Recent Progress in Electrocatalysts for Hydroquinone Electrochemical Sensing Application
Abstract
1. Introduction
2. HQ Electrochemical Sensors
2.1. Metal-Oxide-Based HQ Sensors
2.2. Carbon-Derivatives-Based Sensing Materials for HQ Detection
2.3. Metal-Sulfides-Based Materials for HQ Detection
2.4. MXene-Based Materials for HQ Detection
2.5. MOFs/ZIF/COF-Based Materials for HQ Detection
2.6. LDH/Polymers-Based Materials for HQ Detection
2.7. Other HQ Sensors
3. Challenges, Limitations, and Future Perspectives
- Metal oxides are cost-effective and stable electrode materials but have low conductivity, which can affect the electron transfer process and reduce the sensitivity of the fabricated electrodes for HQ detection.
- Carbon-based electrode materials such as CNTs, rGO, etc., offer high conductivity and larger surface area but agglomeration occurs, which can affect the selectivity.
- MXene-based materials are also considered as highly conducting materials but their synthesis process involves harsh etching conditions and low stability for long-term use.
- MOF and COF are well-known high-surface-area materials with decent functionality for electrochemical sensing applications but often show poor stability in aqueous environments.
- The polymer-based materials demonstrate improved conductivity and flexibility for the construction of electrochemical sensors but suffer from poor long-term stability.
- The selectivity of HQ in the presence of CC or RS may be compromised due to the isomeric properties of CC and RS.
- Real-time monitoring of HQ is still not convincing using standard addition methods, as mentioned in the manuscript.
- Commercial-scale fabrication of HQ sensors remained a key challenge.
- Simple and eco-friendly methods should be developed for the preparation of MXene materials.
- MXene/LDH materials or MXene/MOF materials need to be studied in detail and their mechanism for HQ sensing should be improved. Doping strategies may also be used to prepare the metal-doped MXene/MOF or MXene/LDH composites.
- Cost-effective, surface engineering, and scalable fabrication techniques need to be developed.
- Machine-learning-assisted sensors can be developed for HQ detection, which can be useful for the accurate detection of HQ.
- Wearable HQ sensors may be developed using flexible substrates.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Zn@ZnO | Zinc@zinc oxide |
| N-rGO/CuO-ILCPE | Nitrogen-reduced graphene oxide/copper oxide-ionic liquid modified carbon paste electrode |
| MoO3@KSC | Molybdenum oxide@keratinous sludge biomass derived carbon |
| CoWO4 | cobalt tungstate |
| ZnO@MnO2-rGO | Zinc oxide@manganese dioxide-reduced graphene oxide |
| MnOx/rGO | Manganese oxide/reduced graphene oxide |
| SiO2/Bi2O3 | Silicon dioxide/bismuth oxide |
| Bi2WO6 | Bismuth tungstate |
| SrMnO3/g-CN | Strontium manganese oxide/graphitic carbon nitride |
| ZnFe2O4@f-CNF | Zinc ferrite@functionalized carbon nanofiber |
| g-CN/BN | Graphitic carbon nitride/boron nitride |
| OGO | Oxidative graphene oxide |
| MIP/MWCNTs | Molecularly imprinted polymer/multi-walled carbon nanotubes |
| TT-COF(Co)/N-CNTs | Cobalt porphyrin-based covalent organic framework/nitrogen doped carbon nanotubes |
| Mo, N, S-IPCS | Molybdenum, nitrogen, sulfur doped interconnected porous carbon spheres |
| Co/HNC | Cobalt/N doped carbon nanotube hollow spheres |
| N-MCQDs | Nitrogen doped malic acid carbon quantum dots |
| CoSnS2 | Cobalt tin sulfide |
| CuS/N-CNFs | Copper sulfide/nitrogen doped carbon nanofibrers |
| V2CTx@NiCoMn-OH | Vanadium carbide MXene@nickel-cobalt-manganese hydroxide |
| SSPC/Cu-Me | Shrimp shell-derived porous carbon/copper-melamine MOF |
| TFPB | 1, 3, 5-tris-(4-formylphenyl) benzene |
| BD-COF | Benzidine (BD) based covalent organic framework |
| PtNPs | Platinum nanoparticles |
| NH2-MWCNTs | Amine functionalized multi-walled carbon nanotubes |
| Ni3ZnC0.7/Ni | Ni nanoparticle-supported porous microsphere composites |
| CB/ZIF-8 | Carbon black/zeolite imidazolium framework-8 |
| NiCoFe-LDH NFs | Nickel cobalt iron-layered double hydroxide nanoflowers |
| CC | carbon cloth |
| Cu-CuO@MoOx | Copper/copper oxide@molybdenum oxide |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PET | Polyethylene terephthalate |
| PPy@NCS-5 | Polypyrrole anchored NiCo bi-metal sulfide |
| GCE | glassy carbon electrode |
| NFG-BAC | Nano-flake graphite and bamboo activated carbon composites |
| PC/LLSP800 | Porous carbon/longquan lignite soluble portion |
| PGE | Pencil graphite electrode |
| AuNPs/LYH-47 | Gold nanoparticles anchored layered yttrium hydroxide |
| KB | Ketjen black |
| IL | Ionic liquid |
| CoP/Co2P@NC | Carbon-encapsulated cobalt phosphide nanoparticles and N doped carbon |
| CoFe2Se4 | Cobalt iron selenide |
| PCF | Porous carbon nanofibers |
| CV | cyclic voltammetry |
| DPV | differential pulse voltammetry |
| SWV | square wave voltammetry |
| Amp | amperometry |
References
- Vadurin, K.; Perekrest, A.; Bakharev, V.; Shendryk, V.; Parfenenko, Y.; Shendryk, S. Towards Digitalization for Air Pollution Detection: Forecasting Information System of the Environmental Monitoring. Sustainability 2025, 17, 3760. [Google Scholar] [CrossRef]
- Ge, L.; Li, S.-P.; Lisak, G. Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2020, 179, 112913. [Google Scholar] [CrossRef] [PubMed]
- Naranov, E.; Ramazanov, D.; Agliullin, M.; Sinyashin, O.; Maximov, A. Recent Advances in Aromatic Hydroxylation to Phenol and Hydroquinone Using H2O2. Catalysts 2024, 14, 930. [Google Scholar] [CrossRef]
- Alrashidi, A.; El-Sherif, A.M.; Ahmed, J.; Faisal, M.; Alsaiari, M.; Algethami, J.S.; Moustafa, M.I.; Abahussain, A.A.M.; Harraz, F.A. A Sensitive Hydroquinone Amperometric Sensor Based on a Novel Palladium Nanoparticle/Porous Silicon/Polypyrrole-Carbon Black Nanocomposite. Biosensors 2023, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Sadef, Y.; Shakoor, M.B.; Naeem, M.; Bashir, F.; Ahmad, S.R.; Ali, S.; Abid, I.; Khan, N.; Alyemeni, M.N. Quantitative Estimation of the Hydroquinone, Mercury and Total Plate Count in Skin-Lightening Creams. Sustainability 2021, 13, 8786. [Google Scholar] [CrossRef]
- Gao, W.; Legido-Quigley, C. Fast and sensitive high performance liquid chromatography analysis of cosmetic creams for hydroquinone, phenol and six preservatives. J. Chromatogr. A 2011, 1218, 4307–4311. [Google Scholar] [CrossRef]
- Butwong, N.; Heng, S.; Kunawong, T.; Kunthadong, P.; Mukdasai, S.; Uppachai, P. A novel fluorescence sensor film for hydroquinone based on a graphene quantum dots-nano activated carbon composite. Chem. Eng. J. Adv. 2024, 19, 100623. [Google Scholar] [CrossRef]
- Zhao, L.; Lv, B.; Yuan, H.; Zhou, Z.; Xiao, D. A Sensitive Chemiluminescence Method for Determination of Hydroquinone and Catechol. Sensors 2007, 7, 578–588. [Google Scholar] [CrossRef]
- Upan, J.; Reanpang, P.; Chailapakul, O.; Jakmunee, J. Flow injection amperometric sensor with a carbon nanotube modified screen printed electrode for determination of hydroquinone. Talanta 2016, 146, 766–771. [Google Scholar] [CrossRef]
- Zhou, Q.; Lei, M.; Wu, Y.; Zhou, X.; Wang, H.; Sun, Y.; Sheng, X.; Tong, Y. Magnetic solid phase extraction of bisphenol A, phenol and hydroquinone from water samples by magnetic and thermo dual-responsive core-shell nanomaterial. Chemosphere 2020, 238, 124621. [Google Scholar] [CrossRef]
- Sukma, R.M.; Iswantini, D.; Nurhidayat, N.; Rafi, M.; Ariyanti, D. Antioxidant Determining Using Electrochemical Method. Chemistry 2023, 5, 1921–1941. [Google Scholar] [CrossRef]
- Chen, W.-H.; Maheshwaran, S.; Park, Y.-K.; Ong, H.C. Iron-based electrode material composites for electrochemical sensor application in the environment: A review. Sci. Total Environ. 2024, 953, 176128. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Jin, W. Earth-abundant transition metal and metal oxide nanomaterials: Synthesis and electrochemical applications. Prog. Mater. Sci. 2019, 106, 10057. [Google Scholar] [CrossRef]
- Maheshwaran, S.; Balaji, R.; Chen, S.-M.; Biswadeep, R.; Renganathan, V.; Narendhar, C.; Kao, C.R. Copper sulfide nano-globules reinforced electrodes for high-performance electrochemical determination of toxic pollutant hydroquinone. New J. Chem. 2021, 45, 3215–3223. [Google Scholar] [CrossRef]
- Devi, A.; Yadav, S.; Chahar, M.; Dabas, N. A Review of Recent Advances in Nanostructured Electrode Materials for High-Performance Supercapacitors. J. Electron. Mater. 2025, 1–21. [Google Scholar] [CrossRef]
- Mashhadian, A.; Jian, R.; Tian, S.; Wu, S.; Xiong, G. An Overview of Electrochemical Sensors Based on Transition Metal Carbides and Oxides: Synthesis and Applications. Micromachines 2024, 15, 42. [Google Scholar] [CrossRef]
- Lu, J.-Y.; Yu, Y.-S.; Chen, T.-B.; Chang, C.-F.; Tamulevičius, S.; Erts, D.; Wu, K.C.-W.; Gu, Y. Fabrication of an Extremely Cheap Poly(3,4-ethylenedioxythiophene) Modified Pencil Lead Electrode for Effective Hydroquinone Sensing. Polymers 2021, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Y.; He, Z.; Zhang, L.; Wang, T.; Tang, T.; Chen, J.; Cheng, H. A Carbon Nanofiber Electrochemical Sensor Made of FeMn@C for the Rapid Detection of Tert-Butyl Hydroquinone in Edible Oil. Molecules 2025, 30, 2725. [Google Scholar] [CrossRef]
- Alshahrani, L.A.; Miao, L.; Zhang, Y.; Cheng, S.; Sathishkumar, P.; Saravanakumar, B.; Nan, J.; Gu, F.L. 3D-Flower-Like Copper Sulfide Nanoflake-Decorated Carbon Nanofragments-Modified Glassy Carbon Electrodes for Simultaneous Electrocatalytic Sensing of Co-existing Hydroquinone and Catechol. Sensors 2019, 19, 2289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Li, J.; Xie, Y. Detection of Tert-Butylhydroquinone in Edible Oils Using an Electrochemical Sensor Based on a Nickel-Aluminum Layered Double Hydroxide@Carbon Spheres-Derived Carbon Composite. Foods 2024, 13, 3431. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Y.; Wang, L.; Wang, L.; Chen, S. An ultrafine ZnO/ZnNi2O4@porous carbon@covalent-organic framework for electrochemical detection of paracetamol and tert-butyl hydroquinone. J. Alloys Compd. 2022, 906, 164369. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Min, A.; Choi, M.Y. Fabrication of nonenzymatic electrochemical sensor based on Zn@ZnO core-shell structures obtained via pulsed laser ablation for selective determination of hydroquinone. Environ. Res. 2022, 204, 112340. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, P.; Zhang, L.; Wang, Y.; Fu, Q.; Li, R.; Li, J.; Li, C.; Xie, Y.; Fei, J. Switched electrochemical sensor for hydroquinone based on rGO@Au, monoclinic BiVO4 and temperature-sensitive polymer composite material. Microchem. J. 2022, 179, 107412. [Google Scholar] [CrossRef]
- Jahani, P.M.; Nejad, F.G.; Dourandish, Z.; Zarandi, M.P.; Safizadeh, M.M.; Tajik, S.; Beitollahi, H. A modified carbon paste electrode with N-rGO/CuO nanocomposite and ionic liquid for the efficient and cheap voltammetric sensing of hydroquinone in water specimens. Chemosphere 2022, 302, 134712. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Al-Mubaddel, F.S.; Noman, M.T. A sensitive and economical electrochemical platform for detection of food additive tert-butylhydroquinone based on porous Co3O4 nanorods embellished chemically oxidized carbon black. Food Control. 2022, 136, 108844. [Google Scholar] [CrossRef]
- Ganesan, S.; Sivam, S.; Elancheziyan, M.; Senthilkumar, S.; Ramakrishan, S.G.; Soundappan, T.; Ponnusamy, V.K. Novel delipidated chicken feather waste-derived carbon-based molybdenum oxide nanocomposite as efficient electrocatalyst for rapid detection of hydroquinone and catechol in environmental waters. Environ. Pollut. 2022, 293, 118556. [Google Scholar] [CrossRef]
- Dalkiran, B.; Brett, C.M. Poly(safranine T)-deep eutectic solvent/copper oxide nanoparticle-carbon nanotube nanocomposite modified electrode and its application to the simultaneous determination of hydroquinone and catechol. Microchem. J. 2022, 179, 107531. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, G.; Patel, R.; Gaur, U.K.; Sharma, M. Highly sensitive detection of hazardous hydroquinone and chloramphenicol in the presence of paracetamol using cobalt tungstate (CoWO4) nanoplates modified electrode. J. Environ. Chem. Eng. 2023, 11, 111208. [Google Scholar] [CrossRef]
- Karami-Kolmoti, P.; Beitollahi, H.; Modiri, S. Electrochemical Sensor for Simple and Sensitive Determination of Hydroquinone in Water Samples Using Modified Glassy Carbon Electrode. Biomedicines 2023, 11, 1869. [Google Scholar] [CrossRef]
- Movahed, V.; Arshadi, L.; Ghanavati, M.; Nejad, E.M.; Mohagheghzadeh, Z.; Rezaei, M. Simultaneous electrochemical detection of antioxidants Hydroquinone, Mono-Tert-butyl hydroquinone and catechol in food and polymer samples using ZnO@MnO2-rGO nanocomposite as sensing layer. Food Chem. 2023, 403, 134286. [Google Scholar] [CrossRef] [PubMed]
- Sumanth, G.; Swamy, B.K.; Chetankumar, K. Poly DY 11/Zn/CuO modified electrochemical sensor for the detection of catechol and hydroquinone: A voltammetric study. Mater. Chem. Phys. 2023, 296, 127349. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; Pan, P.; Wang, Z.; Yang, Z.; Wei, J.; Li, P.; Cao, S.; Shen, H.; Zhou, J.; et al. Electrochemical sensor based on laser-induced preparation of MnOx/rGO composites for simultaneous recognition of hydroquinone and catechol. Microchem. J. 2023, 185, 108234. [Google Scholar] [CrossRef]
- Rajpurohit, N.A.; Kaushalya, B.; Meena, N.; Zhang, T.C.; Dinesh, K. An electrochemical sensor made with thermally regulated sheets of Ce2(WO4)3@rGO nanocomposite for aqueous hydroquinone detection. Microchem. J. 2023, 195, 109458. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; Pan, P.; Wang, Z.; Yang, Z.; Wei, J.; Li, P.; Cao, S.; Shen, H.; Zhou, J.; et al. Effective hydroquinone detection using a manganese stannate/functionalized carbon black nanocomposite. J. Ind. Eng. Chem. 2024, 133, 539–549. [Google Scholar]
- Hasan, I.M.A.; Abd-Elsabour, M.; Assaf, F.H.; Abd-Elsabur, K.M. Green synthesized SiO2/Bi2O3 nanocomposite sensor for catechol and hydroquinone detection in water. Sens. Actuators A Phys. 2024, 372, 115310. [Google Scholar] [CrossRef]
- Ahmed, J.; Faisal, M.; Algethami, J.S.; Rahman, M.M.; Harraz, F.A. Electrochemical detection of hydroquinone as an environmental contaminant using Ga2O3 incorporated ZnO nanomaterial. J. Saudi Chem. Soc. 2024, 28, 101875. [Google Scholar] [CrossRef]
- Thenrajan, T.; Malar, M.M.; Kumaravel, S.; Rajaram, R.; Kundu, S.; Wilson, J. Bismuth tungstate nanocomposites for simultaneous detection of hydroquinone and resorcinol. Mater. Adv. 2024, 5, 1691–1701. [Google Scholar] [CrossRef]
- Li, M.; Dai, Y.; Liu, Z.; Liang, S.; Han, Y.; Fan, L.; Li, Z.; Dong, B.; Guo, Y. Bifunctional Cu0.4Co2.6O4 nanocube induced by Cu substitution with superior peroxidase-like activity: Application in hydroquinone multi-mode detection. Sens. Actuators B Chem. 2025, 425, 136980. [Google Scholar] [CrossRef]
- Achache, M.; Ben Seddik, N.; Bouchta, D.; Draoui, K.; Choukairi, M. NiO nanoparticles modified carbon paste electrode for the voltammetric simultaneous detection of catechol and hydroquinone as environmental pollutants. Microchem. J. 2025, 208, 112578. [Google Scholar] [CrossRef]
- Umar, E.; Iqbal, M.W.; Shaheen, F.; Ullah, H.; Wahab, R.; Hassan, R.U. Synergistic effect of redox-active SrMnO3/g-C3N4 electrode materials for supercapattery hybrid energy storage devices and electrochemical sensing of hydroquinone. J. Power Sources 2025, 650, 236761. [Google Scholar] [CrossRef]
- Promsuwan, K.; Chuenjitt, S.; Kongsuwan, A.; Saichanapan, J.; Soleh, A.; Saisahas, K.; Samoson, K.; Wangchuk, S.; Limbut, W. A disposable dual-mode electrochemical/colorimetric paper-based analytical device for simultaneous detection of hydroquinone and mercury ion. Talanta 2025, 294, 128166. [Google Scholar] [CrossRef]
- Yemmi, R.; Swamy, B.K.; Manjunath, K.M.; Yusuf, K.; Aljuwayid, A.M.; Govindasamy, M. Advanced ZnFe2O4@f-CNF electrode: A robust electrochemical sensor for Tert-butyl hydroquinone detection in food and edible oil. Food Chem. 2025, 486, 144648. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, N.; Duraisamy, V.; Kumar, S.M.S.; Thangamuthu, R. N, S dual doped mesoporous carbon assisted simultaneous electrochemical assay of emerging water contaminant hydroquinone and catechol. Chemosphere 2022, 307, 135771. [Google Scholar] [CrossRef]
- Harikrishnan, K.; Singh, G.; Kushwaha, A.; Singh, V.P.; Gaur, U.K.; Sharma, M. 2D/2D heterojunction of graphitic carbon nitride and hexagonal boron nitride nanosheets mediated electrochemical detection of hazardous hydroquinone with high selectivity and sensitivity. J. Environ. Chem. Eng. 2022, 10, 108717. [Google Scholar] [CrossRef]
- Chen, Y.; He, T.; Liao, D.; Li, Q.; Song, Y.; Xue, H.; Zhang, Y. Carbon aerogels with nickel@N-doped carbon core-shell nanoclusters as electrochemical sensors for simultaneous determination of hydroquinone and catechol. Electrochim. Acta 2022, 414, 140199. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Wu, Q.; Zhu, J.; Chen, S.; Lei, Y.; Li, Y.; Yi, H.; Chen, L.; Shi, Z.-Q.; et al. Simultaneous detection of acetaminophen, catechol and hydroquinone using a graphene-assisted electrochemical sensor. RSC Adv. 2022, 12, 23762–23768. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Preparation of Ni-Zn-doped porous carbon materials (Ni@C) as electrochemical sensor for hydroquinone detection. Int. J. Electrochem. Sci. 2022, 17, 221029. [Google Scholar] [CrossRef]
- Al-Shekaili, A.; Al-Shukaili, W.; Khudaish, E.A. A surface network based on oxidative graphene oxide for the determination of hydroquinone and catechol in ground and wastewater samples. J. Electroanal. Chem. 2022, 919, 116509. [Google Scholar] [CrossRef]
- Rao, Q.; Hu, F.X.; Gan, L.-Y.; Guo, C.; Liu, Y.; Zhang, C.; Chen, C.; Bin Yang, H.; Li, C.M. Boron-Nitrogen-Co-Doping Nanocarbons to Create Rich Electroactive Defects toward Simultaneous Sensing Hydroquinone and Catechol. Electrochim. Acta 2022, 402, 139427. [Google Scholar] [CrossRef]
- Liu, X.; He, F.; Bai, L.; Cao, X.; Liu, C.; Lu, W. A two-dimensional G-CoP/N,P-co-doped carbon nanowire electrode for the simultaneous determination of hydroquinone and catechol in domestic wastewater. Anal. Chim. Acta 2022, 1210, 339871. [Google Scholar] [CrossRef]
- Hu, C.; Huang, H.; Sun, H.; Yan, Y.; Xu, F.; Liao, J. Simultaneous analysis of catechol and hydroquinone by polymelamine/CNT with dual-template molecular imprinting technology. Polymer 2022, 242, 124593. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Yang, Y.; Wang, C.; Wang, Y.; Zhao, P.; Xie, Y.; Fei, J. A novel electrochemical sensor based on mesoporous carbon hollow spheres/ZIF-67-derived Co-embedded N-doped carbon nanotubes composite for simultaneous determination of dihydroxybenzene isomers in environmental water samples. Microchem. J. 2023, 191, 108754. [Google Scholar] [CrossRef]
- Li, J.; Hou, L.; Jiang, Y.; Wei, M.-J.; Kong, F.-Y.; Li, H.-Y.; Wang, Z.-X.; Wang, W. Porphyrin-based covalent organic framework integrated with nitrogen doped carbon nanotube as electrode modifier for selective and simultaneous detection of hydroquinone and catechol with high sensitivity. Microchem. J. 2024, 199, 110007. [Google Scholar] [CrossRef]
- Buoro, R.M.; Almeida, J.M.; Brett, C.M. Cresyl violet electropolymerization on functionalized multiwalled carbon nanotubes in carboxylic acid based ternary deep eutectic solvents for hydroquinone sensing. Electrochim. Acta 2024, 490, 144305. [Google Scholar] [CrossRef]
- Qazi, S.; Shaikh, H.; Solangi, A.R.; Khand, N.H.; Mallah, S.A.; Koondhar, M. Fabrication of poly (quinine-co-itaconic acid) incorporated reduced graphene oxide nanocomposite and its application for electrochemical sensing and photocatalysis of hydroquinone. RSC Adv. 2024, 14, 31057–31071. [Google Scholar] [CrossRef]
- Wahyuni, W.T.; Ta’ALia, S.A.H.; Akbar, A.Y.; Elvira, B.R.; Irkham; Rahmawati, I.; Wahyuono, R.A.; Putra, B.R. Electrochemical sensors based on the composite of reduced graphene oxide and a multiwalled carbon nanotube-modified glassy carbon electrode for simultaneous detection of hydroquinone, dopamine, and uric acid. RSC Adv. 2024, 14, 27999–28016. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Ma, W.; Chen, W.; Tan, H.; Yang, C.; Wang, X.; Yao, H.; Cao, H. Detection of hydroquinone in carboxylated multi-walled carbon nanotube/poly(N,N-dimethylacrylamide) binary structured films and study of logic gate system. Polymer 2025, 321, 128145. [Google Scholar] [CrossRef]
- Jiao, S.; Tang, J.; Li, X.; Zhai, Y.; Li, M. A sensor based on Mo, N, S-doped interconnected porous carbon spheres material for simultaneous determination of hydroquinone and catechol. Microchem. J. 2025, 211, 113065. [Google Scholar] [CrossRef]
- Brincoveanu, O.; Geana, E.-I.; Romanitan, C.; Pachiu, C.; Mocanu, A.; State, S.; Ghebaur, A.; Kurbanoglu, S.; Marolt, G.; Dinu, L.A. Prussian blue nanocubes growth by electrochemical deposition on sulfur-doped graphene as nanozyme: Optimization and application in the field of environmental sensors. Appl. Surf. Sci. Adv. 2025, 26, 100716. [Google Scholar] [CrossRef]
- Cheng, X.; Shui, X.; Yang, Q.; Ma, H.; Zhang, Y.; Zeng, T.; Yang, J.; Wu, Z.; Zhang, X.; Yang, N. Highly selective electrochemical sensing of hydroquinone and catechol using Co nanoparticles anchored on N-doped carbon nanotube hollow sphere. Anal. Chim. Acta 2025, 1357, 344074. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, F.; Zou, Q.; Zhang, Y.; Xie, Y.; Ouyang, Y.; Fei, J.; Zhang, L. A novel electrochemical sensor based on a hybrid composed of CoZn/N-doped porous carbon for the simultaneous determination of catechol and hydroquinone. Microchem. J. 2025, 212, 113416. [Google Scholar] [CrossRef]
- Rong, C.; Huang, Y.; Zheng, X. Sensitive determination of hydroquinone and catechol using an electrochemical sensor based on nitrogen-doped malic acid carbon quantum dots. Mater. Chem. Phys. 2025, 329, 130077. [Google Scholar] [CrossRef]
- Jahani, S.; Sedighi, A.; Toolabi, A.; Foroughi, M.M. Development and characterization of La2O3 nanoparticles@snowflake-like Cu2S nanostructure composite modified electrode and application for simultaneous detection of catechol, hydroquinone and resorcinol as an electrochemical sensor. Electrochim. Acta 2022, 416, 140261. [Google Scholar] [CrossRef]
- Ganesh, P.S.; Dhand, V.; Kim, S.-Y.; Kim, S. Design and synthesis of active site rich cobalt tin sulfide nano cubes: An effective electrochemical sensing interface to monitor environmentally hazardous phenolic isomers. Microchem. J. 2024, 200, 110308. [Google Scholar] [CrossRef]
- Jiang, X.; Yuan, Y.; Zhao, X.; Wan, C.; Duan, Y.; Wu, C. Microbial synthesis of antimony sulfide to prepare catechol and hydroquinone electrochemical sensor by pyrolysis and carbonization. Environ. Res. 2024, 252, 118860. [Google Scholar] [CrossRef]
- Yuan, X.; Jin, Y.; Xu, L.; Ou, L.; Guo, J.; Wang, Q.; Xiong, X. Sulfur vacancy-rich CuS nanoparticles anchored on N-doped carbon nanofibers for electrochemical and colorimetric dual-mode sensing of hydroquinone. Microchem. J. 2025, 209, 112629. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Vilian, A.E.; Ghoreishian, S.M.; Umapathi, R.; Hwang, S.-K.; Oh, C.W.; Huh, Y.S.; Han, Y.-K. Hybridized 1D–2D MnMoO4–MXene nanocomposites as high-performing electrochemical sensing platform for the sensitive detection of dihydroxybenzene isomers in wastewater samples. J. Hazard. Mater. 2022, 421, 126775. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, S.; Zhang, L.; Li, F.; Wang, J.; Pan, H.; Zhang, D. In situ growth of ZIF-67-derived nickel-cobalt-manganese hydroxides on 2D V2CTx MXene for dual-functional orientation as high-performance asymmetric supercapacitor and electrochemical hydroquinone sensor. J. Colloid Interface Sci. 2023, 629, 546–558. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Li, S.; Li, F.; Xin, J.; Wang, X.; Liang, M. Controllable selenization strategy for in-situ construction of V2CTx MXene-based multiphase electrode materials for simultaneous detection of hydroquinone and catechol in drinking water and beverages. Food Chem. 2025, 483, 144234. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Zhu, Y.; Zhou, J.; Wang, L.; Zeng, L.; Li, L.; He, S.; Sun, W. Shrimp shell-derived porous carbon and Cu-melamine metal-organic frameworks composite modified electrode for sensitive electrochemical determination of hydroquinone. Int. J. Electrochem. Sci. 2022, 17, 221241. [Google Scholar] [CrossRef]
- Liu, B.; Guo, H.; Sun, L.; Pan, Z.; Peng, L.; Wang, M.; Wu, N.; Chen, Y.; Wei, X.; Yang, W. Electrochemical sensor based on covalent organic frameworks/MWCNT for simultaneous detection of catechol and hydroquinone. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128335. [Google Scholar] [CrossRef]
- Sun, L.; Guo, H.; Pan, Z.; Liu, B.; Zhang, T.; Yang, M.; Wu, N.; Zhang, J.; Yang, F.; Yang, W. In-situ reducing platinum nanoparticles on covalent organic framework as a sensitive electrochemical sensor for simultaneous detection of catechol, hydroquinone and resorcinol. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128114. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Dou, N.; Qu, J. Sensitive simultaneous determination of catechol and hydroquinone based on iron and nitrogen doped carbon nanonets derived from MOFs. J. Electroanal. Chem. 2022, 913, 116290. [Google Scholar] [CrossRef]
- Levshakova, A.; Kaneva, M.; Borisov, E.; Panov, M.; Shmalko, A.; Nedelko, N.; Mereshchenko, A.S.; Skripkin, M.; Manshina, A.; Khairullina, E. Simultaneous Catechol and Hydroquinone Detection with Laser Fabricated MOF-Derived Cu-CuO@C Composite Electrochemical Sensor. Materials 2023, 16, 7225. [Google Scholar] [CrossRef]
- Liao, Y.; Lin, L.; Liu, J.; Zhang, X.; Li, X. Ultrasensitive simultaneous determination of hydroquinone and catechol by Ni3ZnC0.7/Ni porous composites derived from Ni/Zn-MOF. J. Electroanal. Chem. 2024, 954, 118028. [Google Scholar] [CrossRef]
- Niu, Y.; Kang, K.; Wang, B.; Wang, L.; Li, C.; Gao, X.; Zhao, Z.; Ji, X. Ultrasensitive electrochemical sensing of catechol and hydroquinone via single-atom nanozyme anchored on MOF-derived porous carbon. Talanta 2024, 268, 125349. [Google Scholar] [CrossRef]
- Rajpurohit, N.A.; Agrawal, H.; Bhakar, K.; Panchal, K.; Kumar, D. A stable and efficient electrochemical sensor for hydroquinone and catechol detection in real-world water samples using mesoporous CaM@rGO nanocomposite. Sens. Actuators B Chem. 2024, 420, 136481. [Google Scholar] [CrossRef]
- Wang, X.; Fan, L.; Zhang, X.; Liu, J.; Chen, J.; Fan, Y.; Du, X.; Lu, X. Bifunctional ZIF-8/carbon black modified MXene with honeycomb sandwich structure for simultaneous electrochemical sensing of hydroquinone and catechol. Talanta 2025, 288, 127721. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liao, D.; Yu, J.; Jiang, X. An electrochemical sensor based on oxygen-vacancy cobalt–aluminum layered double hydroxides and hydroxylated multiwalled carbon nanotubes for catechol and hydroquinone detection. Microchem. J. 2022, 175, 107216. [Google Scholar] [CrossRef]
- He, H.; Lv, S.; Kang, Y.; Yi, J.; Zhang, Y.; Cong, Y. In situ preparation of NiCoFe-LDH nanoflowers on carbon cloth toward simultaneous detecting hydroquinone and catechol. J. Electroanal. Chem. 2022, 919, 116540. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Heydarzad, M. Development of novel electrochemical sensor based on Co-Fe layered double hydroxide for simultaneous determination of Hydroquinone, Catechol, and Resorcinol. Microchem. J. 2024, 206, 111437. [Google Scholar] [CrossRef]
- Shan, Y.; Han, Y.; Yao, X.; Liu, T.; Liu, Y.; Chu, Z.; Jin, W. A novel Prussian blue/PANI nanostructure-based biosensor for ultrasensitive determination of trace hydroquinone. Sens. Actuators B Chem. 2023, 393, 134137. [Google Scholar] [CrossRef]
- Zuo, J.; Shen, Y.; Wang, L.; Yang, Q.; Cao, Z.; Song, H.; Ye, Z.; Zhang, S. Flexible electrochemical sensor constructed using an active copper center instead of unstable molybdenum carbide for simultaneous detection of toxic catechol and hydroquinone. Microchem. J. 2023, 187, 108443. [Google Scholar] [CrossRef]
- Saleem, Q.; Shahid, S.; Javed, M.; Iqbal, S.; Rahim, A.; Mansoor, S.; Bahadur, A.; Nasser, S.; Awwad Hala, A.; Ibrahium Rasmiah, S.; et al. Synchronized electrochemical detection of hydroquinone and catechol in real water samples using a Co@SnO2–polyaniline composite. RSC Adv. 2023, 13, 10017–10028. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, S.; Zhang, L.; Li, F.; Pan, H.; Zhang, D. Design and construction of conductive polymer PPy anchored NiCo bi-metal sulfide composite electrode materials for high-performance hybrid supercapacitor and electrochemical hydroquinone sensor. J. Energy Storage 2024, 87, 111427. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, L.; Li, S.; Li, F.; Han, Y.; Yu, T.; Lin, Z. In-situ polymerization strategy for conductive polymer poly(3,4-ethylenedioxythiophene) on bimetallic MOF to enhance electrochemical detection of toxic hydroquinone in industrial wastewater and tap water. Colloids Surf. A Physicochem. Eng. Asp. 2025, 717, 136793. [Google Scholar] [CrossRef]
- Chuenjitt, S.; Kongsuwan, A.; Phua, C.H.; Saichanapan, J.; Soleh, A.; Saisahas, K.; Samoson, K.; Wangchuk, S.; Promsuwan, K.; Limbut, W. A poly(neutral red)/porous graphene modified electrode for a voltammetric hydroquinone sensor. Electrochim. Acta 2022, 434, 141272. [Google Scholar] [CrossRef]
- Yang, X.; He, C.; Lin, W.; Qiu, Y.; Li, P.; Chen, Y.; Huang, B.; Zheng, X. Electrochemical sensors for hydroquinone and catechol based on nano-flake graphite and activated carbon sensitive materials. Synth. Met. 2022, 287, 117079. [Google Scholar] [CrossRef]
- Deng, P.; Zhou, C.; Wei, Y.; Yue, X.; Li, J.; Yao, L.; Ding, J.; He, Q. Salicylaldehyde functionalized chitosan for electrochemical sensitive sensor: Simultaneous determination of catechol and hydroquinone. J. Electroanal. Chem. 2022, 918, 116506. [Google Scholar] [CrossRef]
- Fan, Z.-C.; Li, Z.; Wei, X.-Y.; Kong, Q.-Q.; Liu, Z.-Q.; Li, L.; Li, J.-H.; Yin, F.; Lu, K.-L.; Zong, Z.-M. Longquan lignite-derived hierarchical porous carbon electrochemical sensor for simultaneous detection of hazardous catechol and hydroquinone in environmental water samples. Microchem. J. 2022, 182, 107880. [Google Scholar] [CrossRef]
- Maciel, C.C.; de Lima, L.F.; Ferreira, A.L.; de Araujo, W.R.; Ferreira, M. Development of a flexible and disposable electrochemical sensor based on poly (butylene adipate-co-terephthalate) and graphite for hydroquinone sensing. Sens. Actuators Rep. 2022, 4, 100091. [Google Scholar] [CrossRef]
- Cong, W.; Song, P.; Zhang, Y.; Yang, S.; Liu, W.; Zhang, T.; Zhou, J.; Wang, M.; Liu, X. Supramolecular confinement pyrolysis to carbon-supported Mo nanostructures spanning four scales for hydroquinone determination. J. Hazard. Mater. 2022, 437, 129327. [Google Scholar] [CrossRef] [PubMed]
- Naik, T.S.S.K.; Kesavan, A.V.; Swamy, B.E.K.; Singh, S.; Amith, G.; Anil, V.; Madhavi, P. Ramamurthy, Low cost, trouble-free disposable pencil graphite electrode sensor for the simultaneous detection of hydroquinone and catechol. Mater. Chem. Phys. 2022, 278, 125663. [Google Scholar] [CrossRef]
- Mia, M.; Islam, S.; Alfasane, A.; Abu Nayem, S.; Awal, A.; Shah, S.S.; Dafalla, H.D.M.; Aziz, A.; Ahammad, A.S. Sargassum coriifolium seaweed derived sub-micro sphere carbon for simultaneous electrochemical detection of hydroquinone and catechol: Mechanistic and kinetic studies. Mater. Chem. Phys. 2023, 309, 128439. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Li, Y.; Shi, F.; Ai, Y.; Wang, B.; Zhang, S.; Zhang, X.; Sun, W. Electrochemical detection of hydroquinone based on marine biomass carbon from shrimp shells as electrode modifier. Int. J. Electrochem. Sci. 2023, 18, 100063. [Google Scholar] [CrossRef]
- Fan, Z.-C.; Li, Z.; Wei, X.-Y.; Kong, Q.-Q.; Zhao, J.; Li, L.; Li, J.-H.; Liu, Z.-Q.; Zong, Z.-M. Porous carbon fabricated by a residue from Longquan lignite ethanolysis as an electrochemical sensor for simultaneous detection of hydroquinone and catechol in the presence of resorcinol. Microchem. J. 2023, 189, 108543. [Google Scholar] [CrossRef]
- Xue, Y.; Noroozifar, M.; May, R.A.; Sullan Kerman, K. Electrochemical simultaneous determination of hydroquinone, catechol, bisphenol A, and bisphenol S using a novel mesoporous nickel-modified carbon sensor. Chemosphere 2023, 342, 140003. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, X.; Mao, J.; He, S.; Cao, Z. Facile preparation of gold nanoparticles anchored on layered yttrium hydroxide by electrochemical methods for enhanced sensing of hydroquinone and catechol. Mater. Chem. Phys. 2024, 331, 128526. [Google Scholar] [CrossRef]
- Kaneva, M.; Levshakova, A.; Tumkin, I.; Fatkullin, M.; Gurevich, E.; Manshina, A.; Rodriguez, R.D.; Khairullina, E. Simultaneous electrochemical detection of hydroquinone and catechol using flexible laser-induced metal-polymer composite electrodes. Microchem. J. 2024, 204, 111106. [Google Scholar] [CrossRef]
- Trachioti, M.G.; Ch Lazanas, A.; Prodromidis, M.I. Cooperative interaction of Ketjen black coating and electrical discharge post-treatment towards advanced screen-printed electrodes for hydroquinone and catechol. Sens. Actuators B Chem. 2024, 401, 134947. [Google Scholar] [CrossRef]
- Puneeth Swamy, B.E.K.; Sharma, S.C. Environmental contaminants detection by disposable burnt carving-based graphite pencil electrode for hydroquinone and catechol. Microchem. J. 2025, 213, 113554. [Google Scholar] [CrossRef]
- Achache, M.; Bouchta, D.; Draoui, K.; Choukairi, M. Low-cost electrochemical sensor based on montmorillonite for the simultaneous detection of phenolic pollutants in tap and bottled water. J. Environ. Chem. Eng. 2025, 13, 115981. [Google Scholar] [CrossRef]
- Zheng, S.; Tang, W.; Bao, R.; Cui, S.; Yin, F.; Zhang, E.; Tang, J.; Guo, J.; Wang, X. Metal−Organic Frameworks-derived CoP/Co2P@NC for simultaneous detection of dihydroxybenzene isomers, Colloids Surf. A Physicochem. Eng. Asp. 2025, 707, 135928. [Google Scholar] [CrossRef]
- Yin, D.; Liu, J.; Bo, X.; Guo, L. Cobalt-iron selenides embedded in porous carbon nanofibers for simultaneous electrochemical detection of trace of hydroquinone, catechol and resorcinol. Anal. Chim. Acta 2020, 1093, 35–42. [Google Scholar] [CrossRef]
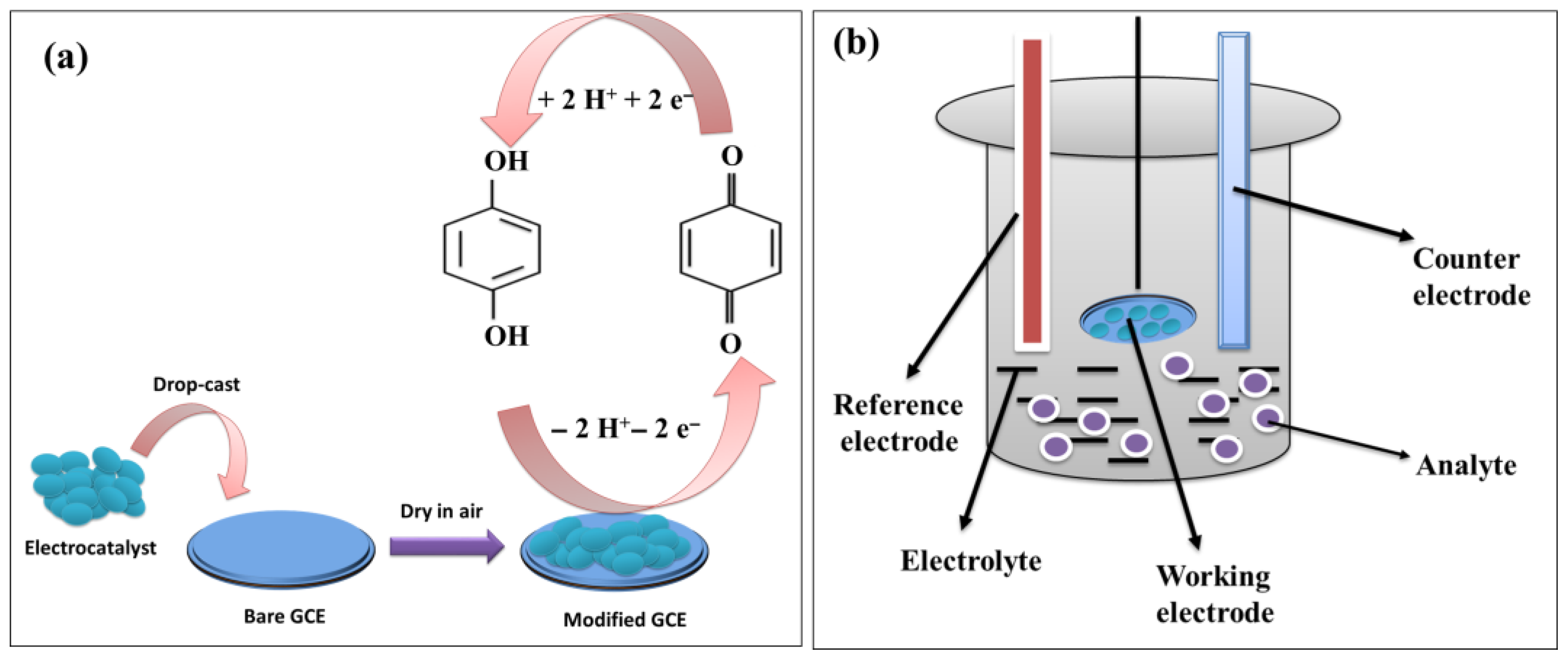
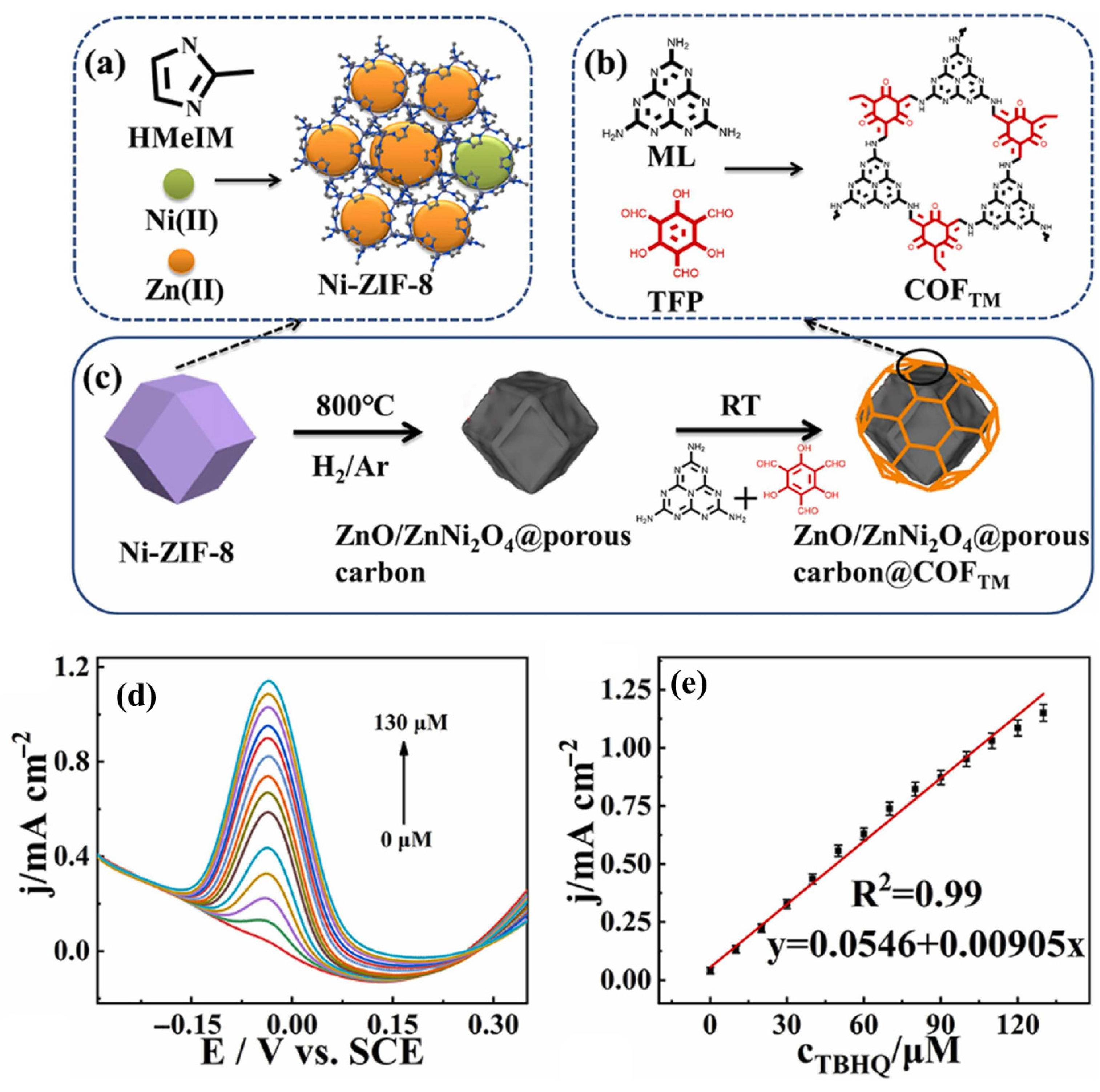




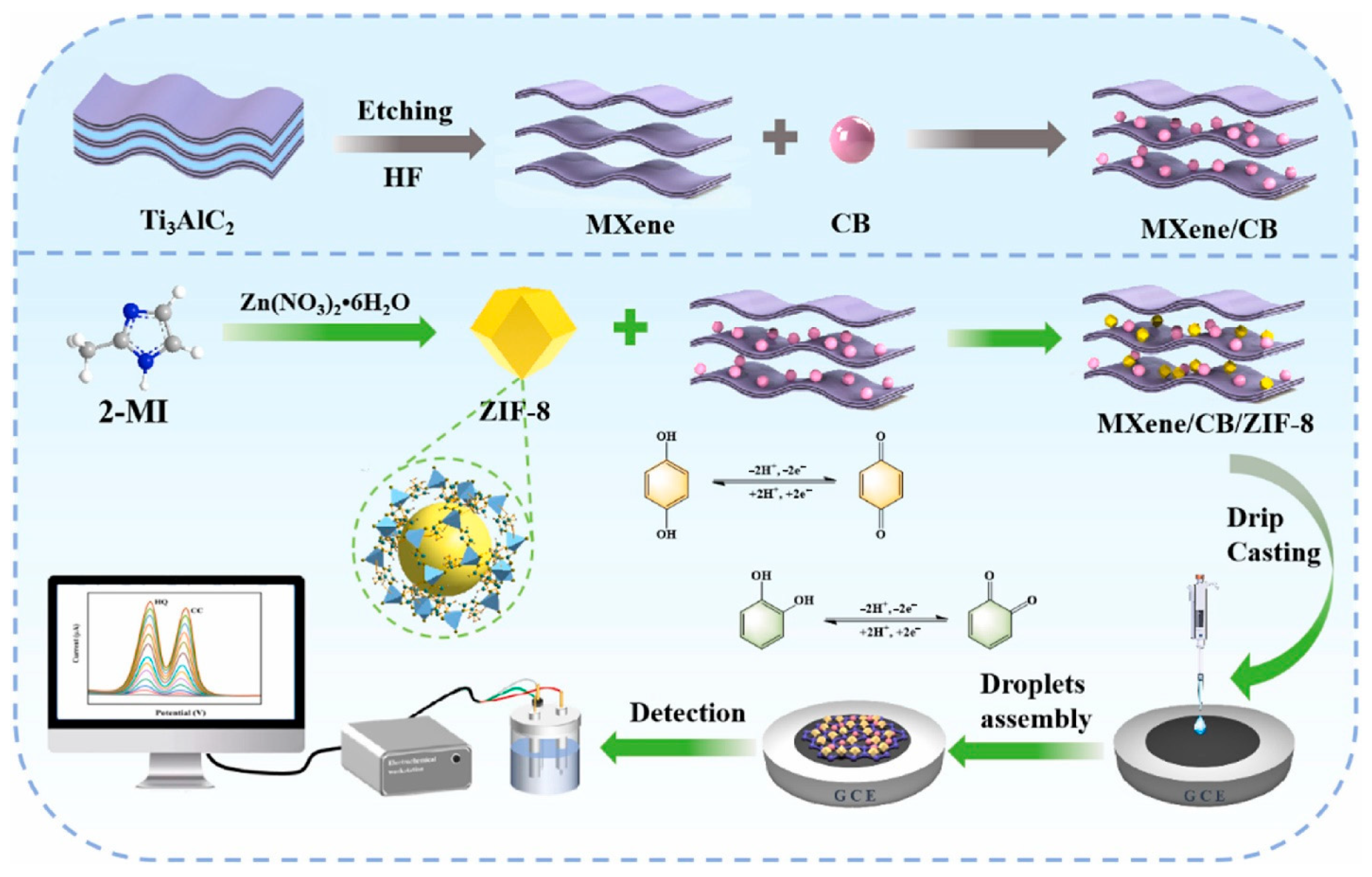


| Electrocatalyst | LOD (µM) | Sensitivity | LR (µM) | Technique | Real Sample | References |
|---|---|---|---|---|---|---|
| Zn@ZnO core shell | 0.10 | 0.5673 μA μM−1 cm−2 | 10–90 | CV | - | [22] |
| N-rGO/CuO-ILCPE | 0.25 | - | 1–600 | DPV | Tap and river water | [24] |
| MoO3@KSC | 0.063 | - | 5 to 176.8 | DPV | River and lake water | [26] |
| CoWO4 nanoplates | 0.00221 | 57.37 μA mM−1 cm−2 | 0.02–0.1 and 0.12–0.32 | Amp | - | [28] |
| ZnO@MnO2-rGO | 0.0012 | - | 0.008–10 and 10–320 | DPV | Soyabean oil, tap water, orange juice, and river water | [30] |
| MnOx/rGO | 0.388 | - | 20–300 | DPV | Lake water | [32] |
| SiO2/Bi2O3 | 0.00075 | - | 0.005–5 | DPV | River, tap, and drinking water | [35] |
| Bi2WO6 | 57 | - | 200–5000 | SWV | Ointment | [37] |
| SrMnO3/g-CN | 6.32 | 0.214 μA μM−1 cm−2 | 1–600 | DPV | Wastewater and tap water | [40] |
| ZnFe2O4@f-CNF | 0.026 | - | 0.2–4013 | DPV | Edible oil and cake | [42] |
| g-CN/BN(1:1) | 0.00923 | - | 0.02–0.08 and 009–0.17 | DPV | Tap water and industrial wastewater | [44] |
| Oxidative graphene oxide (OGO) | 0.114 | - | - | DPV | Ground and wastewater | [48] |
| MIP/MWCNTs | 3.1 | - | 10–100 | DPV | River water | [51] |
| TT-COF(Co)/N-CNTs | 0.81 | - | 0.003–300 | DPV | Lake water | [53] |
| Poly (quinine-co-itaconic acid)@rGO | 0.03 | - | 0.1–40 | DPV | Industrial water, river water, and cream | [55] |
| Mo, N, S-IPCS | 0.047 | - | 5–10,000 | i-t | River water | [58] |
| Co/HNC | 0.023 | - | 1–100 | DPV | Lake water, tap water, orange juice, detergents, and ointment | [60] |
| N-MCQDs | 0.18 | - | 1–500 | DPV | Lake water | [62] |
| CoSnS2 | 0.0125 | - | 5–135 | DPV | Tap water | [64] |
| CuS/N-CNFs | 0.293 | - | 1–614 | Amp | Landfill leachate, storm drain water, and textile mill wastewater | [66] |
| V2CTx@NiCoMn-OH | 0.559 | - | 2–1050 | DPV | Tap water | [68] |
| SSPC/Cu-Me | 1.83 | - | 5–1800 | DPV | Lake water | [70] |
| TFPB-BD-COF/PtNPs/NH2-MWCNTs | 0.022 | - | 0.2–360 | DPV | Tap water, river water, and sanitary sewage | [72] |
| Ni3ZnC0.7/Ni | 0.14 | - | 0.3–100 | DPV | Tap and river water | [75] |
| MXene/CB/ZIF-8 | 0.021 | - | 0.3–160 | DPV | Upstream, midstream, and downstream water | [78] |
| NiCoFe-LDH NFs | 0.15 | - | 5–200 | DPV | Tap and lake water | [80] |
| Cu-CuO@MoOx-PEDOT/PET | 0.221 | - | 5–870 | i-t | Tap water | [83] |
| PPy@NCS-5 | 0.026 | 0.026 μA/μM | 0.5–1923 | DPV | Oilfield wastewater | [85] |
| NFG-BAC | 0.4 | - | 2–1000 | DPV | Lake water | [88] |
| PC/LLSP800 | 0.16 | - | 19.9–318.3 | DPV | Environmental water sample | [90] |
| Pre-treated PGE | 0.85 | - | 40–102 | DPV | Tap water | [93] |
| SSPC | 0.18 | - | 5–25 and 25–1200 | DPV | Lake water | [95] |
| AuNPs/LYH-47 | 0.2 | 1–100 | DPV | Tap and river water | [98] | |
| Sparked KB | 0.04 | - | 0.05–100 | DPV | Tap water | [100] |
| MWNTs-IL-Gel | 0.06 | - | 0.2–35 | DPV | Tap water and mineral bottle water | [102] |
| CoP/Co2P@NC | 0.02 | - | 0.05–80 | DPV | River and lake water | [103] |
| CoFe2Se4/PCF-2 | 0.13 | - | 0.5–200 | DPV | Lake water | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, M.; Ahmad, K.; Ali, S.; Hamdy, K.; Danishuddin. Recent Progress in Electrocatalysts for Hydroquinone Electrochemical Sensing Application. Biosensors 2025, 15, 488. https://doi.org/10.3390/bios15080488
Aslam M, Ahmad K, Ali S, Hamdy K, Danishuddin. Recent Progress in Electrocatalysts for Hydroquinone Electrochemical Sensing Application. Biosensors. 2025; 15(8):488. https://doi.org/10.3390/bios15080488
Chicago/Turabian StyleAslam, Mohammad, Khursheed Ahmad, Saood Ali, Khaled Hamdy, and Danishuddin. 2025. "Recent Progress in Electrocatalysts for Hydroquinone Electrochemical Sensing Application" Biosensors 15, no. 8: 488. https://doi.org/10.3390/bios15080488
APA StyleAslam, M., Ahmad, K., Ali, S., Hamdy, K., & Danishuddin. (2025). Recent Progress in Electrocatalysts for Hydroquinone Electrochemical Sensing Application. Biosensors, 15(8), 488. https://doi.org/10.3390/bios15080488






