AI-Empowered Electrochemical Sensors for Biomedical Applications: Technological Advances and Future Challenges
Abstract
1. Introduction
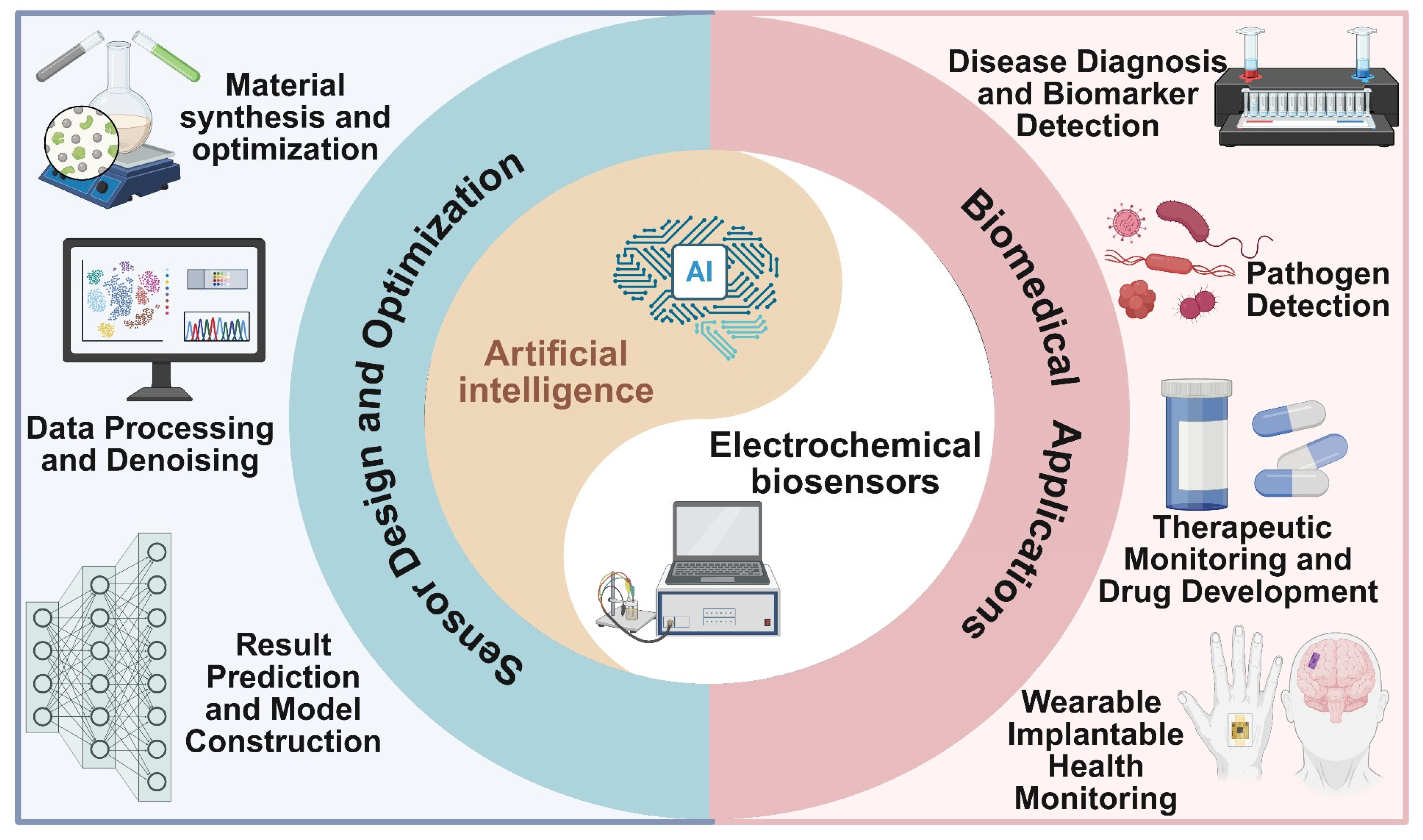
2. Key AI Technologies in Electrochemical Sensors
2.1. Sensor Design and Material Optimization
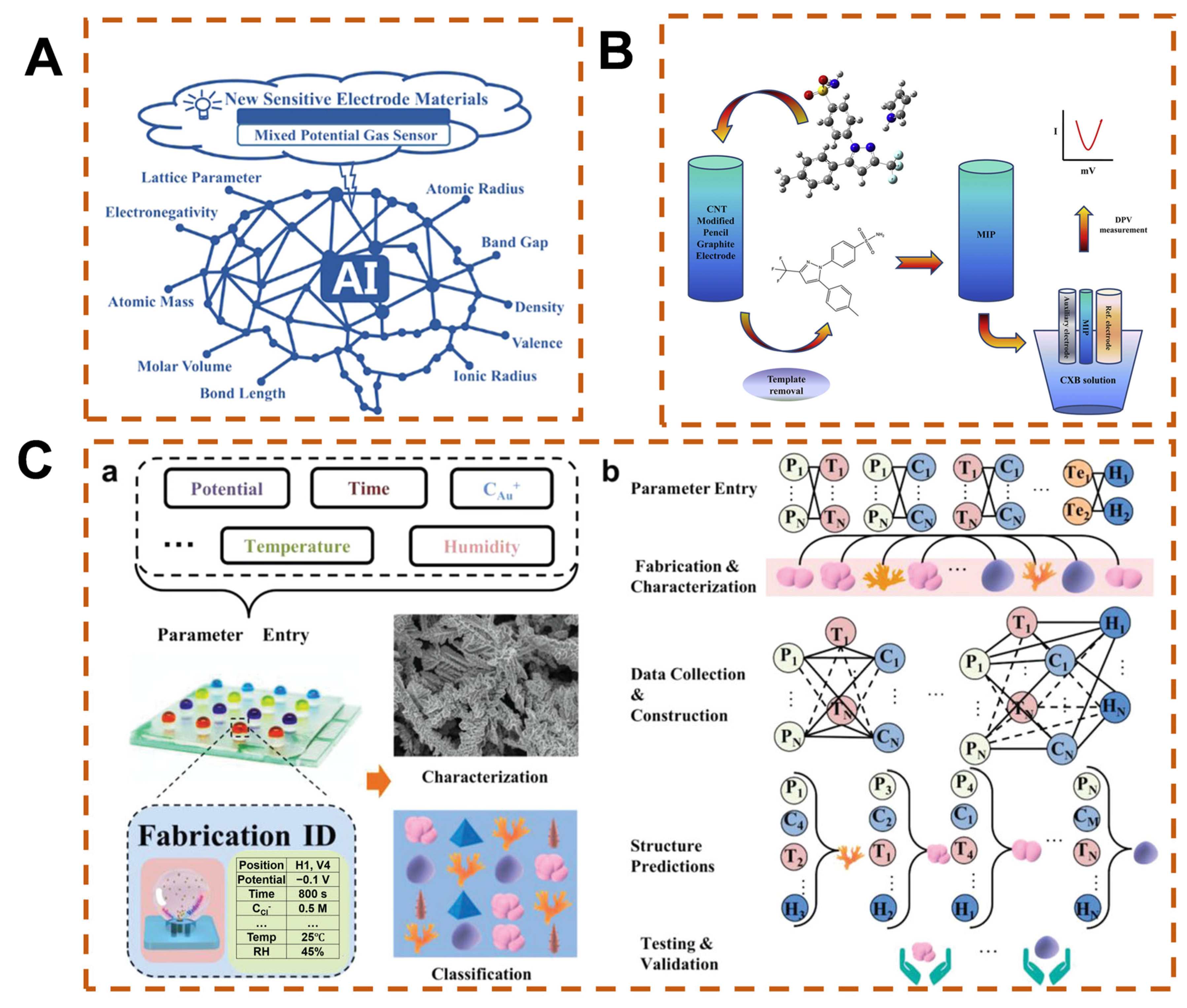
2.2. Data Processing and Denoising
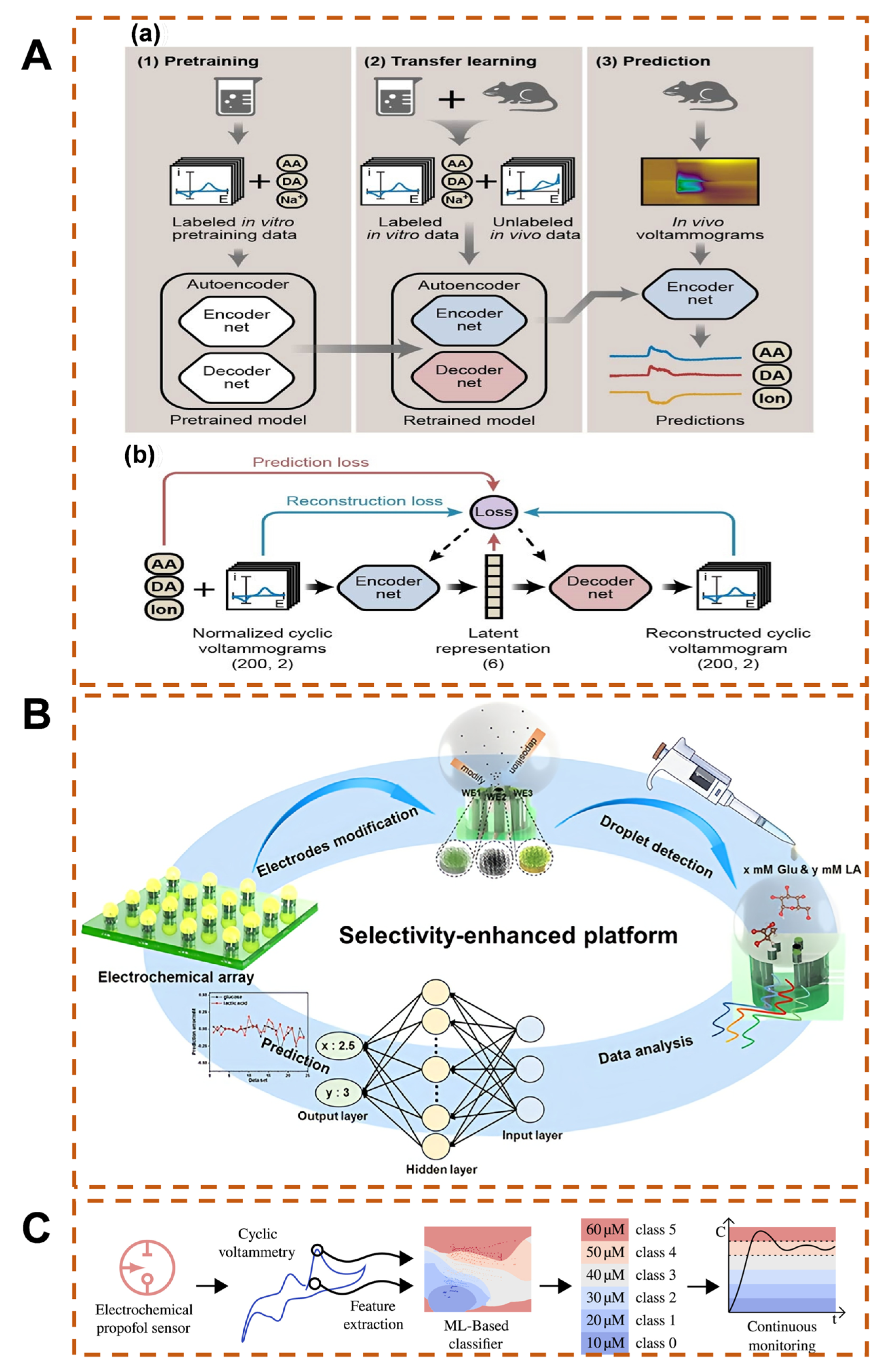
2.3. Result Prediction and Model Construction
3. Key Biomedical Applications of AI-Enabled Electrochemical Sensors
3.1. Disease Diagnosis and Biomarker Detection
3.2. Infectious Diseases and Pathogen Detection
3.3. Therapeutic Monitoring and Drug Development
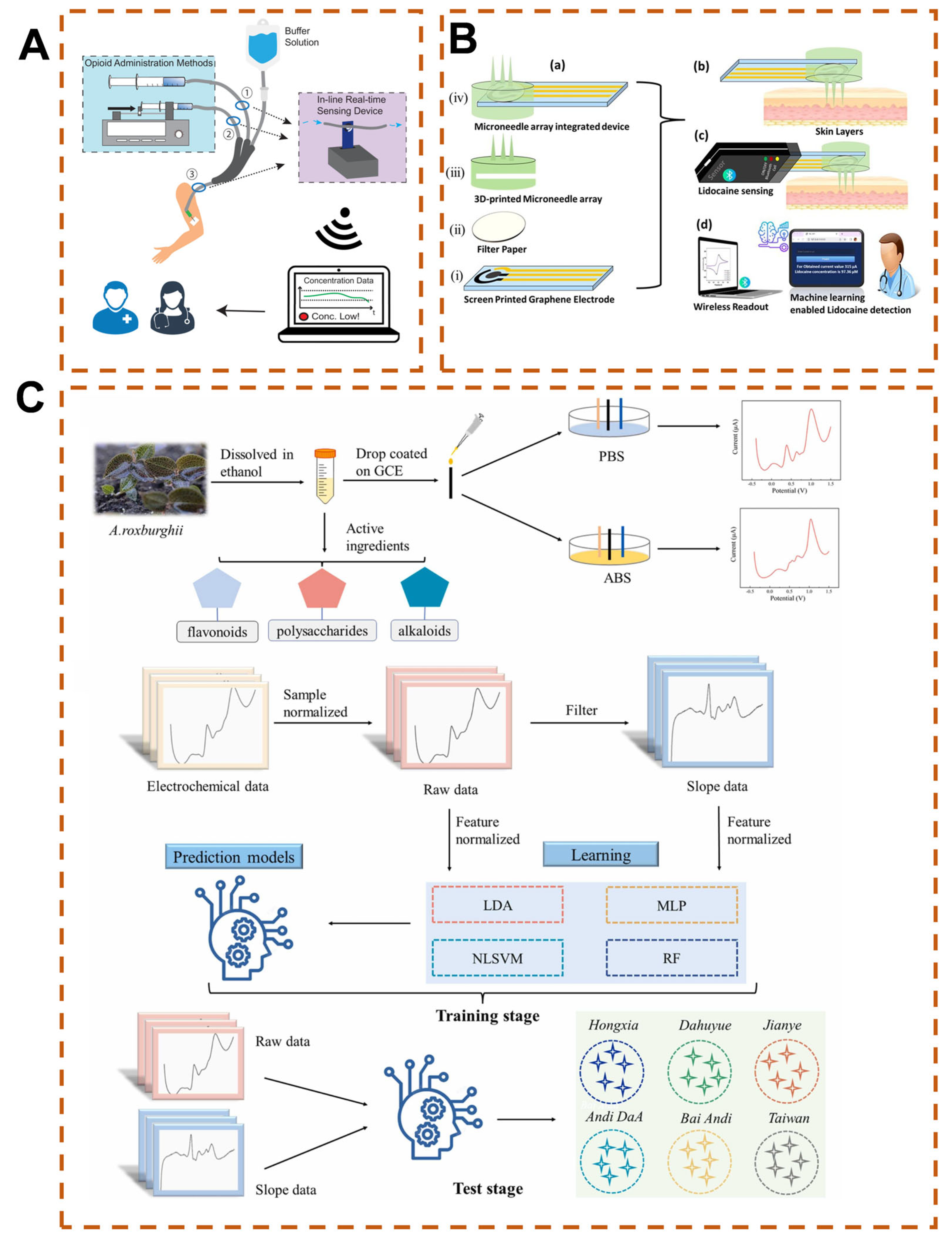
3.4. Wearable and Implantable Health Monitoring

4. Summary and Future Perspectives
- (1)
- Developing self-calibrating electrode materials and biomimetic interfaces, coupled with federated learning to optimize cross-scenario data utilization;
- (2)
- Advancing lightweight NNs and edge computing chips to enable localized real-time analysis in implantable devices;
- (3)
- Integrating electrochemical signals with genomic/proteomic data to construct multimodal AI diagnostic models;
- (4)
- Combining microfluidic technologies with flexible electronics to develop “detection-feedback-therapy” closed-loop systems and intelligent drug delivery systems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| COVID-19 | coronavirus disease 2019 |
| SERS | surface-enhanced Raman spectroscopy |
| FDA | Food and Drug Administration |
| ML | machine learning |
| SVM | support vector machine |
| PPE | poly(p-aryl acetylene) |
| AIE | aggregation-induced emission |
| RF | random forest |
| PCA | principal component analysis |
| HCA | hierarchical cluster analysis |
| LDA | linear discriminant analysis |
| DL | deep learning |
| ANNs | artificial neural networks |
| DNN | deep neural network |
| NNs | neural networks |
| MLP | multi-layer perceptron |
| CNN | convolutional neural network |
| RNN | recurrent neural network |
| MIPs | molecularly imprinted polymers |
| KNN | k-nearest neighbors |
| SNR | signal-to-noise ratio |
| POCT | point-of-care testing |
| BPNN | backpropagation neural network |
| RBF-SVC | radial basis function support vector classifier |
| SISSO | Sure Independence Screening and Sparsifying Operator |
| EIS | electrochemical impedance spectroscopy |
| IVD | in vitro diagnostics |
| RBF | radial basis function |
| VOCs | volatile organic compounds |
| LOD | limit of detection |
| RBF-PLS | radial basis function–partial least squares |
| LS-SVM | least squares–support vector machine |
| RBF-ANN | radial basis function–artificial neural network |
| AMR | antimicrobial resistance |
| RBD | receptor-binding domain |
| IoT | Internet of Things |
| LIGE | laser-induced graphene electrode |
| PVA | polyvinyl alcohol |
References
- Beer, L.; Tie, Y.; Crim, S.M.; Weiser, J.; Taussig, J.; Craw, J.A.; Buchacz, K.A.; Dobbs, A.; Collins, C.B.; Johnston, M.E.; et al. Progress toward achieving national HIV/AIDS strategy goals for quality of life among persons aged ≥50 years with diagnosed HIV—Medical monitoring project, united states, 2017–2023. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Deng, Y.; Zhao, Z.; Mao, B.; Lu, M.; Lin, Y.; Huang, A. Characterization of SARS-CoV-2-specific humoral immunity and its potential applications and therapeutic prospects. Cell. Mol. Immunol. 2022, 19, 150–157. [Google Scholar] [CrossRef]
- Li, X.; Shen, M.; Yang, J.; Liu, L.; Yang, Y. Pillararene-based stimuli-responsive supramolecular delivery systems for cancer therapy. Adv. Mater. 2024, 36, e2313317. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, Z. Progress and challenges in NCD prevention and control in China. BMJ 2024, 387, q2098. [Google Scholar] [CrossRef]
- Nielsen, S.K.N.; Lamberts, M.L.; Nouhravesh, N.N.; Jensen, M.H.J.; Gislason, G.G.; Holt, A.H. Temporal trends of first time antihypertensive treatment among citizens over 75 years—A register-based, cohort study. Eur. Heart J. 2023, 44, 2. [Google Scholar] [CrossRef]
- Danpanichkul, P.; Pang, Y.; Inkongngam, T.; Namsathimaphorn, K.; Rakwong, K.; Kaeosri, C.; Nah, B.; Duangsonk, K.; Tang, N.S.Y.; Mittal, N.; et al. Older adults living with gastrointestinal cancers in 2021. Cancer Commun. 2025, 6, 658–662. [Google Scholar] [CrossRef]
- Bateman, R.J.; Horie, K.; Hansson, O. A fluid biomarker accurately detects tau aggregate pathology in Alzheimer’s disease. Nat. Med. 2023, 29, 1912–1913. [Google Scholar] [CrossRef] [PubMed]
- Mcmackin, R.; Bede, P.; Ingre, C.; Malaspina, A.; Hardiman, O. Biomarkers in amyotrophic lateral sclerosis: Current status and future prospects. Nat. Rev. Neurol. 2023, 19, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, L.; Zheng, H.; Lin, W. Photoacoustic probes for inflammation-related biomarker imaging: Mechanisms, design, and applications. Coord. Chem. Rev. 2024, 517, 215975. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, J.; Zhao, L.; Yang, L.; Fa, H.; Wang, Y.; Huo, D.; Hou, C.; Zhong, D.; Yang, M. MBene nanosheets with DNA adsorbability for circulating tumor DNA assay via fluorescence biosensing and paper-based microfluidic POCT. Adv. Funct. Mater. 2025, 35, 2415074. [Google Scholar] [CrossRef]
- Kakkar, P.; Kakkar, T.; Nampi, P.P.; Jose, G.; Saha, S. Upconversion nanoparticle-based optical biosensor for early diagnosis of stroke. Biosens. Bioelectron. 2025, 275, 117227. [Google Scholar] [CrossRef]
- Daya, N.R.; Fang, M.; Shin, J.; Pankow, J.S.; Lutsey, P.L.; Valint, A.; Echouffo-Tcheugui, J.B.; Zeger, S.; Selvin, E. Detecting hyperglycemia using biomarkers versus continuous glucose monitoring. Diabetes Care 2025, 48, 1446–1452. [Google Scholar] [CrossRef]
- Wulff, A.B.; Nordestgaard, B.G. Residual cardiovascular risk beyond low-density lipoprotein cholesterol: Inflammation, remnant cholesterol, and lipoprotein (a). Eur. Heart J. 2025, 3, ehaf274. [Google Scholar] [CrossRef]
- Lin, J.; Koenig, W.; Zeller, T.; Peters, A.; Thorand, B. Longitudinal and cross-sectional associations of NT-proBNP with kidney function and chronic kidney disease: Results from KORA cohort studies. Eur. Heart J. 2024, 45, 2. [Google Scholar] [CrossRef]
- Gilboa, T.; Maley, A.M.; Ogata, A.F.; Wu, C.; Walt, D.R. Sequential protein capture in multiplex single molecule arrays: A strategy for eliminating assay cross-reactivity. Adv. Healthc. Mater. 2021, 10, e2001111. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and microfluidic biosensors: From fabrication to application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Cao, M.; Cheng, H.; Wang, Y.; He, C.; Shi, X.; Li, T.; Li, Z. Plasmon-stimulated colorimetry biosensor array for the identification of multiple metabolites. ACS Appl. Mater. Interfaces 2024, 16, 6849–6858. [Google Scholar] [CrossRef] [PubMed]
- Toma, K.; Iwasaki, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Sensitive and selective methanol biosensor using two-enzyme cascade reaction and fluorometry for non-invasive assessment of intestinal bacteria activity. Biosens. Bioelectron. 2021, 181, 113136. [Google Scholar] [CrossRef]
- Zhang, X.; Gan, T.; Xu, Z.; Zhang, H.; Wang, D.; Zhao, X.; Huang, Y.; Liu, Q.; Fu, B.; Dai, Z.; et al. Immune-like sandwich multiple hotspots SERS biosensor for ultrasensitive detection of NDKA biomarker in serum. Talanta 2024, 271, 125630. [Google Scholar] [CrossRef]
- Rai, P.; Hoba, S.N.; Buchmann, C.; Kersten, C.; Schirmeister, T.; Bufe, B.; Tarasov, A. Peptide-based biosensor for real-time monitoring of protease biomarker activity using multi-parametric surface plasmon resonance spectroscopy. Biosens. Bioelectron. 2025, 286, 117586. [Google Scholar] [CrossRef]
- Bennett, J.L.; Nguyen, G.T.H.; Donald, W.A. Protein-small molecule interactions in native mass spectrometry. Chem. Rev. 2022, 122, 7327–7385. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, Y.; Lu, J.; Gong, T.; Ibanez, E.; Cifuentes, A.; Lu, W. Microfluidic biosensors for biomarker detection in body fluids: A key approach for early cancer diagnosis. Biomark. Res. 2024, 12, 153. [Google Scholar] [CrossRef]
- Ganbold, E.; Kim, N.Y.; Kim, Y.M.; Sharma, P.K.; Lee, D.N.; Oh, B.; Kim, H.S.; Song, J.; Lee, B.; Kim, E.; et al. Reagentless aptamer based on the ultrasensitive and fast response electrochemical capacitive biosensor for EGFR detection in non-small cell lung cancer. Biosens. Bioelectron. 2025, 278, 117319. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Q.; Qiu, D.; Chen, M.; Zhang, W.; Li, Y.; Huang, J.; Xu, Q. Programming an amplified and self-calibrated electrochemical biosensor with high sensitivity, reproducibility, and stability for ORAOV 1 detection. Anal. Chem. 2025, 97, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Ma, B.; Xu, C.; Liu, H. Emerging tumor-on-chips with electrochemical biosensors. Trac-Trends Anal. Chem. 2022, 153, 116640. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. A review on electrochemical sensors and biosensors used in assessing antioxidant activity. Antioxidants 2022, 11, 584. [Google Scholar] [CrossRef]
- Raza, T.; Qu, L.; Khokhar, W.A.; Andrews, B.; Ali, A.; Tian, M. Progress of wearable and flexible electrochemical biosensors with the aid of conductive nanomaterials. Front. Bioeng. Biotechnol. 2021, 9, 761020. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, H.; Zhuang, X.; Xu, Y.; Liu, J.; Zeng, C.; Ding, W.; Cui, F.; Zhu, S. Multivalent acetylated-sialic acid as recognition elements for the electrochemical sensing of viral antigens. Biosens. Bioelectron. 2025, 268, 116883. [Google Scholar] [CrossRef]
- Abdulbari, H.A.; Basheer, E.A.M. Electrochemical biosensors: Electrode development, materials, design, and fabrication. ChemBioEng Rev. 2017, 4, 92–105. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Darrow, J.J.; van de Wiele, V.; Beran, D.; Kesselheim, A.S. An empirical review of key glucose monitoring devices: Product iterations and patent protection. J. Diabetes Sci. Technol. 2025, 19, 84–90. [Google Scholar] [CrossRef]
- Mihailescu, C.M.; Stan, D.; Savin, M.; Moldovan, C.A.; Dinulescu, S.; Radulescu, C.H.; Firtat, B.; Muscalu, G.; Brasoveanu, C.; Ion, M.; et al. Platform with biomimetic electrochemical sensors for adiponectin and leptin detection in human serum. Talanta 2020, 210, 120643. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, W.; Zhang, L.; Yang, J.; Yao, Z.; He, Y.; Li, Y. Integrated hand-held electrochemical sensor for multicomponent detection in urine. Biosens. Bioelectron. 2021, 193, 113534. [Google Scholar] [CrossRef]
- Wang, X.; He, A.; Yu, B.; Zhang, L.; Pang, W.; Zhang, H.; Niu, P. Uncovering the sweat biofouling components and distributions in electrochemical sensors. Anal. Chem. 2022, 94, 14402–14409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pandey, R.; Mccarthy, M.J.; Raymond, O. Single-use electrochemical aptamer-based sensors for calibration-free measurements in human saliva via dual-frequency approaches: Prospects and challenges. Anal. Chem. 2025, 97, 5234–5243. [Google Scholar] [CrossRef]
- Achache, M.; Garcia-Guzman, J.J.; Seddik, N.B.; Cubillana-Aguilera, L.; Palacios-Santander, J.M.; Bouchta, D.; Choukairi, M. A novel sonogel-leucine electrochemical sensor for the detection of neuroblastoma biomarker in human urine and synthetic cerebrospinal fluid. Talanta 2025, 286, 127452. [Google Scholar] [CrossRef]
- Ma, L.; Sun, B. Machine learning and AI in marketing—Connecting computing power to human insights. Int. J. Res. Mark. 2020, 37, 481–504. [Google Scholar] [CrossRef]
- Glaser, J.I.; Benjamin, A.S.; Farhoodi, R.; Kording, K.P. The roles of supervised machine learning in systems neuroscience. Prog. Neurobiol. 2019, 175, 126–137. [Google Scholar] [CrossRef]
- Blanco-Justicia, A.; Domingo-Ferrer, J.; Martinez, S.; Sanchez, D. Machine learning explainability via microaggregation and shallow decision trees. Knowl.-Based Syst. 2020, 194, 105532. [Google Scholar] [CrossRef]
- Peng, S.; Wang, W.; Chen, Y.; Zhong, X.; Hu, Q. Regression-based hyperparameter learning for support vector machines. IEEE Trans. Neural Netw. Learn. Syst. 2024, 35, 18799–18813. [Google Scholar] [CrossRef]
- Zaadnoordijk, L.; Besold, T.R.; Cusack, R. Lessons from infant learning for unsupervised machine learning. Nat. Mach. Intell. 2022, 4, 510–520. [Google Scholar] [CrossRef]
- Moses, I.A.; Joshi, R.P.; Ozdemir, B.; Kumar, N.; Eickholt, J.; Barone, V. Machine learning screening of metal-ion battery electrode materials. ACS Appl. Mater. Interfaces 2021, 13, 53355–53362. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Wang, L.; Pu, H.; Chan, M.K.Y.; Chen, J. Understanding, discovery, and synthesis of 2d materials enabled by machine learning. Chem. Soc. Rev. 2022, 51, 1899–1925. [Google Scholar] [CrossRef]
- Lu, S.; Jayaraman, A. Machine learning for analyses and automation of structural characterization of polymer materials. Prog. Polym. Sci. 2024, 153, 101828. [Google Scholar] [CrossRef]
- Huang, G.; Huang, F.; Dong, W. Machine learning in energy storage material discovery and performance prediction. Chem. Eng. J. 2024, 492, 152294. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Y.; Zhou, Y.; Fang, D.; Ding, X.; Sun, J.; Xue, D. Machine learning assisted multi-objective optimization for materials processing parameters: A case study in mg alloy. J. Alloys Compd. 2020, 844, 156159. [Google Scholar] [CrossRef]
- Tu, K.; Huang, H.; Lee, S.; Lee, W.; Sun, Z.; Alexander-Katz, A.; Ross, C.A. Machine learning predictions of block copolymer self-assembly. Adv. Mater. 2020, 32, e2005713. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, C.; Yu, Y.; Yang, S.; Shi, F.; Gao, X.; Liu, Y.; Huang, H.; Stewart, C.; Li, F.; et al. Machine learning-assisted nanoenzyme/bioenzyme dual-coupled array for rapid detection of amyloids. Anal. Chem. 2023, 95, 4605–4611. [Google Scholar] [CrossRef]
- Yu, Y.; Ni, W.; Hu, Q.; Li, H.; Zhang, Y.; Gao, X.; Zhou, L.; Zhang, S.; Ma, S.; Zhang, Y.; et al. A dual fluorescence turn-on sensor array formed by poly(para-aryleneethynylene) and aggregation-induced emission fluorophores for sensitive multiplexed bacterial recognition. Angew. Chem. Int. Ed. Engl. 2024, 63, e202318483. [Google Scholar] [CrossRef]
- Materon, E.M.; Gomez, F.R.; Almeida, M.B.; Shimizu, F.M.; Wong, A.; Teodoro, K.B.R.; Silva, F.S.R.; Lima, M.J.A.; Angelim, M.K.S.C.; Melendez, M.E.; et al. Colorimetric detection of SARS-CoV-2 using plasmonic biosensors and smartphones. ACS Appl. Mater. Interfaces 2022, 14, 54527–54538. [Google Scholar] [CrossRef]
- Dizaji, A.N.; Ozek, N.S.; Yilmaz, A.; Aysin, F.; Yilmaz, M. Gold nanorod arrays enable highly sensitive bacterial detection via surface-enhanced infrared absorption (SEIRA) spectroscopy. Colloids Surf. B-Biointerfaces 2021, 206, 111939. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Y.; Zhou, M.; Han, Y.; Zhang, M.; Gao, Z.; Liu, Z.; Chen, P.; Du, W.; Zhang, X.; et al. Smartphone-based platforms implementing microfluidic detection with image-based artificial intelligence. Nat. Commun. 2023, 14, 1341. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, H.; Li, Y.; Li, X. Application of artificial intelligence (AI)-enhanced biochemical sensing in molecular diagnosis and imaging analysis: Advancing and challenges. Trac-Trends Anal. Chem. 2024, 174, 117700. [Google Scholar] [CrossRef]
- Kumar, A.; Jain, D.; Bahuguna, J.; Bhaiyya, M.; Dubey, S.K.; Javed, A.; Goel, S. Machine learning assisted and smartphone integrated homogeneous electrochemiluminescence biosensor platform for sample to answer detection of various human metabolites. Biosens. Bioelectron. 2023, 238, 115582. [Google Scholar] [CrossRef]
- Sadeghi, P.; Noroozizadeh, S.; Alshawabkeh, R.; Sun, N.X. Machine learning-driven d-glucose prediction using a novel biosensor for non-invasive diabetes management. Biosensors 2025, 15, 152. [Google Scholar] [CrossRef]
- Sukjee, W.; Sirisangsawang, P.; Thepparit, C.; Auewarakul, P.; Puttasakul, T.; Sangma, C. MIP-based electrochemical sensor with machine learning for accurate ZIKV detection in protein-and glucose-rich urine. Anal. Biochem. 2025, 702, 115854. [Google Scholar] [CrossRef]
- Wei, Y.; Abbasi, S.M.T.; Mehmood, N.; Li, L.; Qu, F.; Cheng, G.; Hu, D.; Ho, Y.; Yuan, W.; Ho, H. Deep-qGFP: A generalist deep learning assisted pipeline for accurate quantification of green fluorescent protein labeled biological samples in microreactors. Small Methods 2024, 8, e2301293. [Google Scholar] [CrossRef]
- Chavlis, S.; Poirazi, P. Dendrites endow artificial neural networks with accurate, robust and parameter-efficient learning. Nat. Commun. 2025, 16, 943. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, J.; Thorpe, H.; Lee, S.; Kim, S.; Park, H.J. Deep neural network learning biological condition information refines gene-expression-based cell subtypes. Brief. Bioinform. 2024, 25, bbad512. [Google Scholar] [CrossRef]
- Hagen, G.M.; Bendesky, J.; Machado, R.; Nguyen, T.A.; Kumar, T.; Ventura, J. Fluorescence microscopy datasets for training deep neural networks. Gigascience 2021, 10, giab032. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Liu, J. Neural network with multiple connection weights. Pattern Recognit. 2020, 107, 107481. [Google Scholar] [CrossRef]
- Das, S.; Moon, S.; Kaur, R.; Sharma, G.; Kumar, P.; Stangar, U.L. Artificial neural network modeling of photocatalytic degradation of pollutants: A review of photocatalyst, optimum parameters and model topology. Catal. Rev.-Sci. Eng. 2024, 67, 2338131. [Google Scholar] [CrossRef]
- Paoletti, M.E.; Haut, J.M.; Plaza, J.; Plaza, A. A new deep convolutional neural network for fast hyperspectral image classification. ISPRS-J. Photogramm. Remote Sens. 2018, 145, 120–147. [Google Scholar] [CrossRef]
- Shen, J.; Liu, F.; Tu, Y.; Tang, C. Finding gene network topologies for given biological function with recurrent neural network. Nat. Commun. 2021, 12, 3125. [Google Scholar] [CrossRef]
- Xu, R.; Li, Y.; Wang, C.; Xu, S.; Meng, W.; Zhang, X. Instance segmentation of biological images using graph convolutional network. Eng. Appl. Artif. Intell. 2022, 110, 104739. [Google Scholar] [CrossRef]
- Zhang, C.; Correia, C.; Weiskittel, T.M.; Tan, S.H.; Meng-Lin, K.; Yu, G.T.; Yao, J.; Yeo, K.S.; Zhu, S.; Ung, C.Y.; et al. A knowledge-based discovery approach couples artificial neural networks with weight engineering to uncover immune-related processes underpinning clinical traits of breast cancer. Front. Immunol. 2022, 13, 920669. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, H.; Shen, W.; Yang, Y.; Li, Z.; Liu, Y.; Wang, C.; Gu, B.; Zhang, L. Rapid SERS identification of methicillin-susceptible and methicillin-resistant staphylococcus aureus via aptamer recognition and deep learning. RSC Adv. 2021, 11, 34425–34431. [Google Scholar] [CrossRef]
- Cheng, N.; Chen, D.; Lou, B.; Fu, J.; Wang, H. A biosensing method for the direct serological detection of liver diseases by integrating a SERS-based sensor and a CNN classifier. Biosens. Bioelectron. 2021, 186, 113246. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhu, A.; Tian, Y. In vivo electrochemical biosensors: Recent advances in molecular design, electrode materials, and electrochemical devices. Anal. Chem. 2023, 95, 388–406. [Google Scholar] [CrossRef]
- Xu, T.; Geng, Z. Strategies to improve performances of LSPR biosensing: Structure, materials, and interface modification. Biosens. Bioelectron. 2021, 174, 112850. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, H.; Fujiki, S.; Shibata, T.; Oishi, M.; Iiyama, M.; Takayanagi, T.; Lin, Y.; Yeh, M. A flow-based enzyme-free biosensor fabricated using track-etched membrane electrodes: Selective and sensitive detection of uric acid. Sens. Actuator B-Chem. 2023, 383, 133588. [Google Scholar] [CrossRef]
- Lv, C.; Zhou, X.; Zhong, L.; Yan, C.; Srinivasan, M.; Seh, Z.W.; Liu, C.; Pan, H.; Li, S.; Wen, Y.; et al. Machine learning: An advanced platform for materials development and state prediction in lithium-ion batteries. Adv. Mater. 2022, 34, e2101474. [Google Scholar] [CrossRef]
- Huang, J.S.; Liew, J.X.; Ademiloye, A.S.; Liew, K.M. Artificial intelligence in materials modeling and design. Arch. Comput. Method Eng. 2021, 28, 3399–3413. [Google Scholar] [CrossRef]
- Theyagarajan, K.; Kim, Y. Recent developments in the design and fabrication of electrochemical biosensors using functional materials and molecules. Biosensors 2023, 13, 424. [Google Scholar] [CrossRef]
- Kalita, N.; Gogoi, S.; Minteer, S.D.; Goswami, P. Advances in bioelectrode design for developing electrochemical biosensors. ACS Meas. Sci. Au 2023, 3, 404–433. [Google Scholar] [CrossRef]
- Lorente, D.; Martinez-Martinez, F.; Ruperez, M.J.; Lago, M.A.; Martinez-Sober, M.; Escandell-Montero, P.; Martinez-Martinez, J.M.; Martinez-Sanchis, S.; Serrano-Lopez, A.J.; Monserrat, C.; et al. A framework for modelling the biomechanical behaviour of the human liver during breathing in real time using machine learning. Expert Syst. Appl. 2017, 71, 342–357. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Lu, Q.; Zhang, Y.; Yu, H.; Huang, L.; Wang, T.; Liang, X.; Liu, F.; Liu, F.; et al. Machine learning-assisted development of sensitive electrode materials for mixed potential-type NO2 gas sensors. ACS Appl. Mater. Interfaces 2021, 13, 50121–50131. [Google Scholar] [CrossRef]
- Wang, M.; Ceto, X.; Valle, M.D. A sensor array based on molecularly imprinted polymers and machine learning for the analysis of fluoroquinolone antibiotics. ACS Sens. 2022, 7, 3318–3325. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, B.; Hashemianzadeh, S.M.; Hosseini, S.M.M. Machine-learning-based predictions of imprinting quality using ensemble and non-linear regression algorithms. Sci. Rep. 2023, 13, 12111. [Google Scholar] [CrossRef]
- Nezhadali, A.; Sadeghzadeh, S. Experimental design-artificial neural network-genetic algorithm optimization and computer-assisted design of celecoxib molecularly imprinted polymer/carbon nanotube sensor. J. Electroanal. Chem. 2017, 795, 32–40. [Google Scholar] [CrossRef]
- Kaniskan, H.U.; Martini, M.L.; Jin, J. Inhibitors of protein methyltransferases and demethylases. Chem. Rev. 2018, 118, 989–1068. [Google Scholar] [CrossRef]
- Bryan, B.A.; Gao, L.; Ye, Y.; Sun, X.; Connor, J.D.; Crossman, N.D.; Stafford-Smith, M.; Wu, J.; He, C.; Yu, D.; et al. China’s response to a national land-system sustainability emergency. Nature 2018, 559, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.H.; Li, J.; Yoshinaga, K.; Swager, T.M. Dynamically reconfigurable, multifunctional emulsions with controllable structure and movement. Adv. Mater. 2019, 31, e1905569. [Google Scholar] [CrossRef]
- Peng, L.; Xu, D.; Yang, X.; Tang, J.; Feng, X.; Zhang, S.; Yan, H. Organocatalytic asymmetric one-step desymmetrizing dearomatization reaction of indoles: Development and bioactivity evaluation. Angew. Chem. Int. Ed. Engl. 2019, 58, 216–220. [Google Scholar] [CrossRef]
- Song, Y.; Xu, T.; Xiu, J.; Zhang, X. Mini-pillar microarray for individually electrochemical sensing in microdroplets. Biosens. Bioelectron. 2020, 149, 111845. [Google Scholar] [CrossRef]
- Qiu, C.; Xu, Y.; Fan, X.; Xu, D.; Tandiana, R.; Ling, X.; Jiang, Y.; Liu, C.; Yu, L.; Chen, W.; et al. Highly crystalline k-intercalated polymeric carbon nitride for visible-light photocatalytic alkenes and alkynes deuterations. Adv. Sci. 2019, 6, 1801403. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, C.; Zhang, C.; Liu, Y.; Cheng, J.; Lin, J. Bandgap prediction by deep learning in configurationally hybridized graphene and boron nitride. Npj Comput. Mater. 2019, 5, 349–352. [Google Scholar] [CrossRef]
- Song, Y.; Xu, T.; Song, X.; Zhang, X. Integrated microdroplets array for intelligent electrochemical fabrication. Adv. Funct. Mater. 2020, 30, 1910329. [Google Scholar] [CrossRef]
- Tedjo, W.; Nejad, J.E.; Feeny, R.; Yang, L.; Henry, C.S.; Tobet, S.; Chen, T. Electrochemical biosensor system using a CMOS microelectrode array provides high spatially and temporally resolved images. Biosens. Bioelectron. 2018, 114, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Zhu, M.; Kuang, Z.; Li, X.; Xu, F.; Miao, S.; Zhang, Z.; Lou, X.; Li, H.; et al. Electrochemical biosensors for whole blood analysis: Recent progress, challenges, and future perspectives. Chem. Rev. 2023, 123, 7953–8039. [Google Scholar] [CrossRef]
- Rashidi, K.; Mahmoudi, M.; Mohammadi, G.; Zangeneh, M.M.; Korani, S.; Goicoechea, H.C.; Gu, H.; Jalalvand, A.R. Simultaneous co-immobilization of three enzymes onto a modified glassy carbon electrode to fabricate a high-performance amperometric biosensor for determination of total cholesterol. Int. J. Biol. Macromol. 2018, 120, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, T.; Jiang, M.; Wei, C.; Ma, S.; Chen, D.; Tong, W.; Huang, X. Construction of flexible enzymatic electrode based on gradient hollow fiber membrane and multi-wall carbon tubes meshes. Biosens. Bioelectron. 2020, 152, 112001. [Google Scholar] [CrossRef] [PubMed]
- Bocan, A.; Siavash Moakhar, R.; Del Real Mata, C.; Petkun, M.; De Iure-Grimmel, T.; Yedire, S.G.; Shieh, H.; Khorrami Jahromi, A.; Mahshid, S.S.; Mahshid, S. Machine-learning-aided advanced electrochemical biosensors. Adv. Mater. 2025, e2417520. [Google Scholar] [CrossRef]
- Giordano, G.F.; Ferreira, L.F.; Bezerra, I.R.S.; Barbosa, J.A.; Costa, J.N.Y.; Pimentel, G.J.C.; Lima, R.S. Machine learning toward high-performance electrochemical sensors. Anal. Bioanal. Chem. 2023, 415, 3683–3692. [Google Scholar] [CrossRef]
- Kim, Y.; Kanczler, J.M.; Lanham, S.; Rawlings, A.; Roldo, M.; Tozzi, G.; Dawson, J.I.; Cidonio, G.; Oreffo, R.O.C. Biofabrication of nanocomposite-based scaffolds containing human bone extracellular matrix for the differentiation of skeletal stem and progenitor cells. Bio-Des. Manuf. 2024, 7, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, S.; Hanitra, I.N.; Sandri, G.; Totu, T.; Grassi, F.; Criscuolo, F.; De Micheli, G.; Carrara, S.; Demarchi, D. Continuous monitoring of propofol in human serum with fouling compensation by support vector classifier. Biosens. Bioelectron. 2021, 171, 112666. [Google Scholar] [CrossRef]
- Bao, Q.; Li, G.; Cheng, W.; Yang, Z.; Qu, Z.; Wei, J.; Lin, L. Machine learning-assisted flexible wearable device for tyrosine detection. RSC Adv. 2023, 13, 23788–23795. [Google Scholar] [CrossRef]
- Fatima, M.; Hanif, S.; Elsharkawy, E.R.; Zafar, F.; Zulfiqar, A.; Khan, M.A.; Akhtar, N.; Fareed, Z.; El-Bahy, Z.M.; Shafiq, Z.; et al. Design and fabrication of machine learning trained silver nanoparticles-infused multi-walled carbon nanotube-based sensor for antiviral drug monitoring. Microchem. J. 2024, 203, 110921. [Google Scholar] [CrossRef]
- Xue, Y.; Ji, W.; Jiang, Y.; Yu, P.; Mao, L. Deep learning for voltammetric sensing in a living animal brain. Angew. Chem. Int. Ed. Engl. 2021, 60, 23777–23783. [Google Scholar] [CrossRef]
- Staszak, M.; Staszak, K.; Wieszczycka, K.; Bajek, A.; Roszkowski, K.; Tylkowski, B. Machine learning in drug design: Use of artificial intelligence to explore the chemical structure-biological activity relationship. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1568. [Google Scholar] [CrossRef]
- Cabaneros, S.M.; Calautit, J.K.; Hughes, B.R. A review of artificial neural network models for ambient air pollution prediction. Environ. Modell. Softw. 2019, 119, 285–304. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Wang, J.; Liu, C.; Xu, T.; Zhang, X. Machine learning with neural networks to enhance selectivity of nonenzymatic electrochemical biosensors in multianalyte mixtures. ACS Appl. Mater. Interfaces 2022, 14, 52684–52690. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Han, R.; Yu, K.; Li, R.; Luo, X. Antifouling strategies for electrochemical sensing in complex biological media. Mikrochim. Acta 2024, 191, 138. [Google Scholar] [CrossRef]
- Stradolini, F.; Kilic, T.; Taurino, I.; De Micheli, G.; Carrara, S. Cleaning strategy for carbon-based electrodes: Long-term propofol monitoring in human serum. Sens. Actuator B-Chem. 2018, 269, 304–313. [Google Scholar] [CrossRef]
- Cho, S.; Lee, Y.; Lee, S.; Kang, H.; Kim, J.; Choi, J.; Ryu, J.; Joo, H.; Jung, H.; Kim, J. Finding hidden signals in chemical sensors using deep learning. Anal. Chem. 2020, 92, 6529–6537. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.F.; Freitas, V.M.S.; Schleder, G.R.; Santhiago, M.; Gobbi, A.L.; Lima, R.S. Bifunctional metal meshes acting as a semipermeable membrane and electrode for sensitive electrochemical determination of volatile compounds. ACS Appl. Mater. Interfaces 2021, 13, 35914–35923. [Google Scholar] [CrossRef] [PubMed]
- Kammarchedu, V.; Butler, D.; Ebrahimi, A. A machine learning-based multimodal electrochemical analytical device based on eMoSx-LIG for multiplexed detection of tyrosine and uric acid in sweat and saliva. Anal. Chim. Acta 2022, 1232, 340447. [Google Scholar] [CrossRef]
- Silva, A.A.D.; de Oliveira, R.A.G.; Giordano, G.F.; Silva, G.S.D.; Murer, R.C.; Vieira, L.C.S.; Lorevice, M.V.; Gouveia, R.F.; Carvalho, R.M.; Shimizu, F.M.; et al. Ultrafast microfluidic solvent extraction and machine learning-assisted impedimetric sensor for multidetermination of scaling ions in crude oils. Sens. Actuator B-Chem. 2024, 403, 135151. [Google Scholar] [CrossRef]
- Castro, A.C.H.; Bezerra, I.R.S.; Pascon, A.M.; Silva, G.H.D.; Philot, E.A.; de Oliveira, V.L.; Mancini, R.S.N.; Schleder, G.R.; Castro, C.E.; de Carvalho, L.R.S.; et al. Modular label-free electrochemical biosensor loading nature-inspired peptide toward the widespread use of COVID-19 antibody tests. ACS Nano 2022, 16, 14239–14253. [Google Scholar] [CrossRef]
- Barbosa, J.A.; Freitas, V.M.S.; Vidotto, L.H.B.; Schleder, G.R.; de Oliveira, R.A.G.; Rocha, J.F.D.; Kubota, L.T.; Vieira, L.C.S.; Tolentino, H.C.N.; Neckel, I.T.; et al. Biocompatible wearable electrodes on leaves toward the on-site monitoring of water loss from plants. ACS Appl. Mater. Interfaces 2022, 14, 22989–23001. [Google Scholar] [CrossRef]
- Doretto, D.S.; Corsato, P.C.R.; Silva, C.O.; Pessoa, J.C.; Vieira, L.C.S.; de Araujo, W.R.; Shimizu, F.M.; Piazzetta, M.H.O.; Gobbi, A.L.; Ribeiro, I.R.S.; et al. Ultradense electrochemical chip and machine learning for high-throughput, accurate anticancer drug screening. ACS Sens. 2024, 10, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Kalita, D.; Naskar, U.; Mishra, B.K.; Kumar, P.; Mirza, K.B. Prediction of glucose sensor sensitivity in the presence of biofouling using machine learning and electrochemical impedance spectroscopy. IEEE Sens. J. 2023, 23, 18785–18797. [Google Scholar] [CrossRef]
- Sankhala, D.; Sardesai, A.U.; Pali, M.; Lin, K.; Jagannath, B.; Muthukumar, S.; Prasad, S. A machine learning-based on-demand sweat glucose reporting platform. Sci. Rep. 2022, 12, 2442. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.H.; Besharati, Z.; Hashemi, S.A. Salicylic acid solubility prediction in different solvents based on machine learning algorithms. Digit. Chem. Eng. 2024, 11, 100157. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, S.; Lu, F.; Pan, W.; Li, N.; Tang, B. CRISPR/cas-based in vitro diagnostic platforms for cancer biomarker detection. Anal. Chem. 2021, 93, 11899–11909. [Google Scholar] [CrossRef]
- Ganguly, A.; Ebrahimzadeh, T.; Zimmern, P.; De Nisco, N.J.; Prasad, S. Label-free, novel electrofluidic capacitor biosensor for prostaglandin e2 detection toward early and rapid urinary tract infection diagnosis. ACS Sens. 2021, 7, 186–198. [Google Scholar] [CrossRef]
- Ganguly, A.; Gunda, V.; Thai, K.; Prasad, S. Inflammatory stimuli responsive non-faradaic, ultrasensitive combinatorial electrochemical urine biosensor. Sensors 2022, 22, 7757. [Google Scholar] [CrossRef]
- George, H.; Sun, Y.; Wu, J.; Yan, Y.; Wang, R.; Pesavento, R.P.; Mathew, M.T. Intelligent salivary biosensors for periodontitis: In vitro simulation of oral oxidative stress conditions. Med. Biol. Eng. Comput. 2024, 62, 2409–2434. [Google Scholar] [CrossRef]
- Abreu, A.; Oliveira, D.D.S.; Vinagre, I.; Cavouras, D.; Alves, J.A.; Pereira, A.I.; Lima, J.; Moreira, F.T.C. A machine learning approach for enhanced glucose prediction in biosensors. Chemosensors 2025, 13, 52. [Google Scholar] [CrossRef]
- Jalili, F.; Jalalvand, A.R. A novel and intelligent chemometric-electrochemical-enzymatic biosensing procedure and mimicking a clinical condition environment to trick the red blood cells for counting them under physiological conditions: A new connection among chemometry, electrochemistry and hematology. Sens. Bio-Sens. Res. 2024, 43, 100613. [Google Scholar]
- Xu, Y.; Jiang, Y.; Li, C.; Chen, Y.; Yang, Y. Integration of an XGBoost model and EIS detection to determine the effect of low inhibitor concentrations on E. coli. J. Electroanal. Chem. 2020, 877, 114534. [Google Scholar] [CrossRef]
- Garcia-Junior, M.A.; Andrade, B.S.; Lima, A.P.; Soares, I.P.; Notario, A.F.O.; Bernardino, S.S.; Guevara-Vega, M.F.; Honorio-Silva, G.; Munoz, R.A.A.; Jardim, A.C.G.; et al. Artificial-intelligence bio-inspired peptide for salivary detection of SARS-CoV-2 in electrochemical biosensor integrated with machine learning algorithms. Biosensors 2025, 15, 75. [Google Scholar] [CrossRef]
- Shahub, S.; Upasham, S.; Ganguly, A.; Prasad, S. Machine learning guided electrochemical sensor for passive sweat cortisol detection. Sens. Bio-Sens. Res. 2022, 38, 100527. [Google Scholar] [CrossRef]
- Han, S.; Zeng, Q.; Liang, Y.; Xiao, Q.; Chen, Y.; Yan, F.; Xiong, Y.; Yue, J.; Tian, X. Wearable piezoelectric sensors based on BaTiO 3 films for sarcopenia recognition. Adv. Mater. Technol. 2024, 9, 2302172. [Google Scholar] [CrossRef]
- Gupta, S.; Nayak, M.T.; Sunitha, J.D.; Dawar, G.; Sinha, N.; Rallan, N.S. Correlation of salivary glucose level with blood glucose level in diabetes mellitus. J. Oral Maxillofac. Pathol. 2017, 21, 334–339. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Nesterowicz, M.; Zalewska, A.; Biedrzycki, G.; Gerreth, P.; Hojan, K.; Gerreth, K. Salivary xanthine oxidase as a potential biomarker in stroke diagnostics. Front. Immunol. 2022, 13, 897413. [Google Scholar] [CrossRef] [PubMed]
- Liljestrand, J.M.; Paju, S.; Buhlin, K.; Persson, G.R.; Sarna, S.; Nieminen, M.S.; Sinisalo, J.; Mantyla, P.; Pussinen, P.J. Lipopolysaccharide, a possible molecular mediator between periodontitis and coronary artery disease. J. Clin. Periodontol. 2017, 44, 784–792. [Google Scholar] [CrossRef]
- Lai, W.; Lu, Y.; Hsieh, C.; Wei, C.; Tsai, Y.; Chang, F.; Chan, Y. Developing lactic acid bacteria as an oral healthy food. Life 2021, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreno, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Murray, C.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, S.A.; Mirzajani, H.; Carrara, S.; Onbasli, M.C. Advances in biosensor technologies for infectious diseases detection. Trac-Trends Anal. Chem. 2024, 180, 117979. [Google Scholar] [CrossRef]
- Aliev, T.A.; Lavrentev, F.; Dyakonov, A.; Diveev, D.A.; Shilovskikh, V.V.; Skorb, E. Electrochemical platform for detecting escherichia coli bacteria using machine learning methods. Biosens. Bioelectron. 2024, 259, 116377. [Google Scholar] [CrossRef]
- Parmar, J.; Patel, S.K.; Katkar, V.; Natesan, A. Graphene-based refractive index sensor using machine learning for detection of mycobacterium tuberculosis bacteria. IEEE Trans. Nanobiosci. 2023, 22, 92–98. [Google Scholar] [CrossRef]
- O’Meara, S. Antimicrobial resistance. Nature 2020, 586, S49. [Google Scholar] [CrossRef]
- Chen, J.; Lu, N.; Wang, X.; Chen, Y.; Guo, M.; Xu, Y. Time-lapse electrochemical impedance detection of bacteria proliferation for accurate antibiotic evaluation. IEEE Sens. J. 2022, 22, 5504–5513. [Google Scholar] [CrossRef]
- Karasinski, J.; White, L.; Zhang, Y.; Wang, E.; Andreescu, S.; Sadik, O.A.; Lavine, B.K.; Vora, M. Detection and identification of bacteria using antibiotic susceptibility and a multi-array electrochemical sensor with pattern recognition. Biosens. Bioelectron. 2007, 22, 2643–2649. [Google Scholar] [CrossRef]
- Challhua, R.; Prati, R.; Champi, A. Feature engineering and machine learning for electrochemical detection of rabies virus in graphene-based biosensors. Microchem J. 2024, 204, 111074. [Google Scholar] [CrossRef]
- Kadri, A.N.; Nusairat, L.; Griffin, B.; Gordon, S.; Pettersson, G.; Kapadia, S.; Harb, S. National trends and characteristics of infective endocarditis associated with drug abuse. J. Am. Coll. Cardiol. 2019, 73, 1987. [Google Scholar] [CrossRef]
- Angelone, L.M.; Capanna, K.; Gray, T.; Gutowski, S.; Kotarek, J.; Koustova, E.; Pollock, J.; Rorer, E.; Tarver, M.E.; Yi, J. Novel medical devices to address the opioid crisis. Nat. Med. 2024, 30, 1805–1806. [Google Scholar] [CrossRef] [PubMed]
- De Rycke, E.; Stove, C.; Dubruel, P.; De Saeger, S.; Beloglazova, N. Recent developments in electrochemical detection of illicit drugs in diverse matrices. Biosens. Bioelectron. 2020, 169, 112579. [Google Scholar] [CrossRef]
- Hack, T.; Bisarra, J.; Chung, S.; Kummari, S.; Hall, D.A. Mitigating medication tampering and diversion via real-time intravenous opioid quantification. IEEE Trans. Biomed. Circuits Syst. 2024, 18, 756–770. [Google Scholar] [CrossRef]
- Ortiz-Aguayo, D.; Ceto, X.; De Wael, K.; Valle, M.D. Resolution of opiate illicit drugs signals in the presence of some cutting agents with use of a voltammetric sensor array and machine learning strategies. Sens. Actuator B-Chem. 2022, 357, 131345. [Google Scholar] [CrossRef]
- Kadian, S.; Sahoo, S.S.; Kumari, P.; Narayan, R.J. Machine learning enabled onsite electrochemical detection of lidocaine using a microneedle array integrated screen printed electrode. Electrochim. Acta 2024, 475, 143664. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, Z.; Shen, Z.; Chen, J.; Gu, C.; Li, L.; Chen, F.; Liu, H. Electrochemical fingerprinting combined with machine learning algorithm for closely related medicinal plant identification. Sens. Actuator B-Chem. 2023, 375, 132922. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, X.; Chen, Z.; Wang, G.; Yao, L.; Lin, Z. Low-cost electrochemical sensor based on montmorillonite for antibiotic tetracycline hydrochloride detection. J. Mater. Sci.-Mater. Electron. 2022, 33, 427–442. [Google Scholar] [CrossRef]
- Teymourian, H.; Parrilla, M.; Sempionatto, J.R.; Montiel, N.F.; Barfidokht, A.; Van Echelpoel, R.; De Wael, K.; Wang, J. Wearable electrochemical sensors for the monitoring and screening of drugs. ACS Sens. 2020, 5, 2679–2700. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A review of the pharmacological action of astragalus polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Beshbishy, A.M.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; El-Hack, M.E.A.; Taha, A.E.; Abd-Elhakim, Y.M.; Devkota, H.P. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef]
- Lu, L.; Hu, Z.; Hu, X.; Li, D.; Tian, S. Electronic tongue and electronic nose for food quality and safety. Food Res. Int. 2022, 162, 112214. [Google Scholar] [CrossRef]
- Pradhan, S.; Jityen, A.; Osotchan, T. Prediction of the bitterness of pharmaceutical drugs using a taste sensor with representative group data by the center of mass. J. Phys. Conf. Ser. 2019, 1380, 12091. [Google Scholar] [CrossRef]
- Lee, S.; Shi, Q.; Lee, C. From flexible electronics technology in the era of IoT and artificial intelligence toward future implanted body sensor networks. APL Mater. 2019, 7, 31302. [Google Scholar] [CrossRef]
- Yoon, J.; Kwon, N.; Lee, Y.; Kim, S.; Lee, T.; Choi, J. Nanotechnology-based wearable electrochemical biosensor for disease diagnosis. ACS Sens. 2025, 10, 1675–1689. [Google Scholar] [CrossRef]
- Duan, H.; Peng, S.; He, S.; Tang, S.; Goda, K.; Wang, C.H.; Li, M. Wearable electrochemical biosensors for advanced healthcare monitoring. Adv. Sci. 2025, 12, 2411433. [Google Scholar] [CrossRef]
- Cinca-Morros, S.; Garcia-Rey, S.; Alvarez-Herms, J.; Basabe-Desmonts, L.; Benito-Lopez, F. A physiological perspective of the relevance of sweat biomarkers and their detection by wearable microfluidic technology: A review. Anal. Chim. Acta 2024, 1327, 342988. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, S.; Hu, Z.; Zhang, X.; Yi, N.; Tang, K.; Dexheimer, M.G.; Lian, X.; Wang, Q.; Yang, J.; et al. Laser-induced graphene non-enzymatic glucose sensors for on-body measurements. Biosens. Bioelectron. 2021, 193, 113606. [Google Scholar] [CrossRef]
- Zhou, Z.; He, X.; Xiao, J.; Pan, J.; Li, M.; Xu, T.; Zhang, X. Machine learning-powered wearable interface for distinguishable and predictable sweat sensing. Biosens. Bioelectron. 2024, 265, 116712. [Google Scholar] [CrossRef]
- Lin, P.; Sheu, S.; Chen, C.; Huang, S.; Li, B. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef]
- Munje, R.D.; Muthukumar, S.; Prasad, S. Lancet-free and label-free diagnostics of glucose in sweat using zinc oxide based flexible bioelectronics. Sens. Actuator B-Chem. 2017, 238, 482–490. [Google Scholar] [CrossRef]
- Wang, K.; Margolis, S.; Cho, J.M.; Wang, S.; Arianpour, B.; Jabalera, A.; Yin, J.; Hong, W.; Zhang, Y.; Zhao, P.; et al. Non-invasive detection of early-stage fatty liver disease via an on-skin impedance sensor and attention-based deep learning. Adv. Sci. 2024, 11, e2400596. [Google Scholar] [CrossRef]
- Hua, Q.; Li, Y.; Frost, M.W.; Kold, S.; Rahbek, O.; Shen, M. Machine learning-assisted equivalent circuit characterization for electrical impedance spectroscopy measurements of bone fractures. IEEE Trans. Instrum. Meas. 2024, 73, 2001515. [Google Scholar] [CrossRef]
- Lin, M.; Hu, H.; Zhou, S.; Xu, S. Soft wearable devices for deep-tissue sensing. Nat. Rev. Mater. 2022, 7, 850–869. [Google Scholar] [CrossRef]
- Gao, W.; Ota, H.; Kiriya, D.; Takei, K.; Javey, A. Flexible electronics toward wearable sensing. Acc. Chem. Res. 2019, 52, 523–533. [Google Scholar] [CrossRef]
- Ahn, J.; Ahn, J.; Yoon, S.; Son, M.; Cho, S.; Oh, J. Quantification of non-alcoholic fatty liver disease progression in 3d liver microtissues using impedance spectroscopy. Biomaterials 2021, 268, 120599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, N.; Yetisen, A.K. Brain neurochemical monitoring. Biosens. Bioelectron. 2021, 189, 113351. [Google Scholar] [CrossRef]
- Hou, H.; Jin, Y.; Wei, H.; Ji, W.; Xue, Y.; Hu, J.; Zhang, M.; Jiang, Y.; Mao, L. A generalizable and noncovalent strategy for interfacing aptamers with a microelectrode for the selective sensing of neurotransmitters in vivo. Angew. Chem. Int. Ed. Engl. 2020, 59, 18996–19000. [Google Scholar] [CrossRef] [PubMed]
- Saizaki, T.; Kubo, M.; Sato, Y.; Abe, H.; Ohshiro, T.; Mushiake, H.; Sorin, F.; Guo, Y. The development of aptamer-coupled microelectrode fiber sensors (apta-?FS) for highly selective neurochemical sensing. Anal. Chem. 2023, 95, 6791–6800. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Lukas, H.; Wang, M.; Lee, Y.; Gao, W. Nucleic acid-based wearable and implantable electrochemical sensors. Chem. Soc. Rev. 2024, 53, 7960–7982. [Google Scholar] [CrossRef]




| Algorithm Type | Electrode Materials | Test Sample | Detection Target | Sensor Optimization Results | Reference |
|---|---|---|---|---|---|
| RF | Dithiobis(succinimidyl propionate)-modified gold microelectrode | urine | IL-6, IL-8 | Classification accuracy of 98.437% for disease status | [118] |
| Decision tree | Enzyme-based screen-printed electrode | serum | Glucose concentration | Decision criteria of sensor calibration parameters > 0.9; coefficient of determination for glucose concentration = 0.828 | [120] |
| RBF-ANN | Multi-walled carbon nanotube-ionic liquid co-modified graphite electrode | blood | Red blood cell count | Performance comparable to automated blood cell counters | [121] |
| XGBoost | Multi-walled carbon nanotube–Prussian blue–gold nanoparticle composite material | Escherichia coli | Antiseptic antimicrobial efficacy | Prediction error range of bacteriostatic concentration under varying inhibitor concentrations (0.46–4.95%) | [122] |
| SVM | Biomimetic peptide-modified screen-printed electrode | saliva | SARS-CoV-2 | Achieved 100% sensitivity, 80% specificity, and 90% accuracy in viral detection of infected saliva | [123] |
| KNN | Nanoporous polyamide flexible substrate integrated with dual gold electrodes | sweat | Corticosterone concentration | 100% accuracy achieved in detecting corticosterone concentration changes | [124] |
| ANNs | Electrospun barium titanate nanofiber film | skin-mounted wearable devices | Diagnosis of sarcopenia | Diagnostic accuracy of sarcopenia detection reaches 92.9% and 98.1% in male and female populations, respectively | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, X.; Wang, X.; Jiang, H. AI-Empowered Electrochemical Sensors for Biomedical Applications: Technological Advances and Future Challenges. Biosensors 2025, 15, 487. https://doi.org/10.3390/bios15080487
Liu Y, Liu X, Wang X, Jiang H. AI-Empowered Electrochemical Sensors for Biomedical Applications: Technological Advances and Future Challenges. Biosensors. 2025; 15(8):487. https://doi.org/10.3390/bios15080487
Chicago/Turabian StyleLiu, Yafeng, Xiaohui Liu, Xuemei Wang, and Hui Jiang. 2025. "AI-Empowered Electrochemical Sensors for Biomedical Applications: Technological Advances and Future Challenges" Biosensors 15, no. 8: 487. https://doi.org/10.3390/bios15080487
APA StyleLiu, Y., Liu, X., Wang, X., & Jiang, H. (2025). AI-Empowered Electrochemical Sensors for Biomedical Applications: Technological Advances and Future Challenges. Biosensors, 15(8), 487. https://doi.org/10.3390/bios15080487







