Biosensor for Bacterial Detection Through Color Change in Culture Medium
Abstract
1. Introduction
2. Related Work and Theoretical Framework
2.1. Physical Principles of Optical Sensors for Detection
2.2. Optical Biosensors: Principles and Advantages
2.3. Optical Sensors and Colorimetry in Culture Media
2.4. Colorimetric Detection of Bacterial Byproducts
2.5. Types of Optical Sensors for Bacterial Detection
2.6. Mannitol Salt Agar and Staphylococcus aureus Detection
3. Materials and Methods
3.1. Bacterial Strain Activation
3.2. Inoculation Protocols
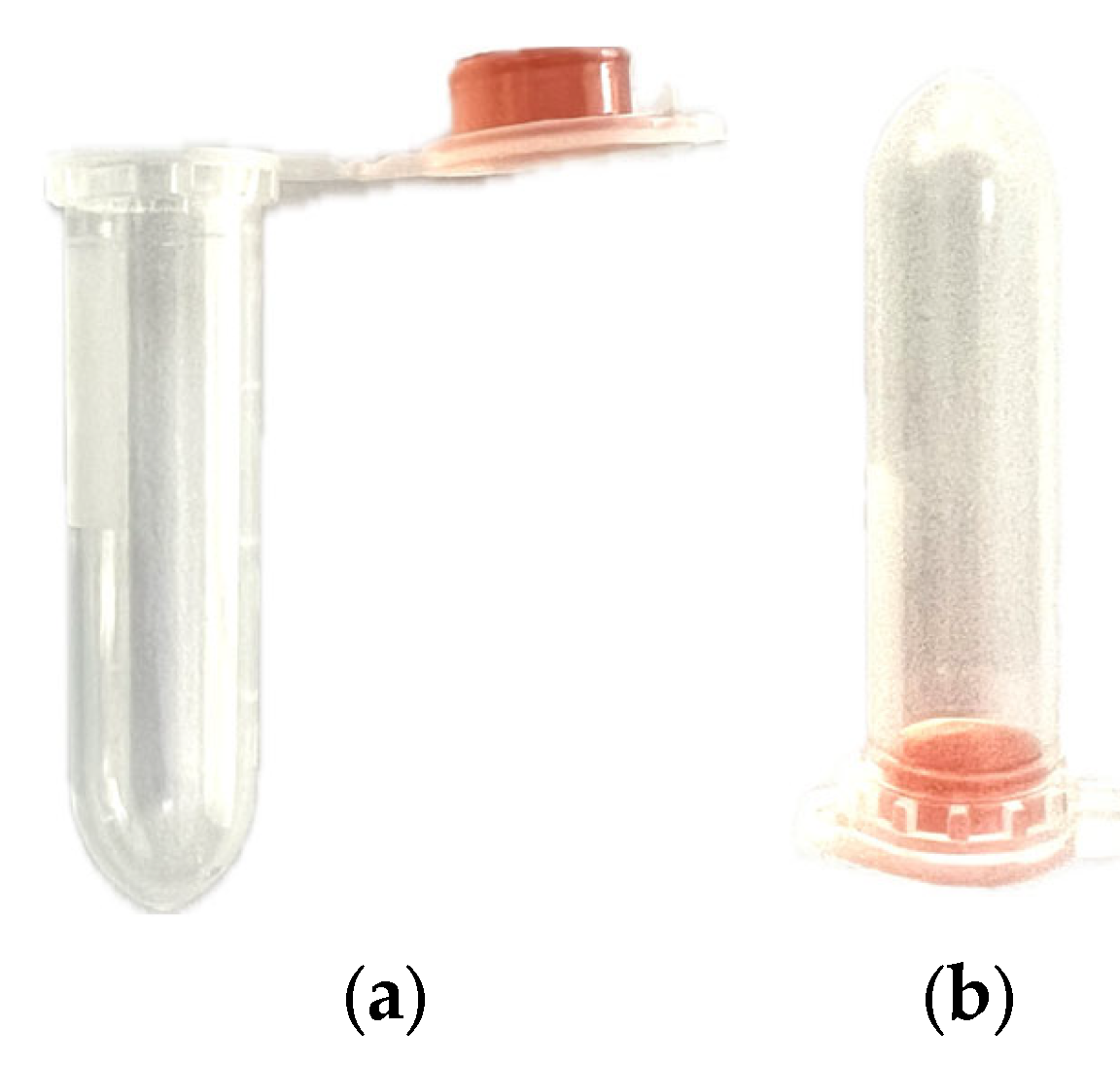
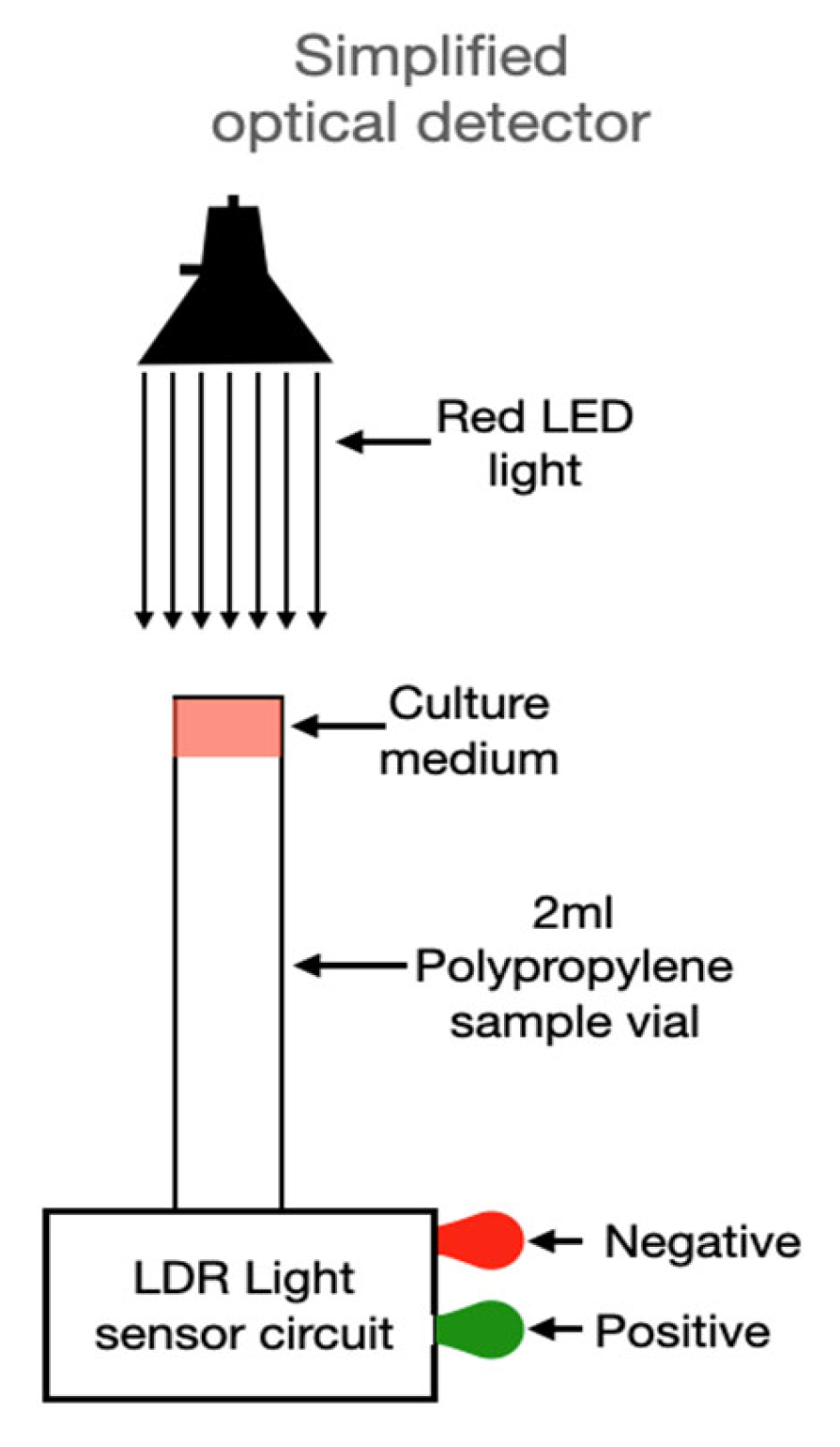
3.3. Prototype Design
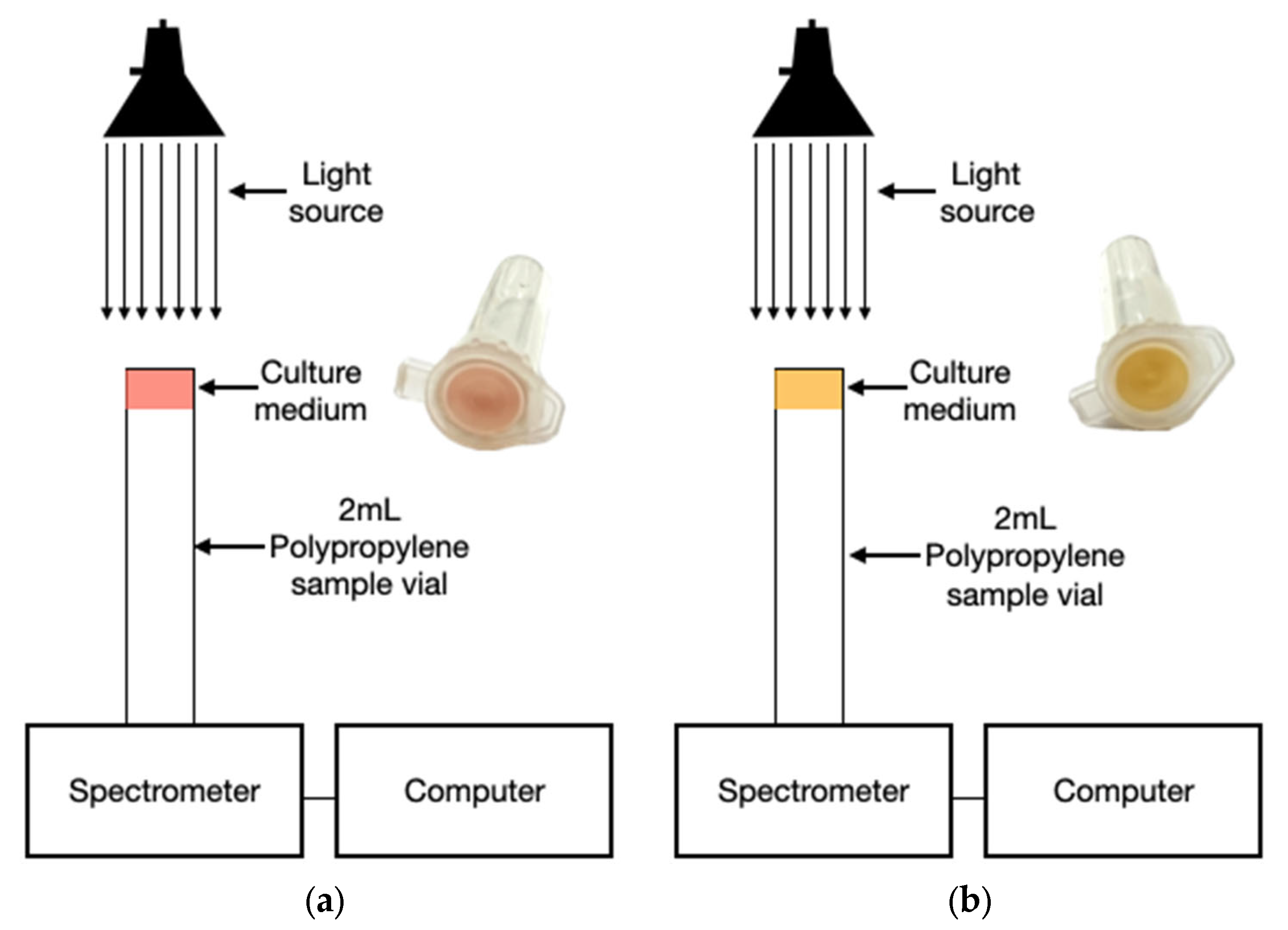
3.4. Optical Sensor Assembly
4. Results
4.1. Sensor Performance
4.2. Interpretation of Optical Signals
5. Discussion
6. Conclusions
- Clinical diagnostics: Early detection in hospitals to prevent nosocomial infections.
- Food industry: Online contamination monitoring in processing environments.
- Environmental monitoring: Field-deployable sensors for water quality analysis.
- Telemedicine: Optical readers integrated into smartphones for remote diagnosis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony-Forming Units |
| RGB | Red Green Blue |
| Ai | Artificial Intelligence |
| IoT | Internet of Things |
| ASM | Agar Salt Mannitol |
| SPR | Superficial Plasmonic Resonance |
| LED | Light-Emitting Diode |
| LDR | Light-Dependent Resistor |
| PCR | Polymerase Chain Reaction |
| NAAT | Nucleic Acid Amplification Test |
| TSA | Tryptic Soy Agar |
| ROI | Region of Interest |
References
- World Health Organization. Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 1 July 2025).
- Murray, C.J.L.; Ikuta, K.S.; Bisignano, C.; Roberts, D.A.; Mott, A.; Robles Aguilar, G.; Wool, E.E.; Han, C.; Sanderson, N.; Reiner, R.C.; et al. Global burden of bacterial infections in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Infect. Dis. 2024, 24, 827–849. [Google Scholar] [CrossRef]
- Tian, T.; Miao, L.; Wang, W.; Zhou, X. Global, Regional and National Burden of Human Cystic Echinococcosis from 1990 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Trop. Med. Infect. Dis. 2024, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, K.S.; Swetschinski, L.R.; Robles Aguilar, G.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Davis Weaver, N.; Wool, E.E.; Han, C.; Gershberg Hayoon, A.; et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Bunin, V.D.; Seregin, A.Y.; Churbanov, M.F. Optical sensors for bacterial detection. Sensors 2023, 23, 9391. [Google Scholar] [CrossRef]
- Idil, N.; Mattiasson, B.; Uzun, L. Recent advances in optical sensing for microbial contaminant detection. Micromachines 2023, 14, 1668. [Google Scholar] [CrossRef]
- Velusamy, V.; Arshak, K.; Korostynska, O.; Oliwa, K.; Adley, C. An overview of foodborne pathogen detection: In the perspective of biosensors. Biotechnol. Adv. 2010, 28, 232–254. [Google Scholar] [CrossRef]
- Guliy, O.I.; Karavaeva, O.A.; Smirnov, A.V.; Eremin, S.A.; Bunin, V.D. Optical Sensors for Bacterial Detection. Sensors 2023, 23, 9391. [Google Scholar] [CrossRef]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef]
- De Acha, N.; Socorro-Leránoz, A.B.; Elosúa, C.; Matías, I.R. Trends in the Design of Intensity-Based Optical Fiber Biosensors (2010–2020). Biosensors 2021, 11, 197. [Google Scholar] [CrossRef]
- di Toma, A.; Brunetti, G.; Chiriacò, M.S.; Ferrara, F.; Ciminelli, C. A Novel Hybrid Platform for Live/Dead Bacteria Accurate Sorting by On-Chip DEP Device. Int. J. Mol. Sci. 2023, 24, 7077. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, M.; Shen, W. Nanofiber-based colorimetric platform for point-of-care detection of E. coli. Chem. Eng. J. 2023, 459, 141685. [Google Scholar] [CrossRef]
- Vázquez, M.; Stortz, C.; Beltrán, M. Rapid chromatic detection of bacteria using biomimetic membranes. Appl. Environ. Microbiol. 2006, 72, 6601–6607. [Google Scholar]
- Pendão, C.; Silva, I. Optical Fiber Sensors and Sensing Networks: Overview of the Main Principles and Applications. Sensors 2022, 22, 7554. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Mohana Shankar, P.; Mutharasan, R. A review of fiber-optic biosensors. Sens. Actuators B Chem. 2007, 125, 688–703. [Google Scholar] [CrossRef]
- Kim, D.-M.; Yoo, S.-M. Colorimetric Systems for the Detection of Bacterial Contamination: Strategy and Applications. Biosensors 2022, 12, 532. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, H.; Chen, X.; Li, W.; Zhang, J.; Wang, M.; Zhang, J.; Wang, S.; Liu, Y. Glucose-metabolism-triggered colorimetric sensor array for point-of-care differentiation and antibiotic susceptibility testing of bacteria. Food Chem. 2024, 438, 137983. [Google Scholar] [CrossRef]
- Zibaii, M.I.; Kazemi, A.; Latifi, H.; Azar, M.K.; Hosseini, S.M.; Ghezelaiagh, M.H. Measuring bacterial growth by refractive index tapered fiber optic biosensor. J. Photochem. Photobiol. B Biol. 2010, 101, 313–320. [Google Scholar] [CrossRef]
- Baljinder, K.; Santosh, K.; Brajesh, K.K. Trends, challenges, and advances in optical sensing for pathogenic bacteria detection. Mater. Today Bio 2023, 20, 100581. [Google Scholar]
- Kim, K.R.; Yeo, W.H. Advances in sensor developments for cell culture monitoring. BMEMat 2023, 1, e12047. [Google Scholar] [CrossRef]
- López-Buenafé, G.D.R.; Alonso-Cabrera, J.A.; Marcuello, C.; Ortiz-Pérez, M.; Benito-López, F.; Colom, A.; Basabe-Desmonts, L.; Saez, J. Fabrication and characterization of PEDOT:PSS-based microstructured electrodes for in vitro cell culture. Adv. Mater. Interfaces 2025, 12, 2500097. [Google Scholar] [CrossRef]
- Sandro, C.O.; Soares, S.; Rodrigues, A.C.M.; Gonçalves, B.V.; Soares, A.M.V.M.; Santos, N.; Kumar, S.; Almeida, P.; Marques, C. Optical fiber immunosensors based on surface plASMon resonance for foodborne pathogens detection. Opt. Express 2023, 32, 10077–10090. [Google Scholar]
- Song, E.; Lee, K.; Kim, J. Tetrazolium-based visually indicating bacteria sensor. ACS Appl. Mater. Interfaces 2022, 14, 45043–45051. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.T.; Stępniewski, G.; Czarnecka, K.H.; Kasztelanic, R.; Long, V.C.; Xuan, K.D.; Shao, L.; Śmietana, M.; Buczyński, R. Optical properties of buffers and cell culture media for optofluidic devices. Appl. Sci. 2019, 9, 1145. [Google Scholar] [CrossRef]
- Crunaire, S.; Marcoux, P.R.; Ngo, K.-Q.; Moy, J.-P.; Mallard, F.; Tran-Thi, T.-H. Discriminating Bacteria with Optical Sensors Based on Functionalized Nanoporous Xerogels. Chemosensors 2014, 2, 171–181. [Google Scholar] [CrossRef]
- Zheng, L.; Qi, P.; Zhang, D. A simple, rapid and cost-effective colorimetric assay based on the 4-mercaptophenylboronic acid functionalized silver nanoparticles for bacteria monitoring. Sens. Actuators B Chem. 2018, 260, 983–989. [Google Scholar] [CrossRef]
- Ligler, F.S.; Ligler, G.T. Forty years of advances in optical biosensors—Are “autonomous” biosensors in our future? Anal. Bioanal. Chem. 2024, 416, 7199–7203. [Google Scholar] [CrossRef]
- Kumari, S.; Puri, P.; Suthar, D.; Kamlesh; Patel, S.L. Himanshu. In Sensing Materials and Devices for Biomarkers; Mutreja, V., Kathuria, D., Sareen, S., Park, J., Eds.; Royal Society of Chemistry: London, UK, 2024; Volume 28, pp. 1–36. [Google Scholar]
- Shrikrishna, N.S.; Sharma, R.; Sahoo, J.; Kaushik, A.; Gandhi, S. Navigating the landscape of optical biosensors. Chem. Eng. J. 2024, 490, 151661. [Google Scholar] [CrossRef]
- Maurya, J.B.; Prajapati, Y.K.; Tripathi, R. Effect of Molybdenum Disulfide Layer on Surface PlASMon Resonance Biosensor for the Detection of Bacteria. Silicon 2018, 10, 245–256. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Z.; Si, C.; Ying, Y. Monitoring of Escherichia coli O157:H7 in food samples using lectin based surface plASMon resonance biosensor. Food Chem. 2013, 136, 1303–1308. [Google Scholar] [CrossRef]
- Simon, A.H. Sputter processing. In Handbook of Thin Film Deposition, 4th ed.; Seshan, K., Schepis, D., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 195–230. [Google Scholar]
- Fu, H.; Zhang, S.; Chen, H.; Weng, J. Graphene enhances the sensitivity of fiber-optic surface plASMon resonance biosensor. IEEE Sens. J. 2015, 15, 5478–5482. [Google Scholar] [CrossRef]
- Kaushik, S.; Tiwari, U.K.; Pal, S.S.; Sinha, R.K. Rapid detection of Escherichia coli using fiber optic surface plASMon resonance immunosensor based on biofunctionalized Molybdenum disulfide (MoS2) nanosheets. Biosens. Bioelectron. 2019, 126, 501–509. [Google Scholar] [CrossRef] [PubMed]
- McGoverin, C.; Steed, C.; Esan, A.; Robertson, J.; Swift, S.; Vanholsbeeck, F. Optical methods for bacterial detection and characterization. APL Photonics 2021, 6, 080903. [Google Scholar] [CrossRef]
- Romphosri, S.; Pissuwan, D.; Wattanavichean, N.; Buabthong, P.; Waritanant, T. Rapid alignment-free bacteria identification via optical scattering with LEDs and YOLOv8. Sci Rep. 2024, 14, 20498. [Google Scholar] [CrossRef] [PubMed]
- Wildeboer, D.; Amirat, L.; Price, R.G.; Abuknesha, R.A. Rapid detection of Escherichia coli in water using a hand-held fluorescence detector. Water Res. 2010, 44, 2621–2628. [Google Scholar] [CrossRef]
- MacFaddin, J.F. Biochemical Tests for Identification of Medical Bacteria, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Perry, J.D. A Decade of Development of Chromogenic Culture Media for Clinical Microbiology in an Era of Molecular Diagnostics. Clin. Microbiol. Rev. 2017, 30. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Luka, G.S.; Nowak, E.; Kawchuk, J.; Hoorfar, M.; Najjaran, H. Portable device for the detection of colorimetric assays. R. Soc. Open Sci. 2017, 4, 171025. [Google Scholar] [CrossRef]
- Vickers, A.A.; Chopra, I.; O’Neill, A.J. Intrinsic Novobiocin Resistance in Staphylococcus saprophyticus. Antimicrob. Agents Chemother. 2007, 51, 4484–4485. [Google Scholar] [CrossRef]

 570 nm,
570 nm,  600 nm, and
600 nm, and  650 nm over 330 min (AU; mean ± SD). Background shading marks the regimes times period discussed in Section 4.2: red, 0–60 min—early attenuation (agar dehydration/thickness change and darkening); amber, 60–110 min—quasi-steady plateau; green, ≥130 min—detection window linked to mannitol-driven acidification (phenol-red shift), showing a peak around ~150 min followed by a gradual decline.
650 nm over 330 min (AU; mean ± SD). Background shading marks the regimes times period discussed in Section 4.2: red, 0–60 min—early attenuation (agar dehydration/thickness change and darkening); amber, 60–110 min—quasi-steady plateau; green, ≥130 min—detection window linked to mannitol-driven acidification (phenol-red shift), showing a peak around ~150 min followed by a gradual decline.
 570 nm,
570 nm,  600 nm, and
600 nm, and  650 nm over 330 min (AU; mean ± SD). Background shading marks the regimes times period discussed in Section 4.2: red, 0–60 min—early attenuation (agar dehydration/thickness change and darkening); amber, 60–110 min—quasi-steady plateau; green, ≥130 min—detection window linked to mannitol-driven acidification (phenol-red shift), showing a peak around ~150 min followed by a gradual decline.
650 nm over 330 min (AU; mean ± SD). Background shading marks the regimes times period discussed in Section 4.2: red, 0–60 min—early attenuation (agar dehydration/thickness change and darkening); amber, 60–110 min—quasi-steady plateau; green, ≥130 min—detection window linked to mannitol-driven acidification (phenol-red shift), showing a peak around ~150 min followed by a gradual decline.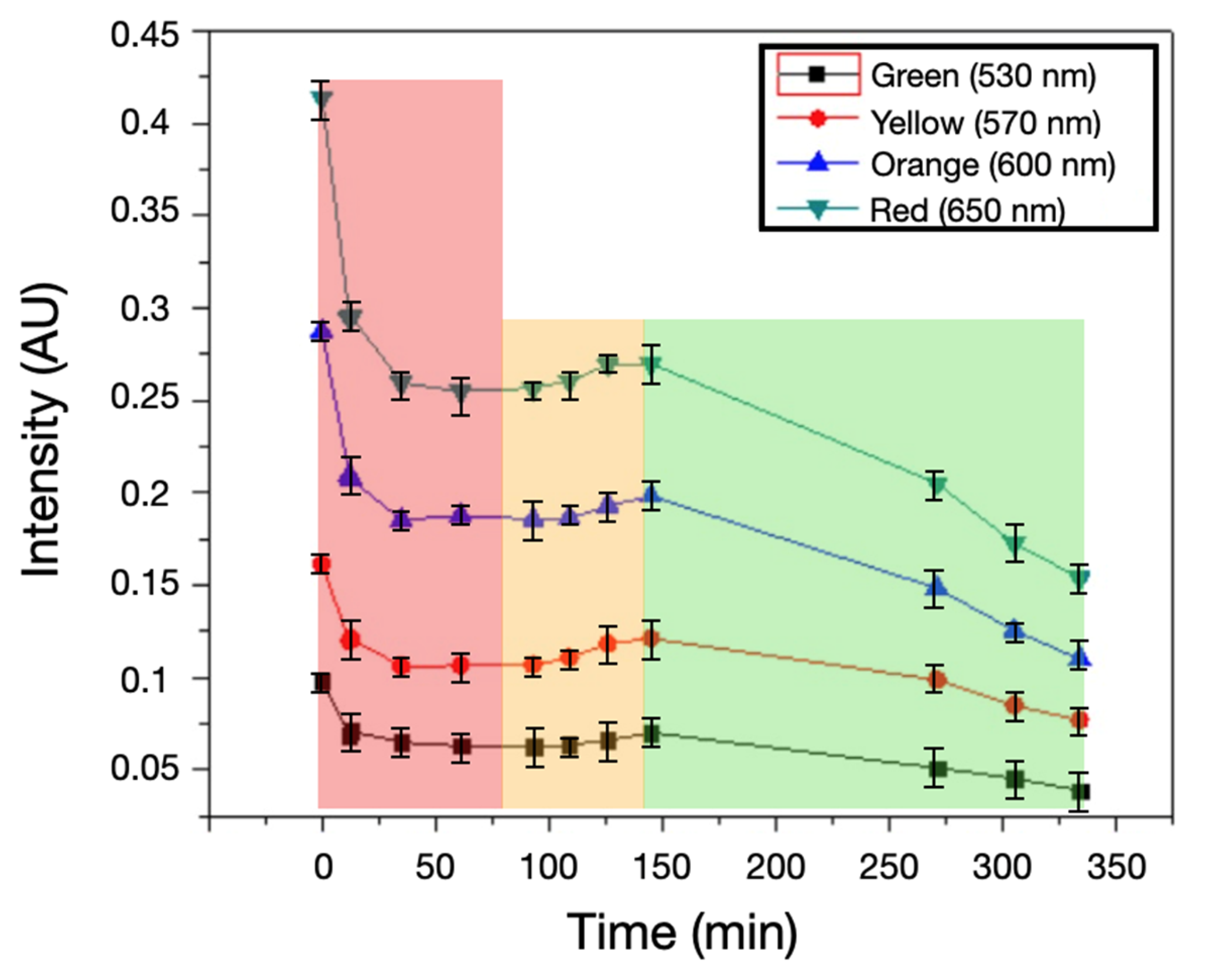
 570 nm,
570 nm,  600 nm, and
600 nm, and  650 nm over ~520 min (mean ± SD; AU). All channels show a small initial decrease followed by stable levels, and notably no late-stage rise at 600–650 nm. This contrasts with inoculated samples (Fig. 4) and confirms that the signal increase is driven by bacterial metabolism, not instrument drift or illumination variability.
650 nm over ~520 min (mean ± SD; AU). All channels show a small initial decrease followed by stable levels, and notably no late-stage rise at 600–650 nm. This contrasts with inoculated samples (Fig. 4) and confirms that the signal increase is driven by bacterial metabolism, not instrument drift or illumination variability.
 570 nm,
570 nm,  600 nm, and
600 nm, and  650 nm over ~520 min (mean ± SD; AU). All channels show a small initial decrease followed by stable levels, and notably no late-stage rise at 600–650 nm. This contrasts with inoculated samples (Fig. 4) and confirms that the signal increase is driven by bacterial metabolism, not instrument drift or illumination variability.
650 nm over ~520 min (mean ± SD; AU). All channels show a small initial decrease followed by stable levels, and notably no late-stage rise at 600–650 nm. This contrasts with inoculated samples (Fig. 4) and confirms that the signal increase is driven by bacterial metabolism, not instrument drift or illumination variability.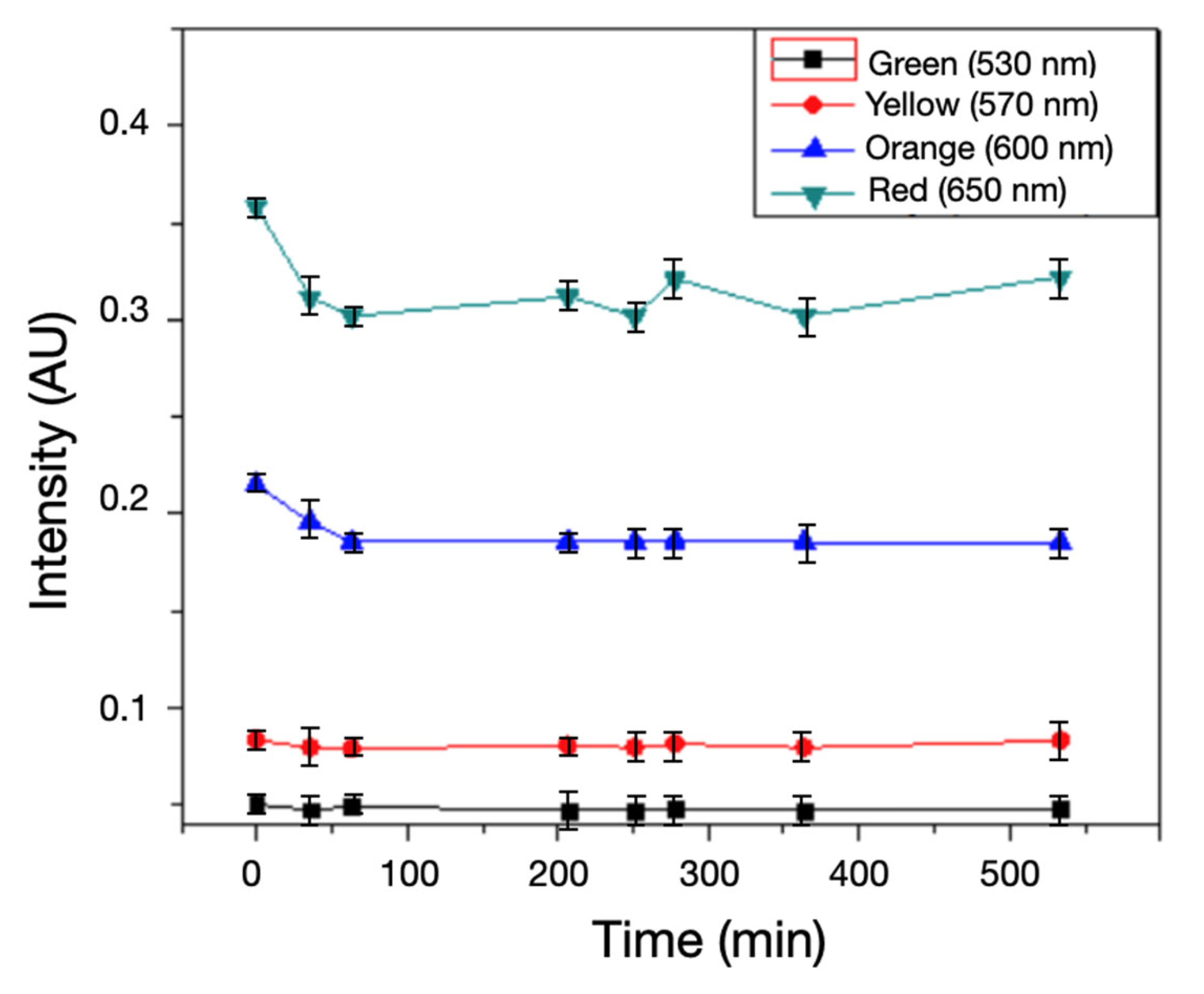

| Type of Sensor | Detection Time (min) | Detection Limit (CFU/mL) | Estimated Cost (USD) | Portability |
|---|---|---|---|---|
| Transmittance | 150 | 1000.0 | 300 | Medium |
| RGB + AI | 90 | 500.0 | 500 | High |
| Optical Fiber | 120 | 100.0 | 700 | Medium |
| LED Photodiode | 100 | 1000.0 | 150 | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, A.A.; Riofrío, G.; Castillo, D.; Padilla-Martínez, J.P.; Lakshminarayanan, V. Biosensor for Bacterial Detection Through Color Change in Culture Medium. Biosensors 2025, 15, 551. https://doi.org/10.3390/bios15080551
Sánchez AA, Riofrío G, Castillo D, Padilla-Martínez JP, Lakshminarayanan V. Biosensor for Bacterial Detection Through Color Change in Culture Medium. Biosensors. 2025; 15(8):551. https://doi.org/10.3390/bios15080551
Chicago/Turabian StyleSánchez, Aramis A., Grettel Riofrío, Darwin Castillo, J. P. Padilla-Martínez, and Vasudevan Lakshminarayanan. 2025. "Biosensor for Bacterial Detection Through Color Change in Culture Medium" Biosensors 15, no. 8: 551. https://doi.org/10.3390/bios15080551
APA StyleSánchez, A. A., Riofrío, G., Castillo, D., Padilla-Martínez, J. P., & Lakshminarayanan, V. (2025). Biosensor for Bacterial Detection Through Color Change in Culture Medium. Biosensors, 15(8), 551. https://doi.org/10.3390/bios15080551







