A Microfluidic Chip-Based Integrated Device Combining Aerosol Sampling and LAMP–CRISPR Detection for Airborne Virus Surveillance
Abstract
1. Introduction
2. Materials and Methods
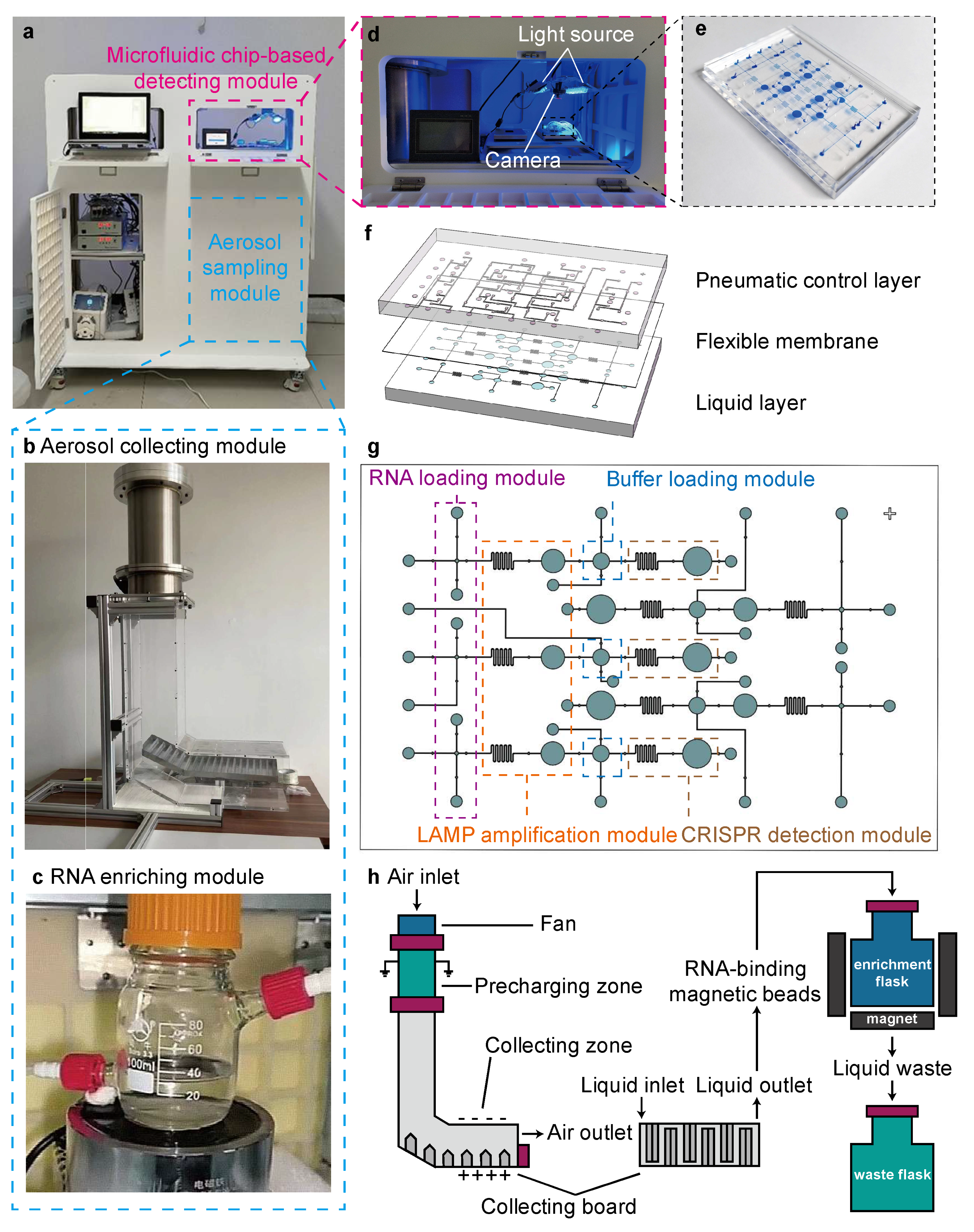
2.1. Fabrication Process of the MLCD Chip
2.2. Theoretical Calculation of the Aerosol Sampling System
2.3. Collection Efficiency Tests
2.4. RNA Sample Preparation
2.5. Aerosol Sampling-Detection Experiments
2.6. On-Chip Detection Experiments
2.7. Statistical Analysis
3. Results
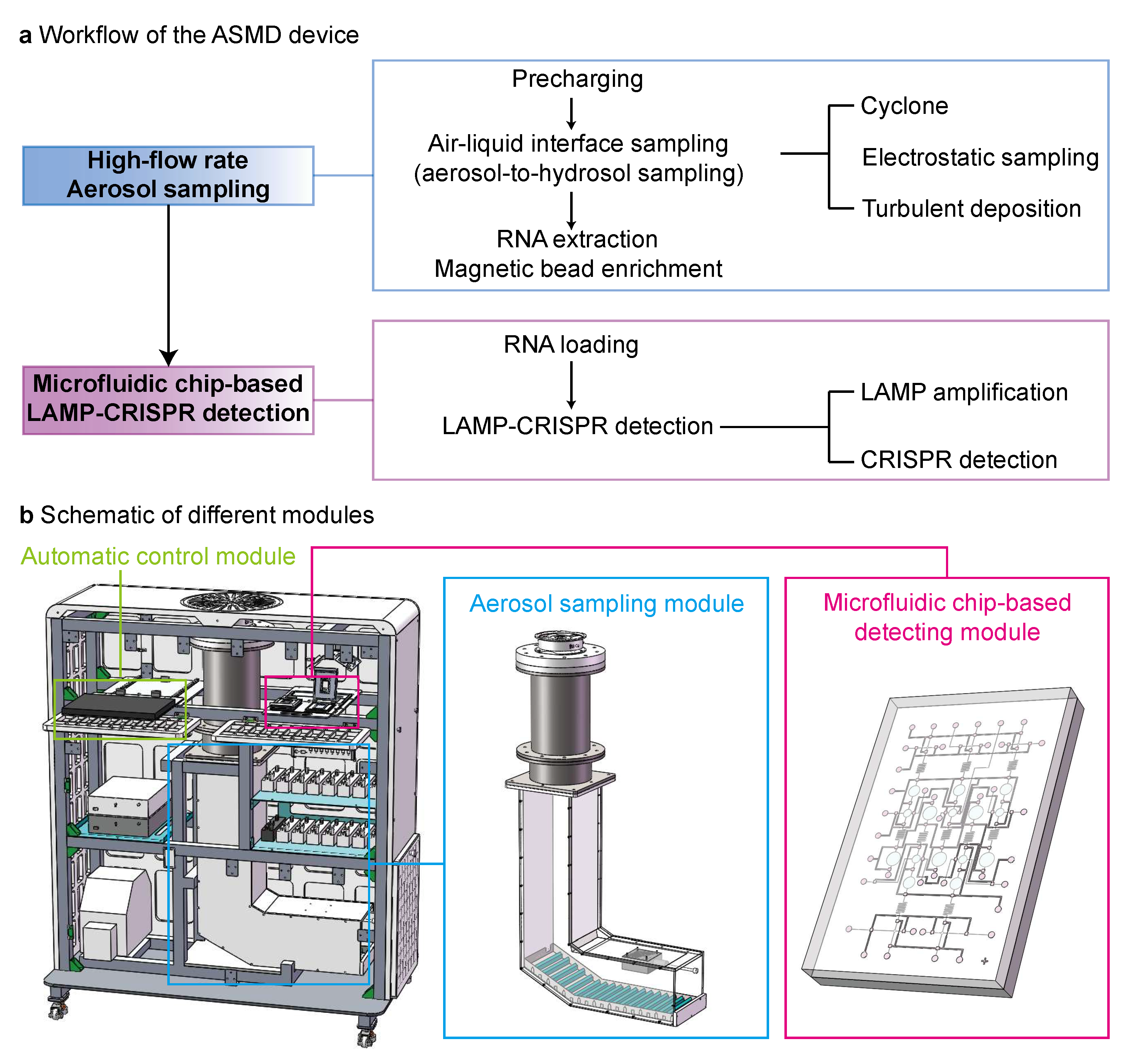
3.1. Combining Aerosol Sampling and Microfluidic Chip-Based LAMP–CRISPR Detection in a Multi-Module Device
3.2. Aerosol Sampling and On-Chip LAMP–CRISPR Detection Using the ASMD Device
4. Discussion
- The use of the high flow-rate aerosol sampling system, HFAS, expands the application scenarios of the device from limited room spaces to relatively large spaces, enabling long-term routine surveillance of SARS-CoV-2 aerosols in public places such as hotels or hospitals;
- The use of a specially designed microfluidic chip, MLCD, enables visualized LAMP–CRISPR detection of multiple SARS-CoV-2 variants on a single chip, which is compatible with the aerosol sampling-detection system;
- The combination of the aerosol sampler with the microfluidic chip leads to an all-in-one automatic aerosol sampling-SARS-CoV-2 detection system, minimizing loss of samples and reagents during the transfer between modules.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASMD | Microfluidic chip-based detection |
| HFAS | High-flow-rate aerosol sampling |
| MLCD | Microfluidic LAMP–CRISPR detection |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | Coronavirus disease 2019 |
| LAMP | Loop-mediated isothermal nucleic acid amplification |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
References
- Zhang, R.; Li, Y.; Zhang, A.L.; Wang, Y.; Molina, M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 14857–14863. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/table (accessed on 25 May 2025).
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Ang, A.X.; Luhung, I.; Ahidjo, B.A.; Drautz-Moses, D.I.; Tambyah, P.A.; Mok, C.K.; Lau, K.J.; Tham, S.M.; Chu, J.J.H.; Allen, D.M.; et al. Airborne SARS-CoV-2 surveillance in hospital environment using high-flowrate air samplers and its comparison to surface sampling. Indoor Air 2022, 32, e12930. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.R.; Farshidi, H.; Alipour, V.; Dindarloo, K.; Azad, M.H.; Jamalidoust, M.; Madani, A.; Aghamolaei, T.; Hashemi, Y.; Fazlzadeh, M.; et al. Detection of SARS-CoV-2 in the indoor air of intensive care unit (ICU) for severe COVID-19 patients and its surroundings: Considering the role of environmental conditions. Environ. Sci. Pollut. Res. Int. 2022, 29, 85612–85618. [Google Scholar] [CrossRef]
- Schuit, M.; Gardner, S.; Taylor, J.; Dabisch, P. Evaluation of four sampling devices for Burkholderia pseudomallei laboratory aerosol studies. PLoS Neglected Trop. Dis. 2021, 15, e0009001. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Spillmann, M.; Tang, J.; Zhao, Y.B.; Tao, Y.; Zhang, X.; Geschwindner, H.; Saleh, L.; Zingg, W.; Wang, J. On-Site Quantification and Infection Risk Assessment of Airborne SARS-CoV-2 Virus Via a Nanoplasmonic Bioaerosol Sensing System in Healthcare Settings. Adv. Sci. 2022, 9, e2204774. [Google Scholar] [CrossRef] [PubMed]
- Passos, R.G.; Silveira, M.B.; Abrahão, J.S. Exploratory assessment of the occurrence of SARS-CoV-2 in aerosols in hospital facilities and public spaces of a metropolitan center in Brazil. Environ. Res. 2021, 195, 110808. [Google Scholar] [CrossRef]
- Kim, H.R.; An, S.; Hwang, J. High air flow-rate electrostatic sampler for the rapid monitoring of airborne coronavirus and influenza viruses. J. Hazard. Mater. 2021, 412, 125219. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Zhang, Z.; Zhu, L.; Liu, Q. A rapid and label-free platform for virus enrichment based on electrostatic microfluidics. Talanta 2022, 242, 122989. [Google Scholar] [CrossRef]
- Hong, S.; Bhardwaj, J.; Han, C.H.; Jang, J. Gentle Sampling of Submicrometer Airborne Virus Particles using a Personal Electrostatic Particle Concentrator. Environ. Sci. Technol. 2016, 50, 12365–12372. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Kim, M.W.; Jang, J. Rapid Airborne Influenza Virus Quantification Using an Antibody-Based Electrochemical Paper Sensor and Electrostatic Particle Concentrator. Environ. Sci. Technol. 2020, 54, 10700–10712. [Google Scholar] [CrossRef]
- Santarpia, J.L.; Herrera, V.L.; Rivera, D.N.; Ratnesar-Shumate, S.; Reid, S.P.; Ackerman, D.N.; Denton, P.W.; Martens, J.W.S.; Fang, Y.; Conoan, N.; et al. The size and culturability of patient-generated SARS-CoV-2 aerosol. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 706–711. [Google Scholar] [CrossRef]
- Ratnesar-Shumate, S.; Bohannon, K.; Williams, G.; Holland, B.; Krause, M.; Green, B.; Freeburger, D.; Dabisch, P. Comparison of the performance of aerosol sampling devices for measuring infectious SARS-CoV-2 aerosols. Aerosol Sci. Technol. J. Am. Assoc. Aerosol Res. 2021, 55, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Kenarkoohi, A.; Noorimotlagh, Z.; Falahi, S.; Amarloei, A.; Mirzaee, S.A.; Pakzad, I.; Bastani, E. Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus. Sci. Total Environ. 2020, 748, 141324. [Google Scholar] [CrossRef]
- Faridi, S.; Niazi, S.; Sadeghi, K.; Naddafi, K.; Yavarian, J.; Shamsipour, M.; Jandaghi, N.Z.S.; Sadeghniiat, K.; Nabizadeh, R.; Yunesian, M.; et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 2020, 725, 138401. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Huang, C.H. High-efficiency electrical charger for nanoparticles. J. Nanopart. Res. 2015, 17, 332. [Google Scholar] [CrossRef]
- Priyamvada, H.; Kumaragama, K.; Chrzan, A.; Athukorala, C.; Sur, S.; Dhaniyala, S. Design and evaluation of a new electrostatic precipitation-based portable low-cost sampler for bioaerosol monitoring. Aerosol Sci. Technol. 2021, 55, 24–36. [Google Scholar] [CrossRef]
- Ma, Z.; Zheng, Y.; Cheng, Y.; Xie, S.; Ye, X.; Yao, M. Development of an integrated microfluidic electrostatic sampler for bioaerosol. J. Aerosol Sci. 2016, 95, 84–94. [Google Scholar] [CrossRef]
- Lu, H.; Lu, L. Effects of rib spacing and height on particle deposition in ribbed duct air flows. Build. Environ. 2015, 92, 317–327. [Google Scholar] [CrossRef]
- Hemmati, Y.; Rafee, R. Effects of the shape and height of artificial 2D roughness elements on deposition of nano and microparticles in the turbulent gas flow inside a horizontal channel. J. Aerosol Sci. 2018, 122, 45–58. [Google Scholar] [CrossRef]
- Zhong, X.; Chung, F.S.; Chan, K.C.; Chao, C.Y.H. Experimental study of particle deposition on patterned microstructured surfaces in a chamber environment. J. Aerosol Sci. 2021, 157, 105802. [Google Scholar] [CrossRef]
- Dritselis, C.D. Numerical study of particle deposition in a turbulent channel flow with transverse roughness elements on one wall. Int. J. Multiph. Flow 2017, 91, 1–18. [Google Scholar] [CrossRef]
- Lecrivain, G.; Sevan, D.M.; Thomas, B.; Hampel, U. Numerical simulation of multilayer deposition in an obstructed channel flow. Adv. Powder Technol. 2014, 25, 310–320. [Google Scholar] [CrossRef]
- Moitra, P.; Alafeef, M.; Dighe, K.; Ray, P.; Chang, J.; Thole, A.; Punshon-Smith, B.; Tolosa, M.; Ramamurthy, S.S.; Ge, X.; et al. Rapid and low-cost sampling for detection of airborne SARS-CoV-2 in dehumidifier condensate. Biotechnol. Bioeng. 2021, 118, 3029–3036. [Google Scholar] [CrossRef]
- Lednicky, J.A.; Lauzard, M.; Fan, Z.H.; Jutla, A.; Tilly, T.B.; Gangwar, M.; Usmani, M.; Shankar, S.N.; Mohamed, K.; Eiguren-Fernandez, A.; et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 2020, 100, 476–482. [Google Scholar] [CrossRef]
- Kwon, H.B.; Yoo, S.J.; Hong, U.S.; Kim, K.; Han, J.; Kim, M.K.; Kang, D.H.; Hwang, J.; Kim, Y.J. MEMS-based condensation particle growth chip for optically measuring the airborne nanoparticle concentration. Lab A Chip 2019, 19, 1471–1483. [Google Scholar] [CrossRef]
- Kwon, H.B.; Yoo, S.J.; Kim, Y.J. Microfluidic condensation nanoparticle counter using water as the condensing liquid for assessing individual exposure to airborne nanoparticles. Lab A Chip 2020, 20, 1092–1102. [Google Scholar] [CrossRef]
- Lim, J.H.; Nam, S.H.; Kim, J.; Kim, N.H.; Park, G.S.; Maeng, J.S.; Yook, S.J. High-volume sampler for size-selective sampling of bioaerosols including viruses. Atmos. Environ. 2021, 265, 118720. [Google Scholar] [CrossRef]
- Puthussery, J.V.; Ghumra, D.P.; McBrearty, K.R.; Doherty, B.M.; Sumlin, B.J.; Sarabandi, A.; Mandal, A.G.; Shetty, N.J.; Gardiner, W.D.; Magrecki, J.P.; et al. Real-time environmental surveillance of SARS-CoV-2 aerosols. Nat. Commun. 2023, 14, 3692. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, Y.; Li, W.; Wei, Z.; Tang, S.; Chen, R. Mechanisms, Techniques and Devices of Airborne Virus Detection: A Review. Int. J. Environ. Res. Public Health 2023, 20, 5471. [Google Scholar] [CrossRef]
- Li, B.; Lin, B.; Wang, Y.; Shi, Y.; Zeng, W.; Zhao, Y.; Gu, Y.; Liu, C.; Gao, H.; Cheng, H.; et al. Multi-scenario surveillance of respiratory viruses in aerosols with sub-single-copy spatial resolution. Nat. Commun. 2024, 15, 8770. [Google Scholar] [CrossRef]
- Xiong, H.; Ye, X.; Li, Y.; Qi, J.; Fang, X.; Kong, J. Efficient Microfluidic-Based Air Sampling/Monitoring Platform for Detection of Aerosol SARS-CoV-2 On-site. Anal. Chem. 2021, 93, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Lin, C.W.; Chuang, H.S. LAMP-Based Point-of-Care Biosensors for Rapid Pathogen Detection. Biosensors 2022, 12, 1068. [Google Scholar] [CrossRef] [PubMed]
- Selvam, K.; Najib, M.A.; Khalid, M.F.; Mohamad, S.; Palaz, F.; Ozsoz, M.; Aziah, I. RT-LAMP CRISPR-Cas12/13-Based SARS-CoV-2 Detection Methods. Diagnostics 2021, 11, 1646. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cao, X.; Meng, Y.; Richards, D.; Wu, J.; Ye, Z.; deMello, A.J. DropCRISPR: A LAMP-Cas12a based digital method for ultrasensitive detection of nucleic acid. Biosens. Bioelectron. 2022, 211, 114377. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Chen, X.; Zhang, X.; Zhu, J.; Li, S.; Wu, C.; Tian, Z.; Sui, G. Fully Automated CRISPR-LAMP Platform for SARS-CoV-2 Delta and Omicron Variants. Anal. Chem. 2022, 94, 15472–15480. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Li, S.; Wang, X.; Liu, G.; Yang, S.; Zhao, F.; Liu, Q.; Chen, X.; He, C.; et al. An integrated dual-layer microfluidic platform for multiple respiratory viruses screening. Anal. Chim. Acta 2023, 1242, 340812. [Google Scholar] [CrossRef]
- Zhang, K.; Xi, J.; Zhao, H.; Wang, Y.; Xue, J.; Liang, N.; Wei, Z. A dual-functional microfluidic chip for guiding personalized lung cancer medicine: Combining EGFR mutation detection and organoid-based drug response test. Lab A Chip 2024, 24, 1762–1774. [Google Scholar] [CrossRef]
- Zhang, K.; Xi, J.; Wang, Y.; Xue, J.; Li, B.; Huang, Z.; Zheng, Z.; Liang, N.; Wei, Z. A Microfluidic Chip-Based Automated System for Whole-Course Monitoring the Drug Responses of Organoids. Anal. Chem. 2024, 96, 10092–10101. [Google Scholar] [CrossRef]
- GB/T 39990-2021; Particulate—Bioaerosol Sampler—Technical Specification. State Administration for Market Regulation: Beijing, China, 2021. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=682B403A98DCF0AC2F5E91214C5D73DF&refer=outter (accessed on 3 July 2025).
- Yu, Z.; Xu, L.; Lyu, W.; Shen, F. Parallel multistep digital analysis SlipChip demonstrated with the quantification of nucleic acid by digital LAMP-CRISPR. Lab A Chip 2022, 22, 2954–2961. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.L.R. The dielectric strength of the vacuum: Electrostatic ionization gradient of metal electrodes. J. Am. Inst. Electr. Eng. 1922, 41, 852–853. [Google Scholar] [CrossRef]
- P-Trak Ultrafine Particle Counter 8525. Available online: https://tsi.com/products/indoor-air-quality-meters-instruments/indoor-air-quality-meters/p-trak-ultrafine-particle-counter-8525 (accessed on 21 June 2025).
- Niculescu, A.G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef] [PubMed]
- Safiabadi Tali, S.H.; LeBlanc, J.J.; Sadiq, Z.; Oyewunmi, O.D.; Camargo, C.; Nikpour, B.; Armanfard, N.; Sagan, S.M.; Jahanshahi-Anbuhi, S. Tools and Techniques for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 Detection. Clin. Microbiol. Rev. 2021, 34, e00228-20. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Chang, Y.; Li, W.; Zhang, Y.; Wang, Y.; Xie, H.; Zuo, T.; Zhang, Y.; Xi, J.; Wu, X.; et al. A Microfluidic Chip-Based Integrated Device Combining Aerosol Sampling and LAMP–CRISPR Detection for Airborne Virus Surveillance. Biosensors 2025, 15, 475. https://doi.org/10.3390/bios15080475
Zhang A, Chang Y, Li W, Zhang Y, Wang Y, Xie H, Zuo T, Zhang Y, Xi J, Wu X, et al. A Microfluidic Chip-Based Integrated Device Combining Aerosol Sampling and LAMP–CRISPR Detection for Airborne Virus Surveillance. Biosensors. 2025; 15(8):475. https://doi.org/10.3390/bios15080475
Chicago/Turabian StyleZhang, Anlan, Yuqing Chang, Wen Li, Yuanbao Zhang, Yuqian Wang, Haohan Xie, Tao Zuo, Yu Zhang, Jiyu Xi, Xin Wu, and et al. 2025. "A Microfluidic Chip-Based Integrated Device Combining Aerosol Sampling and LAMP–CRISPR Detection for Airborne Virus Surveillance" Biosensors 15, no. 8: 475. https://doi.org/10.3390/bios15080475
APA StyleZhang, A., Chang, Y., Li, W., Zhang, Y., Wang, Y., Xie, H., Zuo, T., Zhang, Y., Xi, J., Wu, X., Wei, Z., & Chen, R. (2025). A Microfluidic Chip-Based Integrated Device Combining Aerosol Sampling and LAMP–CRISPR Detection for Airborne Virus Surveillance. Biosensors, 15(8), 475. https://doi.org/10.3390/bios15080475




