Mobile and Wireless Autofluorescence Detection Systems and Their Application for Skin Tissues
Abstract
1. Introduction
2. Materials and Methods
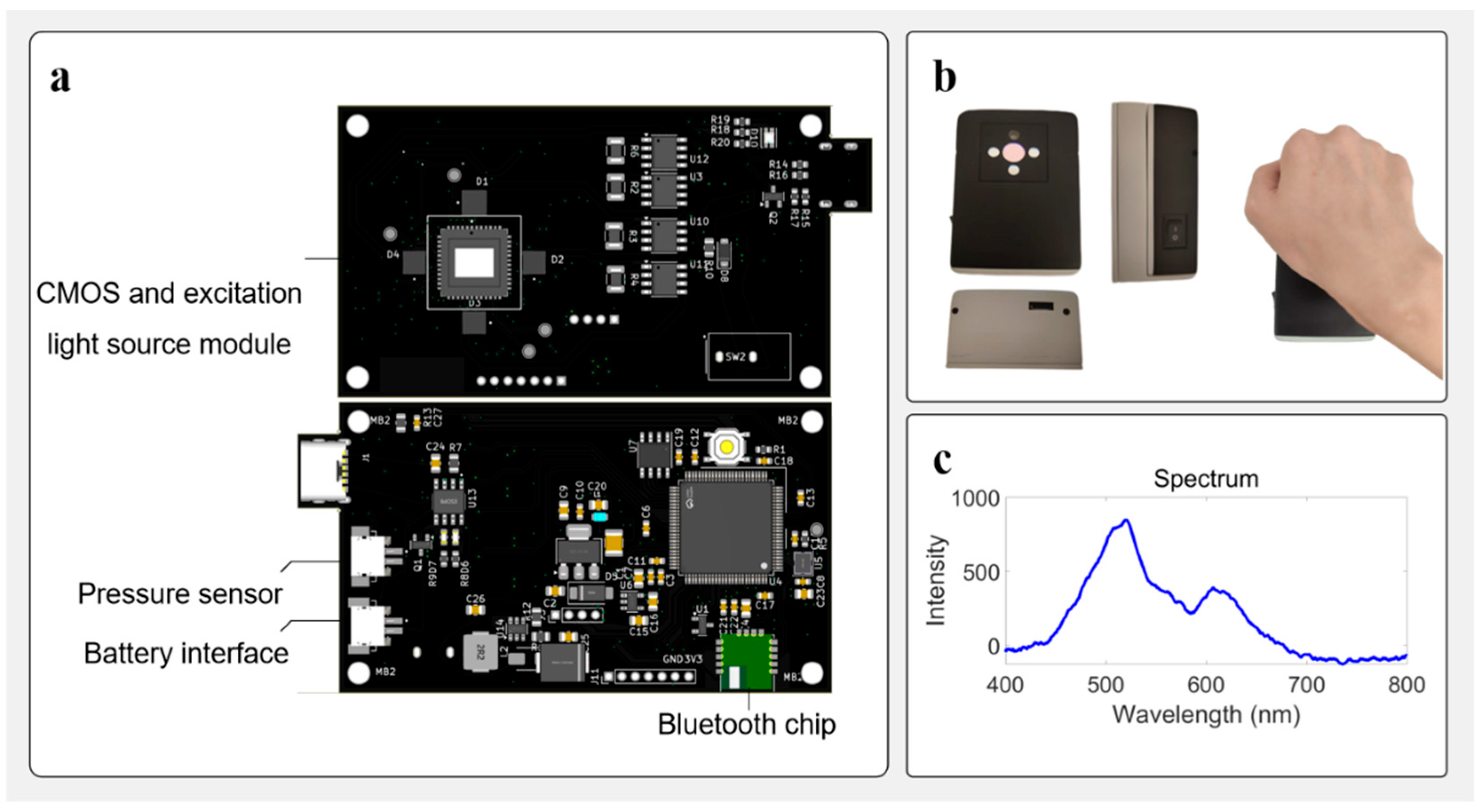
2.1. STM32H750VB-Based Control Device
2.2. Image Acquisition with AR0130 Sensor
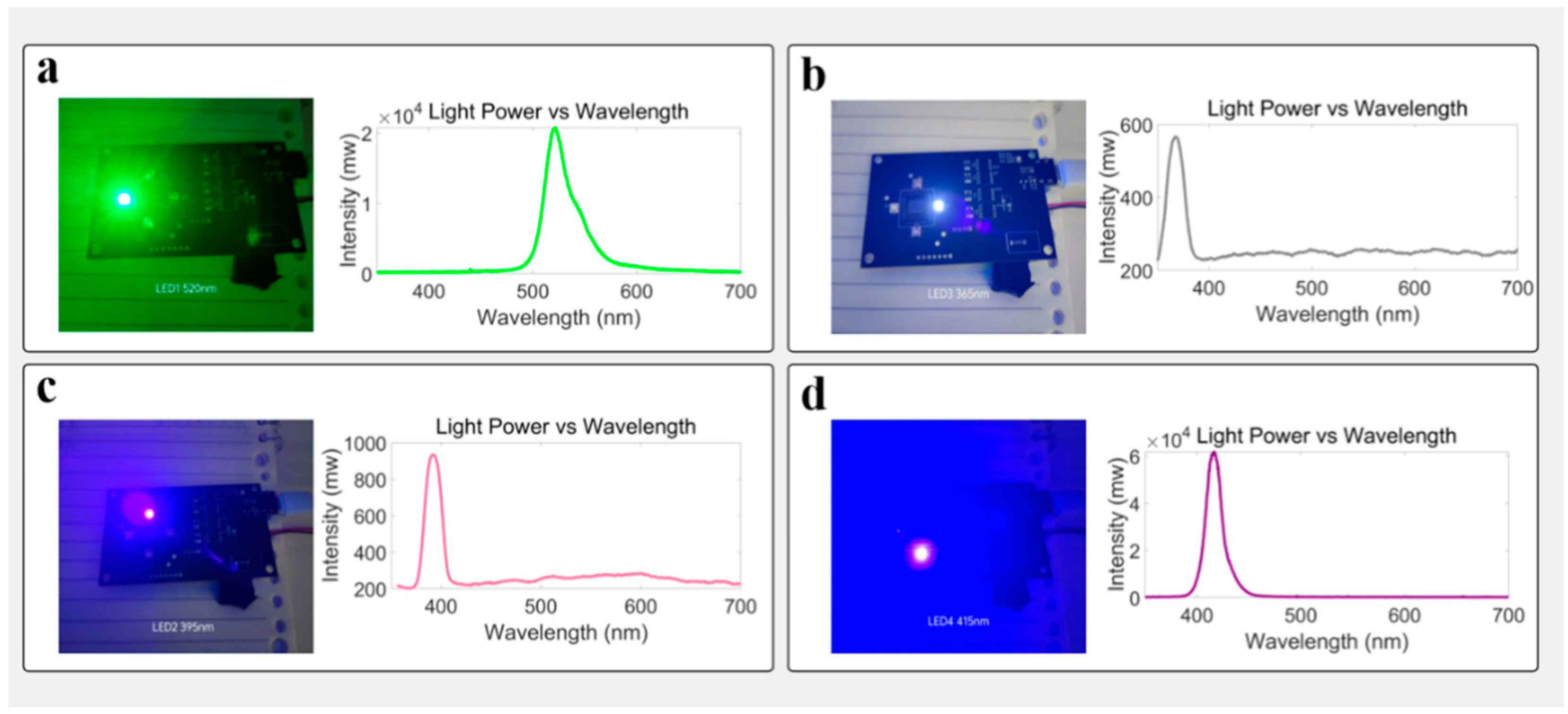
2.3. Multi-Wavelength Excitation and Spectral Matching
2.4. Wireless Communication and BLE Integration
2.5. Pressure Sensing and Contact Calibration
2.6. Closed-Loop Architecture and Data Integration
3. Results
3.1. Skin Autofluorescence Signal Acquisition and Processing
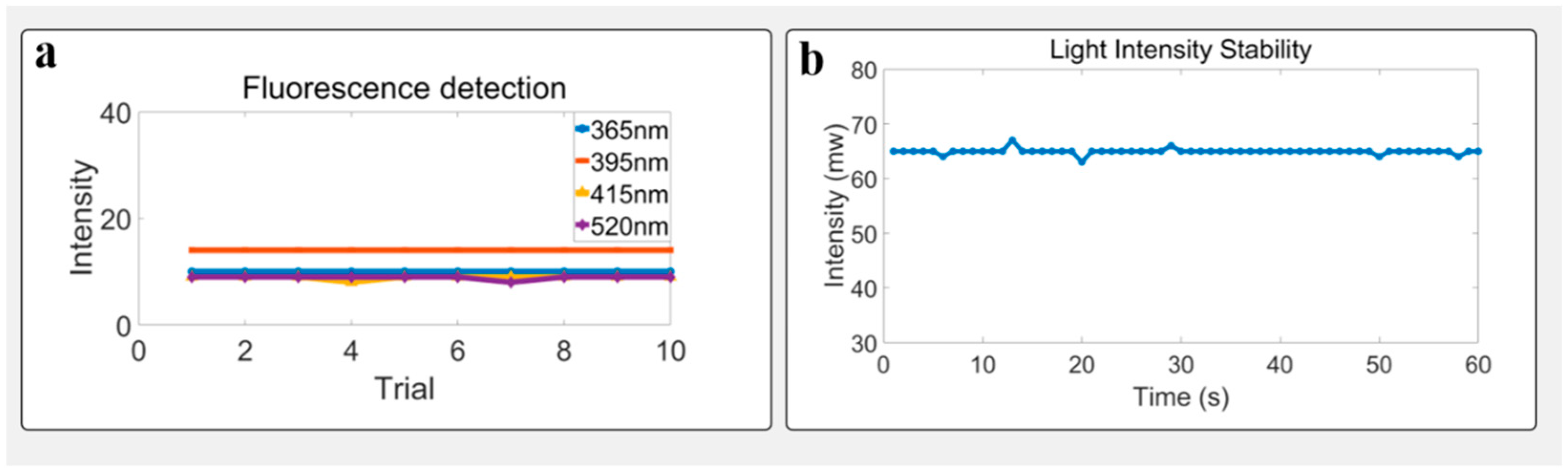
3.2. Light Avoidance Test
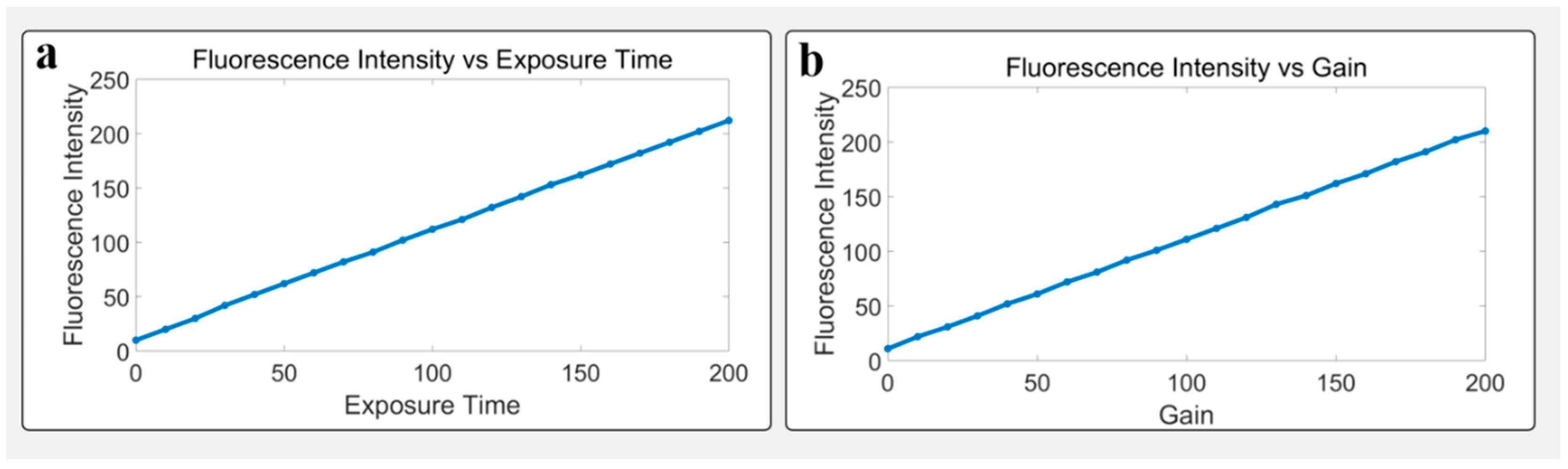
3.3. Parameter Tuning and Fluorescence Response
3.4. Stability Under Static Conditions
3.5. Influence of Age and Body Weight on Fluorescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smit, A.J.; van de Zande, S.C.; Mulder, D.J. Skin Autofluorescence as Tool for Cardiovascular and Diabetes Risk Prediction. Curr. Opin. Nephrol. Hypertens. 2022, 31, 522–526. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Soriano-Cano, A.; Álvarez-Bueno, C.; Cunha, P.G.; Martínez-Hortelano, J.A.; Garrido-Miguel, M.; Berlanga-Macías, C.; Martínez-Vizcaíno, V. Skin Autofluorescence-Indicated Advanced Glycation End Products as Predictors of Cardiovascular and All-Cause Mortality in High-Risk Subjects: A Systematic Review and Meta-analysis. J. Am. Heart Assoc. 2018, 7, e009833. [Google Scholar] [CrossRef]
- Viramontes Hörner, D.; Taal, M.W. Skin Autofluorescence: An Emerging Biomarker in Persons with Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 507–512. [Google Scholar] [CrossRef]
- Etaee, F.; Naguib, T.; Goldust, M.; Daveluy, S.; Maibach, H. Role of Skin Autofluorescence in Managing Renal and Cardiac Diseases in Outpatient Dermatology. Skin Res. Technol. 2022, 28, 889–905. [Google Scholar] [CrossRef]
- Foussard, N.; Larroumet, A.; Rigo, M.; Mohammedi, K.; Baillet-Blanco, L.; Poupon, P.; Monlun, M.; Lecocq, M.; Devouge, A.-C.; Ducos, C.; et al. Skin Autofluorescence Predicts Cancer in Subjects With Type 2 Diabetes. BMJ Open Diabetes Res. Care 2021, 9, e001312. [Google Scholar] [CrossRef]
- Yim, J.H.; Jeong, K.H.; Shin, M.K. Comparative Study of Skin Autofluorescence Expression in Atopic Dermatitis and Psoriasis: A Prospective In Vivo Study. Skin Res. Technol. 2017, 23, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, O.; Abdel-Salam, Z.; Abdel-Harith, M. Optical Characterization of Biological Tissues Based on Fluorescence, Absorption, and Scattering Properties. Diagnostics 2022, 12, 2846. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.D. Confocal Microscopy: Principles and Modern Practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef] [PubMed]
- Luu, P.; Fraser, S.E.; Schneider, F. More Than Double the Fun with Two-Photon Excitation Microscopy. Commun. Biol. 2024, 7, 364. [Google Scholar] [CrossRef]
- Elsnicova, B. Fluorescence Lifetime Imaging Microscopy of Endogenous Fluorophores in Health and Disease. J. Muscle Res. Cell Motil. 2025, 46, 67–82. [Google Scholar] [CrossRef]
- Zhu, X.; Menozzi, L.; Cho, S.-W.; Yao, J. High Speed Innovations in Photoacoustic Microscopy. Npj Imaging 2024, 2, 46. [Google Scholar] [CrossRef]
- Wessler, B.S.; Lai, L.Y.H.; Kramer, W.; Cangelosi, M.; Raman, G.; Lutz, J.S.; Kent, D.M. Clinical Prediction Models for Cardiovascular Disease: Tufts Predictive Analytics and Comparative Effectiveness Clinical Prediction Model Database. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Khanna, A.; Palmer, E.; Wilde, C.; Zaman, A.; Orr, G.; Kumudhan, D.; Lakshmanan, A.; Panos, G.D. Optical Coherence Tomography Angiography: A Review of the Current Literature. J. Int. Med. Res. 2023, 51, 3000605231187933. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Q.; Cai, F.; Liu, Q. Short-wave infrared band spatial-temporal-spectral resolved sensing system and its application in bio-samples measurement. Opt. Commun. 2025, 583, 131674. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Lang, Z.; Cai, F. A High-Frequency and Real-Time Ground Remote Sensing System for Obtaining Water Quality Based on a Micro Hyper-Spectrometer. Sensors 2024, 24, 1833. [Google Scholar] [CrossRef]
- Boersma, H.E.; van der Klauw, M.M.; Smit, A.J.; Wolffenbuttel, B.H.R. A non-invasive risk score including skin autofluorescence predicts diabetes risk in the general population. Sci. Rep. 2022, 12, 21794. [Google Scholar] [CrossRef]
- Alkhami, F.; Borderie, G.; Foussard, N.; Larroumet, A.; Blanco, L.; Barbet-Massin, M.-A.; Ferriere, A.; Ducos, C.; Mohammedi, K.; Fawaz, S.; et al. Skin Autofluorescence of Advanced Glycation End-Products Relates to New Cardiovascular Events in Type 2 Diabetes: A Longitudinal Observational Study. Diabetes Metab. 2024, 50, 101524. [Google Scholar] [CrossRef]
- van Waateringe, R.P.; Slagter, S.N.; van Beek, A.P.; van der Klauw, M.M.; van Vliet-Ostaptchouk, J.V.; Graaff, R.; Paterson, A.D.; Lutgers, H.L.; Wolffenbuttel, B.H.R. Skin Autofluorescence, a Non-Invasive Biomarker for Advanced Glycation End Products, Is Associated with the Metabolic Syndrome and Its Individual Components. Diabetol. Metab. Syndr. 2017, 9, 42. [Google Scholar] [CrossRef]
- Semedo, C.D.M.; Webb, M.; Waller, H.; Khunti, K.; Davies, M. Skin Autofluorescence, a Non-Invasive Marker of Advanced Glycation End Products: Clinical Relevance and Limitations. Postgrad. Med. J. 2017, 93, 289–294. [Google Scholar] [CrossRef]
- Škrha, J., Jr.; Horová, E.; Šoupal, J.; Valeriánová, A.; Malík, J.; Prázný, M.; Zima, T.; Kalousová, M.; Škrha, J. Skin Autofluorescence Corresponds to Microvascular Reactivity in Diabetes Mellitus. J. Diabetes Complicat. 2022, 36, 108206. [Google Scholar] [CrossRef]
- Lutgers, H.L.; Gerrits, E.G.; Graaff, R.; Links, T.P.; Sluiter, W.J.; Gans, R.O.; Bilo, H.J.; Smit, A.J. Skin Autofluorescence Provides Additional Information to the UKPDS Risk Score for the Estimation of Cardiovascular Prognosis in Type 2 Diabetes Mellitus. Diabetologia 2009, 52, 789–797. [Google Scholar] [CrossRef] [PubMed]
- De Pinho Ferreira, N.; Gehin, C.; Massot, B. Ambient Light Contribution as a Reference for Motion Artefacts Reduction in Photoplethysmography. In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020), Valletta, Malta, 24–26 February 2020; SciTePress: Setúbal, Portugal, 2020; Volume 1: BIODEVICES, pp. 23–32. [Google Scholar]
- Lee, J.; Kim, M.; Park, H.-K.; Kim, I.Y. Motion Artifact Reduction in Wearable Photoplethysmography Based on Multi-Channel Sensors with Multiple Wavelengths. Sensors 2020, 20, 1493. [Google Scholar] [CrossRef]
- Burrow, J.A.; Jakachira, R.; Lemaster, G.; Toussaint, K.C., Jr. Smartphone Tristimulus Colorimetry for Skin-Tone Analysis at Common Pulse Oximetry Anatomical Sites. Biophotonics Discov. 2025, 2, 032504. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable Sensors: Modalities, Challenges, and Prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Xiao, K.; Ushakov, N.; Kumar, S.; Li, X.; Min, R. Portable Optical Fiber Biosensors Integrated with Smartphone: Technologies, Applications, and Challenges. Biomed. Opt. Express 2024, 15, 1630–1650. [Google Scholar] [CrossRef]
- Allison, C. Samsung AGEs Index Explained: Understanding the Health Insight and How It’s Measured. Wareable. Available online: https://www.wareable.com/features/samsung-galaxy-watch-ages-index-explained? (accessed on 28 July 2025).
- Samsung Electronics. Unlocking New Possibilities for Preventative Wellness with New Galaxy Watch and BioActive Sensor. Samsung Newsroom. Available online: https://news.samsung.com/global/unlocking-new-possibilities-for-preventative-wellness-with-new-galaxy-watch-and-bioactive-sensor (accessed on 13 June 2025).
- Cai, F.; He, S. Using Graphics Processing Units to Accelerate Perturbation Monte Carlo Simulation in a Turbid Medium. J. Biomed. Opt. 2012, 17, 040502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Y.; Zhang, J.; Cai, F. Developing a Portable Autofluorescence Detection System and Its Application in Biological Samples. Sensors 2024, 24, 3351. [Google Scholar] [CrossRef]
- Lentferink, Y.E.; van Teeseling, L.; Knibbe, C.A.J.; van der Vorst, M.M.J. Skin Autofluorescence in Children with and Without Obesity. J. Pediatr. Endocrinol. Metab. 2019, 32, 41–47. [Google Scholar] [CrossRef]
- Ho, M.Y.; Pham, H.M.; Yiwen, Y.; Saeed, A.; Ma, D. WF-PPG: A Wrist–Finger Dual-Channel Dataset for Studying the Impact of Contact Pressure on PPG Morphology. Sci. Data 2025, 12, 200. [Google Scholar] [CrossRef]
- Sirkiä, J.-P.; Panula, T.; Kaisti, M. Investigating the Impact of Contact Pressure on Photoplethysmograms. Biomed. Eng. Adv. 2024, 7, 100123. [Google Scholar] [CrossRef]
- He, J.; Wu, W. Deformation of Heartbeat Pulse Waveform Caused by Sensor Binding Force. arXiv 2021, arXiv:2108.10014. [Google Scholar] [CrossRef]
- STMicroelectronics. STM32H750VB Datasheet. Available online: https://www.st.com/resource/en/datasheet/stm32h750vb.pdf (accessed on 9 June 2025).
- STMicroelectronics. RM0433 Reference Manual: STM32H742/743/750/753/755/757 and STM32H747/757 MCUs. Available online: https://www.st.com/resource/en/reference_manual/dm00314099.pdf (accessed on 9 June 2025).
- Jacko, P.; Bereš, M.; Kováčová, I.; Molnár, J.; Vince, T.; Dziak, J.; Fecko, B.; Gans, Š.; Kováč, D. Remote IoT Education Laboratory for Microcontrollers Based on the STM32 Chips. Sensors 2022, 22, 1440. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Lopreside, A.; Montali, L.; Zangheri, M.; Evangelisti, L.; D’Elia, M.; Michelini, E. Portable Light Detectors for Bioluminescence Biosensing Applications: A Comprehensive Review from the Analytical Chemist’s Perspective. Anal. Chim. Acta 2022, 1200, 339583. [Google Scholar] [CrossRef]
- Mulberry, G.; White, K.A.; Kim, B.N. Analysis of Simple Half-Shared Transimpedance Amplifier for Picoampere Biosensor Measurements. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 387–395. [Google Scholar] [CrossRef]
- de Borniol, E.; Abedin, M.; Ren, H.; Jackson, J.; Detwiler, K.; Larson, N.; Rogers, J.A.; Holmes, D. Active three-dimensional and thermal imaging with a 30-μm pitch 320 × 256 HgCdTe avalanche photodiode focal plane array. Opt. Eng. 2012, 51, 061305. [Google Scholar] [CrossRef]
- Zhu, B.; Jonathan, H. A Review of Image Sensors Used in Near-Infrared and Shortwave Infrared Fluorescence Imaging Systems. Sensors 2024, 24, 3539. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zang, Z.; Wang, Q.; Jiao, Z.; Mattioli Della Rocca, F.; Chen, Y.; Li, D.D.-U. Smart Wide-Field Fluorescence Lifetime Imaging System with CMOS Single-Photon Avalanche Diode Arrays. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 1887–1890. [Google Scholar]
- Hussain, S.M.; Farrukh, F.U.D.; Su, S.; Wang, Z.; Chen, H. CMOS Image Sensor Design and Image Processing Algorithm Implementation for Total Hip Arthroplasty Surgery. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1383–1392. [Google Scholar] [CrossRef]
- Greffe, T.; Smith, R.; Sherman, M.; Harrison, F.; Earnshaw, H.; Grefenstette, B.; Hennessy, J.; Nikzad, S. Characterization of Low Light Performance of a CMOS Sensor for Ultraviolet Astronomical Applications. arXiv 2021, arXiv:2112.01691. [Google Scholar]
- Layden, C.; Juneau, J.; Pettersson, G.; Lourie, N.; Schneider, B.; LaMarr, B.; Angile, F.E.; Farag, F.; Luo, M.; Zheng, Z.O.; et al. Characterization of the Teledyne COSMOS Camera: A Large Format CMOS Image Sensor for Astronomy. arXiv 2025, arXiv:2502.00101. [Google Scholar] [CrossRef]
- Cannon, T.M.; Lagarto, J.L.; Dyer, B.T.; Garcia, E.; Kelly, D.J.; Peters, N.S.; Lyon, A.R.; French, P.M.W.; Dunsby, C. Characterization of NADH Fluorescence Properties under One-Photon Excitation with Respect to Temperature, pH, and Binding to Lactate Dehydrogenase. OSA Contin. 2021, 4, 1610–1625. [Google Scholar] [CrossRef]
- García, M.J.; Kamaid, A.; Malacrida, L. Label-Free Fluorescence Microscopy: Revisiting the Opportunities with Autofluorescent Molecules and Harmonic Generations as Biosensors and Biomarkers for Quantitative Biology. Biophys. Rev. 2023, 15, 709–719. [Google Scholar] [CrossRef] [PubMed]
- IDEX Health & Science. Selecting Filters for Fluorescence Multiplexing. Available online: https://www.idex-hs.com/resources/resources-detail/selecting-filters-for-fluorescence-multiplexing (accessed on 9 June 2025).
- García-Espinosa, E.; Longoria-Gándara, O.; Pegueros-Lepe, I.; Veloz-Guerrero, A. Power Consumption Analysis of Bluetooth Low Energy Commercial Products and Their Implications for IoT Applications. Electronics 2018, 7, 386. [Google Scholar] [CrossRef]
- Muir, R.; Forbes, S.; Birch, D.J.S.; Vyshemirsky, V.; Rolinski, O.J. Collagen Glycation Detected by Its Intrinsic Fluorescence. J. Phys. Chem. B 2021, 125, 11058–11066. [Google Scholar] [CrossRef] [PubMed]


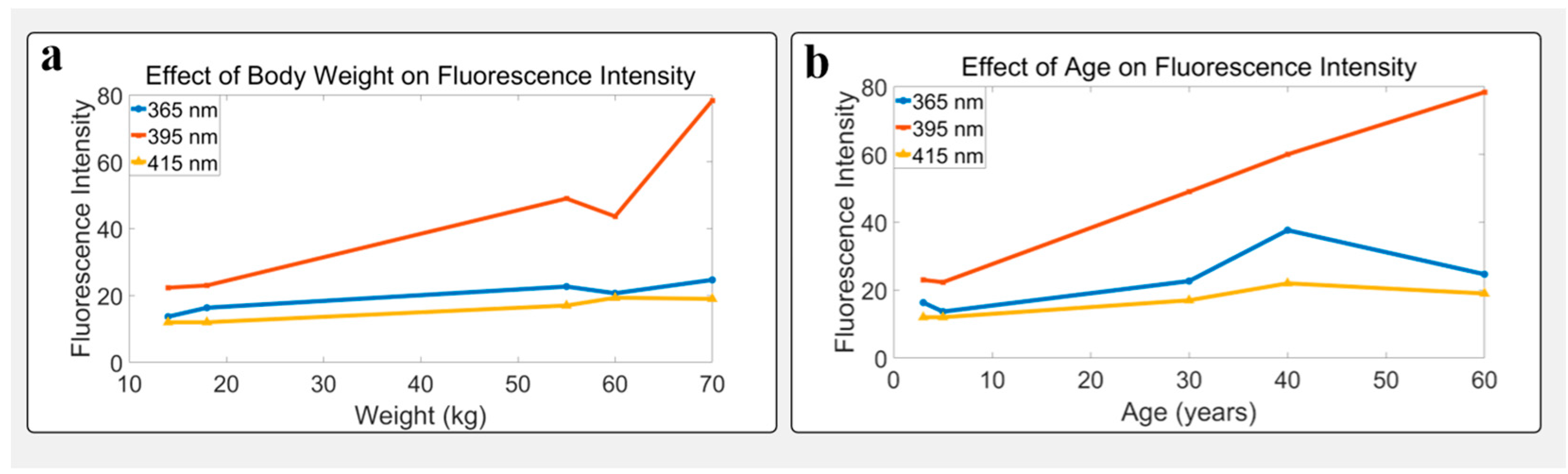
| Age (yrs) | Sex | 365 nm−1 | 365 nm−2 | 365 nm−3 | 365 nm-Mean | 365 nm-Std | 395 nm−1 | 395 nm−2 | 395 nm−3 | 395 nm-Mean | 395 nm-Std | 415 nm−1 | 415 nm−2 | 415 nm−3 | 415 nm-Mean | 415 nm-Std |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | M | 24 | 13 | 12 | 16.33 | 6.51 | 23 | 19 | 27 | 23.00 | 4.00 | 11 | 11 | 14 | 12.00 | 1.73 |
| 5 | F | 14 | 14 | 13 | 13.67 | 0.58 | 19 | 22 | 26 | 22.33 | 3.51 | 11 | 11 | 14 | 12.00 | 1.73 |
| 30 | F | 24 | 17 | 27 | 22.67 | 5.13 | 49 | 43 | 55 | 49.00 | 6.00 | 18 | 14 | 19 | 17.00 | 2.65 |
| 40 | M | 39 | 27 | 47 | 37.67 | 10.07 | 72 | 61 | 47 | 60.00 | 12.75 | 27 | 21 | 18 | 22.00 | 4.58 |
| 60 | M | 23 | 22 | 29 | 24.67 | 3.79 | 82 | 82 | 71 | 78.33 | 6.35 | 19 | 22 | 16 | 19.00 | 3.00 |
| Weight (kg) | Sex | 365 nm−1 | 365 nm−2 | 365 nm−3 | 365 nm-Mean | 365 nm-Std | 395 nm−1 | 395 nm−2 | 395 nm−3 | 395 nm-Mean | 395 nm-Std | 415 nm−1 | 415 nm−2 | 415 nm−3 | 415 nm-Mean | 415 nm-Std |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 | F | 14 | 14 | 13 | 13.67 | 0.58 | 19 | 22 | 26 | 22.33 | 3.51 | 11 | 11 | 14 | 12.00 | 1.73 |
| 18 | M | 24 | 13 | 12 | 16.33 | 6.51 | 23 | 19 | 27 | 23.00 | 4.00 | 11 | 11 | 14 | 12.00 | 1.73 |
| 55 | F | 24 | 17 | 27 | 22.67 | 5.13 | 49 | 43 | 55 | 49.00 | 6.00 | 18 | 14 | 19 | 17.00 | 2.65 |
| 60 | M | 21 | 21 | 20 | 20.67 | 0.58 | 42 | 46 | 43 | 43.67 | 2.08 | 19 | 20 | 19 | 19.33 | 0.58 |
| 70 | M | 23 | 22 | 29 | 24.67 | 3.79 | 82 | 82 | 71 | 78.33 | 6.35 | 19 | 22 | 16 | 19.00 | 3.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, Y.; Li, Y.; Cai, F. Mobile and Wireless Autofluorescence Detection Systems and Their Application for Skin Tissues. Biosensors 2025, 15, 501. https://doi.org/10.3390/bios15080501
Wang Y, Zhang Y, Li Y, Cai F. Mobile and Wireless Autofluorescence Detection Systems and Their Application for Skin Tissues. Biosensors. 2025; 15(8):501. https://doi.org/10.3390/bios15080501
Chicago/Turabian StyleWang, Yizhen, Yuyang Zhang, Yunfei Li, and Fuhong Cai. 2025. "Mobile and Wireless Autofluorescence Detection Systems and Their Application for Skin Tissues" Biosensors 15, no. 8: 501. https://doi.org/10.3390/bios15080501
APA StyleWang, Y., Zhang, Y., Li, Y., & Cai, F. (2025). Mobile and Wireless Autofluorescence Detection Systems and Their Application for Skin Tissues. Biosensors, 15(8), 501. https://doi.org/10.3390/bios15080501





