LAMP-Based 4-Channel Microfluidic Chip for POCT Detection of Influenza A H1N1, H3N2, and Influenza B Victoria Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Primer Design
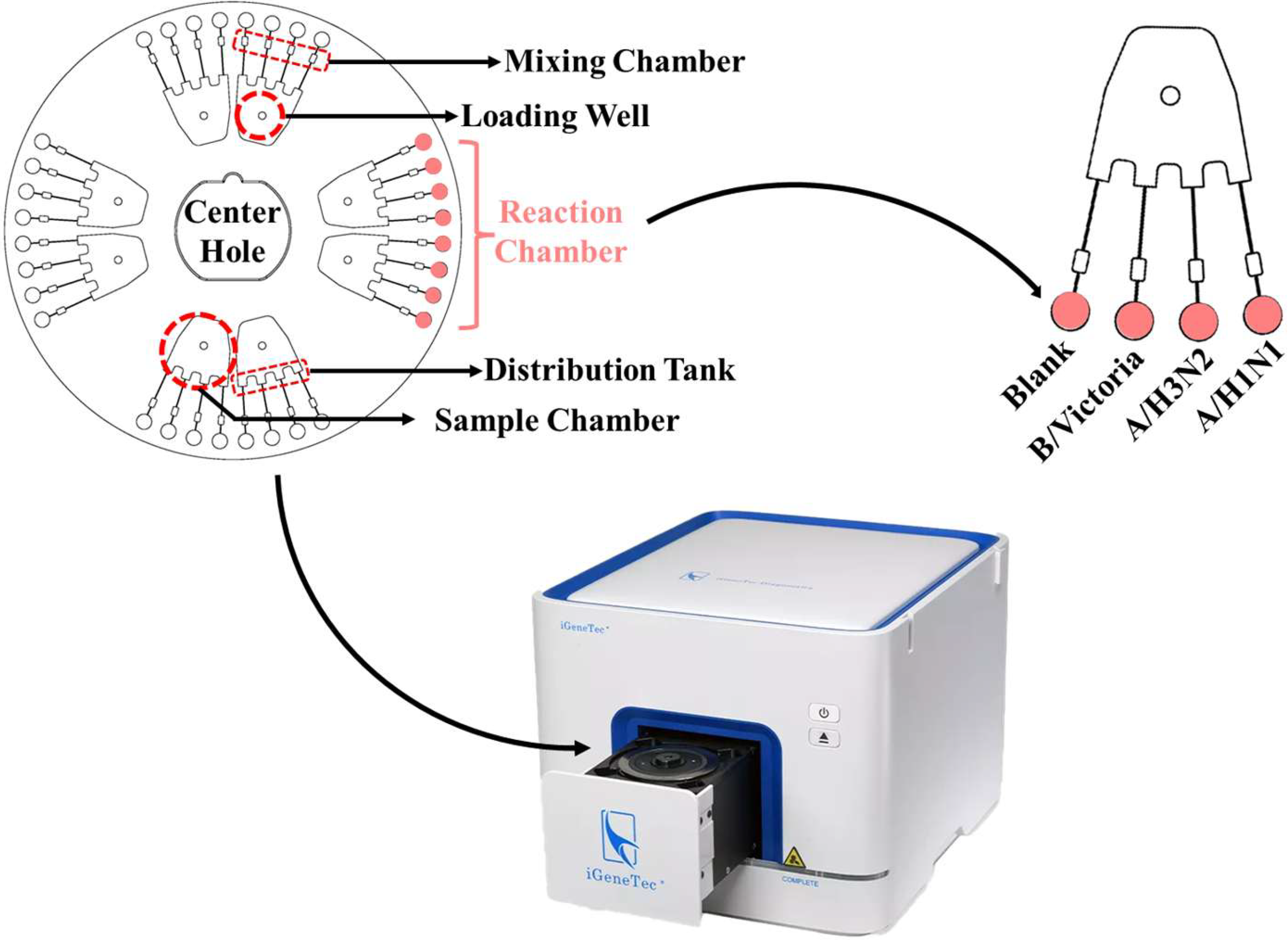
2.2. Amplification and Detection Procedures of LAMP Microfluidic Chip
2.3. Primer Screening
2.4. Amplification Using LAMP Microfluidic Chips
2.5. Methodological Evaluation
2.6. Comparison of Actual Sample Detection
3. Results
3.1. Detection Principle of LAMP Microfluidic Chips
3.2. Primer Sets Screening for LAMP Microfluidic Chips
3.3. Evaluation of Specificity
3.4. Evaluation of Sensitivity
3.5. Evaluation of Reproducibility
3.6. LAMP Microfluidic Chip for Complex Virus Sample Detection
3.7. Simultaneous Detection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LAMP | Loop-mediated isothermal amplification |
| A/H1N1 | Influenza A H1N1 virus |
| A/H3N2 | Influenza A H3N2 virus |
| B/Victoria | Influenza B Victoria virus |
| RSD | Relative standard deviation |
| NAAT | Nucleic acid amplification test |
| NA | Nucleic acid |
| POCT | Point-of-care testing |
References
- Ludwig, S.; Planz, O. Influenza viruses and the NF-κB signaling pathway—Towards a novel concept of antiviral therapy. Biol. Chem. 2008, 389, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Brooks, W.A.; Katz, M.; Roca, A.; Berkley, J.A.; Madhi, S.A.; Simmerman, J.M.; Gordon, A.; Sato, M.; Howie, S.; et al. Global burden of respiratory infections due to seasonal influenza in young children: A systematic review and meta-analysis. Lancet 2011, 378, 1917–1930. [Google Scholar] [CrossRef]
- Thompson, W.W.; Weintraub, E.; Dhankhar, P.; Cheng, P.-Y.; Brammer, L.; Meltzer, M.I.; Bresee, J.S.; Shay, D.K. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir. Viruses 2009, 3, 37–49. [Google Scholar] [CrossRef]
- Bergeron, H.C.; Reneer, Z.B.; Arora, A.; Reynolds, S.; Nagy, T.; Tripp, R.A. Influenza B Virus (IBV) Immune-Mediated Disease in C57BL/6 Mice. Vaccines 2022, 10, 1440. [Google Scholar] [CrossRef]
- Gaymard, A.; Le Briand, N.; Frobert, E.; Lina, B.; Escuret, V. Functional balance between neuraminidase and haemagglutinin in influenza viruses. Clin. Microbiol. Infect. 2016, 22, 975–983. [Google Scholar] [CrossRef]
- Tram, J. Use of Computational Matrix Adjustment to Evaluate the Effectiveness of Common Influenza Vaccines against the Emergence of Drift Variants. Int. J. Appl. Sci. Biotechnol. 2014, 2, 224–228. [Google Scholar] [CrossRef]
- Wikramaratna, P.S.; Sandeman, M.; Recker, M.; Gupta, S. The antigenic evolution of influenza: Drift or thrift? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120200. [Google Scholar] [CrossRef]
- Petrova, V.N.; Russell, C.A. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 2018, 16, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Javanian, M.; Barary, M.; Ghebrehewet, S.; Koppolu, V.; Vasigala, V.; Ebrahimpour, S. A brief review of influenza virus infection. J. Med. Virol. 2021, 93, 4638–4646. [Google Scholar] [CrossRef] [PubMed]
- Garten, R.J.; Davis, C.T.; Russell, C.A.; Shu, B.; Lindstrom, S.; Balish, A.; Sessions, W.M.; Xu, X.; Skepner, E.; Deyde, V.; et al. Antigenic and Genetic Characteristics of Swine-Origin 2009 A (H1N1) Influenza Viruses Circulating in Humans. Science 2009, 325, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Ross, T.M. H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation. Hum. Vaccines Immunother. 2018, 14, 1840–1847. [Google Scholar] [CrossRef]
- Labella, A.M.; Merel, S.E. Influenza. Med. Clin. N. Am. 2013, 97, 621–645. [Google Scholar] [CrossRef]
- Zhao, X.; Gu, Y.; Tang, X.; Jiang, C.; Fang, F.; Chu, W.; Tao, L.; Zhang, X.; Chen, M.; Wu, H.; et al. Whole-genome analysis of circulating influenza A virus (H3N2) strains in Shanghai, China from 2005 to 2023. Emerg. Microbes Infect. 2024, 13, 2396867. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Ross, T.M. Evolution of A (H1N1) pdm09 influenza virus masking by glycosylation. Expert Rev. Vaccines 2021, 20, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Soroka, M.; Wasowicz, B.; Rymaszewska, A. Loop-Mediated Isothermal Amplification (LAMP): The Better Sibling of PCR? Cells 2021, 10, 1931. [Google Scholar] [CrossRef]
- Panno, S.; Matic, S.; Tiberini, A.; Caruso, A.; Bella, P.; Torta, L.; Stassi, R.; Davino, S. Loop Mediated Isothermal Amplification: Principles and Applications in Plant Virology. Plants 2020, 9, 461. [Google Scholar] [CrossRef]
- Moehling, T.J.; Choi, G.; Dugan, L.C.; Salit, M.; Meagher, R.J. LAMP Diagnostics at the Point-of-Care: Emerging Trends and Perspectives for the Developer Community. Expert Rev. Mol. Diagn. 2021, 21, 43–61. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, S.-Y.; Kim, U.; Oh, S.-W. Diverse methods of reducing and confirming false-positive results of loop-mediated isothermal amplification assays: A review. Anal. Chim. Acta 2023, 1280, 341693. [Google Scholar] [CrossRef]
- Schneider, L.; Blakely, H.; Tripathi, A. Mathematical model to reduce loop mediated isothermal amplification (LAMP) false-positive diagnosis. Electrophoresis 2019, 40, 2706–2717. [Google Scholar] [CrossRef]
- Hardinge, P.; Murray, J.A.H. Reduced False Positives and Improved Reporting of Loop-Mediated Isothermal Amplification using Quenched Fluorescent Primers. Sci. Rep. 2019, 9, 7400. [Google Scholar] [CrossRef]
- Liu, K.-Z.; Tian, G.; Ko, A.C.T.; Geissler, M.; Malic, L.; Moon, B.-U.; Clime, L.; Veres, T. Microfluidic methods for the diagnosis of acute respiratory tract infections. Analyst 2025, 150, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, Y.; Fang, X.; Liu, Y.; Du, M.; Lu, X.; Li, Q.; Sun, Y.; Ma, J.; Lan, T. Microfluidic-RT-LAMP chip for the point-of-care detection of emerging and re-emerging enteric coronaviruses in swine. Anal. Chim. Acta 2020, 1125, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, N.; Jing, W.; Liu, Q.; Lu, H.; Zhao, W.; Zhao, W.; Yuan, Z.; Xia, H.; Sui, G. A self-powered rapid loading microfluidic chip for vector-borne viruses detection using RT-LAMP. Sens. Actuators B Chem. 2021, 333, 129521. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, C.; He, Y. Loop-Mediated Isothermal Amplification–Based Microfluidic Platforms for the Detection of Viral Infections. Curr. Infect. Dis. Rep. 2022, 24, 205–215. [Google Scholar] [CrossRef]
- Yuan, H.; Miao, Z.; Wan, C.; Wang, J.; Liu, J.; Li, Y.; Xiao, Y.; Chen, P.; Liu, B.-F. Recent advances in centrifugal microfluidics for point-of-care testing. Lab A Chip 2025, 25, 1015–1046. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Phan, V.M.; Seo, T.S. High-throughput centrifugal microfluidic platform for multiplex respiratory virus diagnostics. Sens. Actuators B Chem. 2024, 399, 134771. [Google Scholar] [CrossRef]
- Dong, X.; Tang, Z.; Jiang, X.; Fu, Q.; Xu, D.; Zhang, L.; Qiu, X. A highly sensitive, real-time centrifugal microfluidic chip for multiplexed detection based on isothermal amplification. Talanta 2024, 268, 125319. [Google Scholar] [CrossRef]
- Ye, X.; Li, Y.; Fang, X.; Kong, J. Integrated Microfluidic Sample-to-Answer System for Direct Nucleic Acid-Based Detection of Group B Streptococci in Clinical Vaginal/Anal Swab Samples. ACS Sens. 2020, 5, 1132–1139. [Google Scholar] [CrossRef]
- Yuan, D.; Kong, J.; Li, X.; Fang, X.; Chen, Q. Colorimetric LAMP microfluidic chip for detecting three allergens: Peanut, sesame and soybean. Sci. Rep. 2018, 8, 8682. [Google Scholar] [CrossRef]
- Oh, S.J.; Park, B.H.; Jung, J.H.; Choi, G.; Lee, D.C.; Kim, D.H.; Seo, T.S. Centrifugal loop-mediated isothermal amplification microdevice for rapid, multiplex and colorimetric foodborne pathogen detection. Biosens. Bioelectron. 2016, 75, 293–300. [Google Scholar] [CrossRef]
- Klein, S.; Müller, T.G.; Khalid, D.; Sonntag-Buck, V.; Heuser, A.-M.; Glass, B.; Meurer, M.; Morales, I.; Schillak, A.; Freistaedter, A.; et al. SARS-CoV-2 RNA Extraction Using Magnetic Beads for Rapid Large-Scale Testing by RT-qPCR and RT-LAMP. Viruses 2020, 12, 863. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Shao, S.; Zhang, Z.; Chen, D. Influenza viruses and SARS-CoV-2 diagnosis via sensitive testing methods in clinical application. Heliyon 2024, 10, e36410. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Gao, J.; Gu, Y.; Teng, Z.; Zhang, X.; Wu, H.; Chen, X.; Chen, M.; Kong, J. LAMP-Based 4-Channel Microfluidic Chip for POCT Detection of Influenza A H1N1, H3N2, and Influenza B Victoria Viruses. Biosensors 2025, 15, 506. https://doi.org/10.3390/bios15080506
Zhao X, Gao J, Gu Y, Teng Z, Zhang X, Wu H, Chen X, Chen M, Kong J. LAMP-Based 4-Channel Microfluidic Chip for POCT Detection of Influenza A H1N1, H3N2, and Influenza B Victoria Viruses. Biosensors. 2025; 15(8):506. https://doi.org/10.3390/bios15080506
Chicago/Turabian StyleZhao, Xue, Jiale Gao, Yijing Gu, Zheng Teng, Xi Zhang, Huanyu Wu, Xin Chen, Min Chen, and Jilie Kong. 2025. "LAMP-Based 4-Channel Microfluidic Chip for POCT Detection of Influenza A H1N1, H3N2, and Influenza B Victoria Viruses" Biosensors 15, no. 8: 506. https://doi.org/10.3390/bios15080506
APA StyleZhao, X., Gao, J., Gu, Y., Teng, Z., Zhang, X., Wu, H., Chen, X., Chen, M., & Kong, J. (2025). LAMP-Based 4-Channel Microfluidic Chip for POCT Detection of Influenza A H1N1, H3N2, and Influenza B Victoria Viruses. Biosensors, 15(8), 506. https://doi.org/10.3390/bios15080506




