Electrochemical Detection of Heavy Metals Using Graphene-Based Sensors: Advances, Meta-Analysis, Toxicity, and Sustainable Development Challenges
Abstract
1. Introduction
2. Electrochemical Applications of Graphene-Based Sensors and Biosensors for the Detection of Heavy Metals
2.1. Metal or Metal Oxide Nanoparticle-Modified Graphene Sensors and Biosensors
2.1.1. Metal Nanoparticle-Modified Graphene Sensors and Biosensors
2.1.2. Metal Oxide Nanoparticle-Modified Graphene Sensors
2.2. Polymer-Modified Graphene-Based Sensors
2.3. Sensors Based on Other Functional Material-Modified Graphene
2.3.1. MOF-Modified Graphene-Based Sensors and Biosensors
2.3.2. COF-Modified Graphene Sensors
2.3.3. Other Functional Material-Modified Graphene Sensors and Biosensors
2.4. Ionic Liquid-Modified Graphene Sensors
| Electrode Substrate | Sensing Materials | Heavy Metal | Method (Accumulation Time) | (Simultaneous) LDR,µg·L−1 | (Simultaneous) LOD, µg·L−1 | Analytical Characteristics | Sensor Characteristics | Matrix | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adv. | Disadv. | Adv. | Disadv. | ||||||||
| CC | EBFC/GDY/GR/AuNPs/DNAzyme/CHA | Pb2+ | EIS (90 min) | 0.0006–1035 | 0.001 | A, B, C, D, F | E | G, H | – | Orange juice, tap water | [54] |
| CFs | Ag/GDs/NCs/CFs | a Cd2+ b Pb2+ | DPV (420 s) | a 1405–28,102 b 2590–51,800 | a 2.8 b 5.2 | A, B, C, D, F | E | H, I | G, J | River water | [59] |
| CPE | IL/GR/L | b Pb2+ d Hg2+ e Tl2+ | SWASV (90 s) | b,d,e 0.3–41 | b 0.09 d 0.08 e 0.07 | A, B, C, D, E, F | – | H, J | G, I | Tap and river water, soil | [58] |
| GCE | L-cys/GR/CS | a Cd2+ b Pb2+ | DPASV (120 s) | a 0.6–67 b 1–62 | a 0.5 b 0.1 | A, B, C, D, E, F | – | G, I | H, J | Honey, rice | [21] |
| N-doped GR | a Cd2+ b Pb2+ c Cu2+ d Hg2+ | DPSV (300 s) | a 11–101 b 2–1863 c 0.6–320 d 40–1809 | a 6 b 1 c 0.3 d 10 | B, D, F | A, C, E | G, H, I, J | – | Tap water | [22] | |
| GRA/UiO-66-NH2 (MOF) | a Cd2+ b Pb2+ c Cu2+ d Hg2+ | DPSV (250 s) | a 1–168 b 0.2–414 c 0.6–102 d 0.2–442 | a 1.1 b 0.21 c 0.5 d 0.1 | A, B, D, F | C, E | J | G, H, I | River water, soil, spinach | [45] | |
| Bi/AuNPs/GR/L-cys | a Cd2+ b Pb2+ | SWASV (800 s) | a,b 0.5–40 | a 0.1 b 0.05 | A, B, D, F | C, E | J | G, H, I | Spring water | [27] | |
| BiNPs@NPGCS | a Cd2+ b Pb2+ | SWASV (180 s) | a 9–90 b 12–124 | a 0.5 b 0.7 | A, D, E, F | B, C | J | G, H, I | Lake and tap water | [30] | |
| Bi/Fe2O3/GR | a Cd2+ b Pb2+ f Zn2+ | DPASV (300 s) | a,b,f 1–100 | a 0.08 b 0.07 f 0.1 | A, D, F | B, C, E | I, J | G, H | Tap water | [31] | |
| PbS/GR | Cu2+ | CV | 1.1–11,200 | 1.1 | A, B, E, F | C, D | – | G, H, I, J | – | [46] | |
| GR/N/Bi | a Cd2+ b Pb2+ f Zn2+ | DPASV (300 s) | a,b,f 5–100 | a 0.07 b 0.05 f 0.57 | A, D, F | B, C, E | I, J | G, H | Tap water | [34] | |
| MgFe-LDH/GR | a Cd2+ b Pb2+ | SWASV (180 s) | a 11–112 b 21–207 | a 0.7 b 0.6 | A, D, E, F | B, C | I, J | F, H | Lake and tap water | [37] | |
| GR/CeO2 | a Cd2+ b Pb2+ c Cu2+ d Hg2+ | DPASV (120 s) | a 22–280 b 41–518 c 13–160 d 40–503 | a 0.02 b 0.02 c 0.01 d 0.06 | A, E, F | B, C, D | G, I, J | H | – | [23] | |
| Sn/poly(p-ABSA)/GR | Cd2+ | SWASV (120 s) | 1–70 | 0.05 | A, B, C, D, E | – | G, H, I, J | – | Lake, farmland irrigation water | [24] | |
| COF/AuNPs/GR | a Cd2+ b Pb2+ c Cu2+ | DPASV (290 s) | a 56–2016 b 104–1242 c 32–384 | a 0.3 b 1.2 c 0.6 | A, C, D, F | B, E | – | G, H, I, J | Baijiu | [47] | |
| Co3O4@NGA-180 | a Cd2+ b Pb2+ | DPASV (360 s) | a 11.2–225 b 20.7–414 | a 2.14 b 2.90 | A, C, D, F | E | G, H, I | – | Pond water | [39] | |
| NMO-GR | b Pb2+ d Hg2+ | SWASV (40 s) | b 290–1596 d 140–1343 | b 10.4 d 5.4 | A, B, C, D, F | – | G, H, I, J | – | River water | [40] | |
| ZMO-GR | b Pb2+ d Hg2+ | SWASV (40 s) | b 290–1596 d 140–1343 | b 16.6 d 8.0 | |||||||
| PEI/CS/GN | Pb2+ | DPASV (360 s) | 0.5–90 | 0.01 | A, B, C, D, F | E | G, H, I | J | Tap water, river, lake | [60] | |
| GNR-PPy | Pb2+ | DPV (180 s) | 0.0207–207.2 | 0.00622 | A, B, C, D, F | – | G, H, I, J | – | Lake water | [44] | |
| EDTA-NH2-UiO-66/G | a Cd2+ b Pb2+ | DPASV (510 s) | a 28.1–4215 b 51.8–7770 | a 9.33 b 17.6 | A, B, C, D, F | E | H, J | G | Tap water | [50] | |
| G/COF-SH | a Cd2+ b Pb2+ c Cu2+ d Hg2+ | SWV (400 s) | a 1–1000 b 1–800 c 1–800 d 5–1000 | a 0.3 b 0.2 c 0.2 d 1.1 | A, C, D, F | E | G, H, J | – | Coastal water | [51] | |
| GR/COFDPTB | a Cd2+ c Pb2+ c Cu2+ | DPASV (260 s) | a 11–2810 b 21–2279 c 6–699 | a 1.24 b 1.81 c 0.41 | A, B, C, D, F | – | G, H, I | – | Baijiu | [52] | |
| GrCFa | Pb2+ | SWV (120 s) | 829–3312 | 740 | C, D, F | A, B | G, I | H, J | Lipstick | [61] | |
| STB/Gs-2 composite | a Cd2+ b Pb2+ d Hg2+ | SWASV (150 s) | a 28.1–337.2 b 51.7–620.4 d 50.2–602.5 | a 0.13 b 0.08 d 0.12 | A, B, C, D, E, F | – | G, I, J | H | River water, tap water | [57] | |
| GE | AgNPs/GrNPs | a Cd2+ b Pb2+ c Cu2+ | SWASV (200 s) | a,b,c 0.5–120 | a 0.005 b 0.001 c 0.0041 | A, B, C, D, F | E | H, I | G, J | Tap water | [62] |
| GP | Bi-NPs@NC | a Cd2+ b Pb2+ f Zn2+ | SWASV (180 s) | a 1–1400 b 1–1400 f 20–1400 | a 0.5 b 0.1 f 10 | A, C, D, F | – | H, I | G, J | Tap water, lake water | [25] |
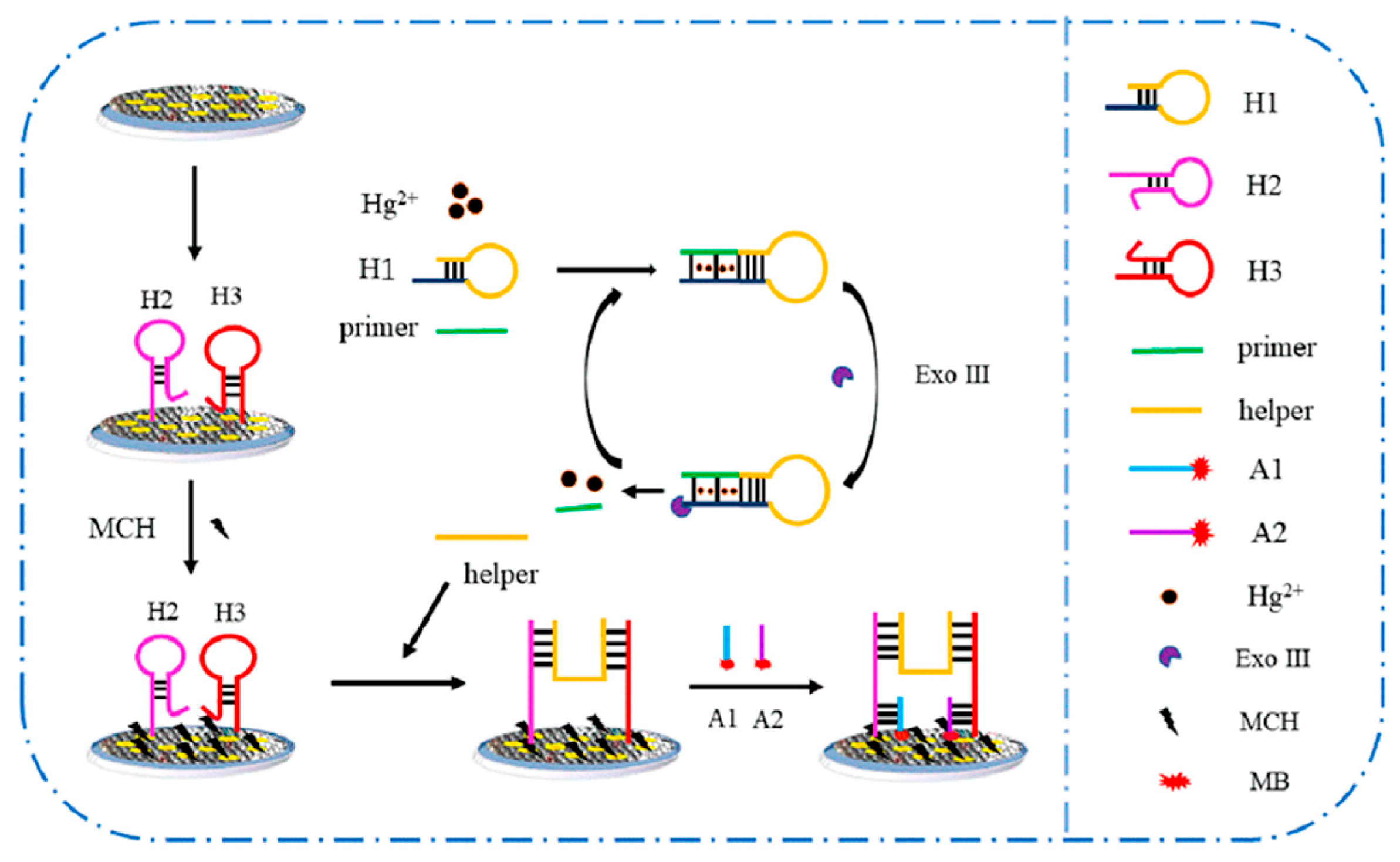
| ITO | GAs-AuNPs, DNA, Exo III, MB | Hg2+ | DPV (225 s) | 2.01 × 10−7–2.01 | 3.21 × 10−8 | A, B, C, D, F | E | H, J | G, I | Milk | [29] |
| Microporous graphene foam | GF/GNFs | As3+ | Chronoamperometry | 1–50 | 1.0 | A, C, D, F | B | I | G, H | Water | [28] |
| Polyimide | Bi/Nafion/LIPG | a Cd2+ b Pb2+ | SWASV (180 s) | a,b 1–10; 10–60 | a 0.25 b 0.41 | A, B, C, D, E, F | – | G, H, I, J | – | Soil, water | [36] |
| N@Bi-LIG | a Cd2+ b Pb2+ | DPSV (300 s) | 1.0–100.0 | a 0.207 b 0.410 | A, B, C, D, F | E | H, I, J | G | Porcelain applique, tea | [63] | |
| LIGBi | a Cd2+ b Pb2+ | SWASV (300 s) | a 2–180 b 2–180 | a 0.914 b 0.916 | A, B, D, F | C | G, I, J | H | Tap water | [64] | |
| LIGBN | a Cd2+ b Pb2+ | SWV (1440 s) | a 899–8993 b 1658–16,576 | a 28.1 b 43.5 | B, C, E, F | – | G, I, J | – | – | [56] | |
| SPCE | Bi film/N/IL/GR | a Cd2+ b Pb2+ f Zn2+ | SWASV (120 s) | a,b,f 0.1–100 | a 0.06 b 0.08 f 0.09 | A, C, E, F | B, D | G, I, J | H | Drinking water | [32] |
| ZnO/GR | a Cd2+ b Pb2+ | ASV (180 s) | a,b 10–200 | a 0.6 b 0.8 | A, C, E, F | B, D | J | G, H, I | Wastewater | [38] | |
| N/GR/PANI | a Cd2+ b Pb2+ f Zn2+ | SWASV (240 s) | a,b,f 1–300 | a 0.1 b 0.1 f 1.0 | A, C, F | B, D, E | J | G, H, I | Human serum | [42] | |
| GR/PANI/PS/nanoporous fiber | a Cd2+ b Pb2+ | SWASV (180 s) | a,b 10–500 | a 4.4 b 3.3 | C, E, F | A, B, D | G, I | H, J | River water | [43] | |
| 3DGO/UiO-66-NH2 | a Cd2+ b Pb2+ c Cu2+ d Hg2+ | DPV (150 s) | a 0.00112–0.0393 b 0.00207–0.0725 c 0.000635–0.0222 d 0.00201–0.0702 | a 0.00123 b 0.00124 c 0.000184 d 0.000622 | A, C, D, E, F | B | G, H, I, J | – | Rice, milk, honey | [49] | |
| GQDs@TPPS (1:6) GQDs@TPPS (1:9) | a Cd2+ c Cu2+ | SWV (180 s) | a 0–900; 675–1463 c 0–510; 382–828 | a 49.0 c 10.9 | A, C, D, F | B, E | G, I, J | H | Seawater | [55] | |
3. Electrochemical Applications of Graphene Oxide-Based Sensors for the Detection of Heavy Metals
3.1. Metal or Metal Oxide Nanoparticle-Modified Graphene Oxide Sensors
3.1.1. Metal Nanoparticle-Modified Graphene Oxide Sensors
3.1.2. Metal Oxide Nanoparticle-Modified Graphene Oxide Sensors
3.2. Polymer-Modified Graphene Oxide Sensors
3.3. MOF-Modified Graphene Oxide Sensors
3.4. Non-Metallic Functional Material-Modified Graphene Oxide Sensors
3.4.1. Sensors Using Active Complex of Ruthenium (II) Bipyridine-Modified Graphene Oxide
3.4.2. Carbon Material-Modified Graphene Oxide Sensors
| Electrode Substrate | Sensing Materials | Heavy Metal | Method (Accumulation time) | (Simultaneous) LDR, µg·L−1 | (Simultaneous) LOD, µg·L−1 | Analytical Characteristics | Sensor Characteristics | Matrix | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adv. | Disadv. | Adv. | Disadv. | ||||||||
| Au | [Ru(bpy)3]2+/GO | a Cd2+ b Pb2+ c As3+ d Hg2+ | DPV | a 6–34 b 10–52 c 4–135 d 20–241 | a 0.3 b 0.3 c 0.2 d 0.3 | A, D, F | B, C | H, I, J | G | Cauvery and tap river water | [77] |
| PEI/CNTs/GO | Pb2+ | Amperometry (30 s) | 207–1532 | 16 | E, F | A, B, C, D | I | G, H, J | Drinking water | [89] | |
| Cu | ZnSe/GO | Cd2+ | DPV (180 s) | 11,200−78,400 | 5101 | D, F | A, B, C, E | G, I | H, J | Wastewater | [69] |
| CPE | AC/GP (1:1) | a Cd2+ b Pb2+ | CV and LSV (510 s) | 1–75 | a 27.68 b 37.78 | A, C, D, F | B | G, I, J | H | Simulated wastewater | [88] |
| AC/RGO (1:1) | a 10.91 b 14.01 | A, B, C, D, F | – | G, I, J | H | ||||||
| AC/RGO/Chitosan (1:1:1) | a 33.11 b 33.70 | A, C, D, F | B | G, I, J | H | ||||||
| GCE | 3DGO/Py10 | Cd2+ | SWASV (600 s) | 5–400 | 3.6 | D, F | A, B, C, E | H, I, J | F | Tap and lake water | [91] |
| GO | a Cd2+ b Pb2+ | DPASV (30 min) | a 0.0001–1.1 b 0.0002–2.1 | a 2.8 × 10−5 b 5.8 × 10−4 | A, D, E, F | B, C | I, J | G, H | Rice, tap water, soya, milk | [92] | |
| GO/Fe3O4/2-CBT | a Cd2+ b Pb2+ | SWASV (180 s) | a ,b 0.08–90 | a 0.03 b 0.02 | A, B, D, E, F | C | I, J | G, H | Tap, river and wastewater | [79] | |
| GO/Fe3O4/ PAMAM | a Cd2+ b Pb2+ | SWASV (160 s) | a 0.2–140 b 0.4–120 | a 0.07 b 0.13 | A, B, C, D E, F | – | H, J | G, I | River water | [76] | |
| GO/Fe3O4/ PMDA/AuNPs | c As3+ e Cu2+ | SWASV (300 s) | c 5–500 e 0.5–750 | c 0.2 e 0.1 | A, D, F | B, C, E | J | G, H, I | Drinking and pool water | [80] | |
| MnFe2O4/GO | Pb2+ | SWASV (150 s) | 41–228 | 18 | D, E, F | A, B, C | I, J | G, H | River water | [66] | |
| GO/MnO2 | a Cd2+ b Pb2+ | DPASV (1800 s) | a 0.001–11 b 0.002–21 | a 1.6 × 10−5 b 2.6 × 10−4 | A, B, C, D, F | E | I, J | G, H | Sea water | [67] | |
| PANI/GO | Pb2+ | SWASV (600 s) | 0.04–52 52–725 | 0.008 | A, B, D, F | C, E | G, H, I, J | – | Industrial effluents, natural water | [70] | |
| PEDOT/GO | Hg2+ | DPSV (360 s) | 2–603 | 0.6 | A, B, D, F | C, E | H, I, J | G | Tap water | [71] | |
| GO/κ-Car/L-cys | a Cd2+ b Pb2+ | SWASV (120 s) | a 0.6–6 b 1–10 | a 0.07 b 0.2 | A, C, D, E, F | B | H, I, J | G | Ground and tap water, raw milk | [72] | |
| PA/PPy/GO | a Cd2+ b Pb2+ | DPV (200 s) | a,b 5–150 | a 2.1 b 0.4 | A, B, D, E, F | C | I, J | G, H | Tap water | [73] | |
| PGA/GO | a Cd2+ d Hg2+ e Cu2+ | DPASV (1600 s) | a 28–616 d 50–1106 e 16–352 | a 1.7 d 6.4 e 1.5 | A, D, F | B, C, E | H, I, J | G | Lake water | [74] | |
| ZIF-8/GO | Pb2+ | DPASV (180 s) | 207–41,400 | 223 | D, E, F | A, B, C | G, I, J | H | Wastewater | [75] | |
| GO/MWCNTs/Bi | a Cd2+ b Pb2+ | DPASV (180 s) | a,b 0.5–30 | a 0.1 b 0.2 | A, B, C, E, F | D | I, J | G, H | Electroplating effluent | [33] | |
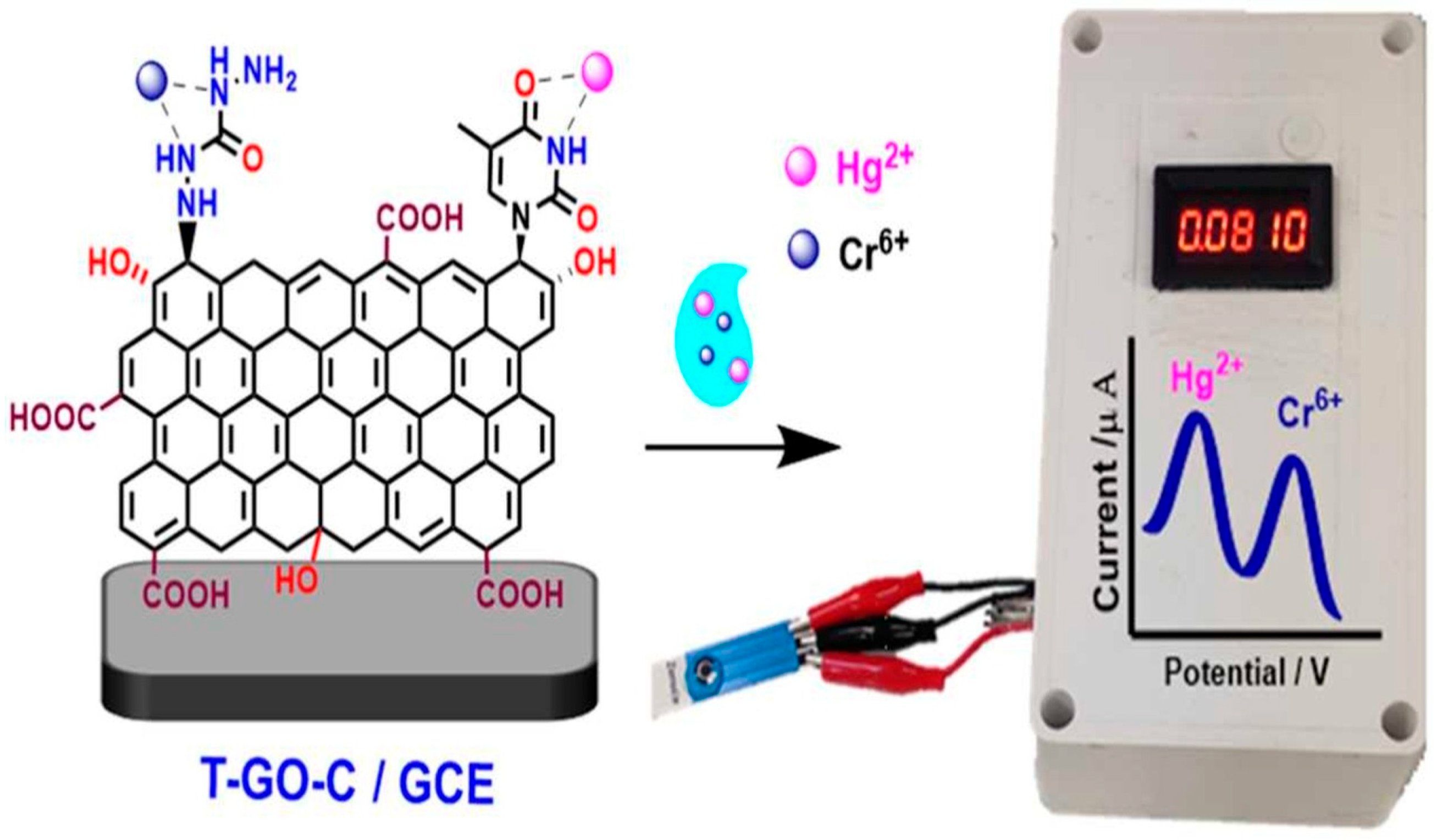
| T-GO-C | d Hg2+ f Cr6+ | SWV (120 s) | d 5–600 f 5–600 | d 1 f 20 | A, B, C, D, E, F | – | G, H, I, J | – | Tap water, tannery water | [90] | |
| Nafion/TEOA@AuNPs-GO-UiO-66-NH2 | a Cd2+ b Pb2+ e Cu2+ | DPV (480 s) | a,b,e 100–3200 | a 11.7 b 27.6 e 33.2 | B, C, D, F | A, E | G, J | H, I | River water | [93] | |
| Sb2WO6/GO | d Hg2+ g UO22+ | DPV (240 s) | d 2006–24,071 g 2700–40,505 | d 0.762 g 37.8 | A, C, D, F | – | G, I | H, J | Aqueous samples | [83] | |
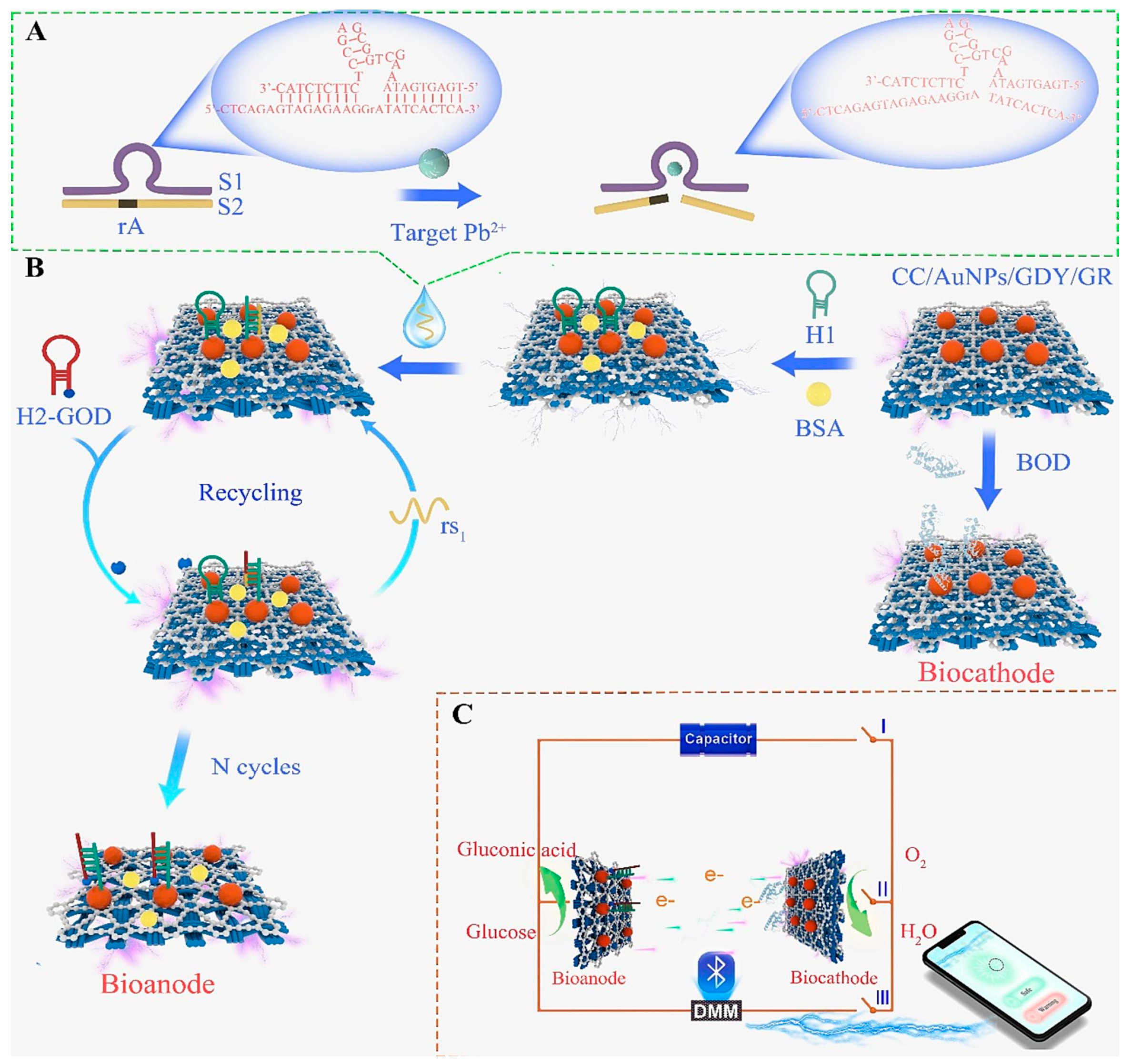
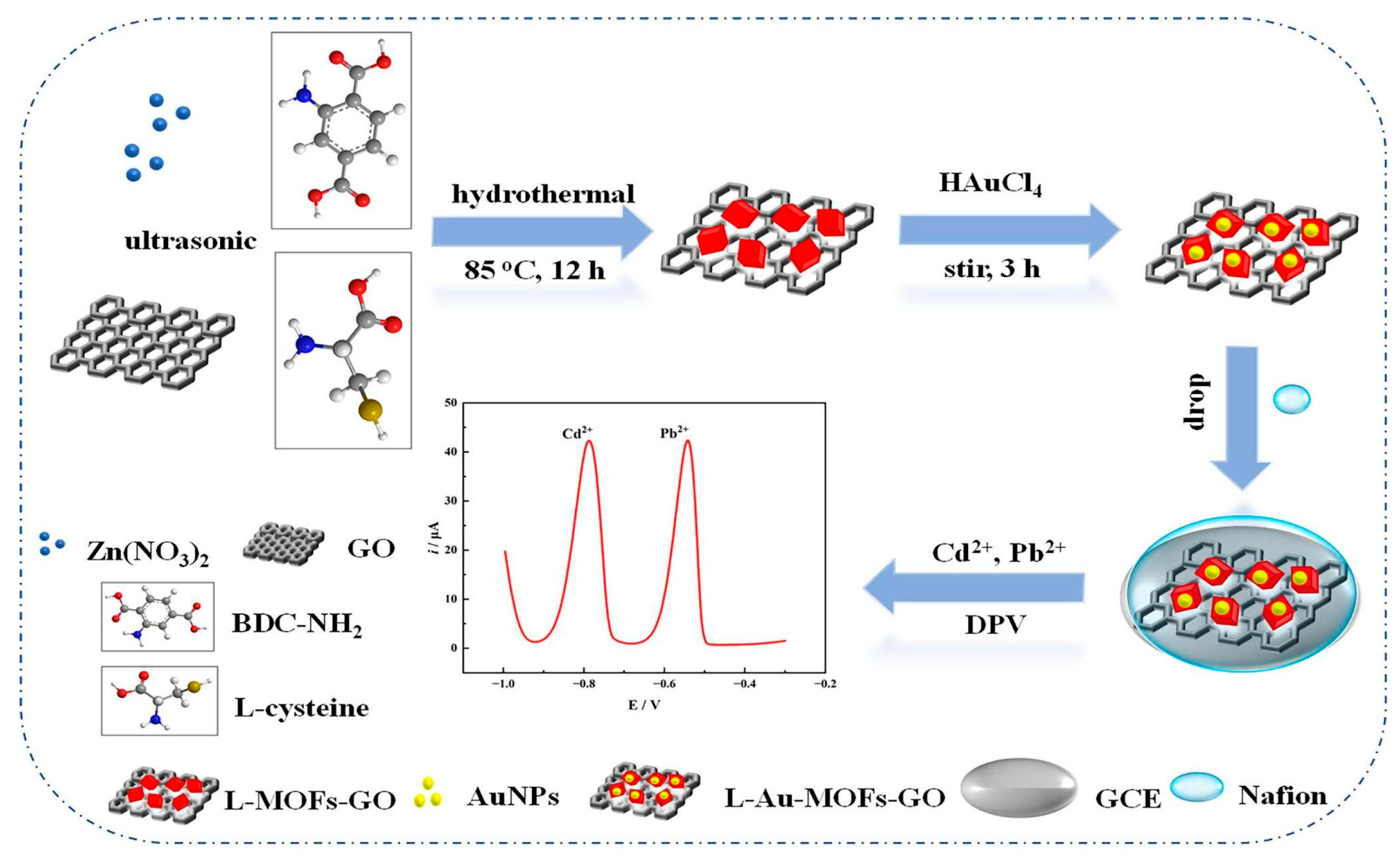
| Nafion/L-Au-MOFs-GO | a Cd2+ b Pb2+ | DPV (300 s) | a,b 80–560 | a 20.8 b 17.3 | B, C, D, F | A, E | G, I, J | H | River water, watermelon | [87] | |
| ZIF-67/GO | b Pb2+ d Hg2+ h Zn2+ i Cr3+ | DPV (280 s) | b 6.21–58.1 d 6–30 h 1.96–9.81 i 1.56–17.16 | b 0.207 d 0.12 h 0.131 i 0.26 | A, C, D, F | B | G, I | J | Real water | [81] | |
| NF@75rGO@ABT | a Cd2+ d Hg2+ e Cu2+ | SWASV (100 s) | a 0.0056–140 d 0.010–270 e 0.0032–80 | a 0.0138 d 0.0173 e 0.00344 | A, B, C, D, E, F | – | – | G, I, J | Cosmetics, water | [82] | |
| GO/UiO-67@PtNPs | As3+ | SWSV (250 s) | 0.202–3.0 | 0.0315 | A, C, D, F | B | G, J | H, I | Chinese herbal medicines | [94] | |
| Co3O4/GO-2/Nafion | a Cd2+ b Pb2+ | DPASV (120 s) | a 157.4–719.4 b 62.2–1326.1 | a 224.8 b 9.32 | A, B, C, D, E, F | – | G, H, I, J | – | Lotus pool water | [95] | |
| PET-based SPCE | CS/PANi–Bi NP@GO–MWCNTs | d Hg2+ e Cu2+ | DPV (460 s) | d 0–200 e 500–16,908 | d 10 e 998 | A, C, D, F | B | G, I | – | Tap water | [96] |
| PGE | MGO-PANI | a Cd2+ b Pb2+ | DPV (384 s) | a 0.5–100; 100–1000 b 0.1–50; 50–1500 | a 0.65 b 0.12 | A, B, C, D, F | – | G, I, J | – | River water | [85] |
| Bi2O3/Fe2O3/GO | Cd2+ | SWV (90 s) | 0.006–1.2 | 0.002 | A, C, E, F | B, D | H, I, J | G | Fruits, water, soil, human serum | [68] | |
| SPGE | [Ru(bpy)3]2+/GO/N | a Cd2+ b Pb2+ | SWASV (120 s) | a,b 50–350 | a 4.2 b 5.3 | A, D, E, F | B, C | H, J | G, I | River and tap water | [78] |
| SPCE | L-cys/GO/PPy | Pb2+ | DPASV (600 s) | 1.4–28 28–280 280–14,000 | 0.07 | A, B, D, F | C, E | H, I, J | G | Spiked and industry water | [86] |
| NH2-MOF@GO15%-NF carbon aerogel | a Cd2+ b Pb2+ | DPASV (300 s) | a 1–150 b 1–150 | a 0.16 b 0.07 | A, B, C, E, F | – | I, J | G, H | Shrimp, clam, sea snail | [97] | |
4. Electrochemical Applications of Reduced Graphene Oxide-Based Sensors and Biosensors for the Detection of Heavy Metals
4.1. Metal or Metal Oxide Nanoparticle-Modified Reduced Graphene Oxide Sensors
4.1.1. Metal Nanoparticle-Modified Reduced Graphene Oxide Sensors
4.1.2. Metal Oxide Nanoparticle-Modified Reduced Graphene Oxide Sensors
4.2. Polymer-Modified Reduced Graphene Oxide Sensors
4.3. Biosensors Based on Reduced Graphene Oxide
| Electrode Substrate | Sensing Materials | Heavy Metal | Method (Accumulation Time) | (Simultaneous) LDR, µg·L−1 | (Simultaneous) LOD, µg·L−1 | Analytical Characteristics | Sensor Characteristics | Matrix | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adv. | Disadv. | Adv. | Disadv. | ||||||||
| Au | 3D-rGO/PANI/DNA | Hg2+ | EIS | 0.02–20 | 0.007 | A, B, C, D, F | – | – | G, H, I, J | River water | [125] |
| rGO/CNT/Bi | a Cd2+ b Pb2+ | SWASV (150 s) | a,b 20–200 | a 0.6 b 0.2 | A, E, F | B, C, D | J | G, H, I | Drinking water | [128] | |
| AuNFs/PEI-rGO | b Pb2+ e Hg2+ | SWV (210 min) | b 0.0002–20.72 e 0.0002–20.06 | e 0.000022 b 0.000019 | A, B, C, D, F | E | H | G, I, J | Tap water | [124] | |
| CC | polyP NPs/rGO | b Pb2+ d Cu2+ | DPV (120 s) | b 2.07–414 d 3.18–158.75 | b 0.50 d 0.07 | A, C, D, E, F | – | G, H, I | – | Tap water, Lake Taiho | [112] |
| C-SPE | PCA-RGO/AuNPs | As3+ | ASV (60 s) | 7.5–7500 | 19 | A, E, F | – | I, J | G | Water | [101] |
| GCE | CAR/rGO | a Cd2+ b Pb2+ c Fe2+ | SWV (25 min) | a 0.01–1.1 b 0.02–2.1 c 0.006–0.6 | a 0.002 b 0.004 c 0.001 | A, C, D, F | B, E | H, J | G, I | – | [114] |
| T/CA/AuNPs/rGO | Hg2+ | DPV (120 s) | 0.01–1 | 0.002 | A, C, E, F | B, D | J | G, H, I | Tap water | [129] | |
| ALA/pDA/rGO | a Cd2+ b Pb2+ c Fe2+ d Cu2+ | DPV (480 s) | a 3–10 b 9–21 c 50–100 d 20–50 | a 1.5 b 2.9 c 50 d 18 | A, D, F | B, C, E | I, J | G, H | Tap water | [130] | |
| rGO/PANI/HBr | a Cd2+ b Pb2+ | DPV (60 s) | a 1–26 b 2–37 | a 0.7 b 1.5 | A, B, C, D, E, F | – | G, I, J | H | Tap, mineral industrial wastewater, plasma | [131] | |
| rGO/CS/PLL | a Cd2+ b Pb2+ d Cu2+ | DPASV (180 s) | a,b,d 0.05–10 | a 0.01 b 0.02 d 0.02 | A, C, D, E, F | B | G, H | I, J | Tap water | [120] | |
| rGO/ALA/PANI | a Cd2+ b Pb2+ d Cu2+ | SWASV (220 s) | a 0.009–11 b 0.02–21 d 0.005–6 | a 0.003 b 0.009 d 0.004 | A, C, D, F | B, E | I, J | G, H | Tap water | [118] | |
| PEI/rGO/CS | Pb2+ | DPASV (120 s) | 1–130 | 0.005 | A, B, D, E, F | C | H, I | G, J | River water | [119] | |
| MB/Apt/MCH/CP/CS/rGO/TiO2 | Pb2+ | DPV (300 s) | 0.001–1 | 0.0003 | A, C, F | B, D, E | – | G, H, I, J | Tea, rice, egg | [123] | |
| Au-Bi/rGO | a Cd2+ b Pb2+ | DPASV (200 s) | a 0.1–300 b 0.1–500 | a 0.02 b 0.05 | A, B, E, F | C, D | J | G, H, I | River water, honey, orange juice | [104] | |
| AuNPs/rGO | Pb2+ | SWASV (180 s) | 2–31 | 0.2 | A, B, E, F | C, D | G, J | H, I | Tap water | [132] | |
| Co3O4/rGO | a Cd2+ b Pb2+ | SWASV (250 s) | a,b 0.1–450 | a 0.062 b 0.034 | A, B, D, F | C, E | H, I, J | G | Pool, well and tap water | [105] | |
| Co:ZnO/rGO | a Cd2+ b Pb2+ | DPV (200 s) | a,b 10–90 | a 0.9 b 0.8 | A, D, E, F | B, C | I, J | G, H | Tap water | [109] | |
| rGO/AgNPs | As3+ | DPASV (120 s) | 0–25 | 0.24 | A, C, D, E, F | B | G, H, I, J | – | Water | [99] | |
| rGO/Zn MOF | As3+ | DPASV (150 s) | 0.2–25 | 0.06 | A, B, C, D, E, F | – | I, J | G, H | Ground, mineral and river water | [133] | |
| TiO2/rGO | a Cd2+ b Pb2+ | DPASV (60 s) | a 5–100 b 5–100 | a 3.12 b 2.38 | A, C, D, E, F | – | H, I | G, J | River water | [107] | |
| ZnO/ErGO | a Cd2+ b Pb2+ | DPASV (120 s) | a,b 2.5–200 | a 1.69 b 0.45 | A, B, C, D, E, F | – | G, H, I, J | – | Tap water, river water, lake water | [110] | |
| NCO/N,S-rGO | a Cd2+ b Cu2+ e Hg2+ | DPASV (120 s) | a 16.8–168 b 9.54–95.4 e 30.1–301 | a 6.63 b 4.89 e 32.8 | A, C, D, E, F | B | I | H | Real water | [108] | |
| rGO/MoS2/CS | Pb2+ | SWASV (180 s) | 1.036–414.4 | 0.3315 | A, B, C, D, E, F | – | H | G, I, J | Tobacco leaves | [111] | |
| MnO2@RGO | a Cd2+ f Zn2+ d Cu2+ | DPASV (330 s) | a 0.05–700 f 0.05–600 d 0.05–600 | a 0.015 f 0.002 d 0.0093 | A, B, C, D, F | E | G, H, I, J | – | Surface water | [106] | |
| Cys-CTS/N-RGO | Cu2+ | DPASV (480 s) | 15.89–8897 | 6.36 | A, B, C, D, E, F | – | G, H, I | J | Tap water, bottled water, river water | [113] | |
| HRP@ZIF-8/THI/Au/IL-rGO | b Pb2+ c Fe2+ d Cu2+ e Hg2+ f Zn2+ g Ba2+ h Co2+ i Cr2+ j Mn2+ | SWV (250 s) | b 0.207–2072 c 0.056–559 d 0.064–636 e 0.201–2006 f 0.065–654 g 0.137–1373 h 0.059–589 i 0.052–520 j 0.055–549 | b 0.0273 c 0.00680 d 0.00871 e 0.0269 f 0.0103 g 0.0155 h 0.00927 i 0.00750 j 0.00639 | A, B, C, D F | – | H | G, J | Real water samples | [126] | |
| rGO/AuNPs/ssDNA | Pb2+ | CV (360 s) | 1.035–10.35 | 0.315 | A, C, D, E, F | B | G, H | J | Tap, pond, river, lake water | [127] | |
| MGCE | AgNPs/rGO | a Cd2+ b Pb2+ d Cu2+ e Hg2+ | SWASV (150 s) | a 6–392 b 10–518 d 3–224 e 101–603 | a 28 b 59 d 11 e 36 | E, F | A, B, C, D | G, I, J | H | – | [100] |
| Ni foam | Ni/TiO2/P-rGO/CS | Cu2+ | DPV (200 s) | 0–286 | 0.014 | A, B, C, D, F | – | H, I | G | Seawater | [116] |
| Polyimide | Nafion/BiNP@LIG | a Cd2+ b Pb2+ | SWASV (200 s) | a 5–50 b 5–50 | a 0.5 b 0.8 | A, C, D, F | – | G, I, J | H | Soil | [103] |
| SPE | rGO/AuNPs | As3+ | DPV (120 s) | 100–1000 | 100 | A, C, D, F | – | G, H | I | Human serum | [102] |
| rGO/Bi film | a Cd2+ b Pb2+ | SWASV (150 s) | a,b 1–60 | a 0.5 b 0.8 | A, B, E, F | C, D | G, I, J | H | Milk | [134] | |
| SPCE | TTU/rGO | Hg2+ | DPV (600 s) | 100–180,000 | 26 | D, F | A, B, C, E | H, I, J | G | River water | [135] |
| AO/rGO | a Cd2+ b Pb2+ | SWASV (120 s) | a 56–1120 b 104–2070 | a 10 b 2 | A, D, E, F | B, C | J | G, H, I | Human plasma | [136] | |
| 4AP/rGO | Pb2+ | DPV (300 s) | 0.0004–0.04 | 0.0001 | A, C, F | B, D, E | I, J | G, H | Tap water | [137] | |
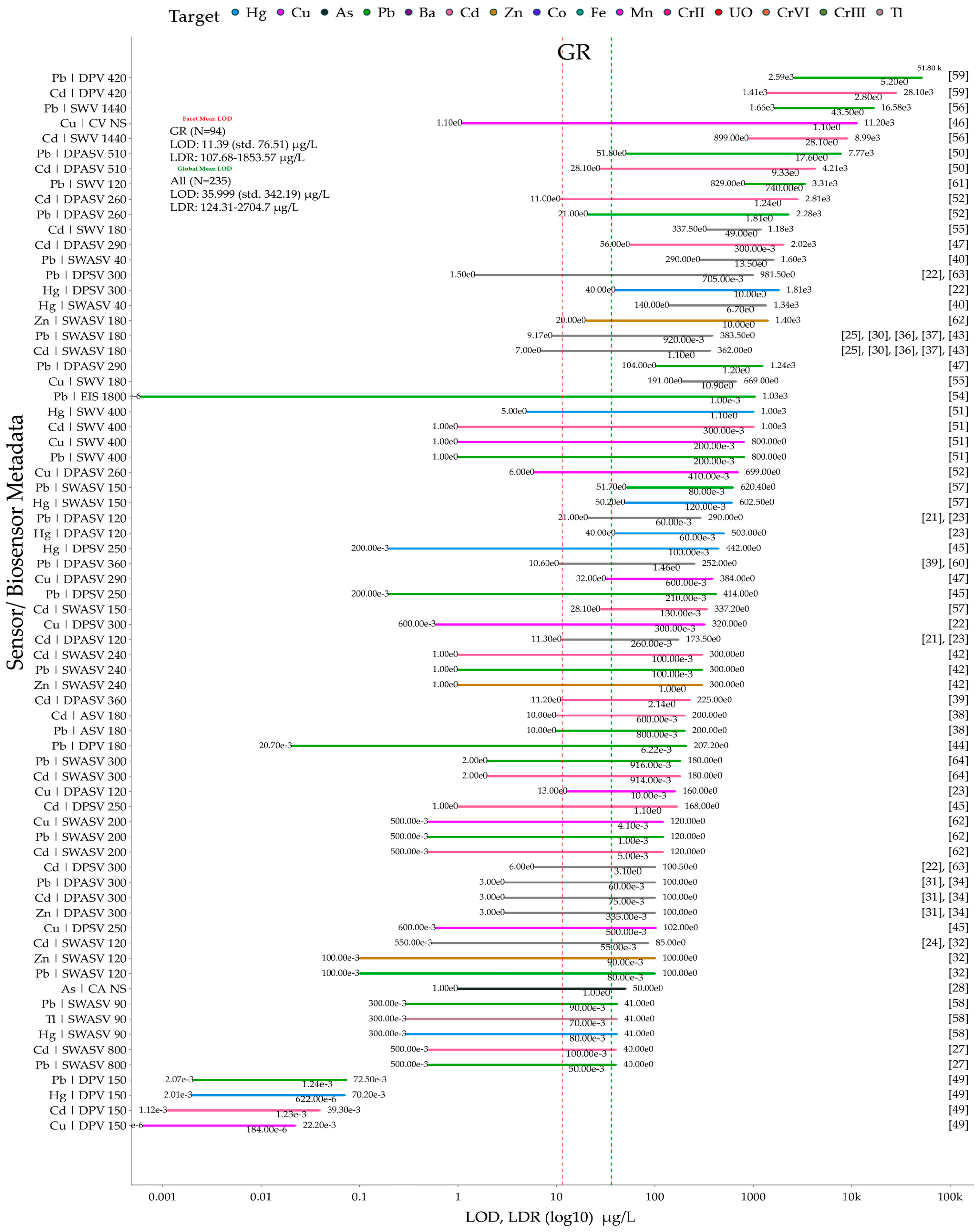
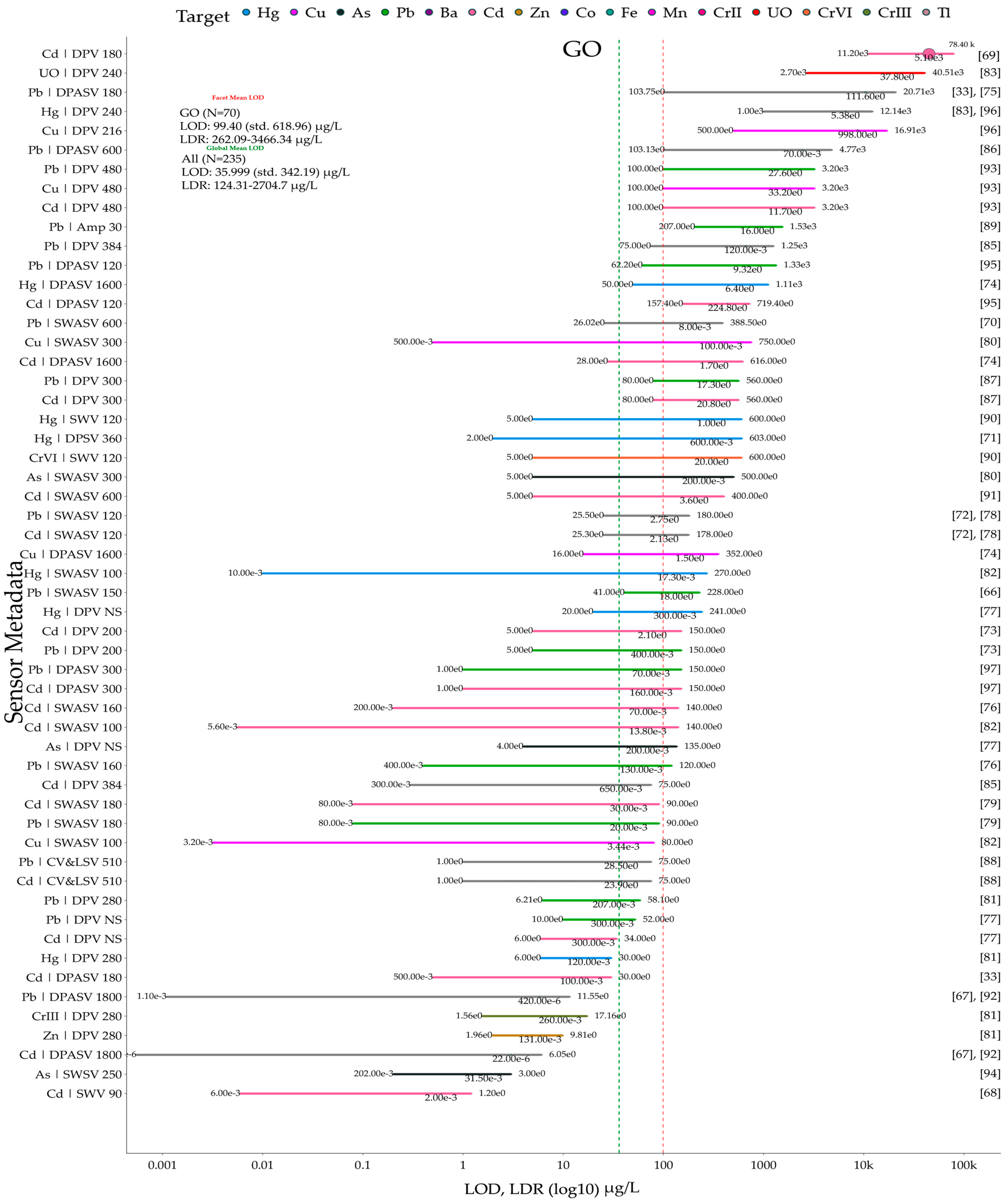
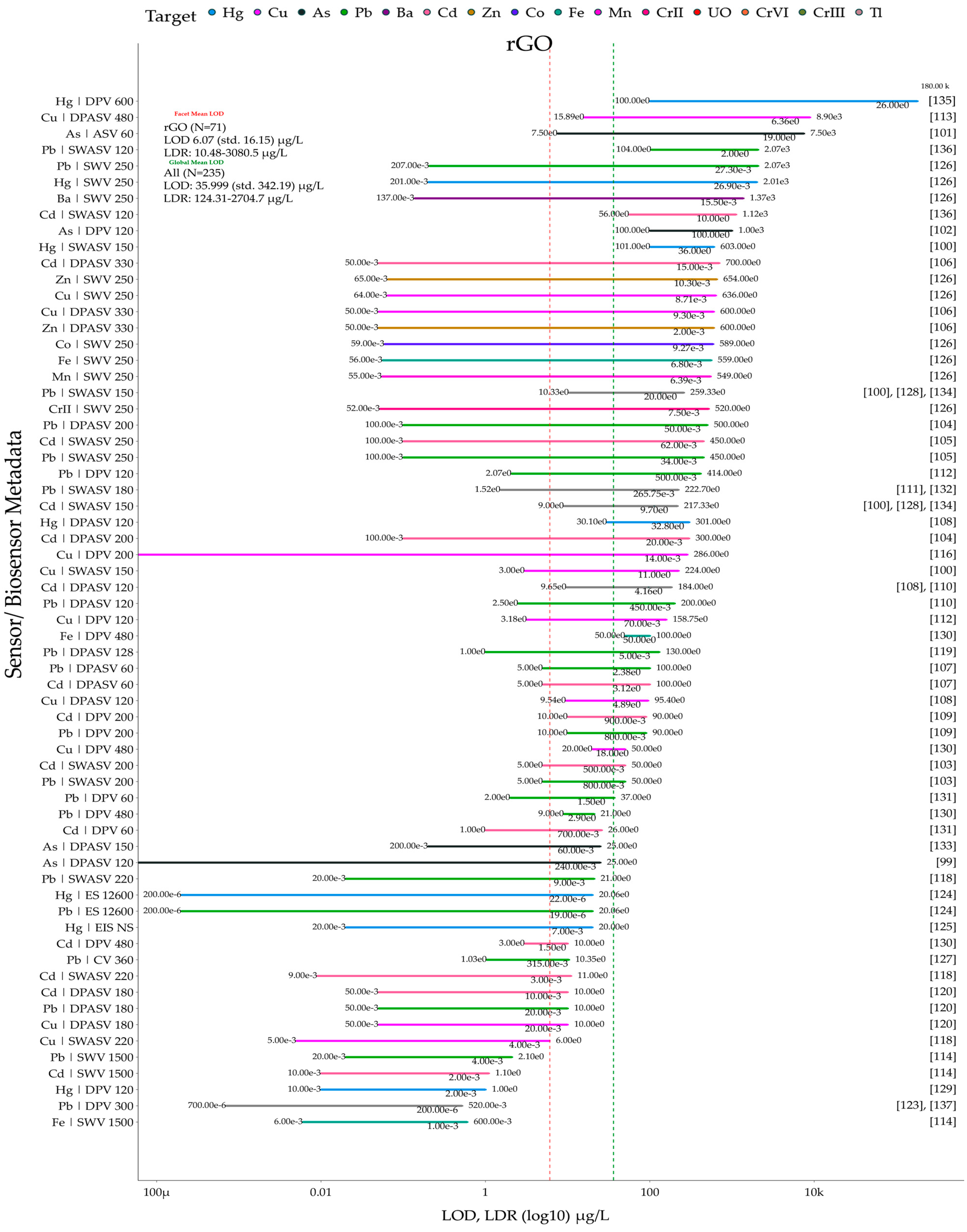
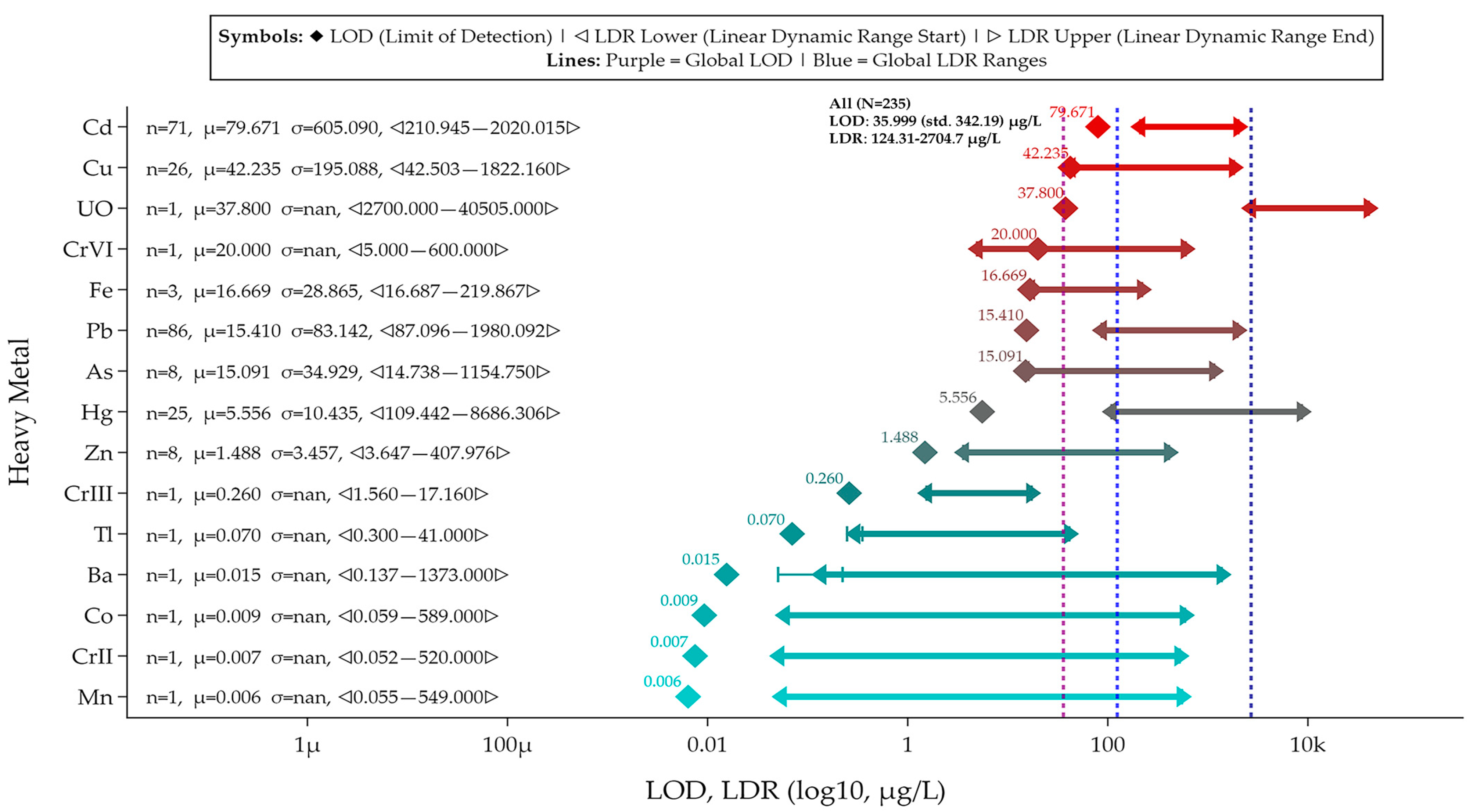
5. Meta-Analysis on Reviewed Graphene and Its Derivative Sensors and Biosensors
5.1. Data and Plotting Pre-Processing and Post-Processing Steps for Method Reproducibility
5.2. Sensor and Biosensor LOD and LDR Comparison Grouped by Material Types and Heavy Metal Ion Types
5.3. Analysis of the Advantages and Disadvantages According to the Coding Scheme
5.4. Identifying Top, Bottom, and Optimum Sensors from the Meta-Analysis
6. Toxicity of Heavy Metals
6.1. Effect of Toxicity of Heavy Metals on Human
6.1.1. Cadmium’s Impact on Human Health
6.1.2. Chromium’s Impact on Human Health
6.1.3. Mercury’s Impact on Human Health
6.1.4. Lead’s Impact on Human Health
6.1.5. Zinc’s Impact on Human Health
6.1.6. Arsenic’s Impact on Human Health
6.2. Effects of Toxicity of Heavy Metals on Plants
6.3. Effects of Toxicity of Heavy Metals on Soil
6.4. Effects of Toxicity of Heavy Metals in Aquatic Life
7. Contribution of Heavy Metals to the SDGs
7.1. Contribution of Heavy Metals in SDG1 (No Poverty)
7.2. Contribution of Heavy Metals in SDG2 (Zero Hunger)
7.3. Contribution of Heavy Metals in SDG3 (Good Health and Well-Being)
7.4. Contribution of Heavy Metals in SDG6 (Clean Water and Sanitation)
7.5. Contribution of Heavy Metals in SDG7 (Affordable and Clean Energy)
7.6. Contribution of Heavy Metals in SDG9 (Industry, Innovation, and Infrastructure)
7.7. Contribution of Heavy Metals in SDG11 (Sustainable Cities and Communities)
7.8. Contribution of Heavy Metals in SDG12 (Responsible Consumption and Production)
7.9. Contribution of Heavy Metals in SDG13 (Climate Action)
7.10. Contribution of Heavy Metals in SDG14 (Life Below Water)
7.11. Contribution of Heavy Metals in SDG15 (Life on Land)
7.12. Contribution of Heavy Metals in SDG17 (Partnerships for the Goals)
8. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1D | One-dimensional |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| 3DGO | Three-dimensional graphene oxide |
| AA | Ascorbic acid |
| ABT | 2-(Anthracen-9-yl)benzothiazole |
| Ag/GDs/NCs/CFs | Silver nanoclusters, graphene quantum dots, N-doped carbon |
| AgNPs | Silver nanoparticles |
| ASV | Anodic stripping voltammetry |
| AuNFs | Gold nanoflowers |
| AuNPs | Gold nanoparticles |
| B/N | Boron/Nitrogen co-doped |
| Bi | Bismuth |
| Bi NPs | Bismuth nanoparticles |
| C3N4 | Carbon nitride |
| CA | Chronoamperometry |
| CAR | Calixarene |
| CBT | Benzothiazole-2-carboxaldehyde |
| CC | Carbon cloth |
| CFs | Carbon fibers |
| CHA | Catalytic hairpin self-assembly |
| CMO | Copper manganate |
| Co3O4 | Cobalt oxide |
| COFs | Covalent organic frameworks |
| COF-SH | Hydrosulphonyl-functionalized covalent organic framework |
| CS | Chitosan |
| CS/PANi–Bi | Chitosan/polyaniline–bismuth nanoparticle |
| DPASV | Differential pulse anodic stripping voltammetry |
| DPV | Differential pulse voltammetry |
| EBFC | Enzyme biofuel cell |
| EDTA | Ethylenediaminetetraacetic acid |
| EIS | Electrochemical impedance spectroscopy |
| ErGO | Electrochemically reduced graphene oxide |
| EUON | European Union Observatory for Nanomaterials |
| Exo | Exouclease |
| FDA | United States Food and Drug Administration |
| Fe3O4 | Iron oxide (magnetite) |
| f-GO | Functionalized graphene oxide |
| GAs | Graphene aerogel |
| GCE | Glassy carbon electrode |
| GDs/GQDs | Graphene quantum dots |
| GDY | Graphdiyne |
| GE | Graphite electrode |
| GF | Graphene foam |
| GF/GNFs | Nanoflower-decorated microporous graphene foam |
| GNFs | Graphene nanoflowers |
| GNR | Graphene nanoribbon |
| GO | Graphene oxide |
| GOMIP | Styrene/EGDMA polymer + functionalized GO |
| GP | Graphene paper |
| GR | Graphene |
| GrCF | Graphene chitosan and Fe composite |
| GrNPs | Graphene nanoplates |
| HMs/HMIs | Heavy metals/heavy metal ions |
| HRP | Horseradish peroxidase |
| HPLC | High-performance liquid chromatography |
| ICP | Inductively coupled plasma |
| IDE | Interdigital electrode |
| IIPs | Ion-imprinted polymers |
| IL | Ionic liquid |
| IL-rGO | Ionic liquid–reduced graphene oxide |
| ITO | Indium tin oxide |
| L-cys | L-cysteine |
| LDR | Linear dynamic range |
| LIG | Laser-induced graphene |
| LIGBN | Bismuth and nitrogen co-doped laser-induced graphene |
| LIPG | Laser-induced porous graphene |
| LOD | Limit of detection |
| LRGO | Laser-reduced graphene oxide |
| MGCE | Magnetic glassy carbon electrode |
| MGO-PANI | Polyaniline/graphene oxide/polyethylene glycol/cysteine |
| MnO2 | Manganese dioxide |
| MOFs | Metal–organic frameworks |
| MoS2 | Molybdenum disulfide |
| MWCNTs | Multi-walled carbon nanotubes |
| N,S-rGO | Nitrogen/sulfur co-doped reduced graphene oxide |
| NF@rGO | Nickel ferrite/reduced graphene oxide |
| NGA | Nitrogen-doped graphene aerogel |
| N-GR | Nitrogen-doped GR |
| NH2-MOF | Amino-functionalized metal–organic framework |
| NiCo2O4 | Nickel cobaltite |
| NMO | Nickel manganate |
| NPC | Nanoporous carbon |
| NPs | Nanoparticles |
| N-rGO | Nitrogen-doped reduced graphene oxide |
| NS | Not specified |
| PAMAM | Poly(amidoamine) |
| PANI | Polyaniline |
| PCA | 1-pyrene carboxylic acid |
| PEI | Polyethylenimine |
| PEI-rGO | Polyethylenimine-reduced graphene oxide |
| PGA | Poly(L-glutamic acid) |
| PGE | Pencil graphite electrode |
| PLL | Poly-L-lysine |
| PMDA | Poly methyldopa |
| PolyP | NPs |
| PPy | Polypyrrole |
| P-rGO | Phosphorus-doped reduced graphene oxide |
| PtNPs | Platinum nanoparticles |
| rGO/RGO | Reduced graphene oxide |
| ROSES | RepOrting standards for Systematic Evidence Syntheses |
| RSD | Relative standard deviation |
| Sb2WO6 | Antimony tungstate |
| SPCE | Screen-printed carbon electrode |
| SPE | Screen-printed electrodes |
| ssDNA | Single-stranded DNA |
| STB/Gs | Sulfur-doped carbon nitride tube bundles/graphene nanosheets |
| SWASV | Square wave anodic stripping voltammetry |
| SWV | Square wave voltammetry |
| TEOA | Triethanolamine-functionalized |
| T-GO-C | Thymine/carbohydrazide-functionalized graphene oxide |
| THI | Thionine |
| TiO2 | Titanium dioxide |
| TPPS | Tetraphenylporphyrin sulfonate |
| UNEP | United Nations Environment Program |
| UN | United Nations |
| WHO | World Health Organization |
| ZIF-67 | Zeolitic imidazolate framework-67 |
| ZIF-8 | Zeolitic imidazolate framework-8 |
| ZMO | Zinc manganate |
| ZnO | Zinc oxide |
| ZnSe | Zinc selenide |
References
- Kapahi, M.; Sachdeva, S. Bioremediation Options for Heavy Metal Pollution. J. Health Pollut. 2019, 9, 191203. [Google Scholar] [CrossRef]
- Houri, T.; Khairallah, Y.; Al Zahab, A.; Osta, B.; Romanos, D.; Haddad, G. Heavy Metals Accumulation Effects on The Photosynthetic Performance of Geophytes in Mediterranean Reserve. J. King Saud. Univ. Sci. 2020, 32, 874–880. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and Environmental Effects of Heavy Metals. J. King Saud. Univ. Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium Toxicity and Health Effects-A Brief Summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Sharma, U.; Sharma, J.G. Nanotechnology for the Bioremediation of Heavy Metals and Metalloids. J. Appl. Biol. Biotechnol. 2022, 10, 34–44. [Google Scholar] [CrossRef]
- Title 21 of the CFR. Available online: https://www.ecfr.gov/current/title-21 (accessed on 26 July 2025).
- Protocol on Heavy Metals. Available online: https://unece.org/environmental-policy/air/protocol-heavy-metals (accessed on 26 July 2025).
- Hu, T.; Lai, Q.; Fan, W.; Zhang, Y.; Liu, Z. Advances in Portable Heavy Metal Ion Sensors. Sensors 2023, 23, 4125. [Google Scholar] [CrossRef]
- Saqib, M.; Dorozhko, E.V.; Barek, J.; Korotkova, E.I.; Semin, V.O.; Kolobova, E.; Erkovich, A. V Sensitive Electrochemical Sensing of Carbosulfan in Food Products on Laser Reduced Graphene Oxide Sensor Decorated with Silver Nanoparticles. Microchem. J. 2024, 207, 112253. [Google Scholar] [CrossRef]
- Dorozhko, E.V.; Gashevskay, A.S.; Korotkova, E.I.; Barek, J.; Vyskocil, V.; Eremin, S.A.; Galunin, E.V.; Saqib, M. A Copper Nanoparticle-Based Electrochemical Immunosensor for Carbaryl Detection. Talanta 2021, 228, 122174. [Google Scholar] [CrossRef]
- Lipskikh, O.I.; Korotkova, E.I.; Barek, J.; Vyskocil, V.; Saqib, M.; Khristunova, E.P. Simultaneous Voltammetric Determination of Brilliant Blue FCF and Tartrazine for Food Quality Control. Talanta 2020, 218, 121136. [Google Scholar] [CrossRef] [PubMed]
- Saqib, M.; Solomonenko, A.N.; Barek, J.; Dorozhko, E.V.; Korotkova, E.I.; Aljasar, S.A. Graphene Derivatives-Based Electrodes for the Electrochemical Determination of Carbamate Pesticides in Food Products: A Review. Anal. Chim. Acta 2023, 1272, 341449. [Google Scholar] [CrossRef]
- Saqib, M.; Dorozhko, E.V.; Barek, J.; Vyskocil, V.; Korotkova, E.I.; Shabalina, A. V A Laser Reduced Graphene Oxide Grid Electrode for the Voltammetric Determination of Carbaryl. Molecules 2021, 26, 5050. [Google Scholar] [CrossRef] [PubMed]
- Dorozhko, E.V.; Solomonenko, A.N.; Saqib, M.; Semin, V.O. Electrochemical Immunosensors Based on Gold Nanoparticles for the Determination of Ovalbumin in Immunobiological Preparations. J. Anal. Chem. 2024, 79, 860–867. [Google Scholar] [CrossRef]
- Dorozhko, E.; Kazachinskaia, E.; Kononova, Y.; Zaikovskaya, A.; Barek, J.; Korotkova, E.; Kolobova, E.; Sheveleva, P.; Saqib, M. Electrochemical Immunoassay of Antibodies Using Freshly Prepared and Aged Conjugates of Silver Nanoparticles. Talanta 2023, 253, 124028. [Google Scholar] [CrossRef]
- Vashisth, M.; Hui, A.L. Cost Effective and Sensitive Electrochemical Detection of Dopamine on Laser Driven Sensor. In Proceedings of the 2025 7th International Youth Conference on Radio Electronics, Electrical and Power Engineering (REEPE), Moscow, Russia, 8–10 April 2025; pp. 1–6. [Google Scholar]
- Dorozhko, E.V.; Solomonenko, A.N.; Erkovich, A.V.; Koltsova, A.V.; Korotkova, E.I.; Kolobova, E.N.; Semin, V.O.; Nikulin, L.G.; Mikhailova, T.V.; Kazachinskaya, E.I.; et al. Copper-Enhanced Electrochemical Immunosensor Based on Gold Nanoparticles for the Quality Control of Hepatitis A Virus Vaccines. Talanta 2025, 297, 128579. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Macura, B.; Whaley, P.; Pullin, A.S. ROSES RepOrting Standards for Systematic Evidence Syntheses: Pro Forma, Flow-Diagram and Descriptive Summary of the Plan and Conduct of Environmental Systematic Reviews and Systematic Maps. Environ. Evid. 2018, 7, 7. [Google Scholar] [CrossRef]
- Zhou, W.; Li, C.; Sun, C.; Yang, X. Simultaneously Determination of Trace Cd2+ and Pb2+ Based on L-Cysteine/Graphene Modified Glassy Carbon Electrode. Food Chem. 2016, 192, 351–357. [Google Scholar] [CrossRef]
- Xing, H.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Wang, W.; Zhang, Y.; Yang, T. Highly Sensitive Simultaneous Determination of Cadmium (II), Lead (II), Copper (II), and Mercury (II) Ions on N-Doped Graphene Modified Electrode. J. Electroanal. Chem. 2016, 760, 52–58. [Google Scholar] [CrossRef]
- Xie, Y.-L.; Zhao, S.-Q.; Ye, H.-L.; Yuan, J.; Song, P.; Hu, S.-Q. Graphene/CeO2 Hybrid Materials for the Simultaneous Electrochemical Detection of Cadmium(II), Lead(II), Copper(II), and Mercury(II). J. Electroanal. Chem. 2015, 757, 235–242. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Zhang, Z.; Yang, X.; Liu, G. Sensitive Electrochemical Determination of Trace Cadmium on a Stannum Film/Poly(p-Aminobenzene Sulfonic Acid)/Electrochemically Reduced Graphene Composite Modified Electrode. Electrochim. Acta 2014, 120, 140–146. [Google Scholar] [CrossRef]
- Hu, M.; He, H.; Xiao, F.; Liu, C. Bi-MOF-Derived Carbon Wrapped Bi Nanoparticles Assembly on Flexible Graphene Paper Electrode for Electrochemical Sensing of Multiple Heavy Metal Ions. Nanomaterials 2023, 13, 2069. [Google Scholar] [CrossRef]
- Gong, J.; Zhou, T.; Song, D.; Zhang, L. Monodispersed Au Nanoparticles Decorated Graphene as an Enhanced Sensing Platform for Ultrasensitive Stripping Voltammetric Detection of Mercury(II). Sens. Actuators B Chem. 2010, 150, 491–497. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, L.; Huang, B.; Jia, N.; Tan, L.; Yao, S. Simultaneous Determination of Cd(II) and Pb(II) Using Square Wave Anodic Stripping Voltammetry at a Gold Nanoparticle-Graphene-Cysteine Composite Modified Bismuth Film Electrode. Electrochim. Acta 2014, 115, 471–477. [Google Scholar] [CrossRef]
- Kosuvun, M.; Danvirutai, P.; Hormdee, D.; Chaosakul, A.; Tanboonchuy, V.; Siritaratiwat, A.; Anutrakulchai, S.; Sharma, A.; Tuantranont, A.; Srichan, C. Nanoflowers on Microporous Graphene Electrodes as a Highly Sensitive and Low-Cost As(III) Electrochemical Sensor for Water Quality Monitoring. Sensors 2023, 23, 3099. [Google Scholar] [CrossRef]
- Peng, G.; Guo, M.; Liu, Y.; Yang, H.; Wen, Z.; Chen, X. Development of a Novel H-Shaped Electrochemical Aptasensor for Detection of Hg2+ Based on Graphene Aerogels–Au Nanoparticles Composite. Biosensors 2023, 13, 932. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Ju, H. Synthesis of Bismuth-Nanoparticle-Enriched Nanoporous Carbon on Graphene for Efficient Electrochemical Analysis of Heavy-Metal Ions. Chem. Eur. J. 2015, 21, 11525–11530. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Oh, J.; Kim, D.; Piao, Y. A Sensitive Electrochemical Sensor Using an Iron Oxide/Graphene Composite for the Simultaneous Detection of Heavy Metal Ions. Talanta 2016, 160, 528–536. [Google Scholar] [CrossRef]
- Chaiyo, S.; Mehmeti, E.; Žagar, K.; Siangproh, W.; Chailapakul, O.; Kalcher, K. Electrochemical Sensors for the Simultaneous Determination of Zinc, Cadmium and Lead Using a Nafion/Ionic Liquid/Graphene Composite Modified Screen-Printed Carbon Electrode. Anal. Chim. Acta 2016, 918, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Chen, T.; Wang, S.; Ma, H. Ultrasensitive and Simultaneous Detection of Heavy Metal Ions Based on Three-Dimensional Graphene-Carbon Nanotubes Hybrid Electrode Materials. Anal. Chim. Acta 2014, 852, 45–54. [Google Scholar] [CrossRef]
- Lee, S.; Bong, S.; Ha, J.; Kwak, M.; Park, S.-K.; Piao, Y. Electrochemical Deposition of Bismuth on Activated Graphene-Nafion Composite for Anodic Stripping Voltammetric Determination of Trace Heavy Metals. Sens. Actuators B Chem. 2015, 215, 62–69. [Google Scholar] [CrossRef]
- Zuo, Y.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Yu, Y. Graphene-Derived Nanomaterials as Recognition Elements for Electrochemical Determination of Heavy Metal Ions: A Review. Microchim. Acta 2019, 186, 171. [Google Scholar] [CrossRef]
- Ye, W.; Liu, N.; Li, S.; Zhao, G.; Liu, G. Methodological Guidance to the Batch Preparation of Graphene-Based Sensor for Low-Cost and High-Performance Cd2+ and Pb2+ Detection. J. Hazard. Mater. 2025, 493, 138331. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Y.; Xie, D.; Gu, Y.; Zhu, X.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Hierarchical MgFe-Layered Double Hydroxide Microsphere/Graphene Composite for Simultaneous Electrochemical Determination of Trace Pb(II) and Cd(II). Chem. Eng. J. 2018, 347, 953–962. [Google Scholar] [CrossRef]
- Yukird, J.; Kongsittikul, P.; Qin, J.; Chailapakul, O.; Rodthongkum, N. ZnO@graphene Nanocomposite Modified Electrode for Sensitive and Simultaneous Detection of Cd (II) and Pb (II). Synth. Met. 2018, 245, 251–259. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, Y.; Chen, S.; Wang, S.; Meng, J.; Xu, H. Nanoarchitectonics of Nest-like Co3O4 Nanowires Embedded Nitrogen-Doped Graphene Aerogel as Electrochemical Sensing Platform for Pb2+ and Cd2+ Trace Detection. Diam. Relat. Mater. 2023, 137, 110132. [Google Scholar] [CrossRef]
- Lei, P.; Zhou, Y.; Zhao, S.; Dong, C.; Shuang, S. Carbon-Supported X-Manganate (X[Dbnd]Ni, Zn, and Cu) Nanocomposites for Sensitive Electrochemical Detection of Trace Heavy Metal Ions. J. Hazard. Mater. 2022, 435, 129036. [Google Scholar] [CrossRef]
- Kumunda, C.; Adekunle, A.S.; Mamba, B.B.; Hlongwa, N.W.; Nkambule, T.T.I. Electrochemical Detection of Environmental Pollutants Based on Graphene Derivatives: A Review. Front. Mater. 2021, 7, 616787. [Google Scholar] [CrossRef]
- Ruecha, N.; Rodthongkum, N.; Cate, D.M.; Volckens, J.; Chailapakul, O.; Henry, C.S. Sensitive Electrochemical Sensor Using a Graphene–Polyaniline Nanocomposite for Simultaneous Detection of Zn(II), Cd(II), and Pb(II). Anal. Chim. Acta 2015, 874, 40–48. [Google Scholar] [CrossRef]
- Promphet, N.; Rattanarat, P.; Rangkupan, R.; Chailapakul, O.; Rodthongkum, N. An Electrochemical Sensor Based on Graphene/Polyaniline/Polystyrene Nanoporous Fibers Modified Electrode for Simultaneous Determination of Lead and Cadmium. Sens. Actuators B Chem. 2015, 207, 526–534. [Google Scholar] [CrossRef]
- Kulkarni, B.B.; Suvina, V.; Balakrishna, R.G.; Nagaraju, D.H.; Jagadish, K. 1D GNR-PPy Composite for Remarkably Sensitive Detection of Heavy Metal Ions in Environmental Water**. ChemElectroChem 2022, 1–9. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Luo, Y.; Lv, J.; Li, T.; Xu, J.; Chen, S.-W.; Wang, J. Graphene Aerogel–Metal–Organic Framework-Based Electrochemical Method for Simultaneous Detection of Multiple Heavy-Metal Ions. Anal. Chem. 2018, 91, 888–895. [Google Scholar] [CrossRef]
- Senthil, T.; Parkavi, R.; Senthil Kumar, P.; Chandramohan, A.; Rangasamy, G.; Srinivasan, K.; Dinakaran, K. PbS/Graphene Hybrid Nanostructures Coated Glassy Carbon Electrode for the Electrochemical Sensing of Copper Ions in Aqueous Solution. Food Chem. Toxicol. 2022, 168, 113375. [Google Scholar] [CrossRef]
- Yu, L.; Chen, X.; Sun, L.; Zhang, Q.; Yang, B.; Huang, M.; Xu, B.; Xu, Q. A Covalent Organic Frameworks@gold Nanoparticles@graphene Nanocomposite Based Electrochemical Sensor for Simultaneous Determination of Trace Cd2+, Pb2+ and Cu2+. React. Funct. Polym. 2024, 194, 105770. [Google Scholar] [CrossRef]
- Gorgolis, G.; Galiotis, C. Graphene Aerogels: A Review. 2D Mater. 2017, 4, 32001. [Google Scholar] [CrossRef]
- Huo, D.; Zhang, Y.; Li, N.; Ma, W.; Liu, H.; Xu, G.; Li, Z.; Yang, M.; Hou, C. Three-Dimensional Graphene/Amino-Functionalized Metal–Organic Framework for Simultaneous Electrochemical Detection of Cd(II), Pb(II), Cu(II), and Hg(II). Anal. Bioanal. Chem. 2022, 414, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liao, S.; Wang, S.; Gao, X.; Liu, Z. Synthesis and Application of EDTA Grafed Metal-Organic Frameworks (MOFs) and Graphene Composite for the Electrochemical Detection of Lead(II). Int. J. Environ. Anal. Chem. 2023, 105, 1373–1391. [Google Scholar] [CrossRef]
- Pan, F.; Tong, C.; Wang, Z.; Han, H.; Liu, P.; Pan, D.; Zhu, R. Nanocomposite Based on Graphene and Intercalated Covalent Organic Frameworks with Hydrosulphonyl Groups for Electrochemical Determination of Heavy Metal Ions. Mikrochim. Acta 2021, 188, 295. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Li, J.; Sun, L.; Zhang, Q.; Yang, B.; Huang, M.; Xu, B. Rapid, Simple, and Simultaneous Electrochemical Determination of Cadmium, Copper, and Lead in Baijiu Using a Novel Covalent Organic Framework Based Nanocomposite. Front. Chem. 2024, 12, 1374898. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Cases, F.; Moretto, L.M. Graphene-Based Materials for the Electrochemical Determination of Hazardous Ions. Anal. Chim. Acta 2016, 946, 9–39. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Luo, X.; Li, Y.; Xing, Y.; Huang, K.J. Visual Self-Powered Platform for Ultrasensitive Heavy Metal Detection Designed on Graphdiyne/Graphene Heterojunction and DNAzyme-Triggered DNA Circuit Strategy. Chem. Eng. J. 2024, 485, 150151. [Google Scholar] [CrossRef]
- Sarà, M.; Abid, K.; Gucciardi, P.G.; Scolaro, L.M.; Neri, G. Electrochemical Determination of Heavy Metals by a Graphene Quantum Dots/Porphyrin-Based Supramolecular System. Synth. Met. 2025, 311, 117824. [Google Scholar] [CrossRef]
- Chaudhary, Y.; Suman, S.; Rakesh, B.; Ojha, G.P.; Deshpande, U.; Pant, B.; Sankaran, K.J. Boron and Nitrogen Co-Doped Porous Graphene Nanostructures for the Electrochemical Detection of Poisonous Heavy Metal Ions. Nanomaterials 2024, 14, 806. [Google Scholar] [CrossRef]
- Wang, J.; Yu, P.; Kan, K.; Lv, H.; Liu, Z.; Sun, B.; Bai, X.; Chen, J.; Zhang, Y.; Shi, K. Efficient Ultra-Trace Electrochemical Detection of Cd2+, Pb2+ and Hg2+ Based on Hierarchical Porous S-Doped C3N4 Tube Bundles/Graphene Nanosheets Composite. Chem. Eng. J. 2021, 420, 130317. [Google Scholar] [CrossRef]
- Bagheri, H.; Afkhami, A.; Khoshsafar, H.; Rezaei, M.; Sabounchei, S.J.; Sarlakifar, M. Simultaneous Electrochemical Sensing of Thallium, Lead and Mercury Using a Novel Ionic Liquid/Graphene Modified Electrode. Anal. Chim. Acta 2015, 870, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.Y.; Jiang, J.C.; Meng, L.Y. Carbons Confined Silver Nanoclusters Decorated Carbon Fiber Electrodes toward Electrochemical Sensor of Dihydroxyphenol and Heavy Metal Ions. Diam. Relat. Mater. 2024, 145, 111074. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, S.; Liu, G.; Li, W.; Liu, L.; Wang, Z.; Dai, X.; Jiang, X. An Electrochemical Sensor Based on PEI/CS/GN Composite–Modified Glassy Carbon Electrode for Determination of Pb(II). Ionics 2023, 29, 2031–2041. [Google Scholar] [CrossRef]
- Pham, H.N.K.; Tran, L.T.; Vu, T.A.; Tran, H.V. A Sensitive Electrochemical Sensor Based on Fe3O4 Magnetic Nanoparticles/Chitosan/Graphene Composite for Detection of Pb2+ in Aqueous Solutions. J. Appl. Electrochem. 2024, 54, 2595–2605. [Google Scholar] [CrossRef]
- Nourbakhsh, A.; Rahimnejad, M.; Asghary, M.; Younesi, H. Simultaneous Electro-Determination of Trace Copper, Lead, and Cadmium in Tap Water by Using Silver Nanoparticles and Graphene Nanoplates as Nanocomposite Modified Graphite Electrode. Microchem. J. 2022, 175, 107137. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, X.; Ling, Y.; Li, S.; Chen, J.; Zhang, Z. In Situ Bismuth Ion Exchange Plating Micro-Electrochemical Sensor Based on Laser-Induced Graphene for Trace Cd2+ and Pb2+ Detection. J. Environ. Chem. Eng. 2024, 12, 112161. [Google Scholar] [CrossRef]
- Chu, G.; Zhang, Y.; Zhou, Z.; Zeng, W.; Chen, D.; Yu, S.; Wang, J.; Guo, Y.; Sun, X.; Li, M. Rapid CO2-Laser Scribing Fabrication of an Electrochemical Sensor for the Direct Detection of Pb2+ and Cd2+. Nano Res. 2023, 16, 7671–7681. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Zhou, S.-F.; Han, X.-J.; Fan, H.-L.; Huang, J.; Liu, Y.-Q. Enhanced Electrochemical Performance for Sensing Pb(II) Based on Graphene Oxide Incorporated Mesoporous MnFe2O4 Nanocomposites. J. Alloys Compd. 2018, 747, 447–454. [Google Scholar] [CrossRef]
- Erkal, A.; Üstündağ, İ.; Yavuz, S.; Üstündağ, Z. An Electrochemical Application of MnO2 Decorated Graphene Supported Glassy Carbon Ultrasensitive Electrode: Pb2+ and Cd2+ Analysis of Seawater Samples. J. Electrochem. Soc. 2015, 162, H213. [Google Scholar] [CrossRef]
- Das, T.R.; Sharma, P.K. Sensitive and Selective Electrochemical Detection of Cd2+ by Using Bimetal Oxide Decorated Graphene Oxide (Bi2O3/Fe2O3@GO) Electrode. Microchem. J. 2019, 147, 1203–1214. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, A.; Sharma, A.; Dubey, A.; Gupta, V.; Abaszade, R.G.O.; Sundramoorthy, A.K.; Sharma, N.; Arya, S. Synthesis and Characterization of Zinc Selenide/Graphene Oxide (ZnSe/GO) Nanocomposites for Electrochemical Detection of Cadmium Ions. Appl. Phys. A 2024, 130, 297. [Google Scholar] [CrossRef]
- Muralikrishna, S.; Nagaraju, D.H.; Balakrishna, R.G.; Surareungchai, W.; Ramakrishnappa, T.; Shivanandareddy, A.B. Hydrogels of Polyaniline with Graphene Oxide for Highly Sensitive Electrochemical Determination of Lead Ions. Anal. Chim. Acta 2017, 990, 67–77. [Google Scholar] [CrossRef]
- Duan, X.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Gao, Y.; Xing, H.; Yang, T.; Ye, G.; Yu, Y. Poly(3,4-Ethylenedioxythiophene) Nanorods/Graphene Oxide Nanocomposite as a New Electrode Material for the Selective Electrochemical Detection of Mercury (II). Synth. Met. 2016, 220, 14–19. [Google Scholar] [CrossRef]
- Priya, T.; Dhanalakshmi, N.; Thennarasu, S.; Thinakaran, N. A Novel Voltammetric Sensor for the Simultaneous Detection of Cd2+ and Pb2+ Using Graphene Oxide/κ-Carrageenan/l-Cysteine Nanocomposite. Carbohydr. Polym. 2018, 182, 199–206. [Google Scholar] [CrossRef]
- Dai, H.; Wang, N.; Wang, D.; Ma, H.; Lin, M.C. An Electrochemical Sensor Based on Phytic Acid Functionalized Polypyrrole/Graphene Oxide Nanocomposites for Simultaneous Determination of Cd(II) and Pb(II). Chem. Eng. J. 2016, 299, 150–155. [Google Scholar] [CrossRef]
- Yi, W.; He, Z.; Fei, J.; He, X. Sensitive Electrochemical Sensor Based on Poly(l-Glutamic Acid)/Graphene Oxide Composite Material for Simultaneous Detection of Heavy Metal Ions. RSC Adv. 2019, 9, 17325–17334. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, H.; Wei, Y.; Feng, H.; Yang, Y.; Lu, Y.; Wei, Y.; Su, J.; Ben, Y.; Yuan, J.; et al. Detection of Heavy Metal Pb2+ by Electrochemical Sensor Based on ZIF-8@GO Composite. J. Appl. Electrochem. 2024, 54, 1397–1407. [Google Scholar] [CrossRef]
- Baghayeri, M.; Alinezhad, H.; Fayazi, M.; Tarahomi, M.; Ghanei-Motlagh, R.; Maleki, B. A Novel Electrochemical Sensor Based on a Glassy Carbon Electrode Modified with Dendrimer Functionalized Magnetic Graphene Oxide for Simultaneous Determination of Trace Pb(II) and Cd(II). Electrochim. Acta 2019, 312, 80–88. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Veerapandian, M.; Krishnan, U.M.; Rayappan, J.B.B. Simultaneous Electrochemical Detection of Cd(II), Pb(II), As(III) and Hg(II) Ions Using Ruthenium(II)-Textured Graphene Oxide Nanocomposite. Talanta 2017, 162, 574–582. [Google Scholar] [CrossRef]
- Albalawi, I.; Hogan, A.; Alatawi, H.; Alsefri, S.; Moore, E. A Novel Comparative Study for Simultaneous Determination of Cd (II) and Pb (II) Based on Ruthenium Complex-Nanoparticles-Nafion Modified Screen-Printed Gold Electrode. Sens. Actuators B Chem. 2023, 380, 133273. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Kilmartin, P.A.; Mousavi, H.Z. Simultaneous Determination of Lead(II) and Cadmium(II) at a Glassy Carbon Electrode Modified with GO@Fe3O4@benzothiazole-2-Carboxaldehyde Using Square Wave Anodic Stripping Voltammetry. J. Mol. Liq. 2018, 249, 1125–1132. [Google Scholar] [CrossRef]
- Nodehi, M.; Baghayeri, M.; Veisi, H. Preparation of GO/Fe3O4@PMDA/AuNPs Nanocomposite for Simultaneous Determination of As3+ and Cu2+ by Stripping Voltammetry. Talanta 2021, 230, 122288. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, M.; Khan, Z.U.; Nawaz, M.H.; Akhtar, N.; Rahim, A.; Riaz, S. In-Situ Synthesized ZIF-67 Graphene Oxide (ZIF-67/GO) Nanocomposite for Efficient Individual and Simultaneous Detection of Heavy Metal Ions. Environ. Monit. Assess. 2023, 195, 423. [Google Scholar] [CrossRef] [PubMed]
- Jyoti; Kaur, R.; Komal; Renu; Singh, P.; Kaur, N.; Rana, S.; Singhal, S. 2-(Anthracen-9-Yl)Benzothiazole–Modified Graphene Oxide–Nickel Ferrite Nanocomposite for Anodic Stripping Voltammetric Detection of Heavy Metal Ions. Microchim. Acta 2022, 189, 186. [Google Scholar] [CrossRef]
- Haq, Z.U.; Nazir, I.; Qureashi, A.; Ganaie, F.A.; Bashir, A.; Fatima, K.; Shah, W.A.; Rizvi, M.A. Design and Development of a Sb2WO6/Graphene Oxide (2D) Nanocomposite as Novel Electrochemical Metal-Ion Sensor and Improved Photocatalyst for the Degradation of Tetracycline. New J. Chem. 2023, 47, 21067–21080. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, D.; He, Y. Graphene Oxide/Polyacrylamide/Carboxymethyl Cellulose Sodium Nanocomposite Hydrogel with Enhanced Mechanical Strength: Preparation, Characterization and the Swelling Behavior. RSC Adv. 2014, 4, 44600–44609. [Google Scholar] [CrossRef]
- Pourbeyram, S.; Fathalipour, S.; Rashidzadeh, B.; Firuzmand, H.; Rahimi, B. Simultaneous Determination of Cd and Pb in the Environment Using a Pencil Graphite Electrode Modified with Polyaniline/Graphene Oxide Nanocomposite. Environ. Sci. 2023, 9, 3355–3365. [Google Scholar] [CrossRef]
- Seenivasan, R.; Chang, W.-J.; Gunasekaran, S. Highly Sensitive Detection and Removal of Lead Ions in Water Using Cysteine-Functionalized Graphene Oxide/Polypyrrole Nanocomposite Film Electrode. ACS Appl. Mater. Interfaces 2015, 7, 15935–15943. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.; Tong, H.; Huang, Y.; Liu, B.; Yang, X.; Su, Z.; Tu, X.; Qin, X. Simultaneous Electrochemical Detection of Cd (II) and Pb (II) Based on L-Cysteine Functionalized Gold Nanoparticles/Metal-Organic Frameworks-Graphene Oxide Nanocomposites. J. Electroanal. Chem. 2023, 943, 117573. [Google Scholar] [CrossRef]
- Aravind, S.P.; Anandhu, T.P.; Jayakumar, K.; Appukuttan, A.; Rahul Krishna, B.; Amala, J.; Bhuvaneshwari, S. Activated Carbon Based Paste Electrodes for the Simultaneous and Effective Detection of Divalent Cadmium and Lead Ions in Wastewater. Environ. Technol. 2024, 45, 3062–3075. [Google Scholar] [CrossRef] [PubMed]
- Martinez Jimenez, M.J.; Avila, A.; de Barros, A.; Lopez, E.O.; Alvarez, F.; Riul, A.J.; Perez-Taborda, J.A. Polyethyleneimine-Functionalized Carbon Nanotube/Graphene Oxide Composite: A Novel Sensing Platform for Pb(II) Acetate in Aqueous Solution. ACS Omega 2021, 6, 18190–18199. [Google Scholar] [CrossRef]
- Jayaraman, N.; Palani, Y.; Jonnalagadda, R.R.; Shanmugam, E. Covalently Dual Functionalized Graphene Oxide-Based Multiplex Electrochemical Sensor for Hg(II) and Cr(VI) Detection. Sens. Actuators B Chem. 2022, 367, 132165. [Google Scholar] [CrossRef]
- Guo, X.; Cui, R.; Huang, H.; Li, Y.; Liu, B.; Wang, J.; Zhao, D.; Dong, J.; Sun, B. Insights into the Role of Pyrrole Doped in Three-Dimensional Graphene Aerogels for Electrochemical Sensing Cd(II). J. Electroanal. Chem. 2020, 871, 114323. [Google Scholar] [CrossRef]
- Yavuz, S.; Erkal, A.; Kariper, İ.A.; Solak, A.O.; Jeon, S.; Mülazımoğlu, İ.E.; Üstündağ, Z. Carbonaceous Materials-12: A Novel Highly Sensitive Graphene Oxide-Based Carbon Electrode: Preparation, Characterization, and Heavy Metal Analysis in Food Samples. Food Anal. Methods 2016, 9, 322–331. [Google Scholar] [CrossRef]
- Tu, X.; Li, X.; Liu, B.; Zhai, C.; Peng, Y.; Wang, B.; Hu, Z.; Su, Z.; Qin, X. Facile One-Pot Synthesis of Triethanolamine-Functionalized AuNPs-GO-UiO-66-NH2 Nanocomposites for Simultaneous Electrochemical Detection of Cd(II), Pb(II), and Cu(II). J. Solid State Electrochem. 2024, 28, 433–444. [Google Scholar] [CrossRef]
- Ru, J.; Wang, X.; Zhou, Z.; Zhao, J.; Yang, J.; Du, X.; Lu, X. Fabrication of Octahedral GO/UiO-67@PtNPs Nanocomposites as an Electrochemical Sensor for Ultrasensitive Recognition of Arsenic (III) in Chinese Herbal Medicine. Anal. Chim. Acta 2022, 1195, 339451. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, C.; Wang, W.; Wang, J.; Chen, S.; Xu, H.; Zhuiykov, S. Self-Assembled Co3O4/GO Composites for Excellent Electrochemical Detection of Heavy-Metal Ions. J. Electrochem. Soc. 2021, 168, 083503. [Google Scholar] [CrossRef]
- Bao, Q.; Li, G.; Yang, Z.; Pan, P.; Liu, J.; Li, R.; Wei, J.; Hu, W.; Cheng, W.; Lin, L. In Situ Detection of Heavy Metal Ions in Sewage with Screen-Printed Electrode-Based Portable Electrochemical Sensors. Analyst 2021, 146, 5610–5618. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Wu, W.; Han, C.; Li, M. Graphene Oxide Supported MOFs-Nanofiber Carbon Aerogel/SPCE for Simultaneous Detection of Cd2+ and Pb2+ in Seafood. Food Chem. 2025, 470, 142643. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Zetterlund, P.B. Strategies for Reduction of Graphene Oxide–A Comprehensive Review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Yeasin Pabel, M.; Yasmin, S.; Shaikh, M.A.A.; Kabir, M.H. Electronic Waste Derived Reduced Graphene Oxide Supported Silver Nanoparticles for the Electrochemical Sensing of Trace Level Arsenite in Aqueous Medium. Sens. Actuators A Phys. 2024, 366, 115028. [Google Scholar] [CrossRef]
- Sang, S.; Li, D.; Zhang, H.; Sun, Y.; Jian, A.; Zhang, Q.; Zhang, W. Facile Synthesis of AgNPs on Reduced Graphene Oxide for Highly Sensitive Simultaneous Detection of Heavy Metal Ions. RSC Adv. 2017, 7, 21618–21624. [Google Scholar] [CrossRef]
- Pifferi, V.; Testolin, A.; Ingrosso, C.; Curri, M.L.; Palchetti, I.; Falciola, L. Au Nanoparticles Decorated Graphene-Based Hybrid Nanocomposite for As(III) Electroanalytical Detection. Chemosensors 2022, 10, 67. [Google Scholar] [CrossRef]
- S, S.; Gautam, V.; Verma, K.L.; Kumar, A.; Jain, V.K.; Nagpal, S. Trace Level Electrochemical Analysis of Arsenite in Human Serum Utilising RGO/AuNPs Based Sensor Platform. Int. J. Environ. Anal. Chem. 2024, 104, 1881–1895. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Liu, G.; Thi Dieu Thuy, N. A Disposable and Flexible Electrochemical Sensor for the Sensitive Detection of Heavy Metals Based on a One-Step Laser-Induced Surface Modification: A New Strategy for the Batch Fabrication of Sensors. Sens. Actuators B Chem. 2022, 350, 130834. [Google Scholar] [CrossRef]
- Wang, W.-J.; Lu, X.-Y.; Kong, F.-Y.; Li, H.-Y.; Wang, Z.-X.; Wang, W. A Reduced Graphene Oxide Supported Au-Bi Bimetallic Nanoparticles as an Enhanced Sensing Platform for Simultaneous Voltammetric Determination of Pb (II) and Cd (II). Microchem. J. 2022, 175, 107078. [Google Scholar] [CrossRef]
- You, J.; Li, J.; Wang, Z.; Baghayeri, M.; Zhang, H. Application of Co3O4 Nanocrystal/RGO for Simultaneous Electrochemical Detection of Cadmium and Lead in Environmental Waters. Chemosphere 2023, 335, 139133. [Google Scholar] [CrossRef]
- Mnyipika, S.H.; Munonde, T.S.; Nomngongo, P.N. Mno2 @reduced Graphene Oxide Nanocomposite-Based Electrochemical Sensor for the Simultaneous Determination of Trace Cd(Ii), Zn(Ii) and Cu(Ii) in Water Samples. Membranes 2021, 11, 517. [Google Scholar] [CrossRef]
- Luyen, N.D.; Toan, T.T.T.; Thanh, N.M. Voltammetry Determination of Cd(II) and Pb(II) at TiO2/Reduced Graphene Oxide Modified Electrodes. Vietnam. J. Chem. 2024, 62, 361–370. [Google Scholar] [CrossRef]
- Pang, J.; Fu, H.; Kong, W.; Jiang, R.; Ye, J.; Zhao, Z.; Hou, J.; Sun, K.; Zheng, Y.; Chen, L. Design of NiCo2O4 Nanoparticles Decorated N, S Co-Doped Reduced Graphene Oxide Composites for Electrochemical Simultaneous Detection of Trace Multiple Heavy Metal Ions and Hydrogen Evolution Reaction. Chem. Eng. J. 2022, 433, 133854. [Google Scholar] [CrossRef]
- Karthik, R.; Thambidurai, S. Synthesis of Cobalt Doped ZnO/Reduced Graphene Oxide Nanorods as Active Material for Heavy Metal Ions Sensor and Antibacterial Activity. J. Alloys Compd. 2017, 715, 254–265. [Google Scholar] [CrossRef]
- Luyen, N.D.; Trang, H.T.; Khang, P.Y.; Thanh, N.M.; Vu, H.X.A.; Phong, N.H.; Khieu, D.Q. Simultaneous Determination of Pb(II) and Cd(II) by Electrochemical Method Using ZnO/ErGO-Modified Electrode. J. Appl. Electrochem. 2024, 54, 917–933. [Google Scholar] [CrossRef]
- Guo, C.; Wang, C.; Sun, H.; Dai, D.; Gao, H. A Simple Electrochemical Sensor Based on RGO/MoS2/CS Modified GCE for Highly Sensitive Detection of Pb(Ii) in Tobacco Leaves. RSC Adv. 2021, 11, 29590–29597. [Google Scholar] [CrossRef]
- Yang, M.; He, D.; Zheng, S.; Yang, L. In Situ Biosynthesized Polyphosphate Nanoparticles/Reduced Graphene Oxide Composite Electrode for Highly Sensitive Detection of Heavy Metal Ions. Environ. Res. 2024, 244, 117966. [Google Scholar] [CrossRef]
- Gong, S.; Liu, X.; Liao, H.; Lin, X.; Huang, Q.; Hasan, M.; Shu, X.; Zhou, X.; Gunasekaran, S. Tailoring of L-Cysteine Conjugated Chitosan Carbon Electrode for Selective and Sensitive Monitor of Copper in the Water. Mater. Today Commun. 2023, 35, 106092. [Google Scholar] [CrossRef]
- Göde, C.; Yola, M.L.; Yılmaz, A.; Atar, N.; Wang, S. A Novel Electrochemical Sensor Based on Calixarene Functionalized Reduced Graphene Oxide: Application to Simultaneous Determination of Fe(III), Cd(II) and Pb(II) Ions. J. Colloid. Interface Sci. 2017, 508, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Elçin, S.; Yola, M.L.; Eren, T.; Girgin, B.; Atar, N. Highly Selective and Sensitive Voltammetric Sensor Based on Ruthenium Nanoparticle Anchored Calix[4]Amidocrown-5 Functionalized Reduced Graphene Oxide: Simultaneous Determination of Quercetin, Morin and Rutin in Grape Wine. Electroanalysis 2016, 28, 611–619. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Pang, K.; Zhao, M. Dual-Modulated Heterojunctions for Anti-Interference Sensing of Heavy Metals in Seawater. Anal. Chem. 2022, 94, 10183–10191. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Dourandish, Z.; Khalilzadeh, M.A.; Jang, H.W.; Venditti, R.A.; Varma, R.S.; Shokouhimehr, M. Recent Developments in Polymer Nanocomposite-Based Electrochemical Sensors for Detecting Environmental Pollutants. Ind. Eng. Chem. Res. 2021, 60, 1112–1136. [Google Scholar] [CrossRef]
- Akhtar, M.; Tahir, A.; Zulfiqar, S.; Hanif, F.; Warsi, M.F.; Agboola, P.O.; Shakir, I. Ternary Hybrid of Polyaniline-Alanine-Reduced Graphene Oxide for Electrochemical Sensing of Heavy Metal Ions. Synth. Met. 2020, 265, 116410. [Google Scholar] [CrossRef]
- Li, W.; Xu, S.; Chen, Y.; Wang, Z.; Cao, M.; Liu, Y. Super-Efficient and Stable Detection of Toxic Heavy Metal by Polyethylenimine-Modified Graphene Electrode Sensor. J. Mater. Sci. Mater. Electron. 2024, 35, 1065. [Google Scholar] [CrossRef]
- Guo, Z.; Li, D.; Luo, X.; Li, Y.; Zhao, Q.-N.; Li, M.; Zhao, Y.; Sun, T.; Ma, C. Simultaneous Determination of Trace Cd(II), Pb(II) and Cu(II) by Differential Pulse Anodic Stripping Voltammetry Using a Reduced Graphene Oxide-Chitosan/Poly-l-Lysine Nanocomposite Modified Glassy Carbon Electrode. J. Colloid. Interface Sci. 2017, 490, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A Review on Graphene-Based Nanocomposites for Electrochemical and Fluorescent Biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Hu, S. Nanocomposites of Graphene and Graphene Oxides: Synthesis, Molecular Functionalization and Application in Electrochemical Sensors and Biosensors. A Review. Microchim. Acta 2017, 184, 1–44. [Google Scholar] [CrossRef]
- Wang, L.; Peng, X.; Fu, H. An Electrochemical Aptasensor for the Sensitive Detection of Pb2+ Based on a Chitosan/Reduced Graphene Oxide/Titanium Dioxide. Microchem. J. 2022, 174, 106977. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; He, B.; Xie, L.; Cao, X.; Wei, M.; Jin, H.; Ren, W.; Suo, Z.; Xu, Y. Simultaneous Hg2+ and Pb2+ Detection in Water Samples Using an Electrochemical Aptasensor with Dual Signal Amplification by Exonuclease III and Metal-Organic Frameworks. Anal. Chim. Acta 2024, 1316, 342800. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, M.; Fang, S.; Wang, M.; He, L.; Zhao, J.; Zhang, H.; Zhang, Z. Electrochemical Biosensor Based on Three-Dimensional Reduced Graphene Oxide and Polyaniline Nanocomposite for Selective Detection of Mercury Ions. Sens. Actuators B Chem. 2015, 214, 63–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, K.; Van Tan, L.; Tan, H.; Zhang, H. Electrochemical Sensing Platform for Detection of Heavy Metal Ions without Electrochemical Signal. Microchim. Acta 2024, 191, 246. [Google Scholar] [CrossRef]
- Kushwah, M.; Yadav, R.; Berlina, A.N.; Gaur, K.; Gaur, M.S. Development of an Ultrasensitive RGO/AuNPs/SsDNA-Based Electrochemical Aptasensor for Detection of Pb2+. J. Solid State Electrochem. 2023, 27, 559–574. [Google Scholar] [CrossRef]
- Xuan, X.; Park, J.Y. A Miniaturized and Flexible Cadmium and Lead Ion Detection Sensor Based on Micro-Patterned Reduced Graphene Oxide/Carbon Nanotube/Bismuth Composite Electrodes. Sens. Actuators B Chem. 2018, 255, 1220–1227. [Google Scholar] [CrossRef]
- Wang, N.; Lin, M.; Dai, H.; Ma, H. Functionalized Gold Nanoparticles/Reduced Graphene Oxide Nanocomposites for Ultrasensitive Electrochemical Sensing of Mercury Ions Based on Thymine–Mercury–Thymine Structure. Biosens. Bioelectron. 2016, 79, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Bisht, N.; Prabhakar, P.; Sen, R.K.; Kumar, P.; Dwivedi, N.; Ashiq, M.; Mondal, D.P.; Srivastava, A.K.; Dhand, C. Ternary Nanocomposite-Based Smart Sensor: Reduced Graphene Oxide/Polydopamine/Alanine Nanocomposite for Simultaneous Electrochemical Detection of Cd2+, Pb2+, Fe2+, and Cu2+ Ions. Environ. Res. 2023, 221, 115317. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.A.; Bahrani, S.; Mousavi, S.M.; Omidifar, N.; Arjmand, M.; Lankarani, K.B.; Ramakrishna, S. Simultaneous Electrochemical Detection of Cd and Pb in Aquatic Samples via Coupled Graphene with Brominated White Polyaniline Flakes. Eur. Polym. J. 2021, 162, 110926. [Google Scholar] [CrossRef]
- Lee, P.M.; Wang, Z.; Liu, X.; Chen, Z.; Liu, E. Glassy Carbon Electrode Modified by Graphene–Gold Nanocomposite Coating for Detection of Trace Lead Ions in Acetate Buffer Solution. Thin Solid Films 2015, 584, 85–89. [Google Scholar] [CrossRef]
- Baghayeri, M.; Ghanei-Motlagh, M.; Tayebee, R.; Fayazi, M.; Narenji, F. Application of Graphene/Zinc-Based Metal-Organic Framework Nanocomposite for Electrochemical Sensing of As(III) in Water Resources. Anal. Chim. Acta 2020, 1099, 60–67. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Wu, J.; Ying, Y. Development of an Electrochemically Reduced Graphene Oxide Modified Disposable Bismuth Film Electrode and Its Application for Stripping Analysis of Heavy Metals in Milk. Food Chem. 2014, 151, 65–71. [Google Scholar] [CrossRef]
- Sapari, S.; Razak, N.H.A.; Hasbullah, S.A.; Heng, L.Y.; Chong, K.F.; Tan, L.L. A Regenerable Screen-Printed Voltammetric Hg(II) Ion Sensor Based on Tris-Thiourea Organic Chelating Ligand Grafted Graphene Nanomaterial. J. Electroanal. Chem. 2020, 878, 114670. [Google Scholar] [CrossRef]
- Kim, C.; Park, J.; Kim, W.; Lee, W.; Na, S.; Park, J. Detection of Cd2+ and Pb2+ Using Amyloid Oligomer–Reduced Graphene Oxide Composite. Bioelectrochemistry 2022, 147, 108214. [Google Scholar] [CrossRef]
- Gunasekaran, B.M.; Rajendran, G.K.; Rayappan, J.B.B.; Sivanesan, J.R.; Nesakumar, N.; Paulraj, A.W. Covalently Grafted 4-Aminopyridine-Reduced Graphene Oxide-Modified Screen-Printed Carbon Electrode for Electrochemical Sensing of Lead Ions. Arab. J. Sci. Eng. 2023, 48, 7721–7738. [Google Scholar] [CrossRef]
- IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man; International Agency for Research on Cancer: Lyon, France, 1977. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Rasin, P.; Ashwathi, A.V.; Basheer, S.M.; Haribabu, J.; Santibanez, J.F.; Garrote, C.A.; Arulraj, A.; Mangalaraja, R.V. Exposure to Cadmium and Its Impacts on Human Health: A Short Review. J. Hazard. Mater. Adv. 2025, 17, 100608. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The Essential Metals for Humans: A Brief Overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Enrique Conti, M. Mineral Components in Foods, Chapter 9, Heavy Metals in Food Packaging, 1st ed.; Szefer, P., Nriagu, J.O., Eds.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Lawless, L.; Xie, L.; Zhang, K. The Inter- and Multi- Generational Epigenetic Alterations Induced by Maternal Cadmium Exposure. Front. Cell Dev. Biol. 2023, 11, 1148906. [Google Scholar] [CrossRef]
- Shin, D.Y.; Lee, S.M.; Jang, Y.; Lee, J.; Lee, C.; Cho, E.-M.; Seo, Y.R. Adverse Human Health Effects of Chromium by Exposure Route: A Comprehensive Review Based on Toxicogenomic Approach. Int. J. Mol. Sci. 2023, 24, 3410. [Google Scholar] [CrossRef] [PubMed]
- Hossini, H.; Shafie, B.; Niri, A.D.; Nazari, M.; Esfahlan, A.J.; Ahmadpour, M.; Nazmara, Z.; Ahmadimanesh, M.; Makhdoumi, P.; Mirzaei, N.; et al. A Comprehensive Review on Human Health Effects of Chromium: Insights on Induced Toxicity. Environ. Sci. Pollut. Res. 2022, 29, 70686–70705. [Google Scholar] [CrossRef] [PubMed]
- Sundseth, K.; Pacyna, J.; Pacyna, E.; Pirrone, N.; Thorne, R. Global Sources and Pathways of Mercury in the Context of Human Health. Int. J. Environ. Res. Public Health 2017, 14, 105. [Google Scholar] [CrossRef]
- Andreoli, V.; Sprovieri, F. Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview. Int. J. Environ. Res. Public Health 2017, 14, 93. [Google Scholar] [CrossRef]
- Uddin, S.; Khanom, S.; Islam, M.R. Mercury Contamination in Food—An Overview. In Mercury Toxicity: Challenges and Solutions; Springer Nature Singapore: Singapore, 2023; pp. 33–70. [Google Scholar] [CrossRef]
- Rolón-Cárdenas, G.A.; Hernández-Morales, A. Phytoremediation of Lead Present in Environment: A Review. In Lead Toxicity Mitigation: Sustainable Nexus Approaches; Springer Nature: Berlin, Germany, 2024; pp. 149–168. [Google Scholar] [CrossRef]
- Heidari, S.; Mostafaei, S.; Razazian, N.; Rajati, M.; Saeedi, A.; Rajati, F. The Effect of Lead Exposure on IQ Test Scores in Children under 12 Years: A Systematic Review and Meta-Analysis of Case-Control Studies. Syst. Rev. 2022, 11, 106. [Google Scholar] [CrossRef]
- Arora, J.; Singal, A.; Jacob, J.; Garg, S.; Aeri, R. A Systematic Review of Lead Exposure on Mental Health. In Lead Toxicity Mitigation: Sustainable Nexus Approaches; Springer Nature: Berlin, Germany, 2024; pp. 51–71. [Google Scholar] [CrossRef]
- Chen, B.; Yu, P.; Chan, W.N.; Xie, F.; Zhang, Y.; Liang, L.; Leung, K.T.; Lo, K.W.; Yu, J.; Tse, G.M.K.; et al. Cellular Zinc Metabolism and Zinc Signaling: From Biological Functions to Diseases and Therapeutic Targets. Signal Transduct. Target. Ther. 2024, 9, 6. [Google Scholar] [CrossRef]
- Fan, Y.-G.; Wu, T.-Y.; Zhao, L.-X.; Jia, R.-J.; Ren, H.; Hou, W.-J.; Wang, Z.-Y. From Zinc Homeostasis to Disease Progression: Unveiling the Neurodegenerative Puzzle. Pharmacol. Res. 2024, 199, 107039. [Google Scholar] [CrossRef]
- Agnew, U.M.; Slesinger, T.L. Zinc Toxicity. Available online: https://europepmc.org/books/n/statpearls/article-31470 (accessed on 26 July 2025).
- Majumdar, S.; R, R.; Muruganantham, L.; Thimmappa, P.; Choudhary, D.; Priya, G.B.; Thakuria, D.; Prakash, N.B. Deciphering the Role of Silicon in Mitigation of Arsenic Toxicity in Soil–Plant Interface–An Overview. Silicon 2025, 17, 1891–1919. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Lou, B.; Wu, R.; Wang, G.; Lu, C.; Wang, H.; Pi, J.; Xu, Y. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules 2020, 10, 240. [Google Scholar] [CrossRef]
- Speer, R.M.; Zhou, X.; Volk, L.B.; Liu, K.J.; Hudson, L.G. Chapter Six–Arsenic and Cancer: Evidence and Mechanisms. In Environmental Carcinogenesis; Costa, M., Ed.; Advances in Pharmacology; Academic Press: London, UK, 2023; Volume 96, pp. 151–202. [Google Scholar]
- Ali, B.; Gill, R.A. Editorial: Heavy Metal Toxicity in Plants: Recent Insights on Physiological and Molecular Aspects, Volume II. Front. Plant Sci. 2022, 13, 1016257. [Google Scholar] [CrossRef]
- Sol-Magdaleno, M.; Aguilar-Aguilar, J.I.; Beltrán-Naturi, E.; Valencia-Ordóñez, L.D.; Díaz-González, A.; Trejo-Flores, P.; Camas-Flores, C.A.; Palacios-Pola, G.; Ali-Sahito, Z.; González-Moscoso, M. Carbon Nanomaterials as an Environmental Technology in the Remediation of Agricultural Soils Contaminated with Heavy Metals: A Review. Discov. Soil 2025, 2, 26. [Google Scholar] [CrossRef]
- Musah, B.I. Effects of Heavy Metals and Metalloids on Plant-Animal Interaction and Biodiversity of Terrestrial Ecosystems-An Overview. Environ. Monit. Assess. 2024, 197, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of Heavy Metals on Microbial Communities in Sediments and Establishment of Bioindicators Based on Microbial Taxa and Function for Environmental Monitoring and Management. Sci. Total Environ. 2020, 749, 141555. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Islam, M.S.; KC, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, Effects and Present Perspectives of Heavy Metals Contamination: Soil, Plants and Human Food Chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Zeng, J.; Tabelin, C.B.; Gao, W.; Tang, L.; Luo, X.; Ke, W.; Jiang, J.; Xue, S. Heterogeneous Distributions of Heavy Metals in the Soil-Groundwater System Empowers the Knowledge of the Pollution Migration at a Smelting Site. Chem. Eng. J. 2023, 454, 140307. [Google Scholar] [CrossRef]
- Sharma, M.; Kant, R.; Sharma, A.K.; Sharma, A.K. Exploring the Impact of Heavy Metals Toxicity in the Aquatic Ecosystem. Int. J. Energy Water Resour. 2025, 9, 267–280. [Google Scholar] [CrossRef]
- Santhosh, K.; Kamala, K.; Ramasamy, P.; Musthafa, M.S.; Almujri, S.S.; Asdaq, S.M.B.; Sivaperumal, P. Unveiling the Silent Threat: Heavy Metal Toxicity Devastating Impact on Aquatic Organisms and DNA Damage. Mar. Pollut. Bull. 2024, 200, 116139. [Google Scholar] [CrossRef]
- Islam, S.M.M.; Rohani, M.F.; Zabed, S.A.; Islam, M.T.; Jannat, R.; Akter, Y.; Shahjahan, M. Acute Effects of Chromium on Hemato-Biochemical Parameters and Morphology of Erythrocytes in Striped Catfish Pangasianodon Hypophthalmus. Toxicol. Rep. 2020, 7, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.D.; Jenkins, S.R.; Boig, C.; Sinfield, C.; Kennington, K.; Brand, A.R.; Lart, W.; Kröger, R. Metal Pollution as a Potential Threat to Shell Strength and Survival in Marine Bivalves. Sci. Total Environ. 2021, 755, 143019. [Google Scholar] [CrossRef]
- Bai, L.; Liu, X.-L.; Hu, J.; Li, J.; Wang, Z.-L.; Han, G.; Li, S.-L.; Liu, C.-Q. Heavy Metal Accumulation in Common Aquatic Plants in Rivers and Lakes in the Taihu Basin. Int. J. Environ. Res. Public Health 2018, 15, 2857. [Google Scholar] [CrossRef]
- The United Nations Department of Economic and Social Affairs. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015; p. 16301. [Google Scholar]
- Bose-O’Reilly, S.; Landrigan, P.J. Chapter 30–Metal Toxicology in Low-Income and Lower-Middle-Income Countries. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: London, UK, 2022; pp. 705–729. ISBN 978-0-12-823292-7. [Google Scholar]
- Panghal, A.; Thakur, A.; Deore, M.S.; Goyal, M.; Singh, C.; Kumar, J. Multimetal Exposure: Challenges in Diagnostics, Prevention, and Treatment. J. Biochem. Mol. Toxicol. 2024, 38, e23745. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shoorei, H.; Mohaqiq, M.; Tahmasebi, M.; Seify, M.; Taheri, M. Counteracting Effects of Heavy Metals and Antioxidants on Male Fertility. BioMetals 2021, 34, 439–491. [Google Scholar] [CrossRef]
- Geron, M.; Cowell, W.; Amarasiriwardena, C.; Andra, S.S.; Carroll, K.; Kloog, I.; Wright, R.O.; Wright, R.J. Racial/Ethnic and Neighborhood Disparities in Metals Exposure during Pregnancy in the Northeastern United States. Sci. Total Environ. 2022, 820, 153249. [Google Scholar] [CrossRef]
- Uddin, S.; Afroz, H.; Hossain, M.; Briffa, J.; Blundell, R.; Islam, M.R. Heavy Metals/Metalloids in Food Crops and Their Implications for Human Health. In Heavy Metal Toxicity and Tolerance in Plants: A Biological, Omics, and Genetic Engineering Approach; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 59–86. ISBN 9781119906506. [Google Scholar]
- Biswas, H.S.; Poddar, S. Heavy Metals in Foodstuffs: Presence, Bioaccumulation, Origins, Health Risk, and Remediation. In Biosorption Processes for Heavy Metal Remova; IGI Global: Hershey, PA, USA, 2024; pp. 85–99. ISBN 9798369316184. [Google Scholar]
- Munir, N.; Jahangeer, M.; Bouyahya, A.; El Omari, N.; Ghchime, R.; Balahbib, A.; Aboulaghras, S.; Mahmood, Z.; Akram, M.; Ali Shah, S.M.; et al. Heavy Metal Contamination of Natural Foods Is a Serious Health Issue: A Review. Sustainability 2022, 14, 161. [Google Scholar] [CrossRef]
- Zhu, Y.; Costa, M. Metals and Molecular Carcinogenesis. Carcinogenesis 2020, 41, 1161–1172. [Google Scholar] [CrossRef]
- Teng, J.; Yu, X. The Effect of Different Heavy Metals on Human Health. Highlights Sci. Eng. Technol. 2023, 74, 875–880. [Google Scholar] [CrossRef]
- Kerna, N.A.; Holets, H.M.; Anderson II, J.; Flores, J.V.; Pruitt, K.D.; McKee, D.; Carsrud, N.D.V.; Ngwu, D.C.; Nnake, I.; Chawla, S.; et al. Heavy Metals and Human Health: From Neurological Disorders to Developmental Delays. Eur. J. Ecol. Biol. Agric. 2024, 1, 152–184. [Google Scholar] [CrossRef]
- Singh, S.; Paswan, S.K.; Kumar, P.; Singh, R.K.; Kumar, L. Chapter 14–Heavy Metal Water Pollution: An Overview about Remediation, Removal and Recovery of Metals from Contaminated Water. In Metals in Water; Shukla, S.K., Kumar, S., Madhav, S., Mishra, P.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 263–284. ISBN 978-0-323-95919-3. [Google Scholar]
- Dashtey, A. Fate and Transport of Heavy Metals in Soil, Surface Water, and Groundwater: Implications for Environmental Management. Int. J. Sci. Res. Manag. 2024, 12, 202–215. [Google Scholar] [CrossRef]
- Matesun, J.; Petrik, L.; Musvoto, E.; Ayinde, W.; Ikumi, D. Limitations of Wastewater Treatment Plants in Removing Trace Anthropogenic Biomarkers and Future Directions: A Review. Ecotoxicol. Environ. Saf. 2024, 281, 116610. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Opp, C.; Evgrafova, A.; Groll, M.; Zitzer, N.; Laufenberg, G. Impacts of Dam Draining on the Mobility of Heavy Metals and Arsenic in Water and Basin Bottom Sediments of Three Studied Dams in Germany. Sci. Total Environ. 2018, 640–641, 1072–1081. [Google Scholar] [CrossRef]
- Singh, R.; Mahandra, H.; Gupta, B. Recovery of Zinc and Cadmium from Spent Batteries Using Cyphos IL 102 via Solvent Extraction Route and Synthesis of Zn and Cd Oxide Nanoparticles. Waste Manag. 2017, 67, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Firincă, C.; Zamfir, L.-G.; Constantin, M.; Răut, I.; Jecu, M.-L.; Doni, M.; Gurban, A.-M.; Șesan, T.E. Innovative Approaches and Evolving Strategies in Heavy Metal Bioremediation: Current Limitations and Future Opportunities. J. Xenobiot. 2025, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Fan, C.; Zhou, X.; Yu, P. Impact of Urbanization on Heavy Metals in Outdoor Air and Risk Assessment: A Case Study in Severe Cold Regions. Sustain. Cities Soc. 2024, 114, 105713. [Google Scholar] [CrossRef]
- Ma, J.; Ullah, S.; Niu, A.; Liao, Z.; Qin, Q.; Xu, S.; Lin, C. Heavy Metal Pollution Increases CH4 and Decreases CO2 Emissions Due to Soil Microbial Changes in a Mangrove Wetland: Microcosm Experiment and Field Examination. Chemosphere 2021, 269, 128735. [Google Scholar] [CrossRef] [PubMed]
- Mok, W.J.; Ghaffar, M.A.; Noor, M.I.M.; Lananan, F.; Azra, M.N. Understanding Climate Change and Heavy Metals in Coastal Areas: A Macroanalysis Assessment. Water 2023, 15, 891. [Google Scholar] [CrossRef]
- Seleghim, M.; Horikawa, A. Heavy Metals in Water and Their Cascading Effects on Ecosystems. Int. J. Soc. Sci. Educ. Res. 2020, 2, 66–69. [Google Scholar] [CrossRef]
- Adesina, F.P. Impacts of Heavy Metals on Aquatic Dwellers: A Literature Review. In Heavy Metals in the Environment–Contamination, Risk, and Remediation; Yoshida, M., Ed.; IntechOpen: Rijeka, Croatia, 2024; ISBN 978-0-85014-846-6. [Google Scholar]
- Sharma, S.; Sharma, M.; Ganjoo, R.; Thakur, A.; Kumar, A. Effect of Heavy Metals on Environment and Flora and Fauna. In Heavy Metals in the Environment: Management Strategies for Global Pollution; American Chemical Society: Washington, DC, USA, 2023; Volume 1456, pp. 103–115. [Google Scholar]
- Jyoti, S.Z. Sources and Pathways of Heavy Metals and Their Persistence in Ecosystems. In Heavy Metal Contamination in the Environment; Singh, V., Kumar, A., Mishra, V., Rai, S.N., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 19–33. ISBN 9781032685793. [Google Scholar]




| Heavy Metal | WHO Thresholds (Drinking Water) (μg·L−1) | IARC Carcinogen Classification |
|---|---|---|
| Cd | 3 | Group 1 |
| Pb | 10 | Group 2B |
| Cu | 2000 | Group 3 |
| Hg | 6 (inorganic) | Group 3 |
| Tl | 0.1 | Group 3 |
| As | 10 | Group 1 |
| Cr3+ and Cr6+ | 50 (total) | Group 1 (Cr6+) |
| Ba | 700 | Group 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saqib, M.; Solomonenko, A.N.; Hazra, N.K.; Aljasar, S.A.; Korotkova, E.I.; Dorozhko, E.V.; Vashisth, M.; Kar, P.K. Electrochemical Detection of Heavy Metals Using Graphene-Based Sensors: Advances, Meta-Analysis, Toxicity, and Sustainable Development Challenges. Biosensors 2025, 15, 505. https://doi.org/10.3390/bios15080505
Saqib M, Solomonenko AN, Hazra NK, Aljasar SA, Korotkova EI, Dorozhko EV, Vashisth M, Kar PK. Electrochemical Detection of Heavy Metals Using Graphene-Based Sensors: Advances, Meta-Analysis, Toxicity, and Sustainable Development Challenges. Biosensors. 2025; 15(8):505. https://doi.org/10.3390/bios15080505
Chicago/Turabian StyleSaqib, Muhammad, Anna N. Solomonenko, Nirmal K. Hazra, Shojaa A. Aljasar, Elena I. Korotkova, Elena V. Dorozhko, Mrinal Vashisth, and Pradip K. Kar. 2025. "Electrochemical Detection of Heavy Metals Using Graphene-Based Sensors: Advances, Meta-Analysis, Toxicity, and Sustainable Development Challenges" Biosensors 15, no. 8: 505. https://doi.org/10.3390/bios15080505
APA StyleSaqib, M., Solomonenko, A. N., Hazra, N. K., Aljasar, S. A., Korotkova, E. I., Dorozhko, E. V., Vashisth, M., & Kar, P. K. (2025). Electrochemical Detection of Heavy Metals Using Graphene-Based Sensors: Advances, Meta-Analysis, Toxicity, and Sustainable Development Challenges. Biosensors, 15(8), 505. https://doi.org/10.3390/bios15080505






