Dual-Mode Optical Detection of Sulfide Ions Using Copper-Anchored Nitrogen-Doped Graphene Quantum Dot Nanozymes
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Synthesis and Characterization of Cu@N-GQDs
2.3. Evaluation of Fluorescent and Peroxidase-Mimetic Properties
2.4. Dual-Mode Optical Detection of Sulfide Ions Using Cu@N-GQDs
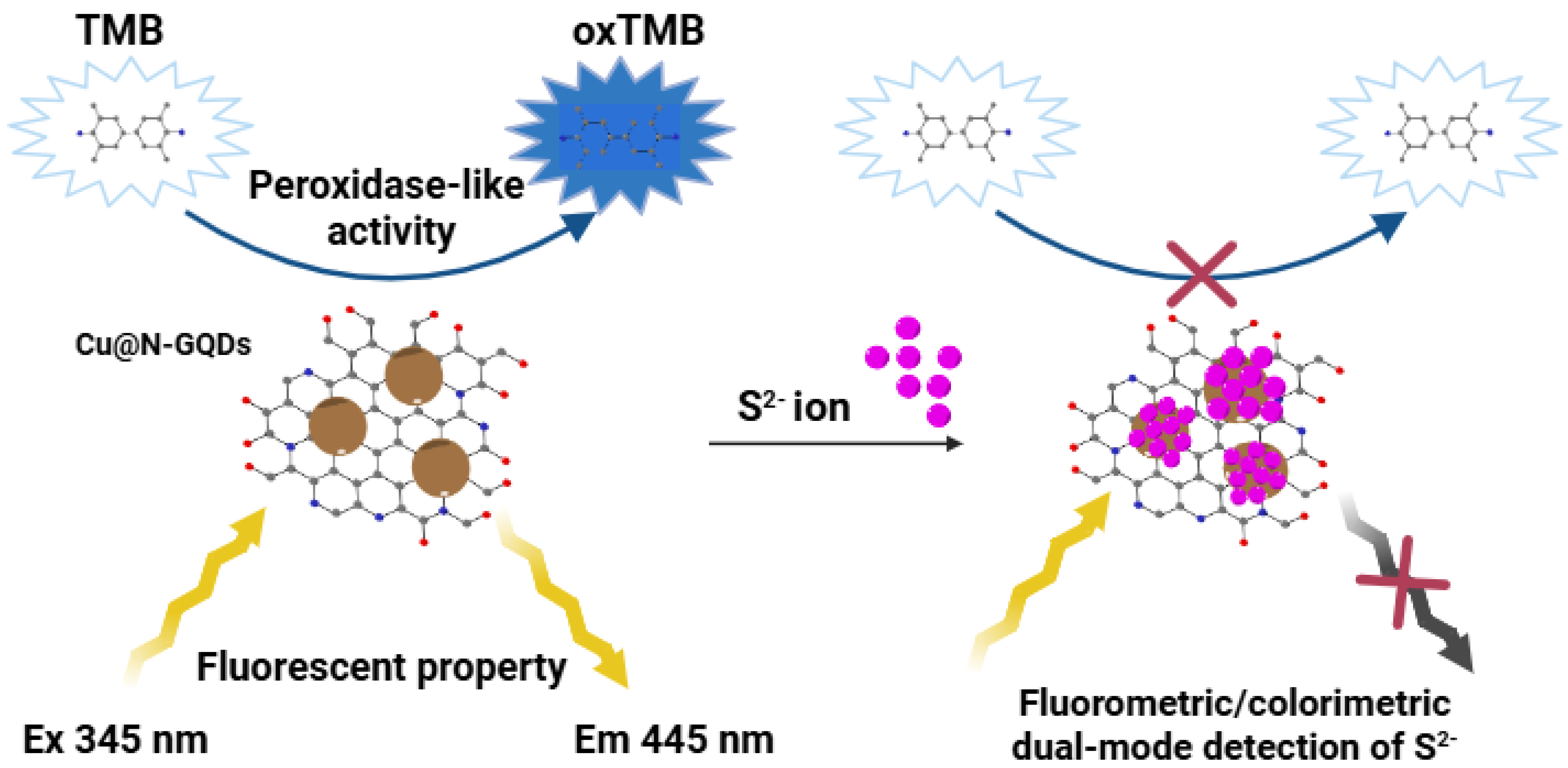
3. Results and Discussion
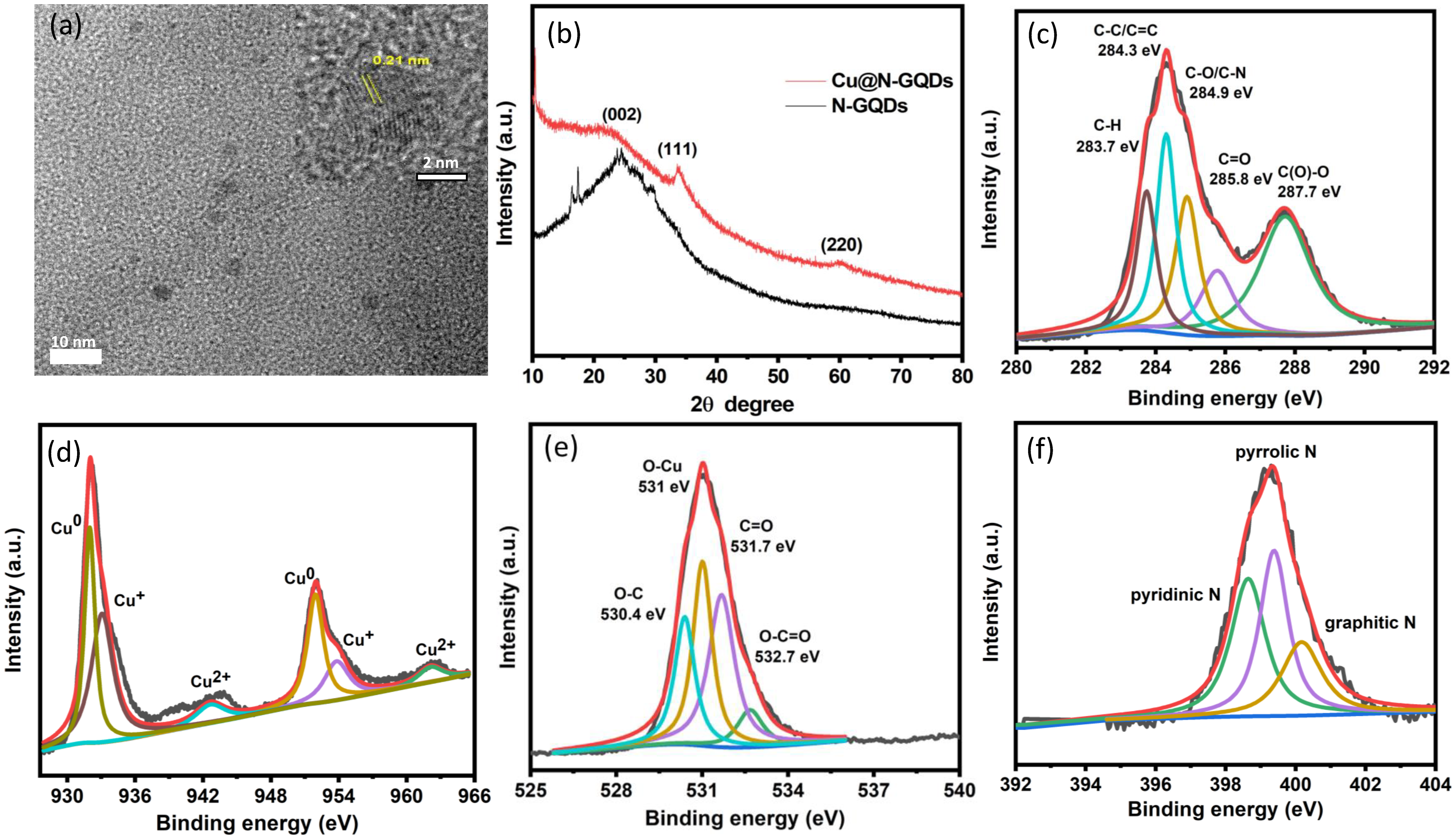
3.1. Synthesis of Bifunctional Cu@N-GQDs
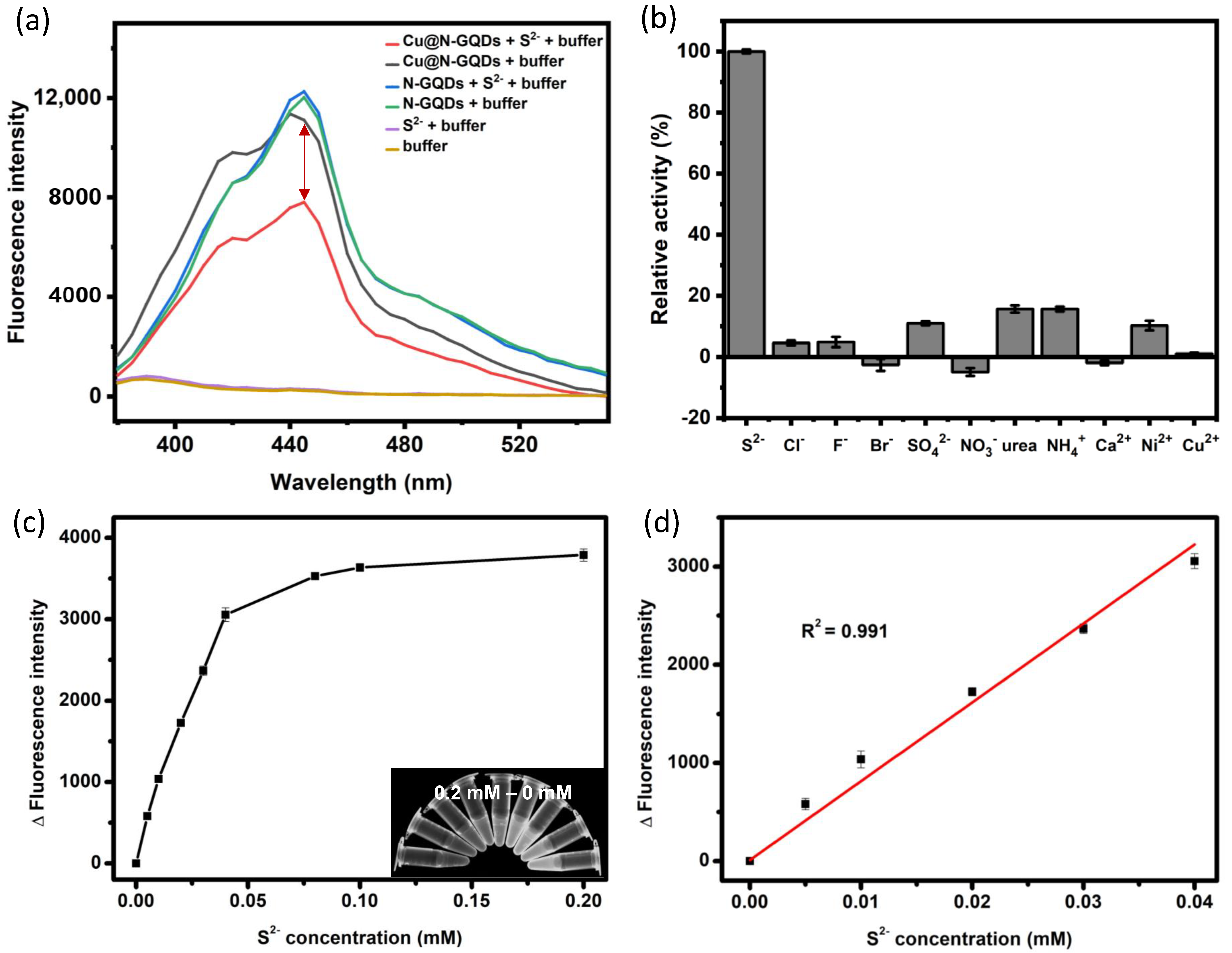
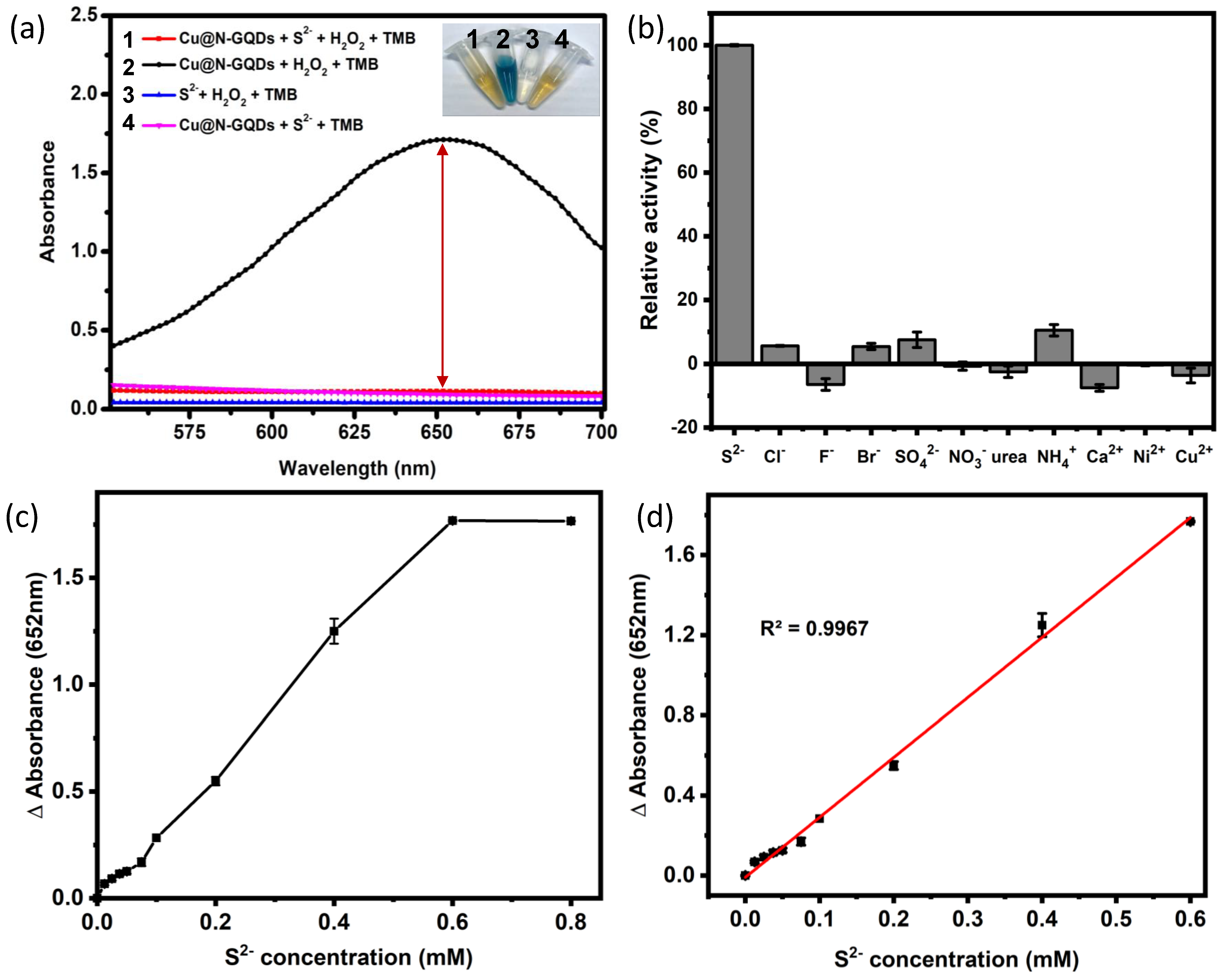
3.2. Evaluation of Fluorescence and Peroxidase-Like Activity of Cu@N-GQDs
3.3. Dual-Mode Optical Detection of Sulfide Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Lancaster, J.R., Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 2013, 35, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Olivera, C.; Tondo, M.L.; Girardi, V.; Fattobene, L.; Herrero, M.S.; Pérez, L.M.; Salvatierra, L.M. Early-stage response in anaerobic bioreactors due to high sulfate loads: Hydrogen sulfide yield and other organic volatile sulfur compounds as a sign of microbial community modifications. Bioresour. Technol. 2022, 350, 126947. [Google Scholar] [CrossRef]
- Karnachuk, O.V.; Rusanov, I.I.; Panova, I.A.; Grigoriev, M.A.; Zyusman, V.S.; Latygolets, E.A.; Kadyrbaev, M.K.; Gruzdev, E.V.; Beletsky, A.V.; Mardanov, A.V. Microbial sulfate reduction by Desulfovibrio is an important source of hydrogen sulfide from a large swine finishing facility. Sci. Rep. 2021, 11, 10720. [Google Scholar] [CrossRef]
- Dai, S.; Korth, B.; Schwab, L.; Aulenta, F.; Vogt, C.; Harnisch, F. Deciphering the fate of sulfate in one-and two-chamber bioelectrochemical systems. Electrochim. Acta 2022, 408, 139942. [Google Scholar] [CrossRef]
- Singh, A.K.; Chandra, R. Pollutants released from the pulp paper industry: Aquatic toxicity and their health hazards. Aquat. Toxicol. 2019, 211, 202–216. [Google Scholar] [CrossRef]
- Pulp and Paper Market-Segmentation. Available online: https://www.fortunebusinessinsights.com/segmentation/pulp-and-paper-market-103447 (accessed on 12 August 2024).
- Global Heavy Vacuum Gas Oil Market Growth Opportunities & Market Dynamics to 2028. Available online: https://www.kenresearch.com/industry-reports/global-heavy-vacuum-gas-oil-market (accessed on 12 August 2024).
- Kimura, H. Hydrogen sulfide (H2S) and polysulfide (H2Sn) signaling: The first 25 years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef]
- Shefa, U.; Kim, M.S.; Jeong, N.Y.; Jung, J. Antioxidant and cell-signaling functions of hydrogen sulfide in the central nervous system. Oxidative Med. Cell. Longev. 2018, 2018, 1873962. [Google Scholar] [CrossRef]
- Kimura, H.; Nagai, Y.; Umemura, K.; Kimura, Y. Physiological roles of hydrogen sulfide: Synaptic modulation, neuroprotection, and smooth muscle relaxation. Antioxid. Redox Signal. 2005, 7, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Casella, I.G.; Guascito, M.R.; Desimoni, E. Sulfide measurements by flow injection analysis and ion chromatography with electrochemical detection. Anal. Chim. Acta 2000, 409, 27–34. [Google Scholar] [CrossRef]
- Mhatre, S.; Rai, A.; Ali, H.; Patil, A.; Singh, N.; Verma, R.; Auden, J.; Chandler, C.; Dash, A.; Opere, C. Comparison of colorimetric, spectroscopic and electrochemical techniques for quantification of hydrogen sulfide. BioTechniques 2024, 76, 71–80. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Wan, Q.; Lu, Q.; Liu, J.; Ren, Y.; Tang, J.; Su, Q.; Luo, Y. Label-free, reusable, equipment-free, and visual detection of hydrogen sulfide using a colorimetric and fluorescent dual-mode sensing platform. Anal. Chem. 2023, 95, 5920–5926. [Google Scholar] [CrossRef] [PubMed]
- Ranjana, M.; Kulkarni, R.M.; Sunil, D. Small molecule optical probes for detection of H2S in water samples: A review. ACS Omega 2024, 9, 14672–14691. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, M.; Liu, T.; Wu, C.; Sun, M.; Su, G.; Wang, X.; Wang, Y.; Yin, H.; Zhou, X. Machine learning system to monitor Hg2+ and sulfide using a polychromatic fluorescence-colorimetric paper sensor. ACS Appl. Mater. Interfaces 2023, 15, 9800–9812. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Wang, Y.; Huo, F.; Karmaker, P.G.; Chen, L.; Yang, X.; Zhao, B. Chameleon-like response mechanism of gold–silver bimetallic nanoclusters stimulated by sulfur ions and their application in visual fluorescence sensing. Anal. Chem. 2024, 96, 5029–5036. [Google Scholar] [CrossRef]
- Auria-Luna, F.; Foss, F.W.; Molina-Canteras, J.; Velazco-Cabral, I.; Marauri, A.; Larumbe, A.; Aparicio, B.; Vázquez, J.L.; Alberro, N.; Arrastia, I. Supramolecular chemistry in solution and solid–gas interfaces: Synthesis and photophysical properties of monocolor and bicolor fluorescent sensors for barium tagging in neutrinoless double beta decay. RSC Appl. Interfaces 2025, 2, 185–199. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, B.; Yuan, B.; Zhou, C.; Zhang, M.; Zhao, Y.; Ye, P.; Li, L.; Li, H. Dual-state fluorescent probe for ultrafast and sensitive detection of nerve agent simulants in solution and vapor. ACS Sens. 2023, 8, 1220–1229. [Google Scholar] [CrossRef]
- Barati, A.; Shamsipur, M.; Abdollahi, H. Metal-ion-mediated fluorescent carbon dots for indirect detection of sulfide ions. Sens. Actuators B Chem. 2016, 230, 289–297. [Google Scholar] [CrossRef]
- Yu, N.; Peng, H.; Xiong, H.; Wu, X.; Wang, X.; Li, Y.; Chen, L. Graphene quantum dots combined with copper (II) ions as a fluorescent probe for turn-on detection of sulfide ions. Microchim. Acta 2015, 182, 2139–2146. [Google Scholar] [CrossRef]
- Zeng, J.; Li, M.; Liu, A.; Feng, F.; Zeng, T.; Duan, W.; Li, M.; Gong, M.; Wen, C.Y.; Yin, Y. Au/AgI dimeric nanoparticles for highly selective and sensitive colorimetric detection of hydrogen sulfide. Adv. Funct. Mater. 2018, 28, 1800515. [Google Scholar] [CrossRef]
- Vu, T.H.; Nguyen, P.T.; Kim, M.I. Polydopamine-coated Co3O4 nanoparticles as an efficient catalase mimic for fluorescent detection of sulfide ion. Biosensors 2022, 12, 1047. [Google Scholar] [CrossRef]
- Van Tam, T.; Trung, N.B.; Kim, H.R.; Chung, J.S.; Choi, W.M. One-pot synthesis of N-doped graphene quantum dots as a fluorescent sensing platform for Fe3+ ions detection. Sens. Actuators B Chem. 2014, 202, 568–573. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xu, T.; Liao, H.; Yao, C.; Liu, Y.; Li, Z.; Chen, Z.; Pan, D.; Sun, L. Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat. Commun. 2014, 5, 5357. [Google Scholar] [CrossRef]
- Stachowska, J.D.; Murphy, A.; Mellor, C.; Fernandes, D.; Gibbons, E.N.; Krysmann, M.J.; Kelarakis, A.; Burgaz, E.; Moore, J.; Yeates, S.G. A rich gallery of carbon dots based photoluminescent suspensions and powders derived by citric acid/urea. Sci. Rep. 2021, 11, 10554. [Google Scholar] [CrossRef]
- Kaviyarasan, K.; Anandan, S.; Mangalaraja, R.V.; Sivasankar, T.; Ashokkumar, M. Sonochemical synthesis of Cu2O nanocubes for enhanced chemiluminescence applications. Ultrason. Sonochem. 2016, 29, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Santiago, S.R.M.; Wong, Y.A.; Lin, T.-N.; Chang, C.-H.; Yuan, C.-T.; Shen, J.-L. Effect of nitrogen doping on the photoluminescence intensity of graphene quantum dots. Opt. Lett. 2017, 42, 3642–3645. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Ghijsen, J.; Tjeng, L.-H.; van Elp, J.; Eskes, H.; Westerink, J.; Sawatzky, G.A.; Czyzyk, M.T. Electronic structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322–11330. [Google Scholar] [CrossRef]
- Wahid, M.; Parte, G.; Phase, D.; Ogale, S. Yogurt: A novel precursor for heavily nitrogen doped supercapacitor carbon. J. Mater. Chem. A 2015, 3, 1208–1215. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, X.; Pang, A.; Yang, J. Facile synthesis and photoluminescence characteristics of blue-emitting nitrogen-doped graphene quantum dots. Nanotechnology 2016, 27, 165704. [Google Scholar] [CrossRef]
- Liu, X.; Han, J.; Hou, X.; Altincicek, F.; Oncel, N.; Pierce, D.; Wu, X.; Zhao, J.X. One-pot synthesis of graphene quantum dots using humic acid and its application for copper (II) ion detection. J. Mater. Sci. 2021, 56, 4991–5005. [Google Scholar] [CrossRef]
- Grommeck, R.; Markakis, P. The effect of peroxidase on anthocyanin pigments. J. Food Sci. 1964, 29, 53–57. [Google Scholar] [CrossRef]
- Kaushal, N.; Jain, A.; Kumar, A.; Sarraf, S.; Basu, A.K.; Raje, C.I.; Saha, A. Solvent-Free Synthesis of S, N-Doped Carbon Dots for Extended Visible-Light-Induced Oxidase-Mimicking Activities and Antimicrobial Applications. ChemPlusChem 2023, 88, e202300125. [Google Scholar] [CrossRef]
- Li, Z.; Deng, X.; Hong, X.; Zhao, S.J.M. Nanozyme based on dispersion of hemin by graphene quantum dots for colorimetric detection of glutathione. Molecules 2022, 27, 6779. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Mahnashi, M.H.; Alhazzani, K.; Az, A.; Algahtani, M.M.; Alaseem, A.; Alyami, B.A.; AlQarni, A.O.; El-Wekil, M.M. Nitrogen doped graphene quantum dots based on host guest interaction for selective dual readout of dopamine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119516. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, X.; Feng, S.; Lei, W.; Xia, M.; Wang, F.; Wang, H. Sensitive fluorescent detection and micromechanism of Mn-doped CuS probe for oxytetracycline hydrochloride. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 284, 121768. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Wang, L.; Li, L.; Xie, W.; Gu, G. Synthesis of CuS nanoparticles decorated Ti3C2T x MXene with enhanced microwave absorption performance. Prog. Nat. Sci. Mater. Int. 2020, 30, 343–351. [Google Scholar] [CrossRef]
- Dai, B.; Dong, F.; Wang, H.; Qu, Y.; Ding, J.; Ma, Y.; Ma, M.; Li, T. Fabrication of CuS/Fe3O4@polypyrrole flower-like composites for excellent electromagnetic wave absorption. J. Colloid Interface Sci. 2023, 634, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, L.; Miao, Y.; Liu, C.; Zhang, C. A colorimetric sensor for the highly selective detection of sulfide and 1,4-dithiothreitol based on the in situ formation of silver nanoparticles using dopamine. Sensors 2017, 17, 626. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Zeng, F.; Zheng, F.; Wu, S. Carbon-dot-based ratiometric fluorescent sensor for detecting hydrogen sulfide in aqueous media and inside live cells. Chem. Commun. 2013, 49, 403–405. [Google Scholar] [CrossRef]
- Nejad, M.A.F.; Bigdeli, A.; Hormozi-Nezhad, M.R. Wide color-varying visualization of sulfide with a dual emissive ratiometric fluorescence assay using carbon dots and gold nanoclusters. Microchem. J. 2020, 157, 104960. [Google Scholar] [CrossRef]
- Gore, A.H.; Vatre, S.B.; Anbhule, P.V.; Han, S.-H.; Patil, S.R.; Kolekar, G.B. Direct detection of sulfide ions [S2-] in aqueous media based on fluorescence quenching of functionalized CdS QDs at trace levels: Analytical applications to environmental analysis. Analyst 2013, 138, 1329–1333. [Google Scholar] [CrossRef] [PubMed]

| Catalytic Materials | Method | Linear Range (μM) | LOD (μM) | Reference |
|---|---|---|---|---|
| Mn-doped ZnS QDs | Fluorometric | 1.2–26 | 0.33 | [41] |
| PDA@Co3O4 | Fluorometric | <200 | 4.3 | [22] |
| DSP-Based Assay | Colorimetric Fluorometric | 0.5–40 0.5–20 | 2.17 2.5 | [13] |
| AuNCs and CDs | Colorimetric Fluorometric | <10 1–50 | 4.0 0.35 | [42] |
| Ag+ and CDs | Fluorometric | 1–100 | 0.43 | [17] |
| 3-Mercaptopropionic acid functionalized CdS QDs | Fluorometric | 3.1–55.7 | 6.5 | [43] |
| Cu@N-GQDs | Colorimetric Fluorometric | 10–600 2–40 | 3.5 0.5 | This work |
| Method | Spiked Level (µM) | Measured a (µM) | Recovery b (%) (n = 3) | CV c (%) |
|---|---|---|---|---|
| Fluorescence | 25 | 24.3 | 97.3 | 2.2 |
| 50 | 52.5 | 104.6 | 3.3 | |
| 100 | 95.4 | 95.4 | 2.2 | |
| Colorimetry | 50 | 47.7 | 95.2 | 1.5 |
| 100 | 99.8 | 99.7 | 1.3 | |
| 150 | 155.5 | 103.3 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.A.N.; Vu, T.H.; Nguyen, P.T.; Kim, M.I. Dual-Mode Optical Detection of Sulfide Ions Using Copper-Anchored Nitrogen-Doped Graphene Quantum Dot Nanozymes. Biosensors 2025, 15, 528. https://doi.org/10.3390/bios15080528
Nguyen VAN, Vu TH, Nguyen PT, Kim MI. Dual-Mode Optical Detection of Sulfide Ions Using Copper-Anchored Nitrogen-Doped Graphene Quantum Dot Nanozymes. Biosensors. 2025; 15(8):528. https://doi.org/10.3390/bios15080528
Chicago/Turabian StyleNguyen, Van Anh Ngoc, Trung Hieu Vu, Phuong Thy Nguyen, and Moon Il Kim. 2025. "Dual-Mode Optical Detection of Sulfide Ions Using Copper-Anchored Nitrogen-Doped Graphene Quantum Dot Nanozymes" Biosensors 15, no. 8: 528. https://doi.org/10.3390/bios15080528
APA StyleNguyen, V. A. N., Vu, T. H., Nguyen, P. T., & Kim, M. I. (2025). Dual-Mode Optical Detection of Sulfide Ions Using Copper-Anchored Nitrogen-Doped Graphene Quantum Dot Nanozymes. Biosensors, 15(8), 528. https://doi.org/10.3390/bios15080528







