2018, 13, 1057–1080. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhu, K.; Ning, L.; Cui, G.; Qu, J.; Cheng, Y.; Xin, Y. Highly sensitive surface enhanced Raman scattering substrates based on Ag decorated Si nanocone arrays and their application in trace dimethyl phthalate detection. Appl. Sci. 2015, 325, 45–51. [Google Scholar] [CrossRef]
- Navarro-Segura, M.E.; Rivera-Rangel, R.D.; Arizmendi-Morquecho, A.; Lopez, I.; Alvarez-Quintana, J.; Sanchez-Dominguez, M. Ultra-high sensitivity surface-enhanced Raman spectroscopy (SERS) substrates based on au nanostructured hollow octahedra. Appl. Mater. Today 2022, 29, 101598. [Google Scholar] [CrossRef]
- Paganoto, G.T.; Temperini, M.L.A.; dos Santos, D.P. Nanoparticle Shape Effects on the SERS Properties of Small Conductive Bridges. J. Phys. Chem. C 2024, 128, 15974–15984. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Z.; Qi, J.; Zhang, S.; Guo, K.; Zhao, S. Modeling and theoretical analysis of the SERS enhancement factor considering the electronic structural energy. IEEE Access 2021, 9, 121279–121287. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A Unified Approach to Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617. [Google Scholar] [CrossRef]
- Adrian, F.J. Charge transfer effects in surface-enhanced Raman scattering. J. Chem. Phys. 1982, 77, 5302–5314. [Google Scholar] [CrossRef]
- Wang, C.; Guo, X.; Fu, Q. TiO2 Thickness-Dependent Charge Transfer in an Ordered Ag/TiO2/Ni Nanopillar Arrays Based on Surface-Enhanced Raman Scattering. Materials 2022, 15, 3716. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chui, K.K.; Fang, Y.; Wen, S.; Zhuo, X.; Wang, J. Metal–Organic Framework-Enabled Trapping of Volatile Organic Compounds into Plasmonic Nanogaps for Surface-Enhanced Raman Scattering Detection. ACS Nano 2024, 18, 11234–11244. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Park, Y.; Park, E.; Jin, S.; Chen, L.; Jung, Y.M. Molecular-Orbital Delocalization Enhances Charge Transfer in π-Conjugated Organic Semiconductors. Angew. Chem. Int. Ed. 2023, 62, e202306709. [Google Scholar] [CrossRef]
- Yonezawa, Y.; Minamimoto, H.; Nagasawa, F.; Takase, M.; Yasuda, S.; Murakoshi, K. In-situ electrochemical surface-enhanced Raman scattering observation of molecules accelerating the hydrogen evolution reaction. J. Electroanal. Chem. 2017, 800, 7–12. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Wang, F.; Zhang, Y.; Wang, H.; Liu, Y. In situ formation of gold nanoparticles decorated Ti3C2 MXenes nanoprobe for highly sensitive electrogenerated chemiluminescence detection of exosomes and their surface proteins. Anal. Chem. 2020, 92, 5546–5553. [Google Scholar] [CrossRef]
- Birke, R.L.; Lombardi, J.R. TDDFT study of charge-transfer Raman spectra of 4-mercaptopyridine on various ZnSe nanoclusters as a model for the SERS of 4-Mpy on semiconductors. J. Phys. Chem. C 2018, 122, 4908–4927. [Google Scholar] [CrossRef]
- Boto, R.A.; Esteban, R.; Candelas, B.; Aizpurua, J. Theoretical Procedure for Precise Evaluation of Chemical Enhancement in Molecular Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2024, 128, 18293–18304. [Google Scholar] [CrossRef]
- Jones, T.; Zhou, D.; Liu, J.; Parkin, I.P.; Lee, T.C. Quantitative multiplexing of uric acid and creatinine using polydisperse plasmonic nanoparticles enabled by electrochemical-SERS and machine learning. J. Mater. Chem. B 2024, 12, 10563–10572. [Google Scholar] [CrossRef]
- Tian, F.; Carvalho, L.F.d.C.e.S.d.; Casey, A.; Nogueira, M.S.; Byrne, H.J. Surface-Enhanced raman analysis of uric acid and hypoxanthine analysis in fractionated bodily fluids. Nanomaterials 2023, 13, 1216. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Abutoama, M.; Abdulhalim, I. 3D nanoplasmonic structure for ultrahigh enhanced SERS with less variability, polarization independence, and multimodal sensing applied to picric acid detection. Nanoscale Adv. 2024, 6, 5681–5693. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yao, H.; Liu, C.; Chen, M.; Zhang, C.; Liu, M.; Wang, J.; Zhang, F.; Yu, J.; Man, B.; et al. High-performance flexible surface-enhanced Raman scattering substrate based on the particle-in-multiscale 3D structure. Nanophotonics 2021, 10, 4045–4055. [Google Scholar] [CrossRef]
- Sahoo, S.R.; Huang, C.T.; Tsai, K.; Wang, G.J.; Chang, C.C. A multiscale 3D hotspot-rich nanostructured substrate for biomolecular detection of SARS-CoV-2. Appl. Phys. Rev. 2023, 10, 041403. [Google Scholar] [CrossRef]
- Bartschmid, T.; Wendisch, F.J.; Farhadi, A.; Bourret, G.R. Recent advances in structuring and patterning silicon nanowire arrays for engineering light absorption in three dimensions. ACS Appl. Energy Mater. 2021, 5, 5307–5317. [Google Scholar] [CrossRef]
- Boles, M.A.; Large, N.; Lee, J.; DeVetter, B.M.; Lee, D.; Liu, Y.; Park, J.; Schatz, G.C.; Mirkin, C.A. Surface-Enhanced Raman Scattering and Surface-Enhanced Infrared Absorption by Plasmon Polaritons in Three-Dimensional Nanoparticle Supercrystals. ACS Nano 2021, 15, 4874–4883. [Google Scholar] [CrossRef]
- Apostolaki, M.-A.; Sakellis, E.; Gardelis, S.; Likodimos, V. Interplay of Plasmonic and Charge Transfer Effects for Ultrasensitive Ag-WO3/TiO2 Photonic Crystal SERS Sensors. Mater. Adv. 2025, 6, 388–399. [Google Scholar] [CrossRef]
- Farid, S.; Dixon, K.; Shayegannia, M.; Ko, R.H.; Safari, M.; Loh, J.Y.; Kherani, N.P. Rainbows at the end of subwavelength discontinuities: Plasmonic light trapping for sensing applications. Adv. Opt. Mater. 2021, 9, 2100695. [Google Scholar] [CrossRef]
- Lv, B.; Wang, Y.; Mei, R.; Li, Y.; Zhao, X.; Yuan, Q.; Xu, H.; Chen, L. Enhanced superhydrophobic substrate-based 3D SERS platform for a portable Raman spectrometer-assisted drug detection in plasma. ACS Appl. Nano Mater. 2024, 7, 14665–14672. [Google Scholar] [CrossRef]
- Eslami, S.; Palomba, S. Integrated enhanced Raman scattering: A review. Nano Converg. 2021, 8, 41. [Google Scholar] [CrossRef]
- Linh, V.T.N.; Ja’farawy, M.S.A.; Koh, E.H.; Lee, M.Y.; Park, S.G.; Kim, D.H.; Jung, H.S. Flexible surface-enhanced Raman scattering substrates toward sampling approaches for on-site sensing and diagnosis applications. Appl. Spectrosc. Rev. 2024, 59, 90–123. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Li, G.; Cui, J.; Jiang, Y.; Li, X.; Wang, Z.; Zhou, X. The efficient and sensitive detection of serum dopamine based on a MOF-199/Ag@Au composite SERS sensing structure. Chemosensors 2024, 12, 187. [Google Scholar] [CrossRef]
- Huang, Z.; Meng, G.; Huang, Q.; Chen, B.; Zhu, C.; Zhang, Z. Large-area Ag nanorod array substrates for SERS: AAO template-assisted fabrication, functionalization, and application in detection PCBs. J. Raman Spectrosc. 2013, 44, 240–246. [Google Scholar] [CrossRef]
- Huang, Z.; Meng, G.; Chen, B.; Zhu, C.; Han, F.; Hu, X.; Wang, X. Surface-enhanced Raman scattering from Au-nanorod arrays with sub-5-nm gaps stuck out of an AAO template. J. Nanosci. Nanotechnol. 2016, 16, 934–938. [Google Scholar] [CrossRef]
- Hsu, C.H.; Hsu, L.C.; Chen, C.H.; Chen, L.Y.; Lai, C.S. Investigation of SERS Studies on Periodic Patterned ZnO Nanorod Array Fabricated Using Silica Inverse Opal Nanostructure as a Template. J. Phys. Chem. C 2024, 128, 8288–8295. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, F.; Zou, S.; Zhu, F.; Zhang, Z.; Wang, W. TiO2 nanorod arrays decorated with Au nanoparticles as sensitive and recyclable SERS substrates. J. Alloys Compd. 2021, 861, 157999. [Google Scholar] [CrossRef]
- Shougaijam, B.; Ngangbam, C.; Lenka, T.R. Plasmon-sensitized optoelectronic properties of Au nanoparticle-assisted vertically aligned TiO 2 nanowires by GLAD technique. IEEE Trans. Electron Devices 2017, 64, 1127–1133. [Google Scholar] [CrossRef]
- Bhardwaj, L.; Yadav, J.; Singh, J.P. Potential-modulated SERS profiling via GLAD-fabricated Ag nanorod arrays for ultrasensitive and label-free spectroelectrochemical sensing. Nanoscale 2025, 17, 13915–13928. [Google Scholar] [CrossRef]
- Yadav, S.; Senapati, S.; Kumar, S.; Gahlaut, S.K.; Singh, J.P. GLAD based advanced nanostructures for diversified biosensing applications: Recent progress. Biosensors 2022, 12, 1115. [Google Scholar] [CrossRef]
- Das, S.; Goswami, L.P.; Gayathri, J.; Tiwari, S.; Saxena, K.; Mehta, D.S. Fabrication of low cost highly structured silver capped aluminium nanorods as SERS substrate for the detection of biological pathogens. Nanotechnology 2021, 32, 495301. [Google Scholar] [CrossRef] [PubMed]
- Plaipichit, S.; Wicharn, S.; Champasee, S.; Kaewyou, T.; Padthaisong, P.; Promjantuk, C.; Chao-Moo, W.; Lertvanithphol, T.; Patthanasettakul, V.; Horprathum, M.; et al. Preparation of TiN nanorods for SERS substrate by controlling pulse frequency of high power impulse magnetron sputtering. Optik 2022, 271, 170081. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, W.; Guo, R.; Zhao, X.; Xie, X.; Xu, T.; Zheng, Y.; Xie, Z.; Liu, Z.; Han, W.; et al. Ag–Au nanorods for the diagnosis of cervical cancer by label-free serum surface-enhanced raman scattering. ACS Appl. Nano Mater. 2024, 7, 10711–10718. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Tao, Y.; Zhang, E.; Ren, X. ZnO nanorods decorated with Ag nanoflowers as a recyclable SERS substrate for rapid detection of pesticide residue in multiple-scenes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 290, 122277. [Google Scholar] [CrossRef] [PubMed]
- Pilyushenko, K.S.; Musaev, A.G.; Mikhailova, P.S.; Atlanov, M.A.; Vershinina, O.V.; Popov, M.A.; Pevtsov, D.N.; Arsenov, P.V. Versatile fabrication of SERS-active substrates with copper nanowires synthesized via hydrothermal method. Mater. Lett. 2025, 388, 138338. [Google Scholar] [CrossRef]
- Jung, Y.; Gwon, G.; Lee, J.-S.; Kim, J.; Lee, J.-H.; Kim, K.-H.; Kim, H.G.; Kim, N.H. Enhancing surface-enhanced Raman scattering intensity through light diffuse reflection on Ag/ZnO nanowire arrays. J. Phys. Chem. C 2024, 128, 6748–6757. [Google Scholar] [CrossRef]
- Gu, H.; Tang, M.; Qin, L.; Kang, S.Z.; Li, X. Aluminum sheet induced flower-like carbon nitride anchored with silver nanowires for highly efficient SERS detection of trace malachite green. Environ. Res. 2022, 204, 112289. [Google Scholar] [CrossRef]
- Tuscharoen, S.; Hicheeranun, W.; Chananonnawathorn, C.; Horprathum, M.; Kaewkhao, J. Effect of sputtered pressure on Au nanoparticles formation decorated ZnO nanowire arrays. Mater. Today Proc. 2021, 43, 2624–2628. [Google Scholar] [CrossRef]
- Li, N.; Xu, G.; Yan, M.; Chen, B.; Yuan, Y.; Zhu, C. Fabrication of Vertically Aligned ZnO Nanorods Modified with Dense Silver Nanoparticles as Effective SERS Substrates. Chemosensors 2023, 11, 210. [Google Scholar] [CrossRef]
- Cong, T.; Huang, Y.; Zhao, Y.; Huang, H.; Zhang, D.; Li, C.; Fan, Z.; Pan, L. Extremely sensitive and reusable surface-enhanced Raman scattering substrate based on 3D Ag-titanium dioxide nanowires. J. Alloys Compd. 2021, 859, 158389. [Google Scholar] [CrossRef]
- Rajesh, Y.; Bharati, M.S.S.; Rao, S.V.; Krishna, M.G. ZnO nanowire arrays decorated with titanium nitride nanoparticles as surface-enhanced Raman scattering substrates. Appl. Phys. A 2021, 127, 270. [Google Scholar] [CrossRef]
- Murugan, D.; Chithravel, A.; Shekhawat, A.S.; Diwan, A.; Sharma, S.; Singh, N.; Kumar, R.; Shrivastav, D.; Srivastava, T.; Saxena, S.K.; et al. Comprehensive survey of plasmonic nano-dendrites: From fabrication to SERS applications. J. Mater. Chem. C 2025, 13, 10507–10528. [Google Scholar] [CrossRef]
- Cheng, Z.-Q.; Li, Z.-W.; Yao, R.; Xiong, K.-W.; Cheng, G.-L.; Zhou, Y.-H.; Luo, X.; Liu, Z.-M. Improved SERS performance and catalytic activity of dendritic Au/Ag bimetallic nanostructures based on Ag dendrites. Nanoscale Res. Lett. 2020, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, L.; Zhu, Z.; Chang, S.; Qian, J.; Jiang, J.; Wang, X.; Li, A.; Jiang, L.; Cao, Y. Simultaneously improved SERS sensitivity and thermal stability on Ag dendrites via surface protection by atomic layer deposition. Appl. Surf. Sci. 2023, 611, 155626. [Google Scholar] [CrossRef]
- Sung, C.L.; Kao, T.T.; Lin, Y.C. Silver Dendrites Decorated AAO Membrane for SERS Sensing of Lactic Acid in Artificial Sweat. IEEE Sens. Lett. 2024, 8, 1501904. [Google Scholar] [CrossRef]
- Shao, Y.; Li, S.; Niu, Y.; Wang, Z.; Zhang, K.; Mei, L.; Hao, Y. Three-Dimensional Dendritic Au–Ag Substrate for On-Site SERS Detection of Trace Molecules in Liquid Phase. Nanomaterials 2022, 12, 2002. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Jiang, Z.; Shi, Z.; Wang, J.; Du, L. Highly sensitive and reproducible SERS substrates based on ordered micropyramid array and silver nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 29222–29229. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ren, Z.; Zhang, M.; Song, Y.; Li, P.; Qiu, Y.; Deng, P.; Li, Z. Three-dimensional porous SERS powder for sensitive liquid and gas detections fabricated by engineering dense “hot spots” on silica aerogel. Nanoscale Adv. 2021, 3, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, X.; Yang, J.; Xi, S.; Jia, M.; Shen, J. Silver nanoparticle-decorated chitosan aerogels as three-dimensional porous surface-enhanced Raman scattering substrates for ultrasensitive detection. ACS Appl. Nano Mater. 2022, 5, 5398–5406. [Google Scholar] [CrossRef]
- Ge, B.; Huang, J.; Qin, H.; Zhao, S.; Yang, F.; Wang, M.; Liang, P. MOF-derived multi-“hotspot” 3D Au/MOF-808 (Zr) nanostructures as SERS substrates for the ultrasensitive determination of thiram. Microchim. Acta 2024, 191, 308. [Google Scholar] [CrossRef]
- Wang, L.; Wang, E.; Tang, J. Facile synthesis of flower-like Ag nanocube@mesoporous SiO2 and their sensitive SERS performance. J. Nanopart Res. 2019, 21, 63. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, W.; Chen, M.; Huang, L.; Shuai, Q.; Ouyang, L. Urchin-like covalent organic frameworks templated Au@Ag composites for SERS detection of emerging contaminants. Chem. Commun. 2024, 60, 8840–8843. [Google Scholar] [CrossRef]
- Xue, W.; Fu, J.; Zhang, Y.; Ren, S.; Liu, G. A core–shell structured AuNPs@ZnCo-MOF SERS substrate for sensitive and selective detection of thiram. Anal. Methods 2024, 11, 315. [Google Scholar] [CrossRef]
- Chen, Y.M.; Zhu, L.X.; Yang, Y.L.; Wu, D. Fabrication of a metal organic framework (MOF)-modified Au nanoparticle array for sensitive and stable SERS sensing of paraquat in cereals. J. Food Sci. 2023, 88, 1769–1780. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, C.; Gu, J.; Wu, Y.; Zhu, C.; Li, L.; Gao, H.; Yin, W.; Wang, Z.; Chen, G. Detection of melamine by using carboxyl-functionalized Ag-COF as a novel SERS substrate. Food Chem. 2023, 401, 134078. [Google Scholar] [CrossRef]
- Liu, W.; Song, Z.; Zhao, Y.; Liu, Y.; He, X.; Cui, S. Flexible porous aerogels decorated with Ag nanoparticles as an effective SERS substrate for label-free trace explosives detection. Anal. Methods 2020, 12, 4123–4129. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Tudorache, D.-I.; Bocioagă, M.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. An Updated Overview of Silica Aerogel-Based Nanomaterials. Nanomaterials 2024, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.; Jia, P.; Feng, Y.; Fu, S.; Yang, J.; Xiong, L.; Su, F.; Wu, Y.; Huang, Y. Ag nanoparticle-decorated mesoporous silica as a dual-mode Raman sensing platform for detection of volatile organic compounds. ACS Appl. Nano Mater. 2021, 4, 1019–1028. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, L.; Kalyinur, K.; Dong, S.; Chang, X.; Yu, Q.; Wang, R.; Pang, B.; Kong, X. Fabrication and Application of Ag@SiO2/Au Core–Shell SERS Composite in Detecting Cu2+ in Water Environment. Molecules 2024, 29, 1503. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Yosef, E.; Mamane, H.; Kumar, R. Engineering gold nanoparticles-infused silica aerogel composite for trace mercury adsorption. RSC Adv. 2025, 15, 23910–23919. [Google Scholar] [CrossRef]
- Fathima, H.; Paul, L.; Thirunavukkuarasu, S.; Thomas, K.G. Mesoporous silica-capped silver nanoparticles for sieving and surface-enhanced Raman scattering-based sensing. ACS Appl. Nano Mater. 2020, 3, 6376–6384. [Google Scholar] [CrossRef]
- Fu, H.; Ding, N.; Ma, D.; Xu, Q.; Lin, B.; Guo, L. Green Synthesis of Three-Dimensional Au Nanorods@TiO2 Nanocomposites as Self-Cleaning SERS Substrate for Sensitive, Recyclable, and In Situ Sensing Environmental Pollutants. Biosensors 2023, 13, 7. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Zha, L.; Lin, D.; Yang, J.; Zhou, J.; Zhang, L. Temperature- and pH-Tunable Plasmonic Properties and SERS Efficiency of Silver Nanoparticles within Dual Stimuli-Responsive Microgels. J. Mater. Chem. C 2014, 2, 7326–7335. [Google Scholar] [CrossRef]
- Li, H.; Men, D.; Sun, Y.; Liu, D.; Li, X.; Li, L.; Li, C.; Cai, W.; Li, Y. Surface enhanced Raman scattering properties of dynamically tunable nanogaps between Au nanoparticles self-assembled on hydrogel microspheres controlled by pH. J. Colloid Interface Sci. 2017, 505, 467–475. [Google Scholar] [CrossRef]
- Borah, R.; Verbruggen, S.W. Silver–Gold Bimetallic Alloy versus Core–Shell Nanoparticles: Implications for Plasmonic Enhancement and Photothermal Applications. J. Phys. Chem. C 2020, 124, 12081–12094. [Google Scholar] [CrossRef]
- Song, D.; Wang, T.; Zhuang, L. Preparation of SiO2@Au nanoparticle photonic crystal array as surface-enhanced Raman scattering (SERS) substrate. Nanomaterials 2023, 13, 2156. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, N.; Park, J.W.; Kim, Z.H. Nanostar probes for tip-enhanced spectroscopy. Nanoscale 2016, 8, 2216–2224. [Google Scholar] [CrossRef]
- Arunbabu, D.; Sannigrahi, A.; Jana, T. Photonic Crystal Hydrogel Material for the Sensing of Toxic Mercury Ions (Hg2+) in Water. Soft Matter 2011, 7, 2592–2599. [Google Scholar] [CrossRef]
- Peyskens, F.; Dhakal, A.; Van Dorpe, P.; Le Thomas, N.; Baets, R. Surface Enhanced Raman Spectroscopy Using a Single Mode Nanophotonic-Plasmonic Platform. ACS Photonics 2016, 3, 102–108. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Z. Photonic-plasmonic resonator for SERS biodetection. Analyst 2024, 149, 3123–3130. [Google Scholar] [CrossRef]
- Mo, J.-Q.; Hou, J.-W.; Lü, X.-Y. Template-Directed Synthesis of Ag Nanowire Arrays by a Simple Paired-Cell Method for SERS. Optoelectron. Lett. 2015, 11, 401–404. [Google Scholar] [CrossRef]
- Feng, Y.; Kim, K.-D.; Nemitz, C.A.; Kim, P.; Pfadler, T.; Gerigk, M.; Polarz, S.; Dorman, J.A.; Weickert, J.; Schmidt-Mende, L. Uniform Large-Area Free-Standing Silver Nanowire Arrays on Transparent Conducting Substrates. J. Electrochem. Soc. 2016, 163, D447–D452. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Ram, R.; Kolaru, R.B.; Jana, A.S.; Sadhu, A.S.; Chu, C.-S.; Lin, Y.-N.; Pal, B.N.; Chang, S.-H.; Biring, S. Ingenious Fabrication of Ag-Filled Porous Anodic Alumina Films as Powerful SERS Substrates for Efficient Detection of Biological and Organic Molecules. Biosensors 2022, 12, 807. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Shi, L.; He, T.; Sun, X.; Mo, Y. SERS enhancement dependence on the diameter and aspect ratio of silver-nanowire array fabricated by anodic aluminium oxide template. Appl. Surf. Sci. 2008, 255, 1901–1905. [Google Scholar] [CrossRef]
- Yang, T.; Fu, X.; Zhang, Q.; Cui, Y.; Yuan, C.; Ge, H.; Chen, Y.; Zhang, W. Fabrication of Ag Nanodot Array over Large Area for Surface-Enhanced Raman Scattering Using Hybrid Nanoimprint Mold Made from AAO Template. Appl. Phys. A 2014, 117, 909–915. [Google Scholar] [CrossRef]
- Cai, J.; Wang, Z.; Wang, M.; Zhang, D. Au nanoparticle-grafted hierarchical pillars array replicated from diatom as reliable SERS substrates. Appl. Surf. Sci. 2021, 541, 148374. [Google Scholar] [CrossRef]
- Ren, L.; Campbell, J.; Gao, W.; Rorrer, G.L.; Wang, A.X. Surface-enhanced Raman spectroscopy sensors from nanobiosilica with self-assembled plasmonic nanoparticles. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 127–132. [Google Scholar] [CrossRef]
- Squire, K.J.; Sivashanmugan, K.; Zhang, B.; Kraai, J.; Rorrer, G.L.; Wang, A.X. Multiscale Photonic Crystal Enhanced Core–Shell Plasmonic Nanomaterial for Rapid Vapor-Phase Detection of Explosives. ACS Appl. Nano Mater. 2020, 3, 1656–1665. [Google Scholar] [CrossRef]
- Faro, M.J.L.; D’andrea, C.; Leonardi, A.A.; Morganti, D.; Irrera, A.; Fazio, B. Fractal Silver Dendrites as 3D SERS Platform for Highly Sensitive Detection of Biomolecules in Hydration Conditions. Nanomaterials 2019, 9, 1630. [Google Scholar] [CrossRef]
- Ye, W.; Chen, Y.; Zhou, F.; Wang, C.; Li, Y. Fluoride-Assisted Galvanic Replacement Synthesis of Ag and Au Dendrites on Aluminum Foil with Enhanced SERS and Catalytic Activities. J. Mater. Chem. 2012, 22, 18327–18334. [Google Scholar] [CrossRef]
- Bai, X.; Gao, Y.; Zheng, L. Galvanic replacement mediated growth of dendritic gold nanostructures with a three-fold symmetry and their applications to SERS. CrystEngComm 2011, 13, 3562–3568. [Google Scholar] [CrossRef]
- Zao, Y.; Chen, S.; Chen, Y.; Luo, J.; Wu, W.; Yougen, Y.; Tang, Y. Preparation of dendritic Ag/Au bimetallic nanostructures and their application in surface-enhanced Raman scattering. Thin Solid Film. 2012, 520, 2178–2184. [Google Scholar] [CrossRef]
- Scandura, G.; Kumari, P.; Palmisano, G.; Karanikolos, G.N.; Orwa, J.; Dumée, L.F. Nanoporous Dealloyed Metal Materials Processing and Applications-A Review. Ind. Eng. Chem. Res. 2023, 62, 1736–1763. [Google Scholar] [CrossRef]
- Shi, S.; Li, Y.; Ngo-Dinh, B.-N.; Markmann, J.; Weissmüller, J. Scaling Behavior of Stiffness and Strength of Hierarchical Network Nanomaterials. Science 2021, 371, 1026–1033. [Google Scholar] [CrossRef]
- Kim, C.; Baek, S.; Ryu, Y.; Kim, Y.; Shin, D.; Lee, C.W.; Park, W.; Urbas, A.M.; Kang, G.; Kim, K. Large-scale nanoporous metal-coated silica aerogels for high SERS effect improvement. Sci. Rep. 2018, 8, 15144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, W.; Wang, J.; Huang, L.; Cui, S.; He, X. Structure-controllable Ag aerogel optimized SERS-digital microfluidic platform for ultrasensitive and high-throughput detection of harmful substances. Sens. Actuators B Chem. 2024, 401, 134934. [Google Scholar] [CrossRef]
- Liu, X.; Li, T.; Liu, Y.; Sun, Y.; Han, Y.; Lee, T.C.; Zada, A.; Yuan, Z.; Ye, F.; Chen, J.; et al. Hybrid plasmonic aerogel with tunable hierarchical pores for size-selective multiplexed detection of VOCs with ultrahigh sensitivity. J. Hazard. Mater. 2024, 495, 133893. [Google Scholar] [CrossRef] [PubMed]
- Juan, A.M.T.S.; Chavva, S.R.; Tu, D.; Tircuit, M.; Coté, G.; Mabbott, S. Synthesis of SERS-active core-satellite nanoparticles using heterobifunctional PEG linkers. Nanoscale Adv. 2021, 4, 258–267. [Google Scholar] [CrossRef]
- Kogikoski, S.; Tapio, K.; von Zander, R.E.; Saalfrank, P.; Bald, I. Raman Enhancement of Nanoparticle Dimers Self-Assembled Using DNA Origami Nanotriangles. Molecules 2021, 26, 1684. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, L.; Shen, X.; Shang, S.; Pan, Y.; Dong, G.; Huo, W.; Zhu, D.; Tang, X. Self-assembled core-shell nanoparticles with embedded internal standards for SERS quantitative detection and identification of nicotine released from snus products. Front. Chem. 2024, 12, 1348423. [Google Scholar] [CrossRef]
- Huang, Z.; Meng, G.; Hu, X.; Pan, Q.; Huo, D.; Zhou, H.; Ke, Y.; Wu, N. Plasmon-tunable Au@Ag core-shell spiky nanoparticles for surface-enhanced Raman scattering. Nano Res. 2019, 12, 449–455. [Google Scholar] [CrossRef]
- Bernard, S.; Felidj, N.; Truong, S.; Peretti, P.; Lévi, G.; Aubard, J. Study of Langmuir–Blodgett phospholipidic films deposited on SERS-active gold nanoparticle monolayers. Biopolymers 2002, 67, 314–318. [Google Scholar] [CrossRef]
- Guselnikova, O.; Mamber, S.; Zaytsev, A.V.; Ipatova, E.; Aleshkevich, P. New trends in nanoarchitectured SERS substrates: Nanospaces, 2D materials, and organic heterostructures. Small 2022, 18, 2107182. [Google Scholar] [CrossRef]
- Fan, X.; Hao, Q.; Li, M.; Zhang, X.; Yang, X.; Mei, Y.; Qiu, T. “Hotspots on the Move”: Active molecular enrichment by hierarchically structured micromotors for ultrasensitive SERS sensing. ACS Appl. Mater. Interfaces 2020, 12, 28783–28791. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Ko, H. Transparent and flexible surface-enhanced Raman scattering (SERS) sensors based on gold nanostar arrays embedded in silicon rubber film. ACS Appl. Mater. Interfaces 2017, 9, 44088–44095. [Google Scholar] [CrossRef]
- Childs, A.; Vinogradova, E.; Ruiz-Zepeda, F.; Velazquez-Salazar, J.J.; Jose-Yacaman, M. Biocompatible gold/silver nanostars for surface-enhanced Raman scattering. J. Raman Spectrosc. 2016, 47, 651–655. [Google Scholar] [CrossRef]
- Chowdury, A.K.M.R.H.; Tavangar, A.; Tan, B.; Venkatakrishnan, K. Biofunctionalized 3-D carbon nano-network platform for enhanced fibroblast cell adhesion. Sci. Rep. 2017, 7, 44250. [Google Scholar] [CrossRef]
- Chowdury, A.K.M.R.H.; Tan, B.; Venkatakrishnan, K. SERS-active 3D interconnected nanocarbon web toward nonplasmonic in vitro sensing of HeLa cells and fibroblasts. ACS Appl. Mater. Interfaces 2018, 10, 35715. [Google Scholar] [CrossRef]
- Yang, C.; Chen, K.; Chen, M.; Hu, X.; Huan, S.-Y.; Chen, L.; Song, G.; Zhang, X. Nanoscale metal−organic framework based two-photon sensing platform for bioimaging in live tissue. Anal. Chem. 2019, 91, 2727–2733. [Google Scholar] [CrossRef]
- Chen, D.-Z.; Li, Y.; Wang, L.-J.; Wang, Y.; Ning, P.; Shum, P.; He, X.; Fu, T. Given the critical role of cytocompatibility in biocompatibility for enhancing Raman scattering signals as enabled by a Mo-Ag film. Analyst 2022, 147, 1385. [Google Scholar] [CrossRef]
- Zhang, Y.; Jimenez de Aberasturi, D.; Henriksen-Lacey, M.; Langer, J.; Liz-Marzán, L.M. Live-cell surface-enhanced Raman spectroscopy imaging of intracellular pH: From two dimensions to three dimensions. ACS Sens. 2020, 5, 3194. [Google Scholar] [CrossRef]
- Schumacher, M.; Jimenez de Aberasturi, D.; Merkl, J.-P.; Scarabelli, L.; Lenzi, E.; Henriksen-Lacey, M.; Liz-Marzán, L.M.; Weller, H. Robust encapsulation of biocompatible gold nanosphere assemblies for bioimaging via surface enhanced Raman scattering. Adv. Opt. Mater. 2022, 10, 2102635. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215. [Google Scholar] [CrossRef]
- Parisi, J.; Su, L.; Lei, Y. In situ synthesis of silver nanoparticle decorated vertical nanowalls in a microfluidic device for ultrasensitive in-channel SERS sensing. Lab Chip 2013, 13, 1501. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Stagon, S.; Huang, H.; Chen, J.; Lei, Y. Functionalized aligned silver nanorod arrays for glucose sensing through surface enhanced Raman scattering. RSC Adv. 2014, 4, 23382. [Google Scholar] [CrossRef]
- Botta, R.; Rajanikanth, A.; Bansal, C. Silver nanocluster films for glucose sensing by Surface Enhanced Raman Scattering (SERS). Sens. Bio-Sens. Res. 2016, 9, 13. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Q.; Wu, G.; Mu, X.; Wang, X.; Wang, Y.; Wu, Y.; Wu, J.; Li, Y. Advances in label-free glucose detection using self-assembled nanoparticles and Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2024, 96, 11533. [Google Scholar] [CrossRef]
- Al-Ogaidi, I.; Gou, H.; Al-Kazaz, A.K.A.; Aguilar, Z.P.; Melconian, A.K.; Zheng, P.; Wu, N. A gold@silica core–shell nanoparticle-based surface-enhanced Raman scattering biosensor for label-free glucose detection. Anal. Chim. Acta 2014, 811, 76. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Zhao, X.; Wu, J.; Muhammad, F.; Lin, S.; He, J.; Zhou, L.; Zhang, C.; Deng, Y.; et al. Surface-Enhanced Raman Scattering active gold nanoparticles with enzyme-mimicking activities for measuring glucose and lactate in living tissues. ACS Nano 2017, 11, 5558. [Google Scholar] [CrossRef]
- Lu, Y.-D.; Zhou, T.; You, R.-Y.; Wu, C.-M.; Shen, Q.; Feng, J.-L.; Su, L. Fabrication and characterization of a highly-Sensitive Surface-Enhanced Raman Scattering nanosensor for detecting glucose in urine. Nanomaterials 2018, 8, 629. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, Y.; Wu, Y.; Guo, X.; Ying, Y.; Wen, Y.; Yang, H. Enzyme-free tandem reaction strategy for Surface-Enhanced Raman Scattering detection of glucose by using the composite of Au nanoparticles and porphyrin-based metal-organic framework. ACS Appl. Mater. Interfaces 2020, 12, 55324. [Google Scholar] [CrossRef]
- Man, B.; Wang, G.; Li, Z.; Xu, S.; Li, C.; Yu, J.; Zhang, C.; Zhao, X. MoS2-spaced bimetal composite structure as SERS-SPR sensor for glucose detection. J. Alloys Compd. 2022, 902, 163789. [Google Scholar] [CrossRef]
- Li, L.; Cui, W.; He, Z.; Xue, W.; He, H. Plasmonic sensor based on silver nanoparticles for the detection of glucose. Plasmonics 2022, 17, 1231. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Li, Y.; Liu, M.; Qiao, J.; Wang, D.; Cao, H.; He, W.; Yang, Z. Detection of glucose in diabetic tears by using gold nanoparticles and MXene composite surface enhanced Raman scattering substrates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 266, 120432. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, G.; Zhang, X.; Gong, H.; Jiang, L.; Sun, G.; Li, Y.; Liang, X. Dual-functional ultrathin wearable 3D particle-in-cavity SF-AAO-Au SERS sensors for effective sweat glucose and lab-on-glove pesticide detection. Sens. Actuators B Chem. 2022, 359, 131512. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, D.; Ma, X.; Huang, R.; Xu, J.; Xu, X.; Cai, L.; Xu, L. Surface enhanced Raman scattering active substrate based on hydrogel microspheres for pretreatment-free detection of glucose in biological samples. Talanta 2023, 260, 124657. [Google Scholar] [CrossRef]
- Atta, S.; Zhao, Y.; Sanchez, S.; Vo-Dinh, T. A Simple and sensitive wearable SERS sensor utilizing plasmonic active gold nanostars. ACS Omega 2024, 9, 38897. [Google Scholar] [CrossRef]
- Yuan, Q.; Fang, H.; Wu, X.; Wu, J.; Luo, X.; Peng, R.; Xu, S.; Yan, S. Self-Adhesive, Biocompatible, Wearable Microfluidics with Erasable Liquid Metal Plasmonic Hotspots for Glucose Detection in Sweat. ACS Appl. Mater. Interfaces 2024, 16, 66810. [Google Scholar] [CrossRef]
- Kamil Reza, K.; Wang, J.; Vaidyanathan, R.; Dey, S.; Wang, Y.; Trau, M. Electrohydrodynamic-induced SERS immunoassay for extensive multiplexed biomarker sensing. Small 2017, 13, 1602902. [Google Scholar] [CrossRef]
- Chen, R.; Liu, B.; Ni, H.; Chang, N.; Luan, C.; Ge, Q.; Dong, J.; Zhao, X. Vertical flow assays based on core–shell SERS nanotags for multiplex prostate cancer biomarker detection. Analyst 2019, 144, 4051. [Google Scholar] [CrossRef]
- Liu, X.J.; Yang, S.K.; Li, Y.; Wang, B.; Guo, J.; Ma, X. Mesoporous nanostructures encapsulated with metallic nanodots for smart SERS sensing. ACS Appl. Mater. Interfaces 2020, 13, 186. [Google Scholar] [CrossRef]
- Basu, S.; Rana, N.; Morgan, D.; Sen, K. Gold nanoparticle incorporated graphene oxide as a SERS platform for ultratrace antibody free sensing of the cancer biomarker CEA. Langmuir 2025, 41, 7886. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, S.; Yu, W.; Wang, L.; Lv, F.; Yang, L.; Yu, H.; Shi, H.; Huang, Y. Hydrogel based flexible wearable sweat sensor for SERS-AI monitoring treatment effect of lung cancer. Sens. Actuators B Chem. 2025, 427, 137155. [Google Scholar] [CrossRef]
- Qiu, C.; Cheng, Z.; Lv, C.; Wang, R.; Yu, F. Development of bioorthogonal SERS imaging probe in biological and biomedical applica-tions. Chin. Chem. Lett. 2021, 32, 2369. [Google Scholar] [CrossRef]
- Bruzas, I.; Lum, W.; Gorunmez, Z.; Sagle, L. Advances in surface-enhanced Raman spectroscopy (SERS) substrates for lipid and protein characterization: Sensing and beyond. Analyst 2018, 143, 3990. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Yang, S.; Xu, M.; Du, S.; Zheng, L.; Wang, X.; Qu, C.; Liu, H. Intrinsic SERS fingerprints of aptamer-peptide conjugates for direct high-specific profiling abnormal protein levels in cancer patients. Anal. Chem. 2023, 95, 12398. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.; Carneiro, M.C.C.G.; Moreira, F.T.C. SERS biosensor with plastic antibodies for detection of a cancer biomarker protein. Microchim. Acta 2024, 191, 238. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xie, W.; Tang, T.; Su, X.; Xiao, S.; Liu, Z.; Li, M.; Liu, J.; Chen, P. Quantitative SERS detection of serum protein biomarkers for assessment of tumor microwave ablation outcomes. Chem. Eng. J. 2024, 496, 154004. [Google Scholar] [CrossRef]
- Chen, H.; Luo, C.X.; Zhang, S.T. Intracellular imaging and concurrent pH sensing of cancer-derived exosomes using surface-enhanced Raman scattering. Anal. Bioanal. Chem. 2021, 413, 4091. [Google Scholar] [CrossRef]
- Kang, T.; Zhu, J.; Luo, X.; Jia, W.; Wu, P.; Cai, C. Controlled self-assembly of a close-packed gold octahedra array for SERS sensing exosomal microRNAs. Anal. Chem. 2021, 93, 2519. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, J.; Liang, J.; Liu, X.; He, X.; Jin, X.; Song, C. CRISPR/Cas13a trans-cleavage and catalytic hairpin assembly cascaded signal amplification powered SERS aptasensor for ultrasensitive detection of gastric cancer-derived exosomes. Anal. Chem. 2024, 96, 18681. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Chen, Z.; He, X.; Yan, C.; Lv, H.; Chen, Z.; Liu, Y.; Wang, L.; Song, C. Branched hybridization chain reaction and tetrahedral DNA-based trivalent aptamer powered SERS sensor for ultra-highly sensitive detection of cancer-derived exosomes. Biosens. Bioelectron. 2025, 267, 116737. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wang, C.; Lu, L.; Wang, C.; Sun, Z.; Xiao, R. Dual-SERS biosensor for one-step detection of microRNAs in exosome and residual plasma of blood samples for diagnosing pancreatic cancer. Biosens. Bioelectron. 2019, 130, 204. [Google Scholar] [CrossRef]
- Tang, C.; Huang, Z.; Li, H.; Zhang, R.; Yu, G.; Kong, J.; Chen, H.; Weng, W. EXPAR and Au–Ag mushroom-shaped SERS probe assisted detection of exosomal miR-375 in prostate cancer. Sens. Diagn. 2023, 2, 1553. [Google Scholar] [CrossRef]
- Shao, D.; Zhang, X.; Liu, W.; Zhang, F.; Zheng, X.; Qiao, P.; Li, J.; Dong, W.; Chen, L. Janus silver-mesoporous silica nanocarriers for SERS traceable and pH-sensitive drug delivery in cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 4303. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, C.; Huang, F.; Yu, Z.; Jiang, L. Efficient interfacial self-assembled MXene/Ag NPs film nanocarriers for SERS-traceable drug delivery. Anal. Bioanal. Chem. 2023, 415, 5379. [Google Scholar] [CrossRef]
- Tian, F.; Conde, J.; Bao, C.; Chen, Y.; Curtin, J.; Cui, D. Gold nanostars for efficient in vitro and in vivo real time SERS detection and drug delivery via plasmonic tunable Raman/FTIR imaging. Biomaterials 2016, 106, 87. [Google Scholar] [CrossRef]
- Song, C.; Dou, Y.; Yuwen, L.; Sun, Y.; Dong, C.; Li, F.; Yang, Y.; Wang, L. Gold nanoflowers-based traceable drug delivery system for intracellular SERS imaging-guided targeted chemo-phototherapy. J. Mater. Chem. B 2018, 6, 3030. [Google Scholar] [CrossRef]
- Samanta, A.; Maiti, K.K.; Soh, K.S.; Liao, X.; Vendrell, M.; Dinish, U.S.; Chang, Y.T. Ultrasensitive near-infrared Raman reporters for SERS-based in vivo cancer detection. Angew. Chem. Int. Ed. 2011, 50, 6089. [Google Scholar] [CrossRef]
- Liu, L.; Tang, Y.; Dai, S.; Kleitz, F.; Qiao, S.Z. Smart surface-enhanced Raman scattering traceable drug delivery systems. Nanoscale 2016, 8, 12803. [Google Scholar] [CrossRef]
- Managò, S.; Tramontano, C.; Delle Cave, D.; Chianese, G.; Zito, G.; De Stefano, L.; Rea, I. SERS quantification of Galunisertib delivery in colorectal cancer cells by plasmonic-assisted diatomite nanoparticles. Small 2021, 17, 2101711. [Google Scholar] [CrossRef]
- Hong, Y.; Ju, Y.; Chen, W.; Liu, Y.; Zhang, M.; Zhao, H. Fabrication of PεCL-AuNP-BSA core-shell-corona nanoparticles for flexible spatiotemporal drug delivery and SERS detection. Biomater. Sci. 2021, 9, 4440. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Zhu, X.; Li, Q.; Yang, Z.; Wang, J. Gold nanobipyramid-directed growth of length-variable silver nanorods with multipolar plasmon resonances. ACS Nano 2015, 9, 7523. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xing, L.; Guo, H.; Luo, C.; Zhang, X. Dual-targeting SERS-encoded graphene oxide nanocarrier for intracellular co-delivery of doxorubicin and 9-aminoacridine with enhanced combination therapy. Analyst 2021, 146, 6893. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sheng, B.; Tian, H.; Chen, Q.; Yang, Y.; Bui, B.; Pi, J.; Cai, H.; Chen, S.; Zhang, L. Real-time SERS monitoring anticancer drug release along with SERS/MR imaging for pHsensitive chemo-phototherapy. Acta Pharm. Sin. B 2023, 13, 1303. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Mei, R.; Lv, B.; Zhao, X.; Bi, L.; Xu, H.; Chen, L. Hydrogel-coated SERS microneedles for drug monitoring in dermal interstitial fluid. ACS Sens. 2024, 9, 2567. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.U.; Song, S.; Kim, S.; Sim, S.J. A shape-code nanoplasmonic biosensor for multiplex detection of Alzheimer’s disease biomarkers. Biosens. Bioelectron. 2018, 101, 96. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, G.; Wang, W.; Ren, Z.; Zhang, C.; Gong, Y.; Zhao, M.; Qu, Y.; Li, W.; Zhou, H.; et al. Bioinspired hot-spot engineering strategy towards ultrasensitive SERS sandwich biosensor for bacterial detection. Biosens. Bioelectron. 2023, 237, 115497. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Kim, W.H.; Lee, H.S.; Park, K.H.; Sim, S.J. Quantitative and specific detection of exosomal miRNAs for accurate diagnosis of breast cancer using a Surface-Enhanced Raman Scattering sensor based on plasmonic head-flocked gold nanopillars. Small 2019, 15, 1804968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, C.; Zhu, Y.; Gan, H.; Fang, X.; Peng, Q.; Xiong, J.; Dong, C.; Han, C.; Wang, L. A novel cascade signal amplification strategy integrating CRISPR/Cas13a and branched hybridization chain reaction for ultra-sensitive and specific SERS detection of disease-related nucleic acids. Biosens. Bioelectron. 2023, 219, 114836. [Google Scholar] [CrossRef]
- Zhan, Y.; Fei, R.; Lu, Y.; Wan, Y.; Wu, X.; Dong, J.; Meng, D.; Ge, Q.; Zhao, X. Ultrasensitive detection of multiple Alzheimer’s disease biomarkers by SERS-LFA. Analyst 2022, 147, 4124. [Google Scholar] [CrossRef]
- Zhang, L.; Su, Y.; Liang, X.; Cao, K.; Luo, Q.; Luo, H. Ultrasensitive and point-of-care detection of plasma phosphorylated tau in Alzheimer’s disease using colorimetric and surface-enhaced Raman scattering dual-readout lateral flow assay. Nano Res. 2023, 16, 7459. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, K.; Su, Y.; Hu, S.; Liang, X.; Luo, Q.; Luo, H. Colorimetric and surface-enhanced Raman scattering dual-mode magnetic immunosensor for ultrasensitive detection of blood phosphorylated tau in Alzheimer’s disease. Biosens. Bioelectron. 2023, 222, 114935. [Google Scholar] [CrossRef]
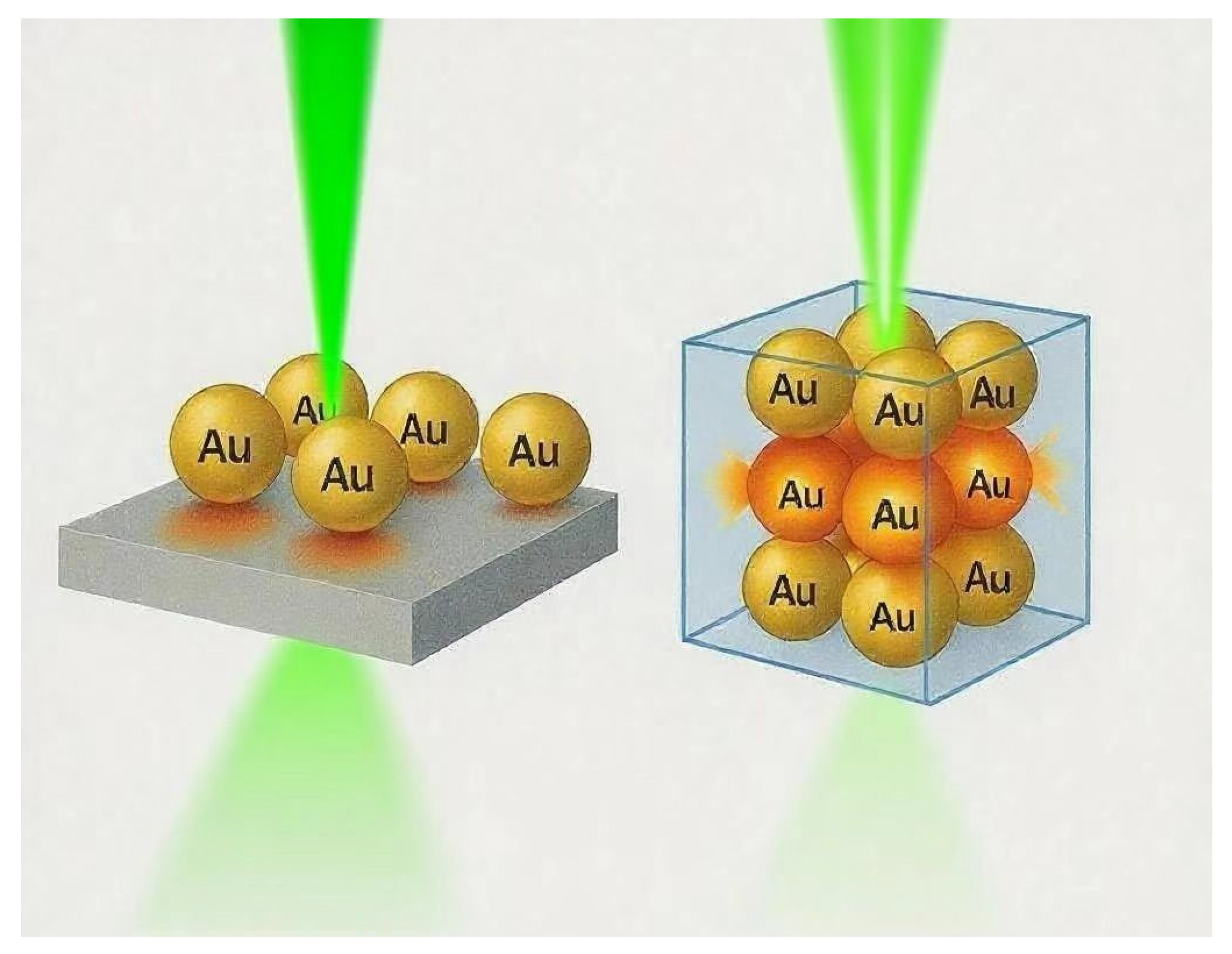
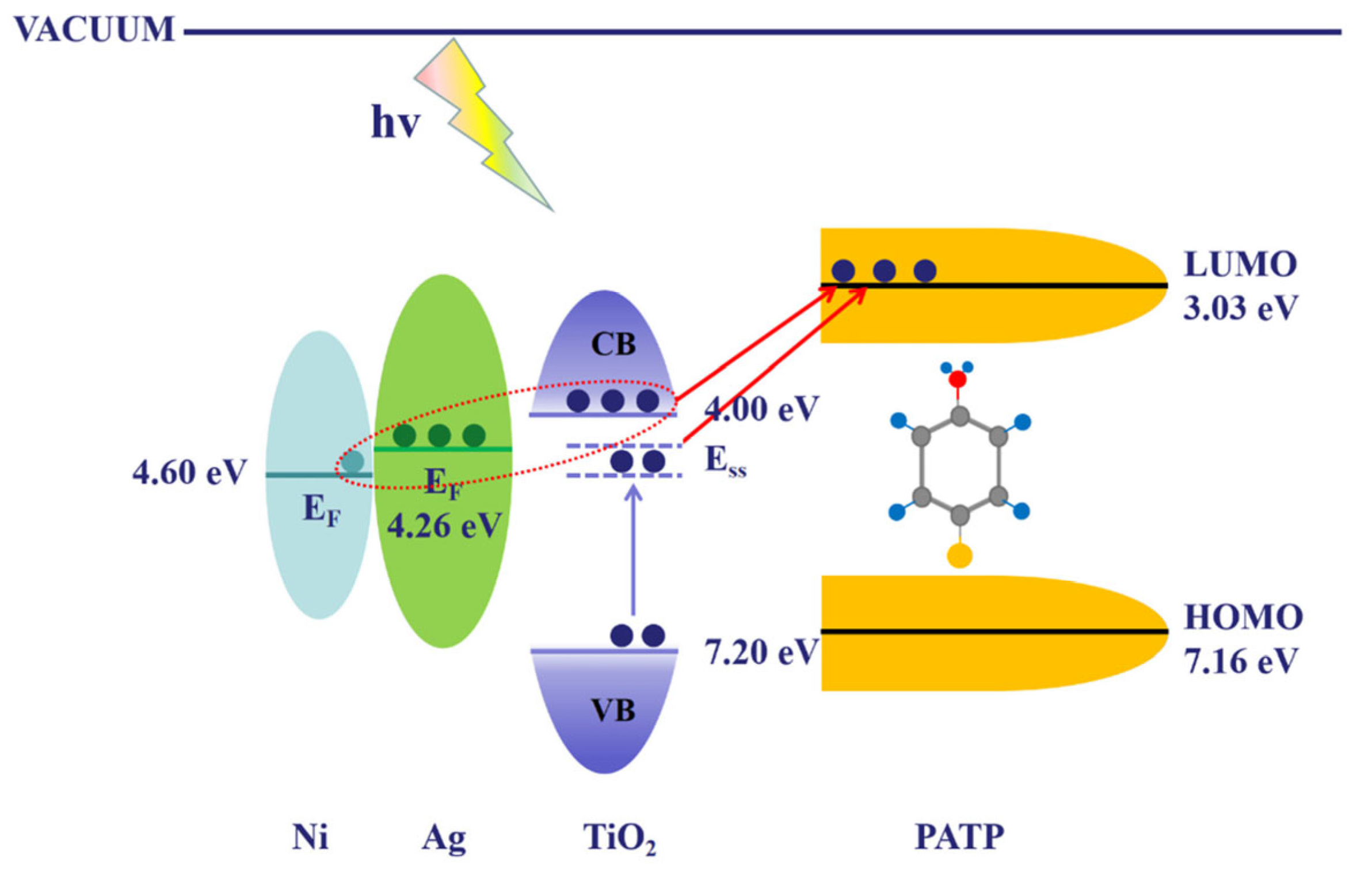

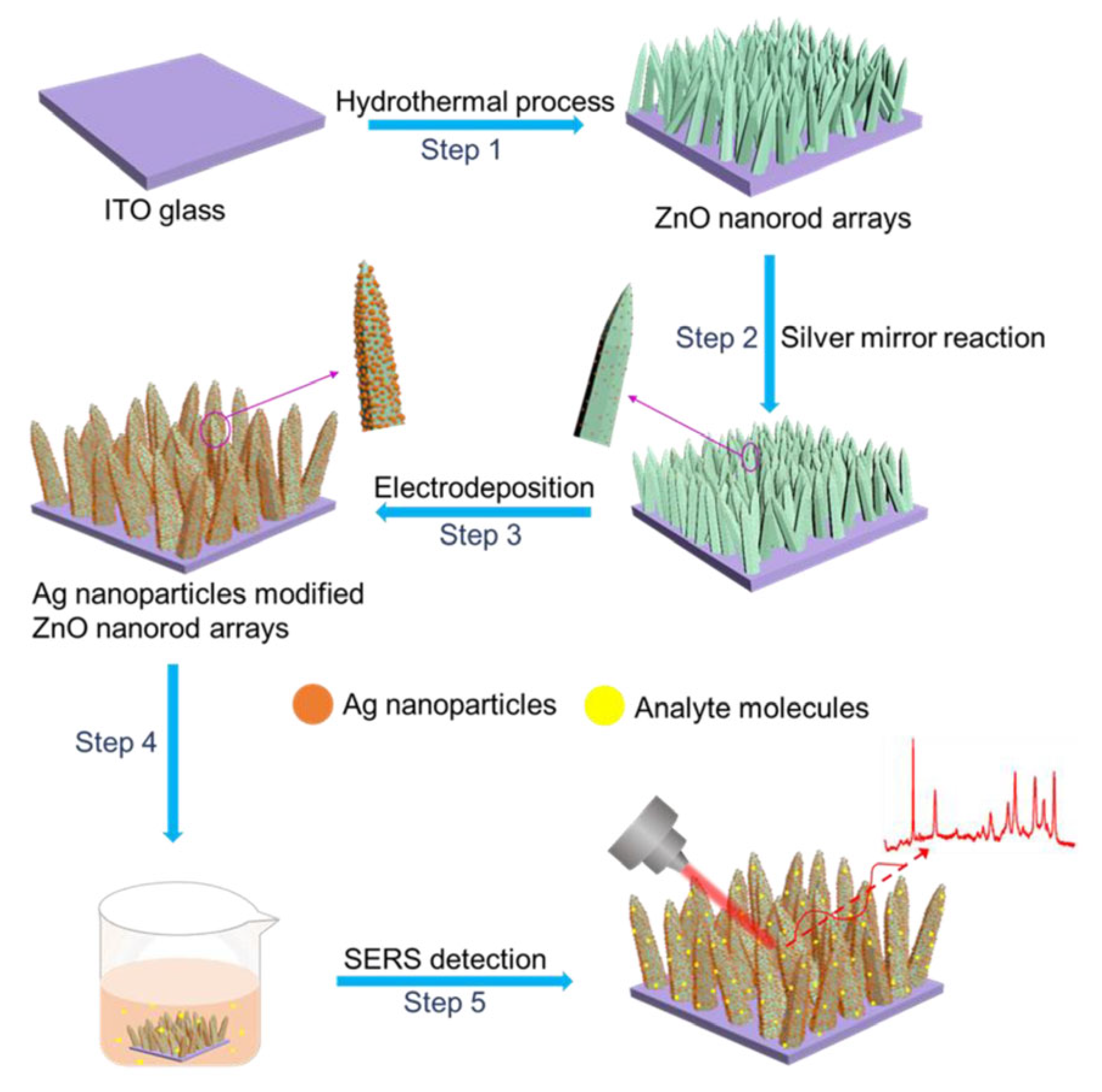

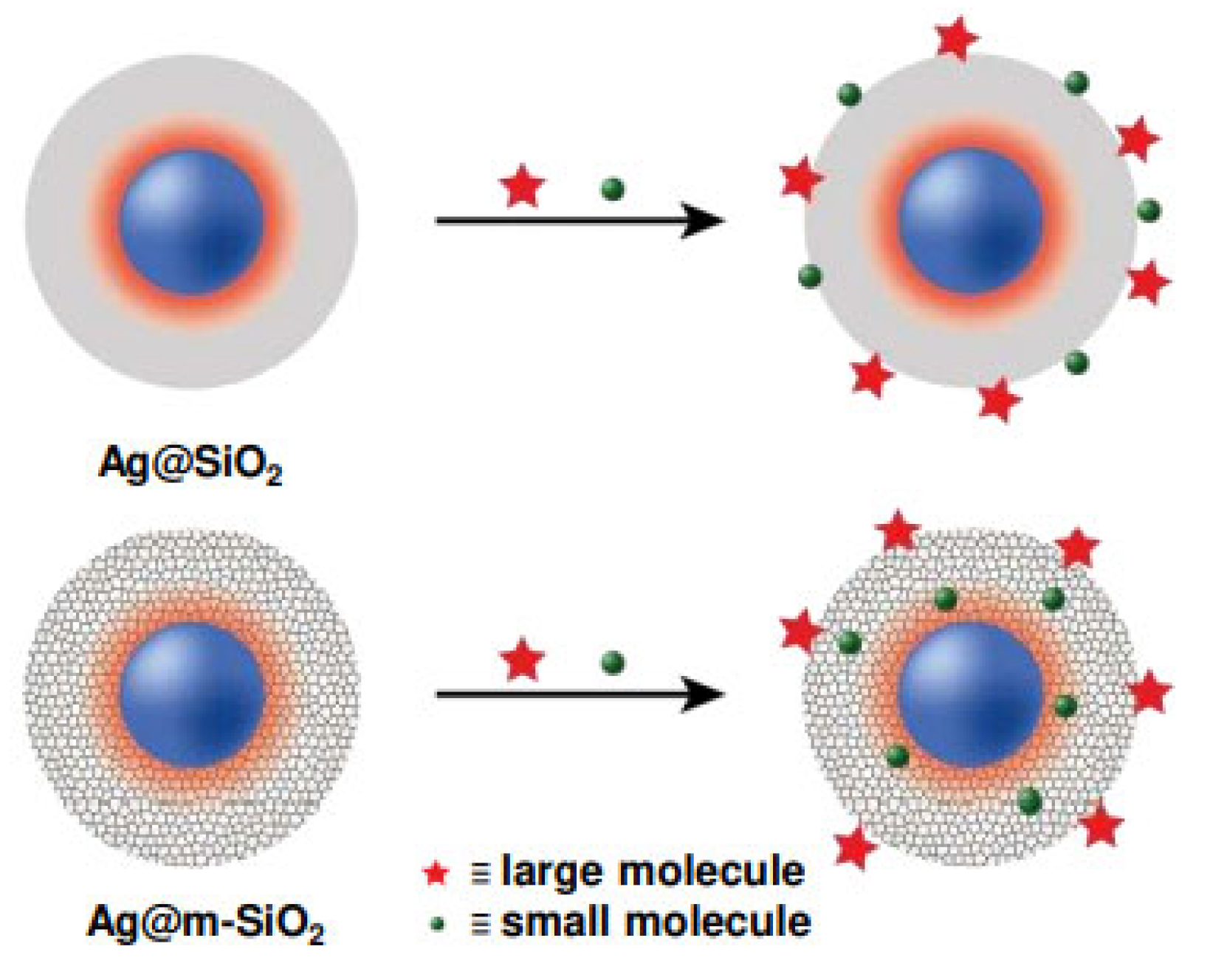

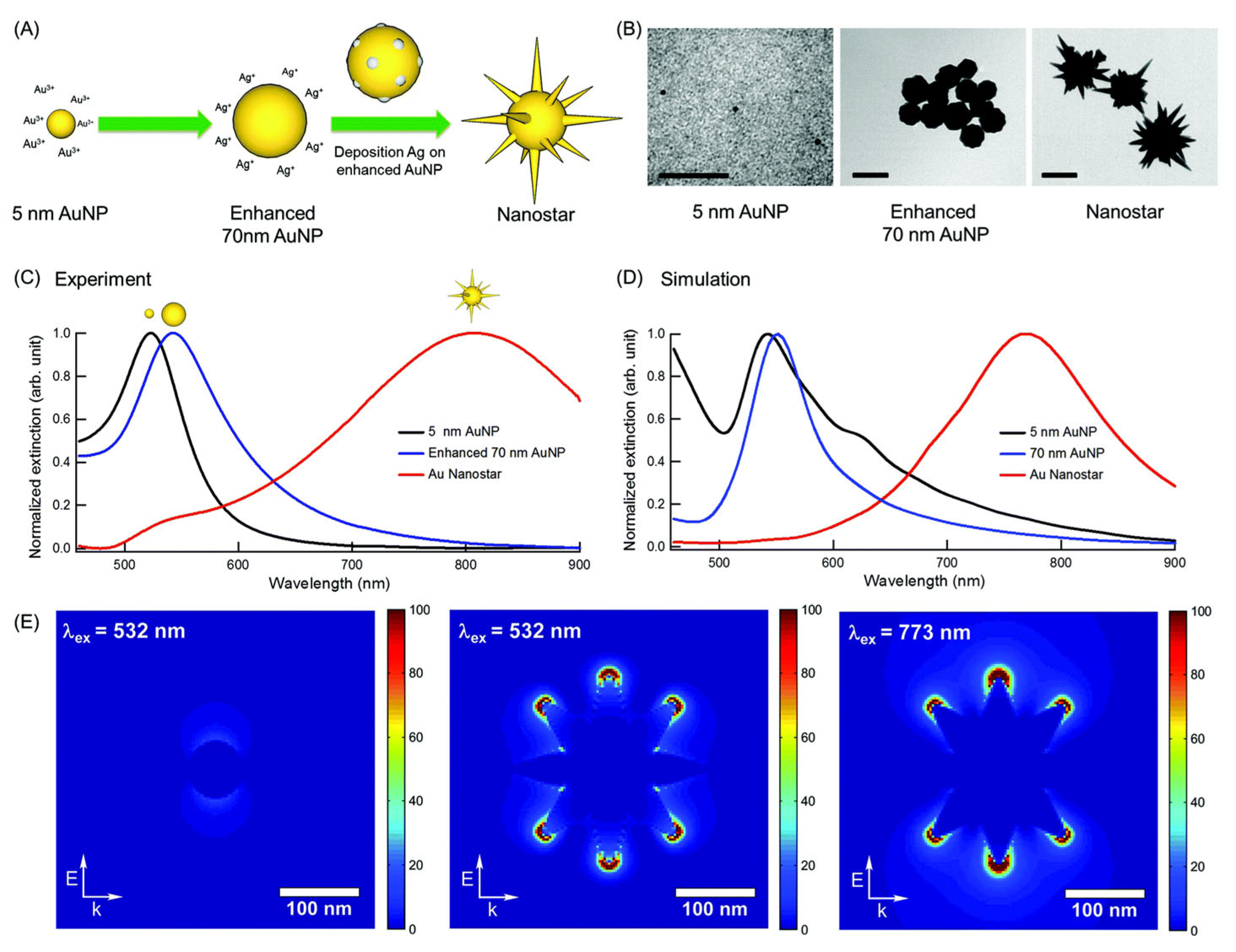


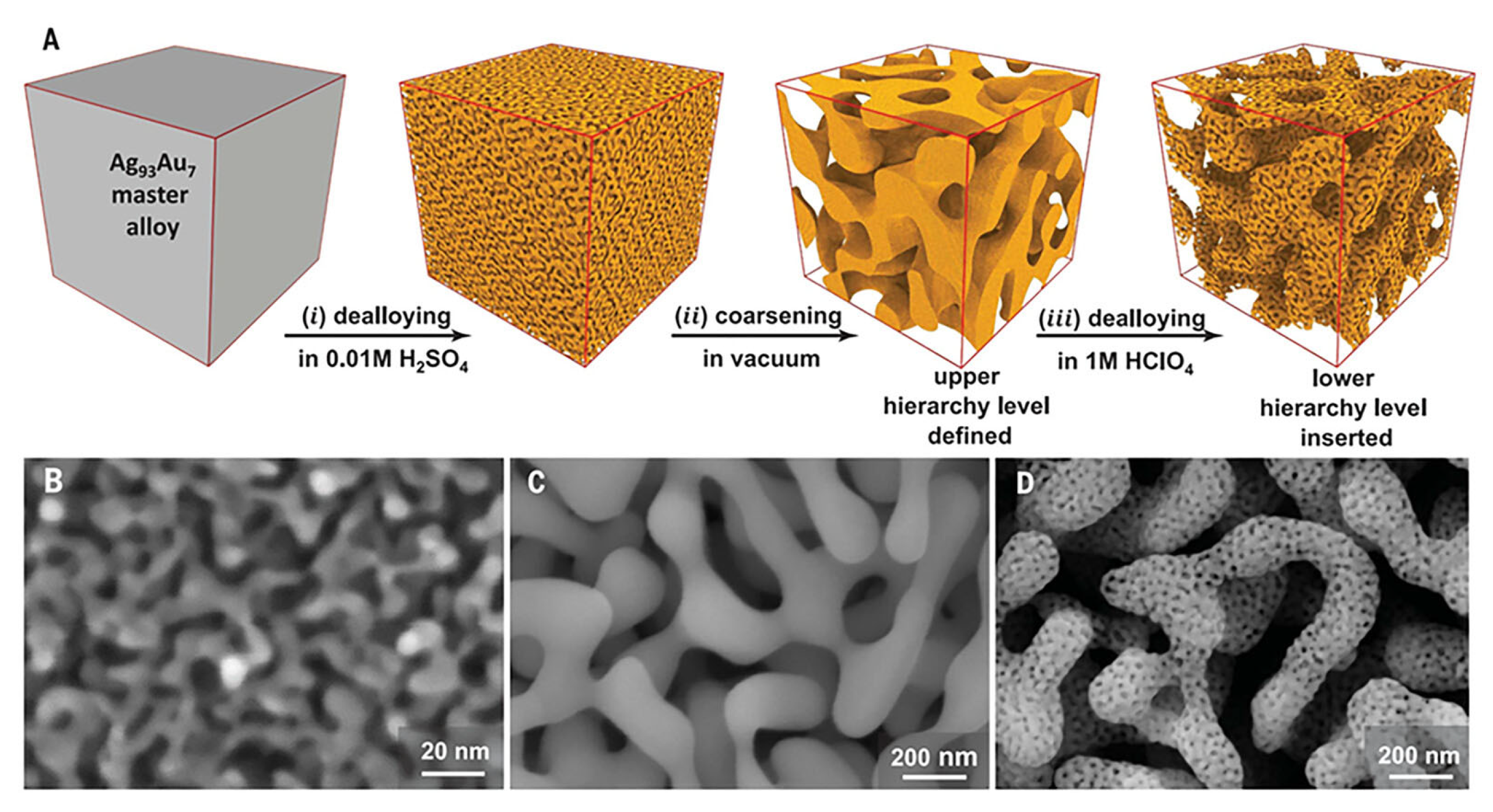

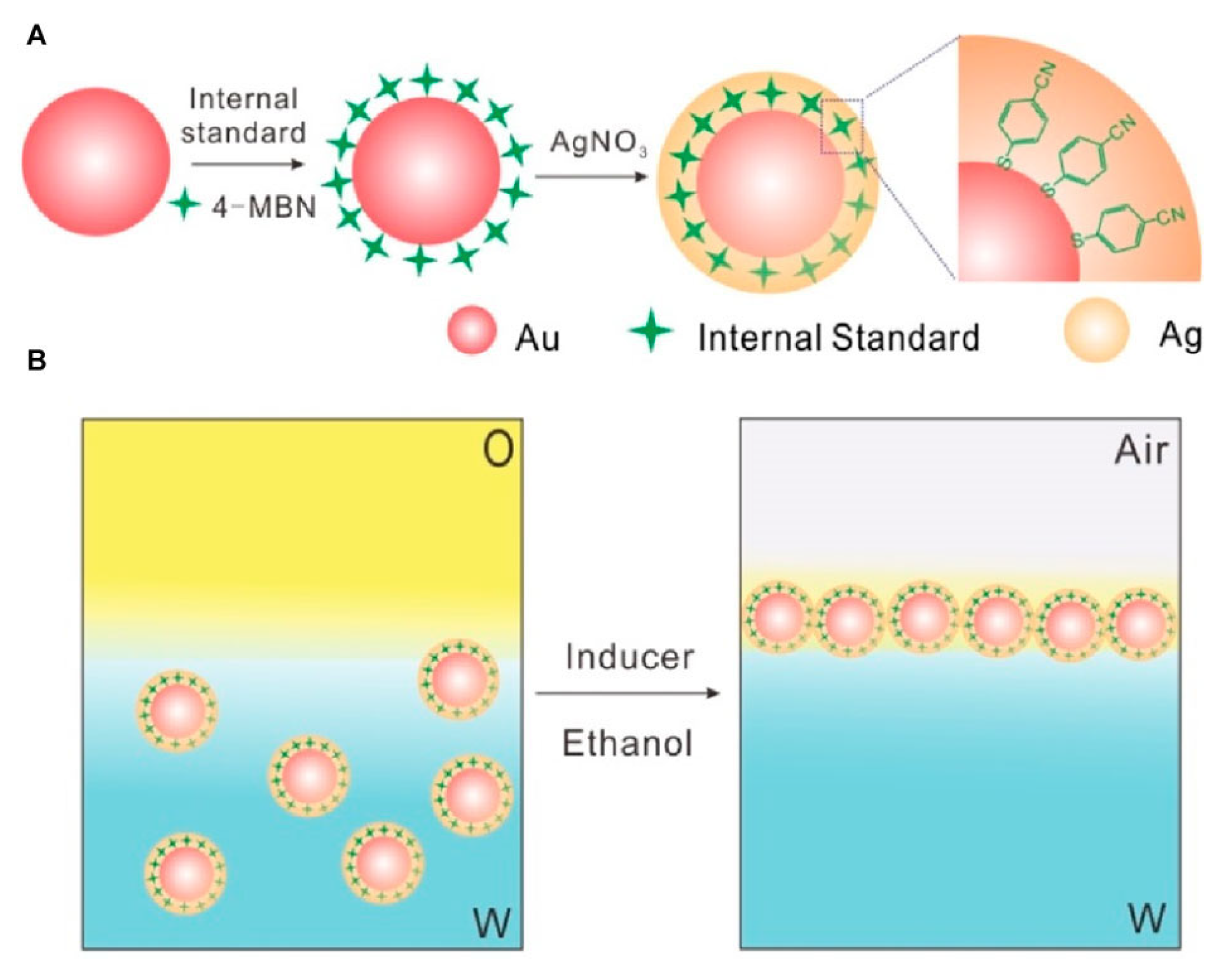
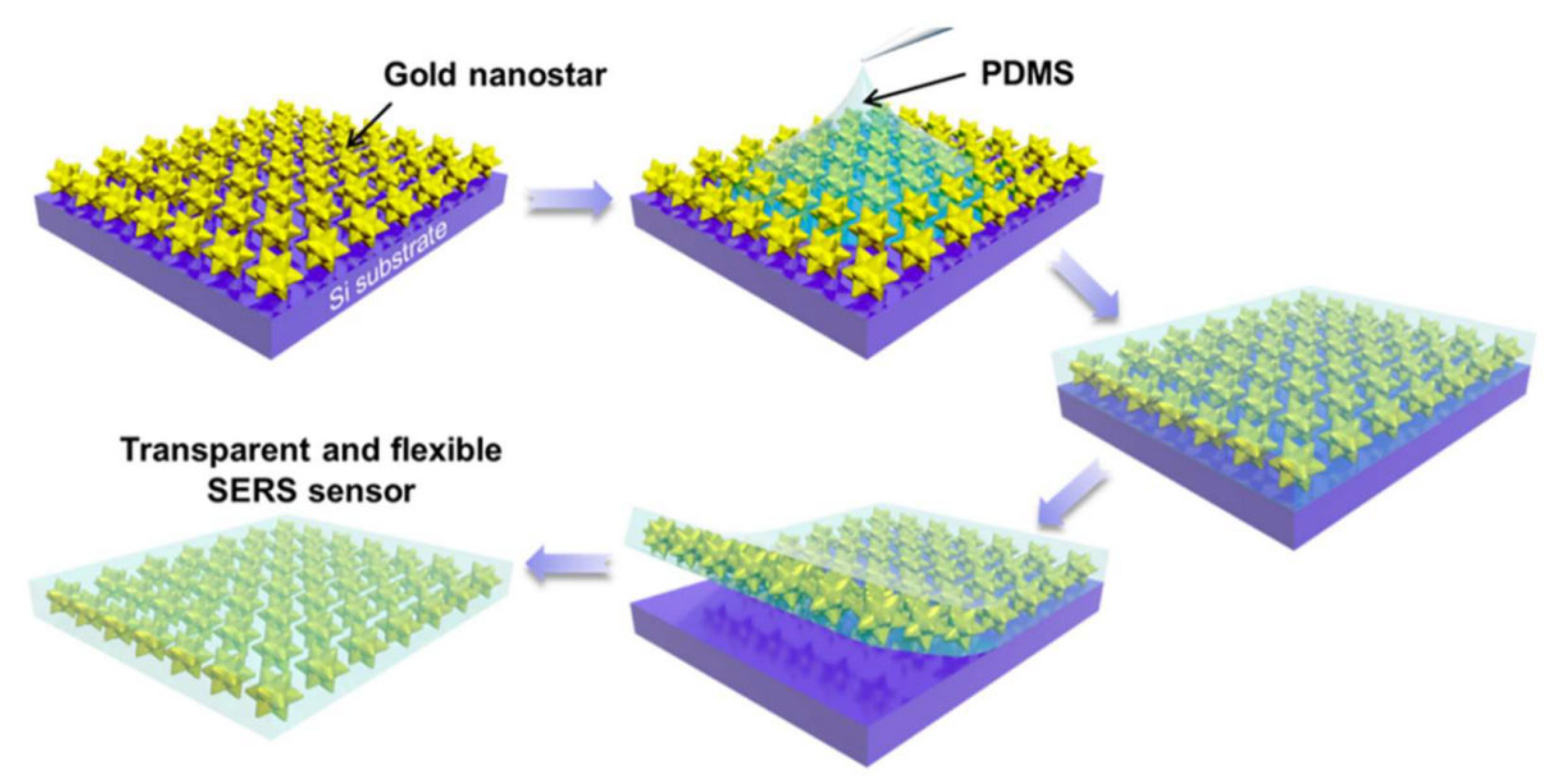
| Feature | 2D SERS Substrates | 3D SERS Substrates |
|---|---|---|
| Hot Spot Dimension | Confined to planar surface | Distributed volumetrically in all dimensions |
| Enhancement Factor | 105–107 | >108 |
| Reproducibility | Moderate | High (RSD typically < 10%) |
| Analyte Accessibility | Limited diffusion on surface | Enhanced diffusion via pores and 3D networks |
| Fabrication Methods | Lithography, self-assembly, etc. | Template growth, dealloying, freeze-drying, etc. |
| Architecture Type | Typical EF | Reproducibility (RSD%) | Structural Features | Advantages |
|---|---|---|---|---|
| Nanowires/Nanorods | 107–108 [27,28] | ≤8% | High aspect ratio; vertical light confinement | Uniform hot spot; scalable fabrication; strong EM |
| Dendritic/Fractal Nanostructures | >108 [25,26] | 5–15% | Multibranched; high-curvature tips; fractal geometry; electrochemically grown | Ultrahigh enhancement; broadband plasmonic response |
| Porous Frameworks/Aerogels | 107–109 [29,30] | ≤10% | Interconnected pores/ligaments; high surface area; tunable porosity | Efficient light trapping; large sensing volume; fast analyte diffusion |
| Core–Shell and Hollow NPs | 106–108 [32,83] | ≤5% | Tunable core–shell interfaces; shell thickness control; cavity modes | High reproducibility; spectral tunability; suitable for multilayer assembly |
| Hierarchical Hybrid Structures | >109 [101] | ≤10% | Multiscale integration | Flexibility; responsiveness; enhanced hot spot density |
| Fabrication Method | Key Features | Advantages | Limitations | EF/RSD |
|---|---|---|---|---|
| Template-Assisted Growth | Uses AAO, colloidal crystals, bio-templates | High uniformity; tunable geometry; good reproducibility; | Limited design freedom; template removal steps; sometimes low throughput | EF > 107; RSD < 7% |
| Electrochemical Dendrite Growth | Self-formed fractal structures on electrodes | simple equipment; broadband EM enhancement; scalable to large areas | Poor reproducibility; random morphology; fragile structure | EF > 108; RSD ~ 15% |
| Dealloying/Freeze-Drying | Nanoporous metals; aerogels from sol–gel or metal–organic systems | High surface area; excellent light trapping; fast analyte access | Brittle (aerogels); pore size hard to control; waste generation | EF > 107~108; RSD < 10% |
| Colloidal Self-Assembly | Langmuir–Blodgett, evaporation-driven, drop-casting of particles | Cost-effective; scalable; tunable interparticle gaps | Sensitive to humidity and solvent; requires precise shell thickness control | EF > 107; RSD < 5% |
| Hybrid/Hierarchical Integration | Combines nanowires, nanostars, hydrogels, photonic crystals | Multifunctional sensing; responsive materials; enhanced light localization via hierarchical design | Structural complexity; reproducibility challenges; difficult to model and fabricate at scale | EF > 109 RSD varies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Liu, S.; Xiang, H.; Li, X.; Wang, C.; Wu, Y.; Li, G. Three-Dimensional SERS Substrates: Architectures, Hot Spot Engineering, and Biosensing Applications. Biosensors 2025, 15, 555. https://doi.org/10.3390/bios15090555
Zhou X, Liu S, Xiang H, Li X, Wang C, Wu Y, Li G. Three-Dimensional SERS Substrates: Architectures, Hot Spot Engineering, and Biosensing Applications. Biosensors. 2025; 15(9):555. https://doi.org/10.3390/bios15090555
Chicago/Turabian StyleZhou, Xiaofeng, Siqiao Liu, Hailang Xiang, Xiwang Li, Chunyan Wang, Yu Wu, and Gen Li. 2025. "Three-Dimensional SERS Substrates: Architectures, Hot Spot Engineering, and Biosensing Applications" Biosensors 15, no. 9: 555. https://doi.org/10.3390/bios15090555
APA StyleZhou, X., Liu, S., Xiang, H., Li, X., Wang, C., Wu, Y., & Li, G. (2025). Three-Dimensional SERS Substrates: Architectures, Hot Spot Engineering, and Biosensing Applications. Biosensors, 15(9), 555. https://doi.org/10.3390/bios15090555





