Electrochemical (Bio)Sensors for Toxins, Foodborne Pathogens, Pesticides, and Antibiotics Detection: Recent Advances and Challenges in Food Analysis
Abstract
1. Introduction
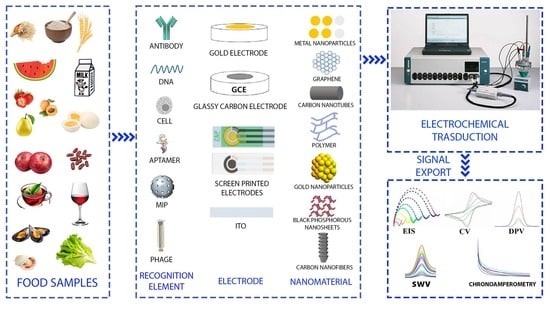
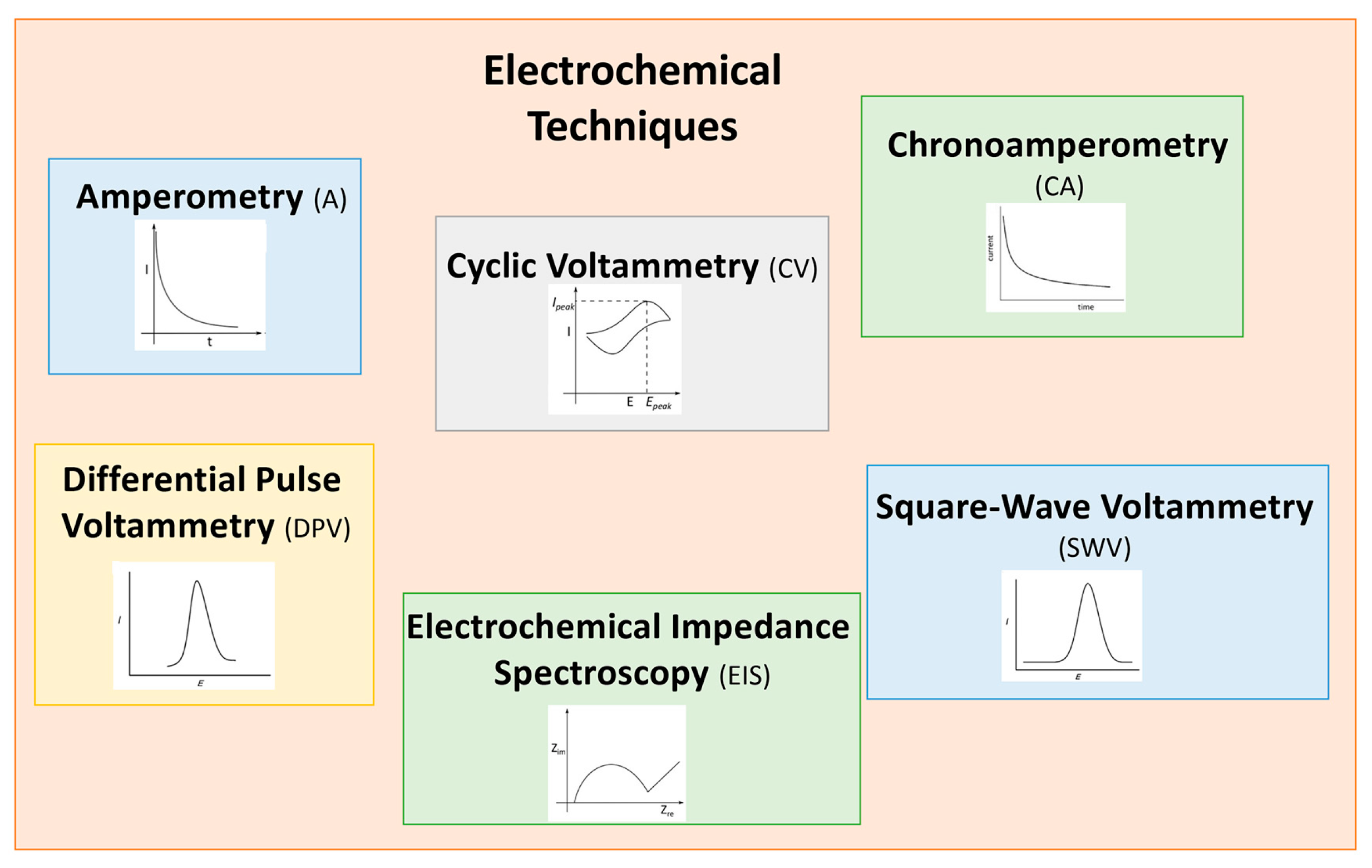
2. Electrochemical (Bio)Sensors
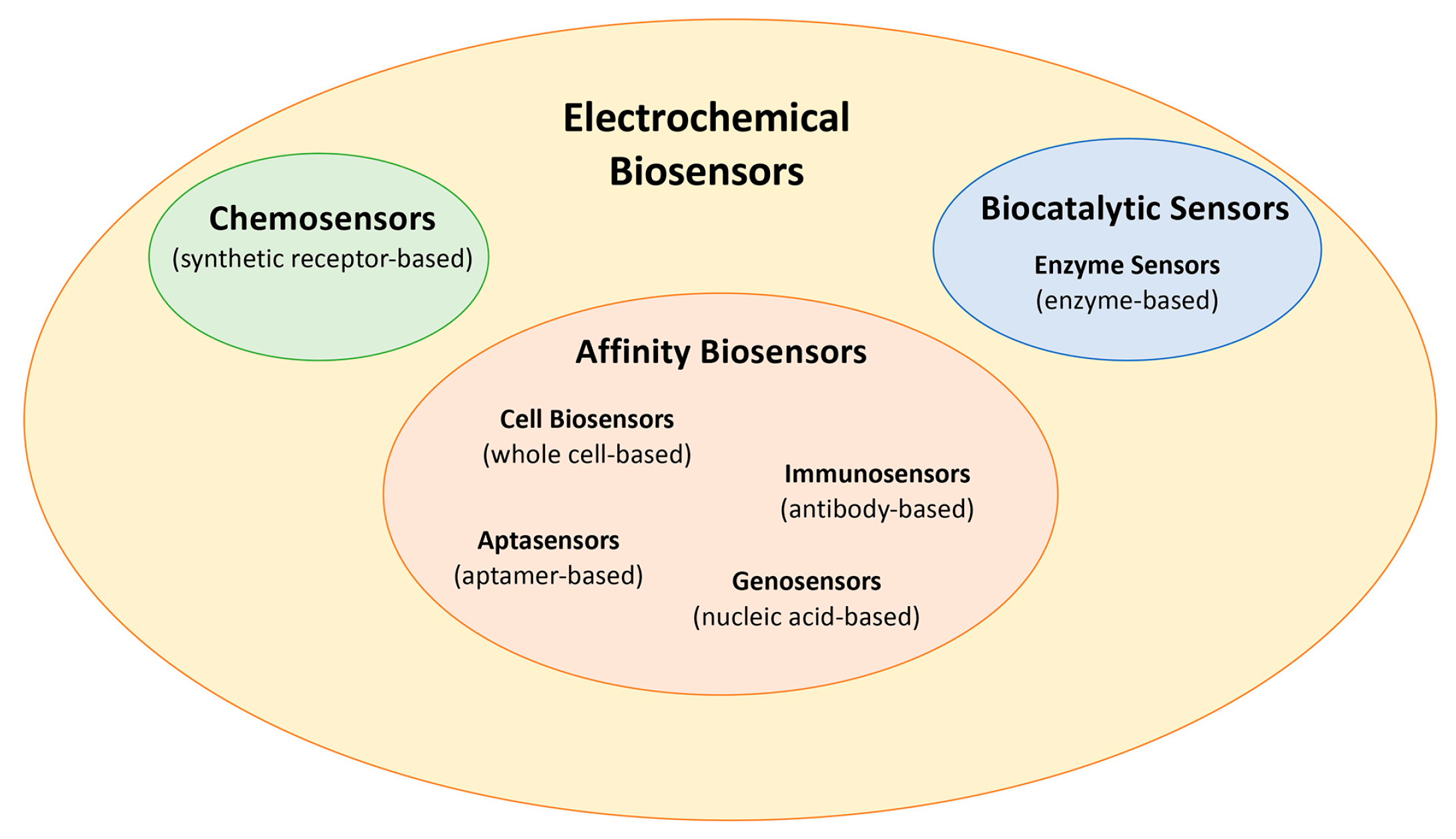
2.1. Biosensors Classification and (Bio)Recognition Element
2.2. Electrode Materials for Food Safety Analysis
3. Application of Electrochemical Biosensors in Food Analysis
3.1. Toxins
3.1.1. Pyrrolizidine Alkaloids (Pas)
3.1.2. Mycotoxins
3.1.3. Microcystins (MCs)
3.1.4. Marine Toxins
3.2. Foodborne Pathogens
3.2.1. Staphylococcus aureus
3.2.2. Listeria monocytogenes
3.2.3. Salmonella
3.2.4. Escherichia coli
3.3. Pesticides
Organophosphate Pesticides (OPs)
3.4. Antibiotics
4. Advances in Nanozyme-Based Electrochemical Sensors for Food Safety
4.1. Mycotoxins
4.2. Antibiotics
4.3. Foodborne Pathogens
4.4. Pesticides
5. Conclusions, Challenges, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D-NPS-doped CNS | 3D nitrogen-, phosphorus-, and sulfur-doped carbon nanosheets |
| A | Amperometry |
| AA | Ascorbic acid |
| AAs | aminoglycoside antibiotics |
| Ab | Antibody |
| AChE | Acetylcholinesterase |
| AF | Aflatoxin |
| AMP | antimicrobial peptide |
| ATCL | Acetylthiocholine chloride |
| AuE | Gold electrode |
| B | Biochar |
| BC | Bacterial cellulose |
| BChE | Butyrylcholinesterase |
| BHL | (S)-N-butyryl-L-homoserine lactone |
| BIP | bacteria-imprinted polymers |
| BPNS | Black phosphorus nanosheets |
| BUHNPS | Bimetallic silver-gold sea urchin-like hollow nanoparticles |
| CA | Chronoamperometry |
| CB | Carbon black |
| CBZ | Carbendazim |
| CDs | Carbon dots |
| CFGO | Conjugated carboxyl graphene oxide |
| CFP | Chlorpyrifos |
| CFU | Colony-forming unit |
| CHI | Chitosan |
| CJ | Campylobacter jejuni |
| CNF | Carbon nanofiber |
| CNTs | Carbon nanotubes |
| COF | Covalent organic framework |
| CQDs | Carbon quantum dots |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CS | Chondroitin sulfate |
| CV | coefficient of variation percentage |
| CV | Cyclic voltammetry |
| DA | Dopamine |
| DDVP | Dichlorvos |
| DFC | Diethofencarb |
| DMSO | Dimethyl sulfoxide |
| DON | Deoxynivalenol |
| DPV | Differential pulse voltammetry |
| dsDNA | Double-stranded DNA |
| E-AB | Methylene blue (MB)-modified aptamer |
| EDC | Entropy-driven amplification reaction |
| EFSA | European Food Safety Authority |
| EIS | Electrochemical impedance spectroscopy |
| ELISA | Enzyme-linked immunosorbent assay |
| ErGO | Electrochemically reduced graphene oxide |
| ERY | Erythromycin |
| Exo III | Exonuclease III |
| f-BN | functionalized boron nitride |
| FM | Fumonisin |
| g-C3N4 | Graphitic carbon nitride |
| GC | Gas chromatography |
| GCE | Glassy carbon electrode |
| GLP | Glyphosate, N-(phosphonomethyl) glycine |
| GO | Graphene oxide |
| HCR | Hybrid chain reaction |
| hp DNA | Hairpin DNA |
| HPG | Highly porous gold |
| HPLC | High-pressure liquid chromatography |
| HS hpDNA | Hydrosulfuryl-modified hairpin DNA |
| IL | Ionic liquid |
| ITOE | Indium tin oxide electrode |
| IUPAC | International union of pure and applied chemistry |
| L | Leucine |
| L.M. | Listeria monocytogenes |
| LC–MS | Liquid chromatography- mass spectrometry |
| LOD | Limit of detection |
| LSV | Linear sweep voltammetry |
| mAb | Monoclonal antibody |
| MAL | Malathion |
| MB | Methylene blue |
| MC | Microcystin |
| Mfps | Marine mussel foot proteins |
| MIP | Molecularly imprinted polymer |
| MOF | Metal–organic framework |
| NEMA | Nicking enzyme-mediated |
| NPs | Nanopartcles |
| NRs | Nanorods |
| NW | Nanowire |
| OA | Okadaic acid |
| OPs | Organophosphate pesticides |
| OT | Ochratoxin |
| OTC | Oxytetracycline |
| PAs | Pyrrolizidine alkaloids |
| PAT | Patulin |
| PB | Prussian blue |
| PCR | Polymerase chain reaction |
| PDA | Polydopamine |
| PEF | Pefloxacin |
| PEI | Polyethyleneimine |
| PGA | Polyglutamic acid |
| PGE | Pencil graphite electrode |
| PLL | Poly(lysine) |
| PPY | Polypyrrole |
| PSA | Pseudomonas aeruginosa |
| PZDA | 3,5-pyrazoledicarboxylic acid |
| QSS | Quorum-sensing system |
| R | Arginine |
| RBP | Receptor-binding phage |
| RBPP | Receptor-binding phage proteins |
| RCA | Rolling circle amplification |
| RPA | Recombinase polymerase amplification |
| RSD | Relative standard deviation |
| RT-qPCR | Quantitative reverse transcription polymerase chain reaction |
| SA | Salmonella |
| scMOF | Semiconductive metal–organic framework |
| SDZ | Sulfadiazine |
| SEN | Senecionine |
| SPAuE | Screen-printed gold electrode |
| SPCE | Screen-printed carbon electrode |
| SPE | Screen-printed electrode |
| SRCA | Saltatory rolling circle amplification |
| ssDNA | Single-stranded DNA |
| ST | Staphylococcus aureus |
| SWASV | Square-wave anodic stripping voltammetry |
| SWCNTs | Single-walled carbon nanotubes |
| SWV | Square-wave voltammetry |
| TA | Tannic acid |
| TBZ | Thiabendazole |
| TC | Tetracycline |
| TDN | tetrahedral scaffold |
| TICA | Trigging isothermal amplification |
| TM | Ti3C2 MXene |
| TMD | Transition metal dichalcogenide |
| TTX | Tetrodotoxin |
| UA | Uric acid |
| USFDA | United States Food and Drug Administration |
| VP | Vibrio parahaemolyticus |
| WHO | World Health Organization |
| β-CD | β-cyclodextrin |
References
- Curulli, A. Electrochemical Biosensors in Food Safety: Challenges and Perspectives. Molecules 2021, 26, 2940. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lin, X.; Zhang, M.; Li, Y.; Luo, C.; Wu, J. Review of Electrochemical Biosensors for Food Safety Detection. Biosensors 2022, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Shruti, A.; Bage, N.; Kar, P. Nanomaterials based sensors for analysis of food safety. Food Chem. 2024, 433, 137284. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Strategy for Food Safety 2022–2030: Towards Stronger Food Safety Systems and Global Cooperation; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240057685 (accessed on 24 March 2025).
- International Finance Corporation. Food Safety Handbook: A Practical Guide for Building a Robust Food Safety Management System; World Bank: Washington, DC, USA, 2020. [Google Scholar] [CrossRef]
- USFDA. 2022 Food Code Health Recommendations of the United States Public Service Food and Drug Administration; Department of Health and Human Services, US FDA, US Public Health Services College Park: Silver Spring, MD, USA, 2022. Available online: https://www.fda.gov/food/fda-food-code/food-code-2022 (accessed on 1 April 2025).
- Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No. 1881/2006. Official Journal of the European Union; pp. 104–119. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj/eng (accessed on 1 April 2025).
- Curulli, A. Recent Advances in Electrochemical Sensing Strategies for Food Allergen Detection. Biosensors 2022, 12, 503. [Google Scholar] [CrossRef]
- Elumalai, A.; Natarajan, V. Advancements in analytical technologies for ensuring food quality and authentication: A comprehensive review. J. Food Compos. Anal. 2025, 139, 107075. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, C.; Long, Y.; Weimin Zhang, Q.-C.; Liu, G. Food additives: From functions to analytical methods. Crit. Rev. Food Sci. Nutr. 2022, 62, 8497–8517. [Google Scholar] [CrossRef]
- Tarannum, N.; Gautam, A.; Chauhan, T.; Kumar, D. Nanomaterial based sensors for detection of food contaminants: A prospect. Sens. Technol. 2024, 2, 2373196. [Google Scholar] [CrossRef]
- Fuente-Ballesteros, A.; Ares, A.M.; Bernal, J. Paving the way towards green contaminant analysis: Strategies and considerations for sustainable analytical chemistry. Green Anal. Chem. 2025, 12, 100221. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Pingarron, J.M.; Labuda, J.; Barek, J.; Brett, C.M.A.; Camoes, M.F.; Fojta, M.; Hibbert, D.B. Terminology of electrochemical methods of analysis (IUPAC Recommendations 2019). Pure Appl. Chem. 2020, 92, 641–694. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Leech, D. Affinity Biosensors. Chem. Soc. Rev. 1994, 23, 205–213. [Google Scholar] [CrossRef]
- Ul Haq, I.; Rahim, K.; Maryam, S.; Parre Paker, N. Bacteriophage-based biosensors technology: Materials, fabrications, efficiencies and shortcomings. Biotechnol. Rep. 2025, 45, e00872. [Google Scholar]
- Petrucci, R.; Pasquali, M.; Scaramuzzo, F.A.; Curulli, A. Recent Advances in Electrochemical Chitosan-Based Chemosensors and Biosensors: Applications in Food Safety. Chemosensors 2021, 9, 254. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry, 2nd ed.; Wiley/VCH: New York, NY, USA, 2000. [Google Scholar]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and-Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef] [PubMed]
- Bockris, J.O.M.; Reddy, A.K.N.; Gamboa-Aldeco, M. Modern Electrochemistry 2A: Fundamentals of Electrodics, 2nd ed.; Kluwer Academic: New York, NY, USA; Plenum Publishers: New York, NY, USA, 2000; Volume 2. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Mizuhashi, M. Electrical Properties of Vacuum-Deposited Indium Oxide and Indium Tin Oxide Films. Thin Solid Film. 1980, 70, 91–100. [Google Scholar] [CrossRef]
- Benck, J.D.; Pinaud, B.A.; Gorlin, Y.; Jaramillo, T.F. Substrate Selection for Fundamental Studies of Electrocatalysts and Photoelectrodes Inert Potential Windows in Acidic, Neutral, and Basic Electrolyte. PLoS ONE 2014, 9, e107942. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Raghavender Suresh, R.; Lakshmanakumar, M.; Arockia Jayalatha, J.B.B.; Rajan, K.S.; Sethuraman, S.; Maheswari Krishnan, U.; Balaguru Rayappan, J.B. Fabrication of screen-printed electrodes: Opportunities and challenges. J. Mater. Sci. 2021, 56, 8951–9006. [Google Scholar] [CrossRef]
- Beitollahi, H.; Mohammadi, S.Z.; Safaeia, M.; Tajik, S. Applications of electrochemical sensors and biosensors based on modified screen-printed electrodes: A review. Anal. Methods 2020, 12, 1547–1560. [Google Scholar] [CrossRef]
- Curulli, A. Functional Nanomaterials Enhancing Electrochemical Biosensors as Smart Tools for Detecting Infectious Viral Diseases. Molecules 2023, 28, 3777. [Google Scholar] [CrossRef]
- Petrucci, R.; Bortolami, M.; Di Matteo, P.; Curulli, A. Gold Nanomaterials-Based Electrochemical Sensors and Biosensors for Phenolic Antioxidants Detection: Recent Advances. Nanomaterials 2022, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Rocco, D.; Moldoveanu, V.G.; Feroci, M.; Martina Bortolami, M.; Vetica, F. Electrochemical Synthesis of Carbon Quantum Dots. ChemElectroChem 2023, 10, e202201104. [Google Scholar] [CrossRef] [PubMed]
- Bortolami, M.; Curulli, A.; Di Matteo, P.; Petrucci, R.; Feroci, M. Carbon Dots in Enantioselective Sensing. Sensors 2024, 24, 3945. [Google Scholar] [CrossRef] [PubMed]
- Lei, O.; Liu, C.; Nan, X.; Zhu, Y.; Fu, L.; Lin, X.; Zhang, H.; Yang, M.; Fang, X.; Luo, Y.; et al. Carbon dots-based electrochemical and fluorescent biosensors for the detection of foodborne pathogens: Current advance and challenge. Coord. Chem. Rev. 2025, 529, 216457. [Google Scholar] [CrossRef]
- Nguyen, D.H.H.; El-Ramady, H.; Prokisch, J. Food safety aspects of carbon dots: A review. Environ. Chem. Lett. 2025, 23, 337–360. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Baig, N. Two-dimensional nanomaterials: A critical review of recent progress, properties, applications, and future directions. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107362. [Google Scholar] [CrossRef]
- Di Matteo, P.; Petrucci, R.; Curulli, A. Not Only Graphene Two-Dimensional Nanomaterials: Recent Trends in Electrochemical (Bio)sensing Area for Biomedical and Healthcare Applications. Molecules 2024, 29, 172. [Google Scholar] [CrossRef]
- Raja, I.S.; Vedhanayagam, M.; Preeth, D.R.; Kim, C.; Lee, J.H.; Han, D.W. Development of Two-Dimensional Nanomaterials Based Electrochemical Biosensors on Enhancing the Analysis of Food Toxicants. Int. J. Mol. Sci. 2021, 22, 3277. [Google Scholar] [CrossRef]
- Manoj, D.; Rajendran, S.; Murphy, M.; Jalil, A.A.; Sonne, C. Recent progress and perspectives of metal organic frameworks (MOFs) for the detection of food contaminants. Chemosphere 2023, 340, 139820. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.-N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2022, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Bhanderi, D.; Lakhani, P.; Modi, C.K. Graphitic carbon nitride (g-C3N4) as an emerging photocatalyst for sustainable environmental applications: A comprehensive review. RSC Sustain. 2024, 2, 265. [Google Scholar] [CrossRef]
- Geng, L.; Huang, J.; Fang, M.; Wang, H.; Liu, J.; Wang, G.; Hu, M.; Sun, J.; Guo, Y.; Sun, X. Recent progress of the research of metal-organic frameworks-molecularly imprinted polymers (MOFs-MIPs) in food safety detection field. Food Chem. 2024, 458, 140330. [Google Scholar] [CrossRef] [PubMed]
- Dayananda, N.R. Natural Toxins in Diverse Foodstuffs and Foodomics. In Biotoxins: Biotechnological and Therapeutic Applications; Narasimha, M., Sarada Devi Tetali, V.P., Bennetau-Pelissero, C., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 217–288. [Google Scholar]
- Lis-Cieplak, A.; Trzesniowska, K.; Stolarczyk, K.; Stolarczyk, E.U. Pyrrolizidine Alkaloids as Hazardous Toxins in Natural Products: Current Analytical Methods and Latest Legal Regulations. Molecules 2024, 29, 3269. [Google Scholar] [CrossRef]
- EFSA Opinion of the scientific panel on contaminants in the food chain on a request from the European Commission related to pyrrolizidine alkaloids as undesirable substances in animal feed. EFSA J. 2007, 5, 447.
- Senturk, H.; Eksin, E.; Zeybek, U.; Erdem, A. Detection of Senecionine in Dietary Sources by Single-Use Electrochemical Sensor. Micromachines 2021, 12, 1585. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Palumbo, R.; Crisci, A.; Venancio, A.; Cortinas Abrahantes, J.; Dorne, J.-L.; Battilani, P.; Toscano, P. Occurrence and Co-Occurrence of Mycotoxins in Cereal-Based Feed and Food. Microorganisms 2020, 8, 74. [Google Scholar] [CrossRef]
- da Silva, J.L.; Oreste, E.Q.; Dias, D.; Garda-Bffon, J. Electrochemistry Applied to Mycotoxin Determination in Food and Beverages. Food Anal. Methods 2023, 16, 541–566. [Google Scholar] [CrossRef]
- Zhai, W.; Wei, D.; Cao, M.; Wang, Z.; Wang, M. Biosensors based on core–shell nanoparticles for detecting mycotoxins in food: A review. Food Chem. 2023, 429, 136944. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Alhachami, F.R.; Imran, K.; Majdi, H.S.; Nisar, N.; Yaser, M.H.; Sivaraman, R.; Romero Parra, R.-M.; Al Mashhadani, Z.I.; Fakri, M.Y. Recent Progress in Aptamer-Functionalized Metal-Organic Frameworks-Based Optical and Electrochemical Sensors for Detection of Mycotoxins. Crit. Rev. Anal. Chem. 2024, 54, 1707–1728. [Google Scholar] [CrossRef] [PubMed]
- Jubeen, F.; Batool, A.; Naz, I.; Sehar, S.; Sadia, H.; Hayat, A.; Kazi, M. Mycotoxins detection in food using advanced, sensitive and robust electrochemical platform of sensors: A review. Sens. Actuators A Phys. 2024, 367, 115045. [Google Scholar] [CrossRef]
- Afzali, Z.; Mohadesi, A.; Karimi, M.A.; Fathirad, F. A highly selective and sensitive electrochemical sensor based on graphene oxide and molecularly imprinted polymer magnetic nanocomposite for patulin determination. Microchem. J. 2022, 177, 107215. [Google Scholar] [CrossRef]
- Razaa, A.; Niazia, S.; Shoaibe, M.; Khand, I.M.; Ul, H.-F.; Alia, K.; Khand, I.; Zhang, Y.; Wang, Z. T-2 mycotoxin: From occurrence and toxicokinetics to recent advances in aptasensor-based detection strategies and future perspectives for enhanced food safety. Trends Food Sci. Technol. 2025, 156, 104784. [Google Scholar] [CrossRef]
- Fernandez Solis, L.N.; Silva, G.J., Jr.; Bertotti, M.; Angnes, L.; Pereira, S.V.; Fernandez-Baldo, M.A.; Regiart, M. Electrochemical microfluidic immunosensor with graphene-decorated gold nanoporous for T-2 mycotoxin detection. Talanta 2024, 273, 125971. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, B.; Cui, X.; Li, Y.; Tang, J.; Wang, H.; Zhang, D.; Li, Z. Recent advances in aptasensors for mycotoxin detection: On the surface and in the colloid. Talanta 2021, 223, 121729. [Google Scholar] [CrossRef]
- Wang, N.; Hu, W.; Jiang, H.; Jiang, D.; Wang, L. A portable micro-nanochannel bio-3D printed liver microtissue biosensor for DON detection. Biosens. Bioelectron. 2025, 267, 116810. [Google Scholar] [CrossRef]
- Ozcelikay, G.; Cetinkaya, A.; Kaya, S.I.; Yence, M.; Canavar Eroğlu, P.E.; Unal, M.A.; Ozkan, S.A. Novel Sensor Approaches of Aflatoxins Determination in Food and Beverage Samples. Crit. Rev. Anal. Chem. 2022, 54, 982–1001. [Google Scholar] [CrossRef]
- Doddanagowada, S.; Palakollu, V.N.; Prabhakar Vattikuti, S.V.; Shim, J.; Mameda, N. Recent progress, challenges, and future perspectives of electrochemical biosensing of aflatoxins. Microchim. Acta 2025, 192, 17. [Google Scholar]
- Zhong, T.; Li, S.; Li, X.; JiYe, Y.; Mo, Y.; Chen, L.; Zhang, Z.; Wu, H.; Meiliang, L.; Luo, Q. A label-free electrochemical aptasensor based on AuNPs-loaded zeolitic imidazolate framework-8 for sensitive determination of aflatoxin B1. Food Chem. 2022, 384, 132495. [Google Scholar] [CrossRef] [PubMed]
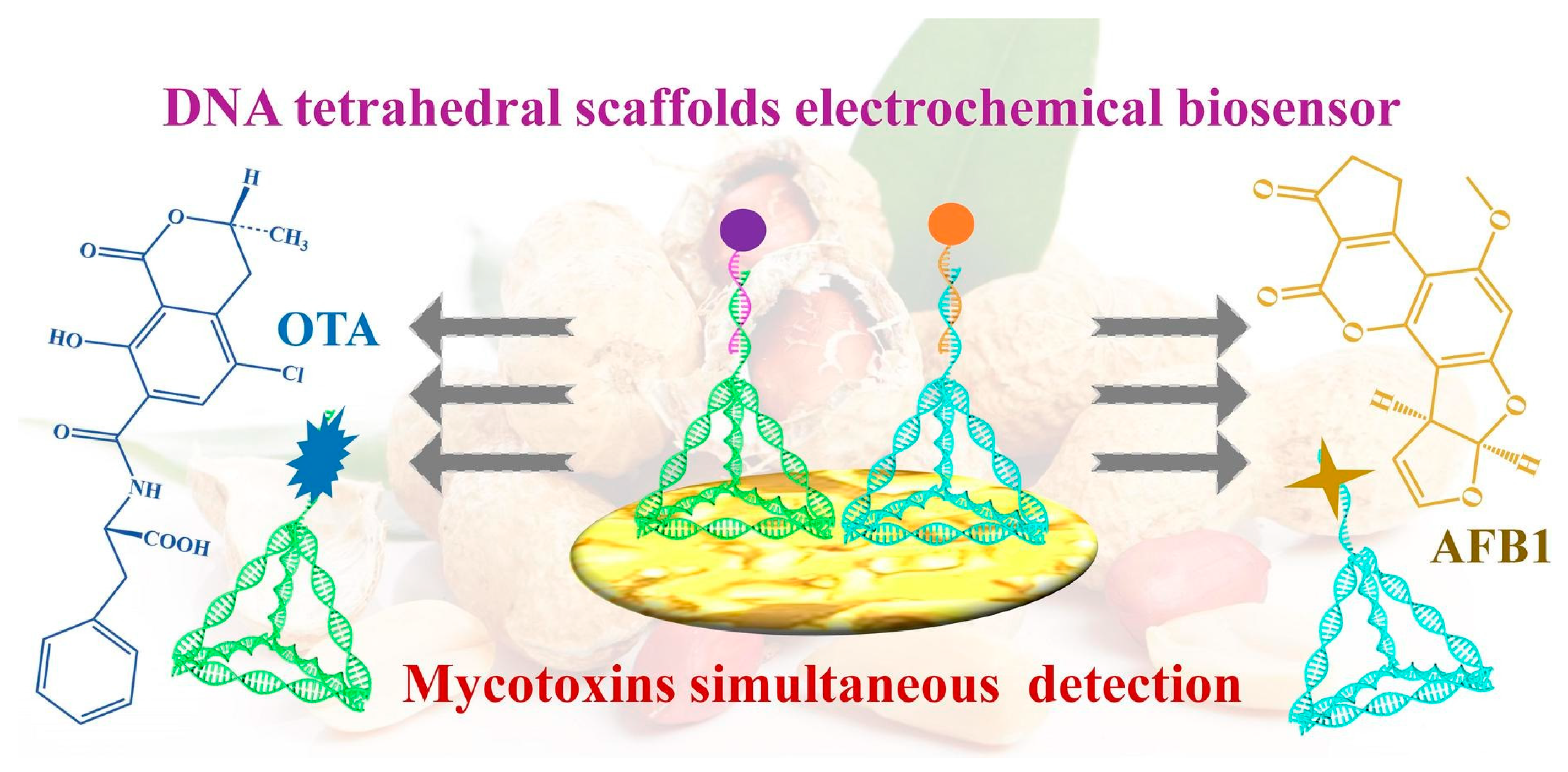
- Li, W.; Shi, Y.; Zhang, X.; Hu, X.; Huang, X.; Liang, N.; Shen, T.; Zou, X.; Shi, J. A DNA tetrahedral scaffolds-based electrochemical biosensor for the simultaneous detection of AFB1 and OTA. Food Chem. 2024, 442, 138312. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, H.; Majidi, M.R.; Arbabzadeh, O.; Khaaki, P.; Pourmohammad, S.; Khataee, A.; Orooji, Y. Recent advances in the highly sensitive determination of zearalenone residues in water and environmental resources with electrochemical biosensors. Environ. Res. 2022, 204, 112082. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, M.; Li, Z.; Liao, W.; Chen, B.; Yang, T.; Hu, R.; Yang, Y.; Meng, S. Highly sensitive and convenient aptasensor based on Au NPs@Ce-TpBpy COF for quantitative determination of Zearalenone. RSC Adv. 2022, 12, 17312. [Google Scholar] [CrossRef]
- Zheng, Q.; Chen, Y.; Cao, Y.; He, Y.; Lu, K.; Jia, N. U-disk portable photoelectrochemical sensor based on bifunctional poly (o-phenylenediamine) for on-site detection of erythromycin. Sens. Actuators B Chem. 2024, 408, 135531. [Google Scholar] [CrossRef]
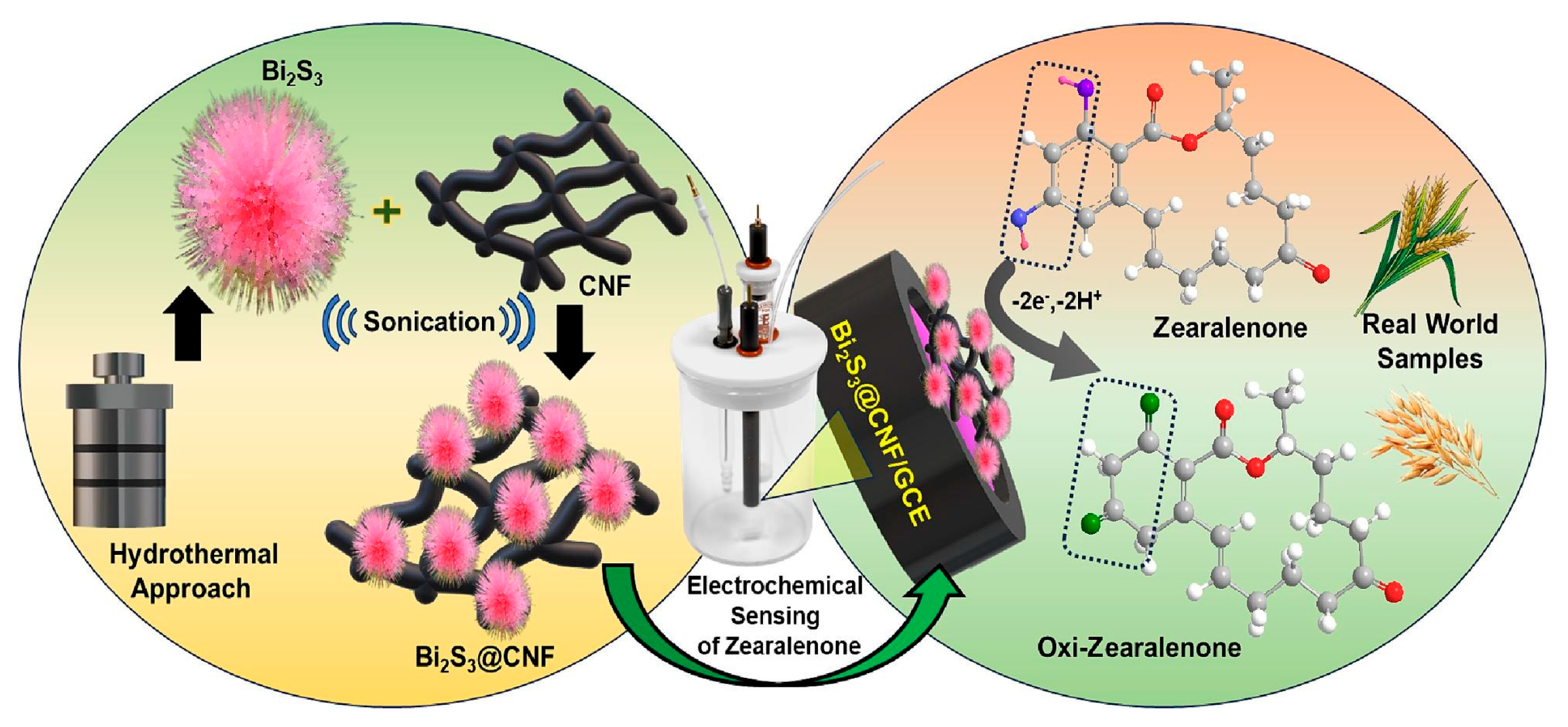
- Huang, S.-J.; Gokulkumar, K.; Mani, G.; Lee, Y.-Y.; Kogularasu, S.; Chang-Chien, G.-P. Synthesis and characterization of Bi2S3-embedded carbon nanofibers as a novel electrochemical biosensor for the detection of mycotoxin zearalenone in food crops. FlatChem 2024, 45, 100652. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, X.; Deng, B.; Chen, H.; Wu, S.; Wu, Y.; Wang, Y.; Ning, G. Dual-signal ratiometric electrochemical aptasensor for Zearalenone detection based on magnetic-assisted enrichment and hybridization chain reaction. Food Chem. 2025, 465, 141963. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Qi, C.; Xu, H.; Lu, X.; Ma, X.; Zhang, W. Detection of zearalenone by electrochemical aptasensor based on enzyme- target recycling assisted and DNAzymes release strategy. Talanta 2025, 286, 127533. [Google Scholar] [CrossRef]
- Dutta, T.; Mandal, S.K.; Biswas, J.K. Natural Biosensors for the Detection of Biotoxins in Finfish and Shellfish. In Biotoxins: Biotechnological and Therapeutic Applications; Narasimha, M., Sarada Devi Tetali, V.P., Bennetau-Pelissero, C., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 27–53. [Google Scholar]
- Wei, X.; Wang, S.; Zhan, Y.; Kai, T.; Ding, P. Sensitive Identification of Microcystin-LR via a Reagent-Free and Reusable Electrochemical Biosensor Using a Methylene Blue-Labeled Aptamer. Biosensors 2022, 12, 556. [Google Scholar] [CrossRef]
- Stoll, S.; Hwang, J.-H.; Fox, D.W.; Kim, K.; Zhai, L.; Lee, W.H. Cost-effective screen-printed carbon electrode biosensors for rapid detection of microcystin-LR in surface waters for early warning of harmful algal blooms. Environ. Sci. Pollut. Res. 2023, 30, 124854–124865. [Google Scholar] [CrossRef] [PubMed]
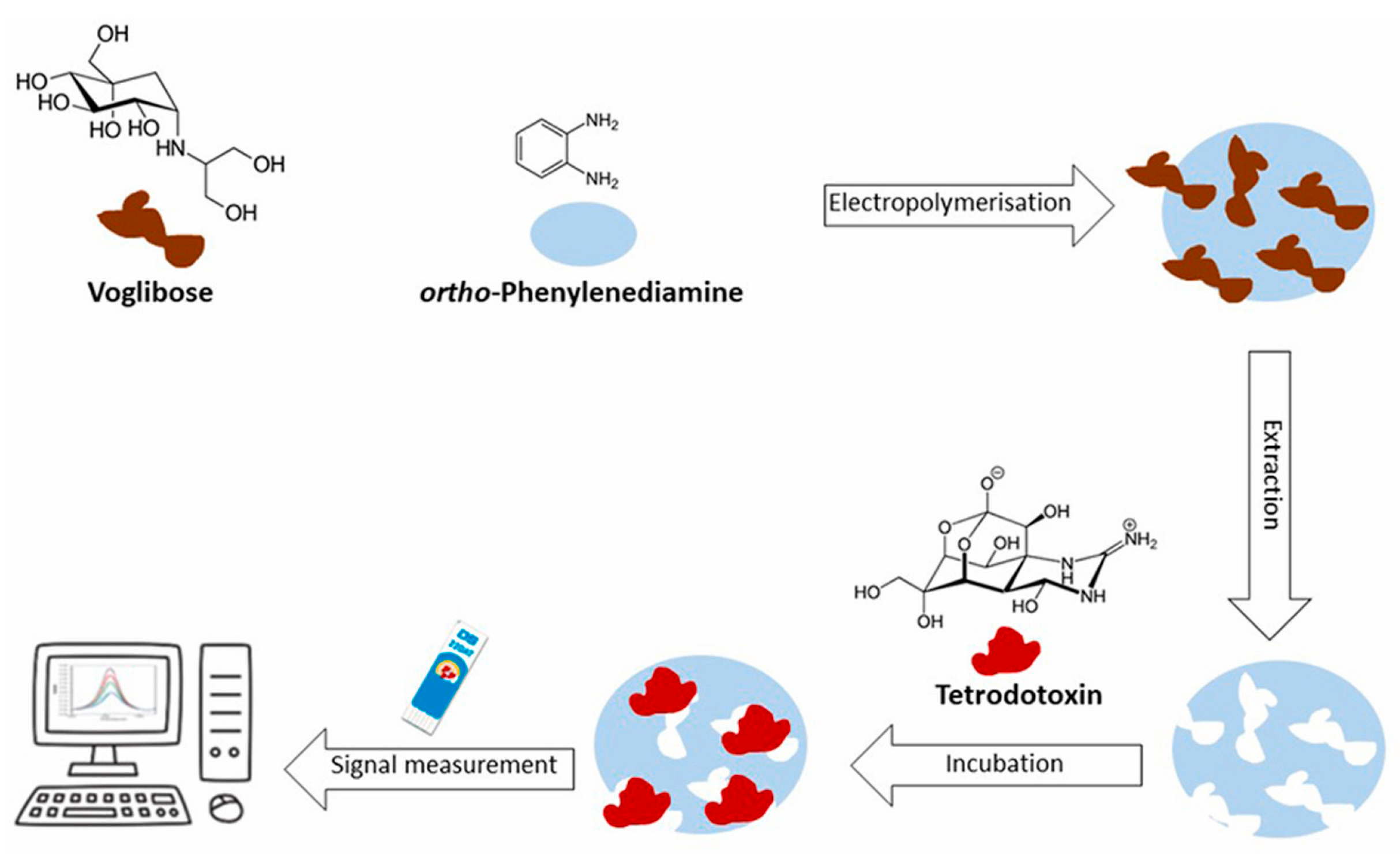
- Rocha, P.; Rebelo, P.; Pacheco, J.G.; Geraldo, D.; Bento, F.; Leao-Martins, J.M.; Delerue-Matos, C.; Nouws, H.P.A. Electrochemical molecularly imprinted polymer sensor for simple and fast analysis of tetrodotoxin in seafood. Talanta 2025, 282, 127002. [Google Scholar] [CrossRef] [PubMed]
- Min Kim, S.; Xu, P.; Seop Hyun, M.; Pil Park, J.; Yeong Park, C.; Jung Park, T. Development of an Electrochemical Biosensor for Tetrodotoxin Using Specific Binding Peptide on Polypyrrole/Au Nanoparticle-Modified Electrodes. BioChip J. 2024, 18, 495–504. [Google Scholar] [CrossRef]
- Janczuk, M.; Niedziółka-Jönsson, J.; Szot-Karpinska, K. Bacteriophages in electrochemistry: A review. J. Electroanal. Chem. 2016, 779, 207–219. [Google Scholar] [CrossRef]
- Zeng, N.; Wang, X.; Dong, Y.; Yang, Y.; Yin, Y.; Zhao, L.; Wang, X. Aptasensor Based on Screen-Printed Carbon Electrodes Modified with CS/AuNPs for Sensitive Detection of Okadaic Acid in Shellfish. J. Anal. Test. 2023, 7, 128–135. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kumar Kaushik, B. Trends, challenges, and advances in optical sensing for pathogenic bacteria detection (PathoBactD). Biosens. Bioelectron. X 2023, 14, 100352. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, Z.; Guo, Q.; Weng, X. Microfluidic thread-based electrochemical aptasensor for rapid detection of Vibrio parahaemolyticus. Biosens. Bioelectron. 2021, 182, 113191. [Google Scholar] [CrossRef]
- Suganthan, B.; Rogers, A.M.; Crippen, C.S.; Asadi, H.; Zolti, O.; Szymanski, C.M.; Ramasamy, R.P. A Bacteriophage Protein-Based Impedimetric Electrochemical Biosensor for the Detection of Campylobacter jejuni. Biosensors 2024, 14, 402. [Google Scholar] [CrossRef]
- Patel, D.; Zhou, Y.; Ramasamy, R.P. A bacteriophage-based electrochemical biosensor for detection of methicillin-resistant Staphylococcus aureus. J. Electrochem. Soc. 2021, 168, 057523. [Google Scholar] [CrossRef]
- Zolti, O.; Suganthan, B.; Nagdeve, S.N.; Maynard, R.; Locklin, J.; Ramasamy, R.P. Investigation of the Efficacy of a Listeria monocytogenes Biosensor Using Chicken Broth Samples. Sensors 2024, 24, 2617. [Google Scholar] [CrossRef]
- Frigoli, M.; Lowdon, J.W.; Donetti, N.; Crapnell, R.D.; Banks, C.E.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Electrochemical Detection of Pseudomonas aeruginosa Quorum Sensing Molecule (S)-N-Butyryl Homoserine Lactone Using Molecularly Imprinted Polymers. ACS Omega 2024, 9, 36411–36420. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q. Seizing the Hidden Assassin: Current Detection Strategies for Staphylococcus aureus and Methicillin-Resistant S. aureus. J. Agric. Food Chem. 2024, 72, 16569–16582. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dou, L.; Zhang, Y.; Luo, L.; Yang, H.; Wen, K.; Yu, X.; Shen, J.; Wang, Z. A comprehensive review on the detection of Staphylococcus aureus enterotoxins in food samples. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13264. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, R.; Wang, G.; Liu, S. An electrochemical biosensor for Staphylococcus aureus detection based on a multilevel surface 3D micro/nanostructure. Analyst 2024, 149, 2594. [Google Scholar] [CrossRef]
- Zhen, D.; Zhang, S.; Yang, A.; Ma, Q.; Deng, Z.; Fang, J.; Cai, Q.; He, J. A supersensitive electrochemical sensor based on RCA amplification-assisted “silver chain”-linked gold interdigital electrodes and CRISPR/Cas9 for the detection of Staphylococcus aureus in food. Food Chem. 2024, 440, 138197. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Naderi-Manesh, H.; Khajeh, K.; Soudi, M.R.; Asghari, S.M.; Sharifzadeh, M. Isolation and characterization of a novel gamma-radiation-resistant bacterium from hot spring in Iran. J. Basic Microbiol. 2009, 49, 119–127. [Google Scholar] [CrossRef]
- Guo, Y.; Li, C.; Guo, W.; Zhang, X.; Wang, L.; Zhang, W.; Zou, X.; Sun, Z. Advanced Electrochemical Biosensing toward Staphylococcus aureus Based on the RPA-CRISPR/Cas12a System and Conductive Nanocomposite. J. Agric. Food Chem. 2024, 72, 22918–22925. [Google Scholar] [CrossRef]
- Lin, X.; Liu, C.; Lei, Q.; Nan, X.; Zhu, Y.; Liao, J.; Du, Z.; Ye, C.; Xiong, Y.; Yang, M.; et al. A novel ratiometric electrochemical aptasensor based on graphene quantum dots/Cu-MOF nanocomposite for the on-site determination of Staphylococcus aureus. J. Hazard. Mater. 2025, 485, 136845. [Google Scholar] [CrossRef]
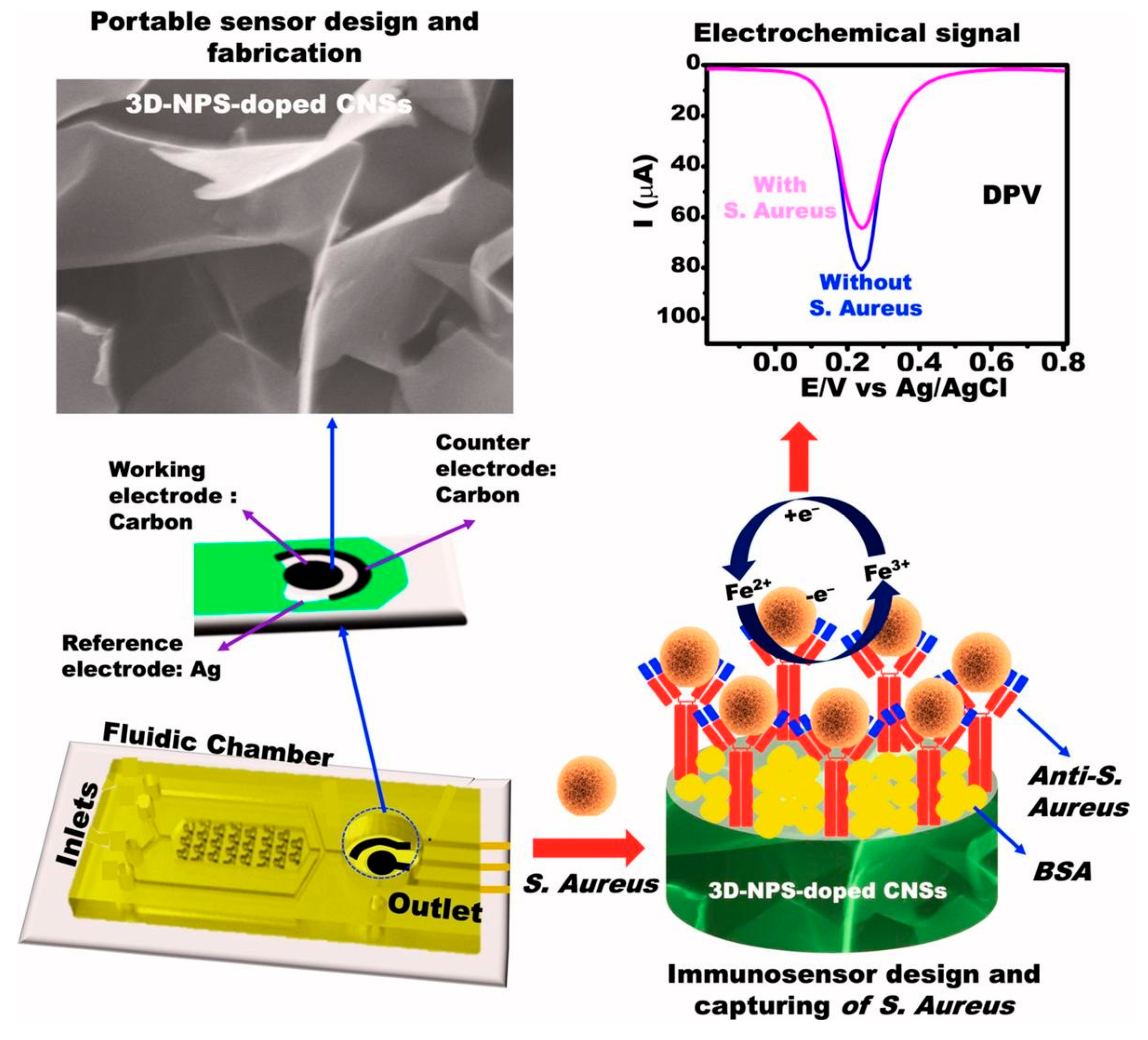
- Emran, M.Y.; Shenashen, M.A.; Fadl, A.M.M.; Saleh, M.O.; Baz, A.A.; Ganganboina, A.B.; Zulfiqur, A.; Sun, M.; Zhou, M.; Kotb, A. Design of portable biosensor assay using multifunctional carbon nanosheets for on-site monitoring of Staphylococcus aureus in water and food samples. Microchem. J. 2025, 208, 112482. [Google Scholar] [CrossRef]
- Mehrannia, L.; Khalilzadeh, B.; Rahbarghazi, R.; Milani, M.; Saydan Kanberoglu, G.; Yousefi, H.; Erk, N. Electrochemical Biosensors as a Novel Platform in the Identification of Listeriosis Infection. Biosensors 2023, 13, 216. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Liu, S.G.; Shi, X. Highly sensitive detection of L. monocytogenes using an electrochemical biosensor based on Si@MB/AuNPs modified glassy carbon electrode. Microchem. J. 2023, 194, 109357. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Xu, H.; Qi, C.; Yang, H.; Lu, X.; Yang, Q.; Zhang, W. A novel electrochemical biosensor based on SRCA-NEMA-G-quadruplex for sensitive detection of Listeria monocytogenes in food. Food Control 2024, 161, 110386. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Shi, X.; Wu, Z. Bacteria imprinted electrochemical sensor based on bimetallic silver-gold sea urchin-like hollow nanoparticles for ultrasensitive detection of Listeria monocytogenes. Microchem. J. 2024, 205, 111206. [Google Scholar] [CrossRef]
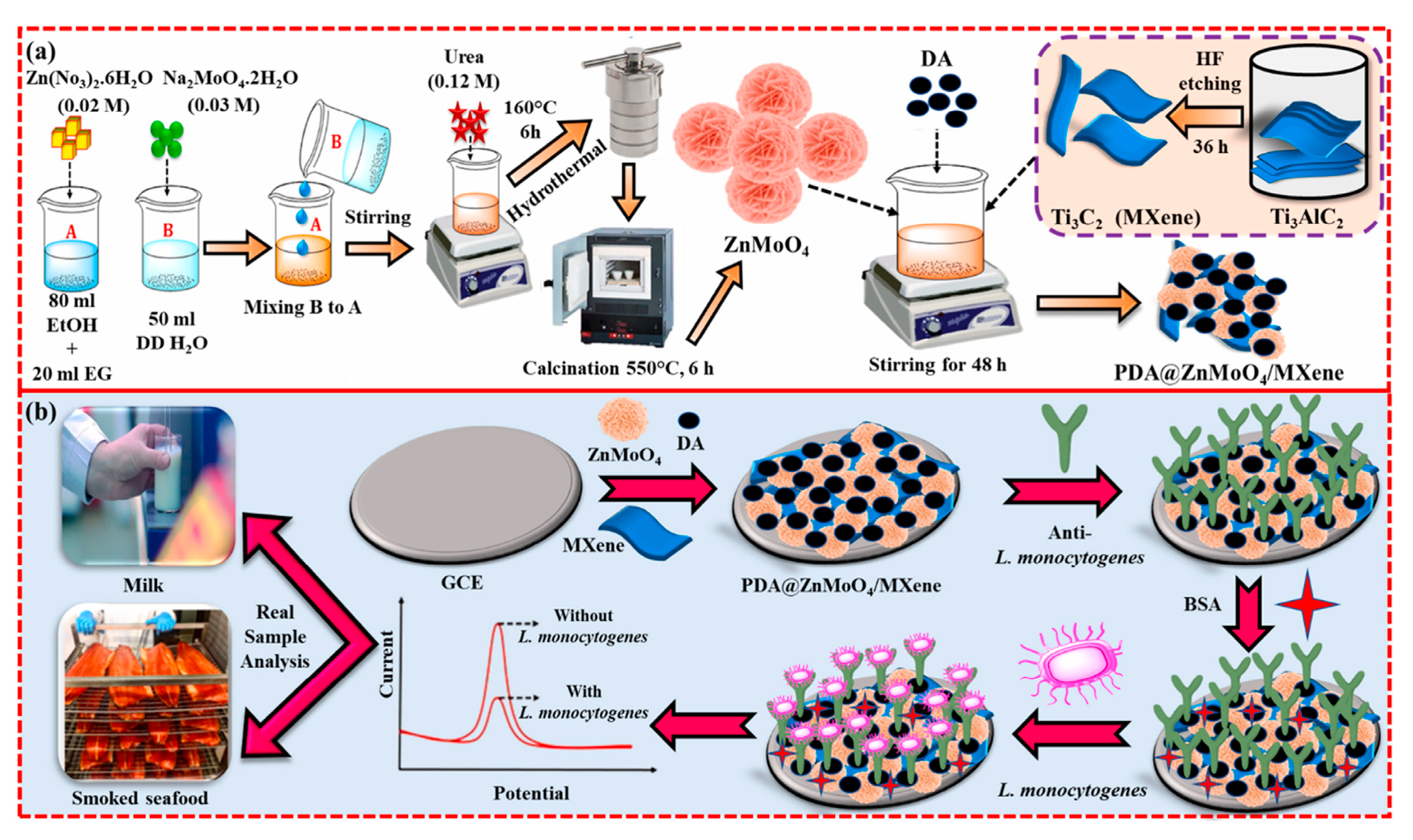
- Baskaran, N.; Sakthivel, R.; Shanthakumar Karth, C.; Lin, Y.-C.; Liu, X.; Wen, H.-W.; Yang, W.; Chung, R.-J. Polydopamine-modified 3D flower-like ZnMoO4 integrated MXene-based label-free electrochemical immunosensor for the food-borne pathogen Listeria monocytogenes detection in milk and seafood. Talanta 2025, 282, 127008. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.; Conte-Junior, C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: A meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef]
- Aparna Garg, M.; Vishwakarma, N.; Mizaikoff, B.; Singh, S. Molecularly imprinted conducting polymer based sensor for Salmonella typhimurium detection. Bioelectrochemistry 2022, 147, 108211. [Google Scholar] [CrossRef]
- He, Y.; Jia, F.; Sun, Y.; Fang, W.; Li, Y.; Chen, J.; Fu, Y. An electrochemical sensing method based on CRISPR/Cas12a system and hairpin DNA probe for rapid and sensitive detection of Salmonella Typhimurium. Sens. Actuators B Chem. 2022, 369, 132301. [Google Scholar] [CrossRef]
- Yan, J.; Liu, W.; Wang, J.; Liu, H.; Wang, L.; Li, X.; Li, Y. Enhancing public health safety: Development and application of a MoS2@CNT-Chit electrochemical DNA biosensor for rapid and accurate detection of S. typhi. Biochem. Eng. J. 2024, 210, 109416. [Google Scholar] [CrossRef]
- Hussain, W.; Wang, H.; Yang, X.; Ullah, M.W.; Hussain, J.; Ullah, N.; Ul-Islam, M.; Awad, M.F.; Wang, S. Ultrasensitive Electrochemical Detection of Salmonella typhimurium in Food Matrices Using Surface-Modified Bacterial Cellulose with Immobilized Phage Particles. Biosensors 2024, 14, 500. [Google Scholar] [CrossRef]
- Yi, Z.; Zhang, Y.; Guo, M.; Li, H.; Liu, Y.; Ding, L.; Xiong, C.; Huang, G.; Zhang, J. Label-free electrochemical immunosensor based on an internal and external dual-signal synergistic strategy for the sensitive detection of Salmonella. Food Biosci. 2024, 61, 105006. [Google Scholar] [CrossRef]
- Gong, L.; Liang, J.; Zhang, Y.; Zhang, M.; Ao, H.; Yang, T. An antifouling electrochemical biosensor using self-signal for Salmonella typhimurium direct detection in food sample. Food Chem. 2024, 452, 139536. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.; Li, Q.; Guo, X.; Guan, Q.; Jiao, K. Electrochemically reduced graphene oxide-enhanced electropolymerization of poly-xanthurenic acid for direct, “signal-on” and high sensitive impedimetric sensing of DNA. Polym. Chem. 2013, 4, 1228. [Google Scholar] [CrossRef]
- Yang, J.; Yin, X.; Xia, M.; Zhang, W. Tungsten disulfide nanosheets supported poly(xanthurenic acid) as a signal transduction interface for electrochemical genosensing applications. RSC Adv. 2018, 8, 39703. [Google Scholar] [CrossRef] [PubMed]
- Zhan, K.; Chen, L.; Li, S.; Yu, Q.; Zhao, Z.; Li, J.; Xing, Y.; Ren, H.; Wang, N.; Zhan, G. A novel metal–organic framework based electrochemical immunosensor for the rapid detection of Salmonella typhimurium detection in milk. Food Chem. 2024, 444, 138672. [Google Scholar] [CrossRef] [PubMed]
- Alsharabi, R.M.; Singh, J.; Saxen, P.S.; Srivastava, A. Ultra-sensitive electrochemical immunosensor based on 2D vanadium diselenide (VSe2) for efficient detection of pathogens: Salmonella typhimurium. Luminescence 2024, 39, e4896. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Wang, S.; Ma, J.; Hu, D.; Liang, H.; Ma, C.; Jin, Y.; Chen, X.Q.; Xu, G.; et al. Rapid detection of Salmonella typhimurium in food samples using electrochemical sensor. LWT 2024, 206, 116567. [Google Scholar] [CrossRef]
- Zamzami, M.; Ahmad, A.; Alamoudi, S.; Choudhry, H.; Hosawi, S.; Rabbani, G.; Shalaan, E.-S.; Arkook, B. A highly sensitive and specific Gold Electrode-Based electrochemical immunosensor for rapid On-Site detection of Salmonella enterica. Microchem. J. 2024, 199, 110190. [Google Scholar] [CrossRef]
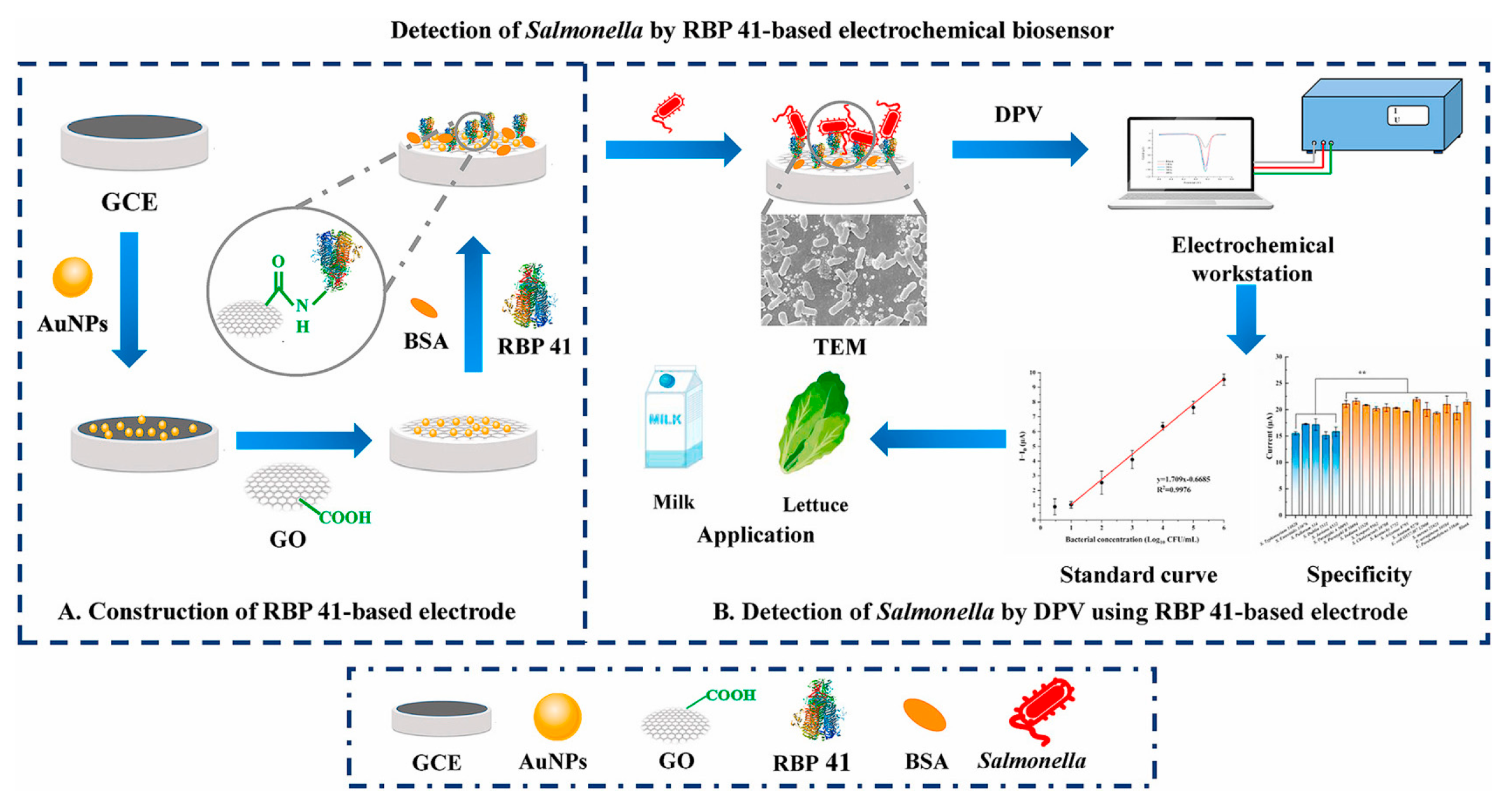
- Ding, Y.; Zhang, Y.; Huang, C.; Wang, J.; Li, H.; Wang, X. An electrochemical biosensor based on phage-encoded protein RBP 41 for rapid and sensitive detection of Salmonella. Talanta 2024, 270, 125561. [Google Scholar] [CrossRef]
- Duan, M.; Li, B.; He, Y.; Zhao, Y.; Liu, Y.; Zou, B.; Liu, Y.; Chen, J.; Dai, R.; Li, X.; et al. A CG@MXene nanocomposite-driven E-CRISPR biosensor for the rapid and sensitive detection of Salmonella typhimurium in food. Talanta 2024, 266, 125011. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Guo, M.; Yi, Z.; Hu, M.; Xiong, C.; Huang, G.; Zhang, J. Rapid detection of Salmonella in milk by labelling-free electrochemical immunosensor based on an Fe3O4–ionic liquid-modified electrode. Talanta 2024, 270, 125576. [Google Scholar] [CrossRef]
- Qazi, R.A.; Aman, N.; Ullah, N.; Jamila, N.; Bibi, N. Recent advancement for enhanced E. coli detection in electrochemical biosensors. Microchem. J. 2024, 196, 109673. [Google Scholar] [CrossRef]
- Oushyani Roudsari, Z.; Karami, Y.; Khoramrooz, S.S.; Rouhi, S.; Ghasem, H.; Khatami, S.H.; Alizadeh, M.; Khosravi, N.A.; Mansoriyan, A.; Ghasemi, E.; et al. Electrochemical and optical biosensors for the detection of E. coli. Clin. Chim. Acta 2025, 565, 119984. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Z.; Huang, J.; Wu, Y.; Mao, X.; Tan, Y.; Liu, H.; Ma, D.; Li, X.; Wang, X. Development of a phage-based electrochemical biosensor for detection of Escherichia coli O157: H7 GXEC-N07. Bioelectrochemistry 2023, 150, 108345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fan, Z.; Ding, Y.; Zhu, S.; Xie, M.; Hao, N. Exploring the entropy-driven amplification reaction and trans-cleavage activity of CRISPR-Cas12a for the development of an electrochemiluminescence biosensor for the detection of the SARS-CoV-2 RdRp gene in real samples and environmental surveillance. Environ. Sci. Nano 2022, 9, 162–172. [Google Scholar] [CrossRef]
- Cui, J.; Luo, Q.; Wei, C.; Deng, X.; Liang, H.; Wei, J.; Gong, Y.; Tang, Q.; Zhang, K.; Liao, X. Electrochemical biosensing for E. coli detection based on triple helix DNA inhibition of CRISPR/Cas12a cleavage activity. Anal. Chim. Acta 2024, 1285, 342028. [Google Scholar] [CrossRef]
- Kim, J.E.; Shin, J.H.; Park, J.P. An engineered antimicrobial peptide as an alternative bioreceptor for the detection of pathogenic Escherichia coli O157:H7. J. Electroanal. Chem. 2024, 953, 118003. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Yang, L.; Huang, B.; Lu, K.; Wen, H.; Ren, J. Ultrasensitive electrochemical biosensor for bacteria detection based on Fe3O4@COF-AuNPs and trigging isothermal circular amplification. Sens. Actuators B. Chem. 2025, 422, 136609. [Google Scholar] [CrossRef]
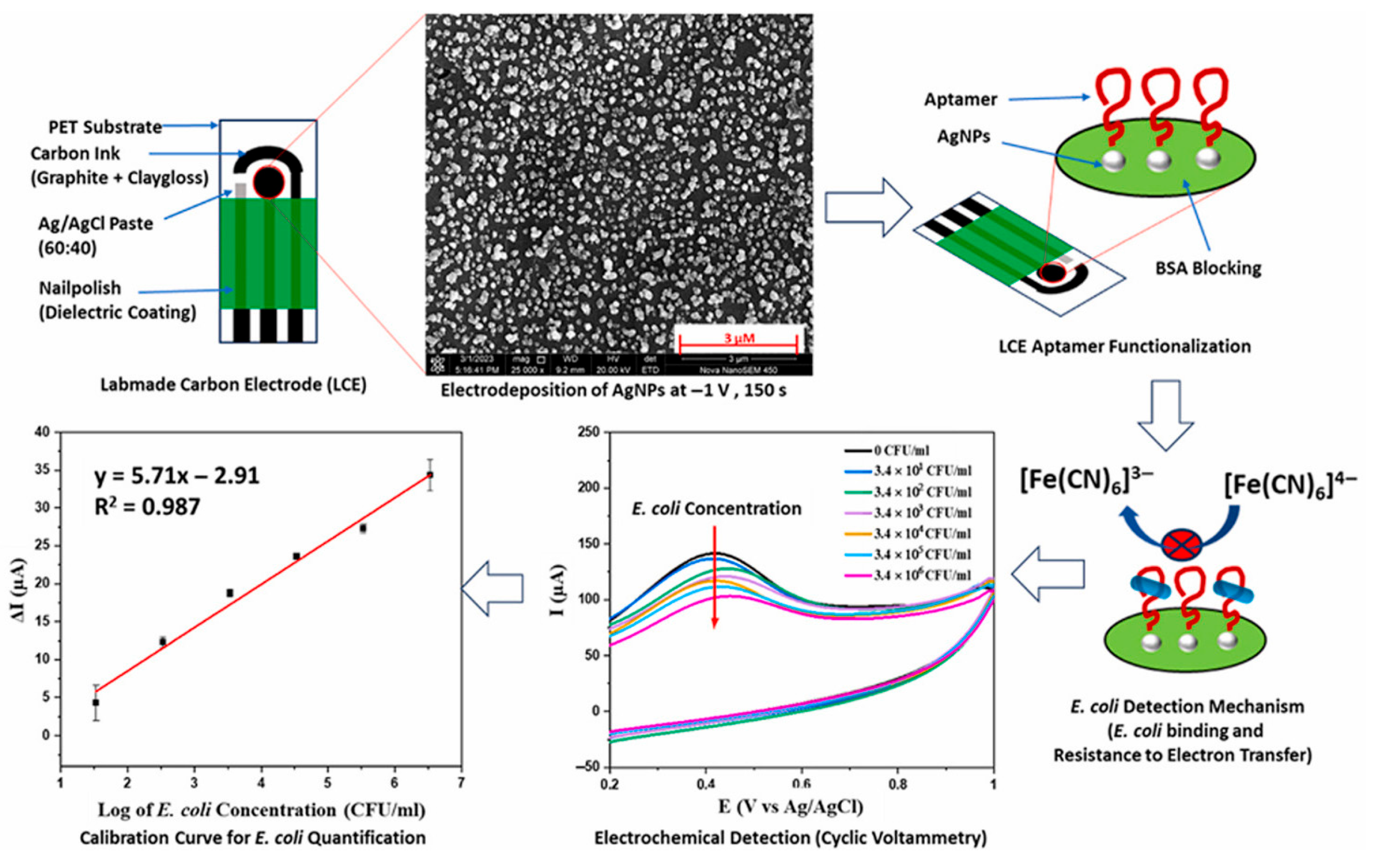
- Dabhade, A.H.; Paramasivan, B.; Kumawat, A.S.; Saha, B. Miniature lab-made electrochemical biosensor: A promising sensing kit for rapid detection of E. coli in water, urine and milk. Talanta 2025, 285, 127306. [Google Scholar] [CrossRef]
- Yang, L.; Ding, Y.; Ma, Y.; Wen, J.; Wang, J.; Dai, G.; Mo, F. An electrochemical sensor based on 2D Zn-MOFs and 2D C-Ti3C2Tx composite materials for rapid and direct detection of various foodborne pathogens. Food Chem. 2025, 462, 140922. [Google Scholar] [CrossRef]
- Xu, P.; Ghosh, S.; Rana Gul, A.; Bhamore, J.R.; Park, J.P.; Park, T.J. Screening of specific binding peptides using phage-display techniques and their biosensing applications. Trends Anal. Chem. 2021, 137, 116229. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Huang, H.; Ma, Y.; Zhao, X. An overview of signal amplification strategies and construction methods on phage-based biosensors. Food Res. Int. 2024, 191, 114727. [Google Scholar] [CrossRef]
- Çelik, M.; Kucuk, I.; Sadak, S.; Uslu, B. Combination of miniature electrode systems via nanomaterials: Pesticide analysis. Trends Environ. Anal. Chem. 2025, 45, e00251. [Google Scholar] [CrossRef]
- Mazuryk, J.; Klepacka, K.; Kutner, W.; Sindhu Sharma, P. Glyphosate Separating and Sensing for Precision Agriculture and Environmental Protection in the Era of Smart Materials. Environ. Sci. Technol. 2023, 57, 9898–9924. [Google Scholar] [CrossRef]
- Dey, B.; Shivangee Kushwaha, K.; Choudhury, A.; Ahmad, M.W.; Datta, P.; Syed, A.; AL-Shwaiman, H.A.; Subramaniam, M. Novel non-enzymatic electrochemical sensing platform based on copper metal organic frameworks for detection of glyphosate herbicide in vegetables extract. Microchem. J. 2025, 298, 112407. [Google Scholar] [CrossRef]
- Mutlu, E.; Senocak, A.; Demirbaş, E.; Koca, A.; Akyüz, D. Selective and sensitive molecularly imprinted polymer-based electrochemical sensor for detection of deltamethrin. Food Chem. 2025, 463, 141121. [Google Scholar] [CrossRef]
- Zheng, X.; Guo, L.; Zhu, C.; Hu, T.; Gong, X.; Wu, C.; Wang, G.; Dong, H.; Hou, Y. A robust electrochemical sensor based on AgNWs@MoS2 for highly sensitive detection of thiabendazole residues in food samples. Food Chem. 2024, 433, 137304. [Google Scholar] [CrossRef]
- Mei, X.; Wang, X.; Huang, W.; Zhu, J.; Liu, K.; Wang, X.; Cai, W.; He, R. A novel polycaprolactone/polypyrrole/β-cyclodextrin electrochemical flexible sensor for dinotefuran pesticide detection. Food Chem. 2024, 434, 137194. [Google Scholar] [CrossRef] [PubMed]
- Tharuman, S.; Nataraj, N.; Chen, S.-M. Advanced electrochemical detection of carbendazim in staple food samples using cobalt hydroxide/titanium dioxide nanocomposite. Food Chem. 2025, 464, 141616. [Google Scholar] [CrossRef] [PubMed]
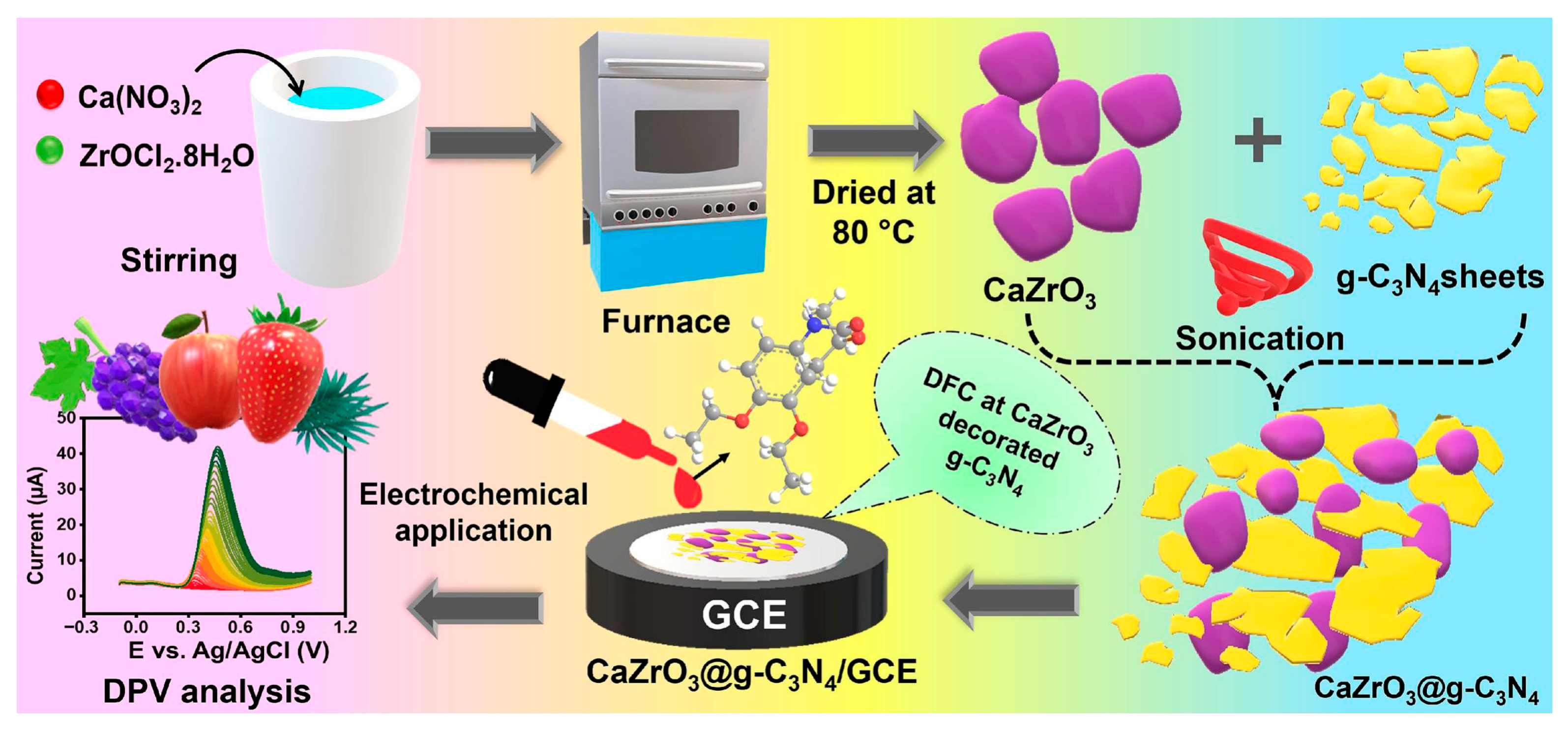
- Radha, A.; Wang, S.-F. Construction of calcium zirconate nanoparticles confined on graphitic carbon nitride: An electrochemical detection of diethofencarb in food samples. Food Chem. 2025, 465, 141929. [Google Scholar] [CrossRef] [PubMed]
- Teshome Wondimu, K.; Kelecha Geletu, A.; Meka Kedir, W. Recent developments in monitoring of organophosphorus pesticides in food samples. J. Agric. Food Res. 2025, 19, 101709. [Google Scholar] [CrossRef]
- Ding, R.; Jiang, W.; Ma, Y.; Yang, Q.; Han, X.; Hou, X. A highly sensitive MXene/AuPt/AChE-based electrochemical platform for the detection of chlorpyrifos. Microchem. J. 2023, 187, 108425. [Google Scholar] [CrossRef]
- Suwannachat, J.; Saenchoopa, A.; Shweyi Thet Tun, W.; Patramanon, R.; Daduang, S.; Daduang, J.; Kulchat, S. An electrochemical AChE-based biosensor for organophosphate pesticides using a modified CuNWs/rGO nanocomposite on a screen-printed carbon electrode. Food Chem. 2024, 434, 137431. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Qian, L.; Yin, Y.; Yuan, Z.; Dai, Y.; Zhang, T.; Yang, D.; Qiu, F. Lamellar Ti3C2 MXene composite decorated with platinum-doped MoS2 nanosheets as electrochemical sensing functional platform for highly sensitive analysis of organophosphorus pesticides. Food Chem. 2024, 459, 140379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, H.; Yang, L. Ultra-highly sensitive and stable acetylcholinesterase biosensor based on TiO2-NRs and rGO. Microchem. J. 2021, 168, 106435. [Google Scholar] [CrossRef]
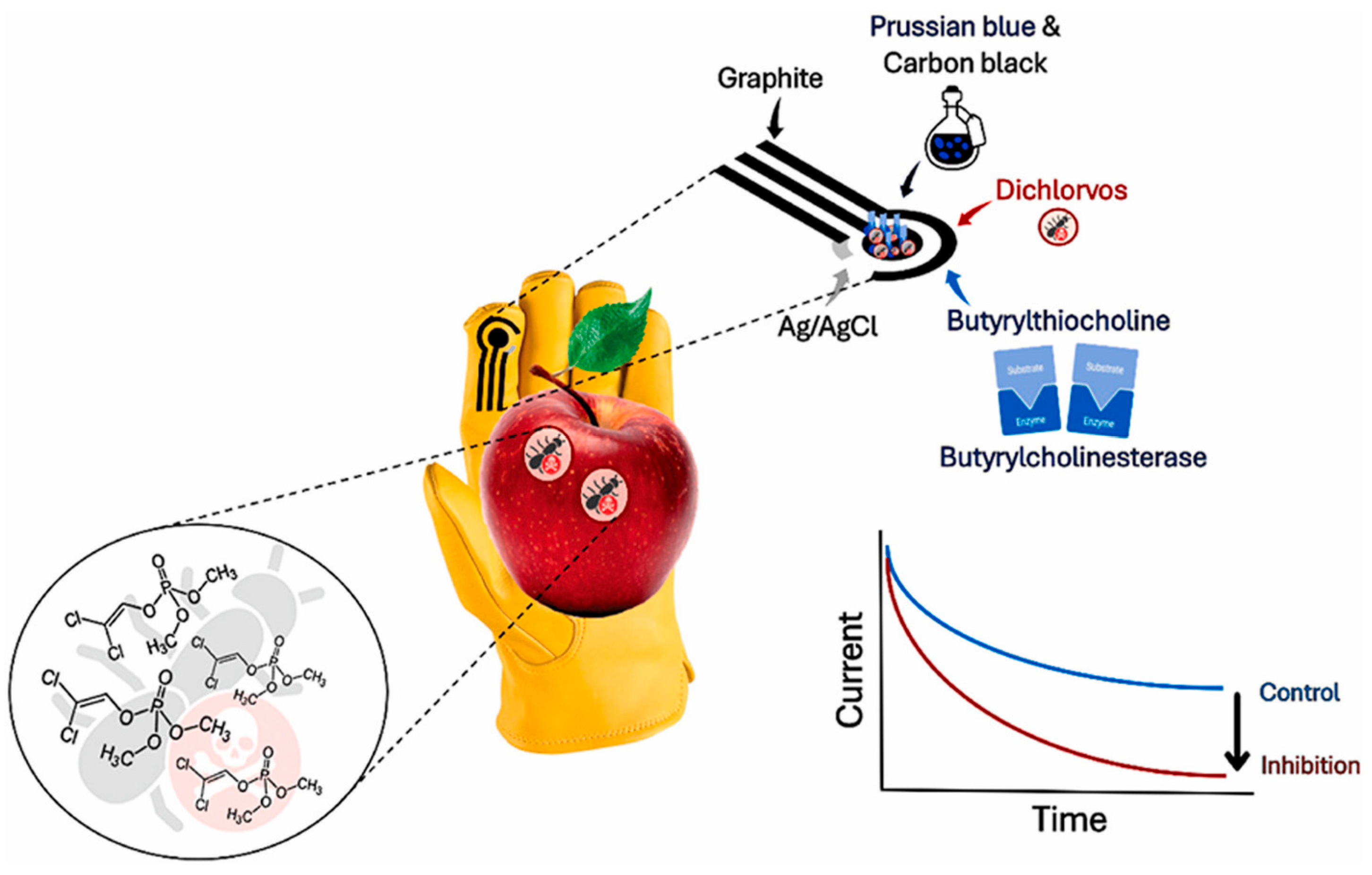
- Miglione, A.; Raucci, A.; Mancini, M.; Gioia, V.; Frugis, A.; Cinti, S. An electrochemical biosensor for on-glove application: Organophosphorus pesticide detection directly on fruit peels. Talanta 2025, 283, 127093. [Google Scholar] [CrossRef]
- Beigmoradi, F.; Moghadam, M.R.; Garkani-Nejad, Z.; Bazmandegan-Shamilia, A.; Masoodia, H.R. Dual-template imprinted polymer electrochemical sensor for simultaneous determination of malathion and carbendazim using graphene quantum dots. Anal. Methods 2023, 15, 5027. [Google Scholar] [CrossRef]
- Devi, W.S.; Kaur, R.; Sharma, A.; Thakur, S.; Mehta, S.K.; Raja, V.; Ataya, F.S. Non-enzymatic g-C3N4 supported CuO derived-biochar based electrochemical sensors for trace level detection of malathion. Biosens. Bioelectron. 2025, 267, 116808. [Google Scholar] [CrossRef]
- Aris, A.; Tri Wahyuni, W.; Riza Putra, B.; Hermawan, A.; Anggoro Ardy Nugroho, F.; Wei Seh, Z.; Khalil, M. Ultrasensitive non-enzymatic electrochemical detection of paraoxon-ethyl in fruit samples using 2D Ti3C2Tx/MWCNT-OH. Nanoscale 2025, 17, 2554. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Y.; Zhang, Y.; Gao, X.; Wang, H.; Niu, B.; Li, W. ZnO-rGO-based electrochemical biosensor for the detection of organophosphorus pesticides. Bioelectrochemistry 2024, 156, 108599. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.; Zhang, J.; Guo, X.; Xu, Y.; Chen, L. Ti3C2 MXene/MoS2@AuNPs ternary nanocomposite for highly sensitive electrochemical detection of phoxim residues in fruits. Food Chem. 2025, 462, 140939. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Tsekenis, G.; Oravczova, V.; Hianik, T. Electrochemical Aptasensors for Antibiotics Detection: Recent Achievements and Applications for Monitoring Food Safety. Sensors 2022, 22, 3684. [Google Scholar] [CrossRef]
- Banan, K.; Hatamabadi, D.; Afsharara, H.; Mostafiz, B.; Sadeghi, H.; Rashidi, S.; Beirami, A.D.; Shahbazi, M.-A.; Keçili, R.; Hussain, C.M.; et al. MIP-based extraction techniques for the determination of antibiotic residues in edible meat samples: Design, performance and recent developments. Trends Food Sci. Technol. 2022, 199, 164–179. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Q.; Liu, Y.; Jiang, B.; Yuan, R.; Xiang, Y. A sensitive tobramycin electrochemical aptasensor based on multiple signal amplification cascades. Bioelectrochemistry 2024, 160, 108797. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, J.; Liu, Y.; Chen, Y.; Liu, H.; Zhu, X.; Ding, P.; Liang, C.; Liu, E.; Wu, S.; et al. A novel label-free electrochemical immunosensor based on Ce-MOF@AgAuNPs for highly sensitive detection of monensin. Food Control 2025, 168, 110927. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Jie, Y.; Ding, Z.; Wang, X.; Wang, J.; Hou, X. MnO2 nanoparticles enhance the activity of the Zr-MOF matrix electrochemical sensor for efficiently identifying ultra-trace tetracycline residues in food. Microchim. Acta 2025, 192, 12. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Dong, Y.; Wang, L.; Cai, D.; Guo, Y.; Zhao, S.; Sheng, Q. An electrochemical aptamer sensor based on AuNPs/ErGO/Cu-MOF nanocomposites for the detection of oxytetracycline in foodstuff. Microchem. J. 2025, 208, 112579. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Y.; Wu, M.; Shang, X.; Duan, F.; Guo, C.; Zhang, S.; Zhang, Z. Construction of a portable and sensitive electrochemical immunosensor for the rapid detection of erythromycin based on semiconductive bimetallic MOF. Talanta 2025, 283, 127187. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Wu, J.; Wan, X.; Wang, T.; Liu, Y.; Chen, Y.; Xia, Y. Highly stable electrochemical sensing platform for the selective determination of pefloxacin in food samples based on a molecularly imprinted-polymer-coated gold nanoparticle/black phosphorus nanocomposite. Food Chem. 2024, 436, 137753. [Google Scholar] [CrossRef]
- Scholar, E. Sulfadiazine. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–5. [Google Scholar]
- Balasubramaniyan, N.G.; Ravikumar, N.K.; Perumal, P. Exploring the synergistic effect of functionalized boron nitride on zinc copper sulfide for enhanced electrochemical detection of sulfadiazine. Surf. Interfaces 2025, 58, 105839. [Google Scholar] [CrossRef]
- Gao, W.; He, J.; Chen, L.; Meng, X.; Ma, Y.; Cheng, L.; Yan, X. Deciphering the catalytic mechanism of superoxide dismutase activity of carbon dot nanozyme. Nat. Commun. 2023, 14, 160. [Google Scholar] [CrossRef]
- Białas, K.; Moschou, D.; Marken, F.; Estrela, P. Electrochemical sensors based on metal nanoparticles with biocatalytic activity. Microchim. Acta 2022, 189, 172. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, J.; Wang, L.; Zhang, J.; Ding, L.; Liu, H.; Yu, X. Nanozyme-enhanced electrochemical biosensors: Mechanisms and applications. Small 2024, 20, 2307815. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Lu, Y.; Zhou, P.; Lu, L.; Lv, H.; Hai, X. Recent advances in the immunoassays based on nanozymes. Biosensors 2022, 12, 1119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, S.; Wei, H. Recent advances on nanozyme-based electrochemical biosensors. Electroanalysis 2022, 35, e202100684. [Google Scholar] [CrossRef]
- Ghosh, S.; Sagayam, K.; Haldar, D.; Jone, A.; Acharya, B.; Gerogiannis, V.; Kanavos, A. A review on the types of nanomaterials and methodologies used for the development of biosensors. Adv. Nat. Sci. Nanosci. Nanotechnol. 2024, 15, 013001. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, T.; Wang, J.; Guo, G.; Chen, Z.; Li, H.; Gao, Y. Determination of deoxynivalenol (DON) by a label-free electrochemical immunosensor based on NiFe PBA nanozymes. Food Chem. 2025, 463, 141436. [Google Scholar] [CrossRef]
- Nie, D.; Zhu, X.; Liu, M.; Cheng, M.; Fan, K.; Zhao, Z.; Han, Z. Molecularly imprinted polymer-based electrochemical sensor for rapid detection of masked deoxynivalenol with Mn-doped CeO2 nanozyme as signal amplifier. J. Hazard. Mater. 2024, 477, 135366. [Google Scholar] [CrossRef]
- Xing, Z.; Wang, C.; Fan, Z.; Qi, S.; Sun, Q.; Song, R.B.; Li, Z. Substrate-Switched Dual-Signal Self-Powered Sensing System Based on Dual-Nanozyme Activity of Bimetal-Doped CeO2 Nanospheres for Electrochemical Assay of Aflatoxin B1. Anal. Chem. 2024, 96, 14944–14952. [Google Scholar] [CrossRef]
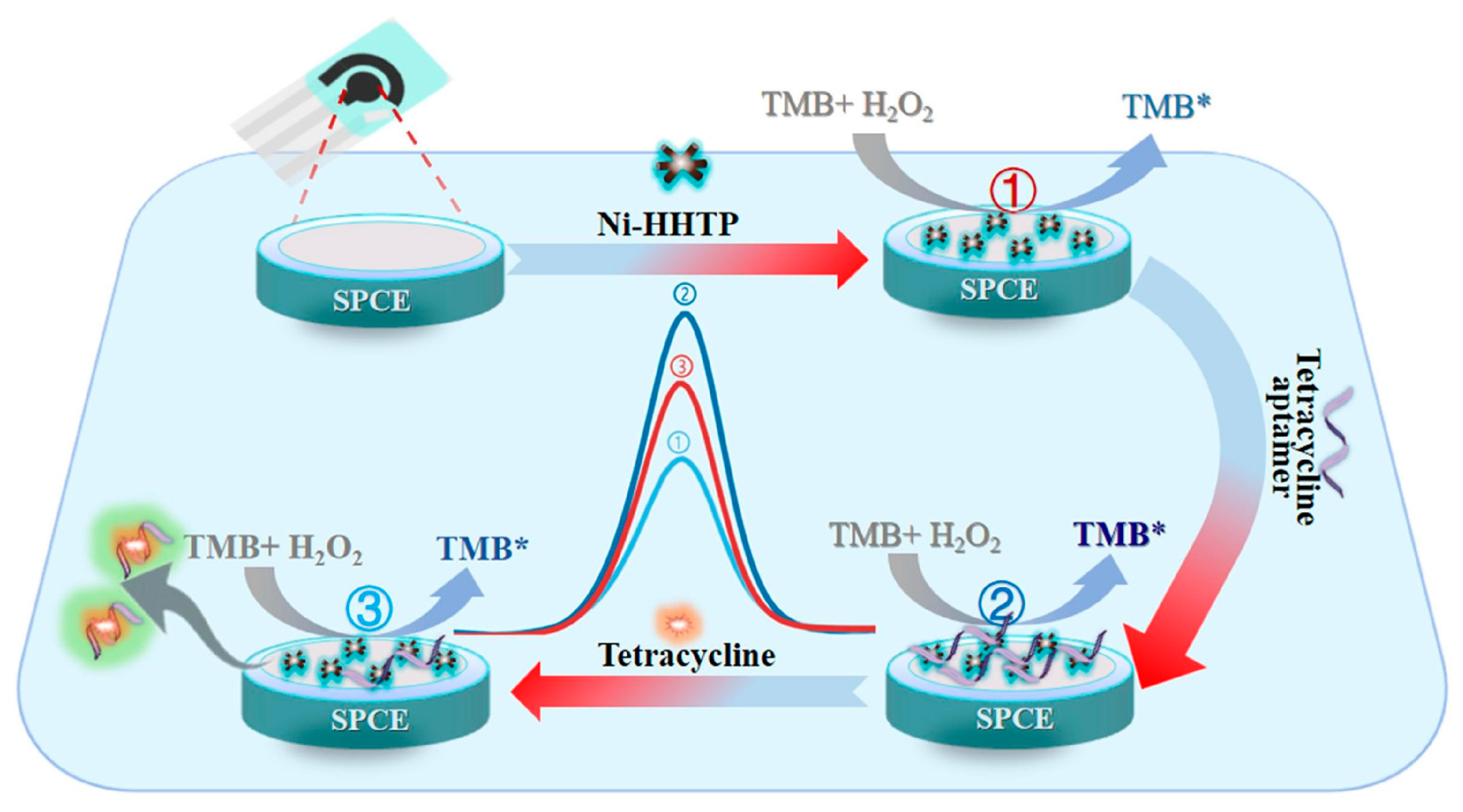
- Gao, F.; Zhao, Y.; Dai, X.; Xu, W.; Zhan, F.; Liu, Y.; Wang, Q. Aptamer tuned nanozyme activity of nickel-metal–organic framework for sensitive electrochemical aptasensing of tetracycline residue. Food Chem. 2024, 430, 137041. [Google Scholar] [CrossRef]
- Yue, F.; Hu, M.; Bai, M.; Guo, Y.; Sun, X.; Zhao, G. An exonuclease III-driven dual-mode aptasensor based on Au-Pd@Fc nanozyme and magnetic separation pretreatment for aminoglycoside antibiotics detection. Food Chem. 2024, 460, 140480. [Google Scholar] [CrossRef]
- Ma, Y.; Lin, X.; Xue, B.; Luan, D.; Jia, C.; Feng, S.; Zhao, J. Ultrasensitive and highly selective detection of Staphylococcus aureus at the single-cell level using bacteria-imprinted polymer and vancomycin-conjugated MnO2 nanozyme. Anal. Chem. 2024, 96, 8641–8647. [Google Scholar] [CrossRef]
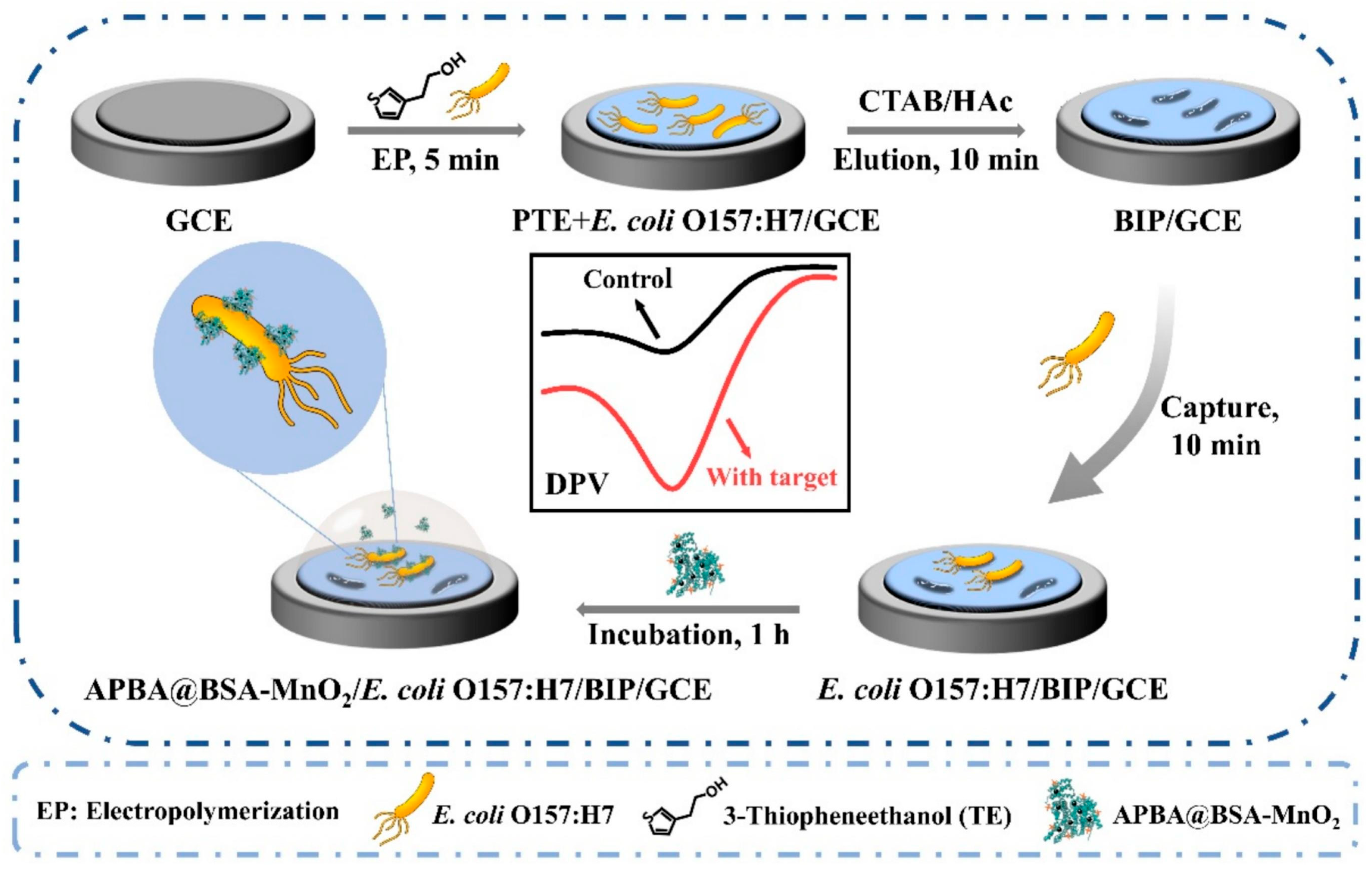
- Ma, Y.; Niu, X.; Shi, L.; Sun, S.; Luan, D.; Yan, J.; Bian, X. Highly selective and sensitive electrochemical detection of Escherichia coli O157:H7 using a bacteria-imprinted polymer and 3-aminophenylboronic acid-conjugated MnO2 nanozyme. Microchem. J. 2025, 213, 113588. [Google Scholar] [CrossRef]
- Geng, L.; Sun, X.; Wang, L.; Liu, F.; Hu, S.; Zhao, S.; Ye, F. Analyte-induced laccase-mimicking activity inhibition and conductivity enhancement of electroactive nanozymes for ratiometric electrochemical detection of thiram. J. Hazard. Mater. 2024, 463, 132936. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, B.; Li, G.; Shen, Q.; Zou, L. NiCoFeS/rGO nanozyme-mediated multifunctional homogeneous sensing system for ultrasensitive electrochemical assay of pesticides residues in fruits and vegetables. Sens. Actuators B Chem. 2025, 422, 136664. [Google Scholar] [CrossRef]
- Yu, L.; Chang, J.; Zhuang, X.; Li, H.; Hou, T.; Li, F. Two-dimensional cobalt-doped Ti3C2 MXene nanozyme-mediated homogeneous electrochemical strategy for pesticides assay based on in situ generation of electroactive substances. Anal. Chem. 2022, 94, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhang, J.; Huang, H.; Wang, X.; He, X.; Luo, Y.; Cheng, N. Single-atom Ce-NC nanozyme bioactive paper with a 3D-printed platform for rapid detection of organophosphorus and carbamate pesticide residues. Food Chem. 2022, 387, 132896. [Google Scholar] [CrossRef]
- Hao, C. Recent Progress in Detecting Enantiomers in Food. Molecules 2024, 29, 1106. [Google Scholar] [CrossRef]
- Bonomo, M.; Bortolami, M.; Curulli, A.; Di Matteo, P.; Feroci, M.; Simonis, B.; Trani, A.; Vetica, F.; Zollo, G. L-Pro functionalized carbon nanoclusters inducing an enantioselective voltammetric response to Trp and Tyr. Electrochim. Acta 2025, 523, 145989. [Google Scholar] [CrossRef]
- Grasso, G.; Zane, D.; Dragone, R. Microbial nanotechnology: Challenges and prospects for green biocatalytic synthesis of nanoscale materials for sensoristic and biomedical applications. Nanomaterials 2019, 10, 11. [Google Scholar] [CrossRef]
- Grasso, G.; Zane, D.; Dragone, R. Precision Microbial Nanobiosynthesis: Knowledge, Issues, and Potentiality for the In Vivo Tuning of Microbial Nanomaterials. In Microbial Nanobiotechnology, 1st ed.; Lateef, A., Gueguim-Kana, E.B., Dasgupta, N., Ranjan, S., Eds.; Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2021; pp. 75–112. [Google Scholar] [CrossRef]
- Mekonnen, E.G.; Shitaw, K.N.; Hwang, B.J.; Workie, Y.A.; Abda, E.M.; Mekonnen, M.L. Copper nanoparticles embedded fungal chitosan as a rational and sustainable bionanozyme with robust laccase activity for catalytic oxidation of phenolic pollutants. RSC Adv. 2023, 13, 32126–32136. [Google Scholar] [CrossRef]
- Mansouri, S.; Al Omary, A. Recent development of nanozymes for dual and multi-mode biosensing applications in food safety and environmental monitoring: A review. J Environ Chem Eng. 2025, 13, 116832. [Google Scholar] [CrossRef]
- McCourt, K.M.; Cochran, J.; Abdelbasir, S.M.; Carraway, E.R.; Tzeng, T.-R.J.; Tsyusko, O.V.; Vanegas, D.C. Potential Environmental and Health Implications from the Scaled-Up Production and Disposal of Nanomaterials Used in Biosensors. Biosensors 2022, 12, 1082. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Yan, X.; Fan, K. Machine learning facilitating the rational design of nanozymes. J. Mater. Chem. B 2023, 11, 6466–6477. [Google Scholar] [CrossRef]
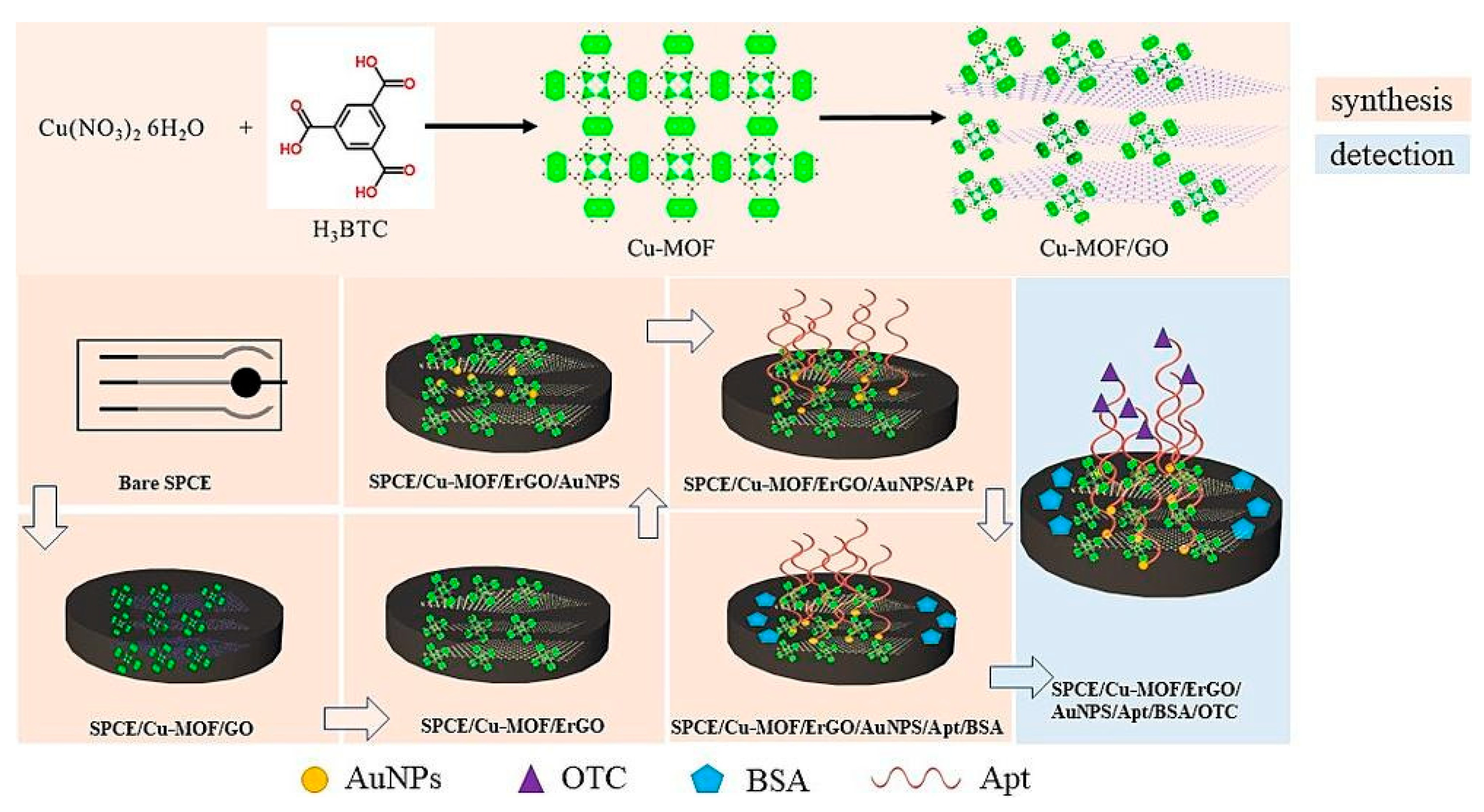
| Electrode | Format | Technique | Sample | Linearity | LOD | Recovery % | Reference Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| PGE | Electrochemical sensor | DPV | SEN/flour, herbal tea | 25–125 μg/mL | 5.45 μg/mL, | - | - | [46] |
| GCE | Electrochemical sensor utilizing MIP/Fe3O4/GO/GCE | SWV | PAT/apple and pear juice | 0.001 nM–250.0 nM | 3.33 × 10−4 nM | 94.0–103 | - | [53] |
| Pt | Microfluidic immunosensor based on | A | T-2/wheat samples | 0–1000 μg/kg | 0.10 μg/kg | 97.4–101.6 | ELISA | [54] |
| SPCE | Bio-3D-printed liver microtissue biosensor based on COX/AuNPs/AAO/SPCE | CV | DON/- | 2~40 μg/mL | 1.229 μg/mL | - | - | [57] |
| GCE | Aptasensor based on MCH/Apt/AuNPs/ZIF-8/GCE | DPV | AFB1/corn and peanut oil | 10.0–1.0 × 105 pg/mL | 1.82 pg/mL | 93.49–106.9 | - | [60] |
| AuE | Bifunctional genosensor based on TDNs/HPG/AuE | DPV | AFB1/peanut OTA/peanut | 0.05–360 ng/mL 0.05–420 ng/mL | 3.5 pg/mL 2.4 pg/mL | 96–102 99–102 | [61] | |
| SPCE | Aptasensor based on Au NPs@Ce-TpBpy COF | CA | ZEN/corn flour | 1 pg/mL–10.0 ng/mL | 0.389 pg/mL | 93.0–104.7 | [63] | |
| GCE | Electrochemical sensor based on Bi2S3@CNF/GCE | A | ZEN/wheat and oats | 0.125–375.5 μM 438–1951 μM | 0.61 μM | 98.9–99.15 | - | [65] |
| GCE | Dual-signal ratiometric electrochemical aptasensor based on MoS2-Thj | DPV | ZEN/corn flour | 1.0 × 10−10–1.0 × 10−6 M | 4.4 × 10−11 M | 99.4–109.5 | - | [66] |
| GCE | Aptasensor based on HP1-MB/AuNPs/GCE | SVW | ZEN/corn flour, peanut oil, and wine | 100 fg/mL–50 ng/mL | 89 fg/mL | 93.52–110.85 | HPLC | [67] |
| AuE | Aptasensor based on E-AB/AuE | SWV | MC-LR/tap and pond water | 1.0–750.0 ng/L | 0.53 ng/L | 96.11–105.60 | - | [69] |
| SPCE | Immunosensor based on anti-MC-LR/MC-LR/cysteamine/SPCE | EIS | MC-LR/surface water | 0.1–100 μg/L | 0.69 ng/L | - | ELISA | [70] |
| SPCE | Electrochemical biosensor based on Specific Binding Peptide/PPy/AuNPs/SPE | EIS | TTX/- | 2–1000 ppb | 2.80 ppb | - | - | [71] |
| SPAuE | Electrochemical sensor based on MIP/SPAuE | DPV | TTX/mussel | 5.0–25.0 μg/mL | 1.14 μg/mL | 81.0–110.2 | HPLC-TMS | [72] |
| SPCE | Aptasensor based on CHI/AuNPs | CV | OA/mussel and scallop | 0.01–100 ng/mL | 6.7 pg/mL | 92.3–116 | - | [74] |
| Electrode | Format | Technique | Sample | Linearity | LOD | Recovery % | Reference Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| Nylon threads | Microfluidic aptasensor based on-PLL MoS2/Nylon threads | DPV | VP/seafood | 10–106 CFU/mL | 5.74 CFU/mL | - | Counting plate | [76] |
| GCE | Receptor-binding phage proteins RBPP-based biosensor, including MWCNTS and PBSE | EIS | CJ/chicken cecal | 102–109 CFU/mL | 102CFU/mL | - | [77] | |
| SPCE | Electrochemical sensor based on MIP | EIS | PSA via BHL/Tap water | 10–1 × 103 nM | 31.78 nM | . | . | [80] |
| Si mold coated with Cr and Au | Immunosensor based on 3D multilevel micro/nano protrusions, including Au nanoclusters | EIS | ST/milk | 10–105 CFU/mL | 10 CFU mL | - | Counting plate | [83] |
| AuIDE | Genosensor using CRISPR/Cas9 system and rolling circle amplification (RCA)-assisted “silver chain” | EIS | ST/milk, beef, fish, lettuce, bean skin | 10–107 CFU/mL | 7 CFU/mL | 90.63–113.00 | - | [84] |
| SPCE | Genosensor based on RPA-CRISPR/Cas12a | DPV | ST/- | 1.04–1.04 × 108 CFU/mL | 3 CFU/mL | [86] | ||
| SPCE | Aptasensor based on GQDs/Cu-MOF nanocomposite | DPV | ST/tap and river water, milk, Lonicera japonica | 5.0–5.0 × 108 CFU/mL | 0.97 CFU/mL | 97.30–106.80 | ELISA | [87] |
| SPCE | Portable immunosensor based on 3D-NPS-doped CNSs | DPV | ST/tap water, guava juice | 1.0 × 102–5.0 × 102 CFU/mL | 24 CFU/mL | - | ELISA | [88] |
| GCE | Aptasensor based on Si@MB and AuNPs | DPV | L.M./lettuce, fresh-cut fruit | 102–107 CFU/mL | 2.6 CFU/mL | 80.0–116.0 | Counting plate | [90] |
| GCE | Genosensor based on SRCA and NEMA, including AuNPs | DPV | L.M./food samples | 5.4–5.4 × 107 fg/μL | 2.13 fg/μL | 91.4–111.1 | RT-qPCR | [91] |
| GCE | Aptasensor based on MIP and BUHNPs | DPV | L.M./drinking water, orange juice | 10–106 CFU/mL | 1.0 CFU/mL | 90.2–105.9 | - | [92] |
| GCE | Immunosensor based on PDA@ZnMoO4/MXene nanocomposite | DPV | L.M./milk, smoked seafood | 10–107 CFU/mL | 12 CFU/mL | 98.0–126.0 | [93] | |
| ITOE | Electrochemical sensor based on MIP | A | SA/water | 1–108 CFU/mL | 3.42 CFU/mL | 96.94–108.25 | - | [95] |
| AuE | Genosensor based on PCR and CRISPR/Cas12a | DPV | SA/poultry | 67–6.7 × 105 CFU/mL | 55 CFU/mL | - | - | [96] |
| GCE | Genosensor based on MoS2@CNT/CHIT nanocomposite | DPV | SA/water, milk | 1.0 × 104–1.0 × 1011 CFU/mL | 1.0 × 104 CFU/mL | 92.05–99.58 | - | [97] |
| BC | Electrochemical biosensor based on BC/PPy/RGO-phage | DPV | SA/milk, chicken | 1–107 CFU/mL | 1 CFU/mL | 97.7–99.5 | Counting plate | [98] |
| AuE | Immunosensor based on Fe3O4@PB nanocomposite | DPV | SA/milk | 73.75–7.375 × 107 CFU/mL | 9.912 CFU/mL | 99.74–106.40 | Counting plate | [99] |
| GCE | Aptasensor based on Apt/CS/PDA/PXA | DPV | SA/milk, orange juice | 10–107 CFU/mL | 3CFU/mL | - | - | [100] |
| GCE | Immunosensor based on Co/Zn-ZIF-8–400@C-MWCNTs nanocomposite | DPV | SA/milk | 1.3 × 102–1.3 × 108 CFU/mL | 94 CFU/mL | 94.07–105.76 | - | [103] |
| ITO | Immunosensor based on VSe2 nanosheets | DPV | SA/sugarcane | 13–107 CFU/mL | 0.096 CFU/mL | 96.2–99.0 | - | [104] |
| SPAuE | Electrochemical MIP sensor | CV | SA/milk, pork | 10–105 CFU/mL | 10 CFU/mL | - | - | [105] |
| SPAuE | Immunosensor using a SAM | EIS | SA/- | 10–106 CFU/mL | 10 CFU/mL | - | - | [106] |
| GCE | Electrochemical biosensor based on phage-encoded protein RBP 41, GO and AuNPs | DPV | SA/milk, lettuce | 3–106 CFU/mL | 0.2984 Log10 CFU/mL | 84.0–120.0 | - | [107] |
| GCE | Genosensor based on CRISPR/Cas12a and CG@MXene nanocomposite | DPV | SA/chicken | 1.6 × 102–1.6 × 107 CFU/mL | 160 CFU/mL | 100.46–106.37 | - | [108] |
| AuE | Immunosensor based on Fe3O4–IL composite | DPV | SA/milk | 3.65 × 102–3.65 × 108 CFU/mL | 1.12 × 102 CFU/mL | 99.40–110.13 | - | [109] |
| GCE | Electrochemical biosensor based on CFGO and CB | EIS | E. coli/milk, pork | 102–107 CFU/mL | 11.8 CFU/mL | 60.8–114.2 | - | [112] |
| AuE | Aptasensor using the triple helix DNA | SVW | E. coli/water, milk | 100–1.0 × 107 CFU/mL | 5.2 CFU/mL | 95.76–101.20 | - | [114] |
| AuE | Electrochemical biosensor based on engineered antimicrobial peptide | DPV | E. coli/milk | 10–105 CFU/mL | 3.4 CFU/mL | 89.1–122.0 | [115] | |
| AuE | Genosensor involving Fe3O4@COF-AuNPs nanocomposite and TICA | CV | E. coli/orange juice, milk | 102–109 CFU/mL | 10 CFU/mL | 92.0–109.0 | - | [116] |
| SPCE | Aptasensor using AgNPs | CV | E. coli/milk, tap water | 34–3.4 × 106 CFU/ml | 34 CFU/mL | [117] | ||
| SPCE | Aptasensor based on 2D Zn-MOFs and 2D C-Ti3C2Tx composite | DPV | E. coli, ST, SA/milk, egg | 10–106 CFU/mL | E. coli 6 CFU/mL STA 5 CFU/mL SA 5 CFU/mL | E. coli 82.54–132.0 | Counting plate | [118] |
| Electrode | Format | Technique | Sample | Linearity | LOD | Recovery % | Reference Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| GCE | Electrochemical sensor based on Cu-PZDA/CNFs | DPV | GLP/beetroot juice, lettuce extracts | 0.01–200 μM | 3.12 μM | 98.01–101.83 | HPLC- | [123] |
| ITOE | Electrochemical sensor based on Co3O4 electrochromic film and MIP | CV | Deltamethrin/tomato, grapefruit, salad, orange | 2.82–56.5 nM | 1.53 nM | 97.20–105.33 | HPLC | [124] |
| GCE | Electrochemical sensor based on Ag NWs@MoS2 nanocomposite | SWV | TBZ/pear, apple | 0.05–10 μM | 1.75 nM | 95.5–103.6% | HPLC | [125] |
| PCL fibers | Electrochemical sensor based on PCL/PPy/β-CD composite | DPV | DNF/rice | 0.2–5 μM 5–50 μM | 0.05 μM | 96.67–103.65 | - | [126] |
| GCE | Electrochemical sensor based on Co(OH)2/TiO2 composite | CV | CBZ/apple, orange, cabbage, carrot, and tomatoes | 0.039–0.399 μM 0.399–2630.1 μM | 0.007 μM | 96.84–106.6 | - | [127] |
| GCE | Electrochemical sensor based on CaZrO3@g-C3N4 composite | DPV | DFC/strawberry, grapes, spinach, and apple | 0.01–230.04 μM | 1.8 nM | 98.20–99.80 | - | [128] |
| GCE | Biosensor based on MXene/AuPt | DPV | CFP/apple, cabbage | 10−8–10−3 mg mL | 1.55 pg/mL | 95.44–102.81 | - | [130] |
| SPCE | Biosensor based on CuNWs/rGO | CV | CFP/drinking water, orange juice | 10–200 μg/L | 3.1 μg/L | 96.67–105.65 | - | [131] |
| GCE | Biosensor based on CHI/Pt/MoS2/TM | DPV | CFP/strawberry, pakchoi, Chinese chive | 10−12–10−6 M | 4.71 × 10−13M | 94.81–104.0 | - | [132] |
| GCE | Biosensor based on TiO2-NRs/AuNPs/CHIT@rGO | DPV | DDPV/cabbage orange juice | 2.26–565 nM | 2.23 nM | 90.3–101.6 | - | [133] |
| SPCE | Biosensor based on PB/CBs | CA | DDPV/apple and orange peels | 0 up to 20 ppb | 1nM (0.3 ppb) | Apple 106–97 Orange 91–115 | - | [134] |
| GECE | Electrochemical sensor based on GQDs@MIP (MAL) GQDs@MIP (CBZ) | DPV | MAL, CBZ/cucumber, tomato, grape juices | MAL 0.02–55.00 μM CBZ 0.02–45.00 μM | MAL 2 nM CBZ 1 nM | 97.75–109.6 | - | [135] |
| GCE | Electrochemical sensor based on B-CuO/g-C3N4 | SWASV | MAL/apple, tomato | 0.18–5.66 pg/mL | 1.2 pg/mL | 87.64–120.59 | - | [136] |
| GCE | Electrochemical sensor based on Ti3C2Tx/MWCNT-OH | DPV | Paraoxon-ethyl/red and green grapes | 1–100 μM | 10 nM | - | - | [137] |
| GCE | Biosensor based on ZnO-rGO | DPV | MP/cucumber, apple | 0.01–1000 ng/mL | 0.00463 ng/mL | 90.92–108.4 | [138] | |
| GCE | Biosensor based on MXene/MoS2@AuNPs | DPV | Phoxim/winter jujube, red date, raisin, and apricot | 1 × 10−13–1 × 10−7 M | 5.29 × 10−15 M | 99–107 | HPLC | [139] |
| Electrode | Format | Technique | Sample | Linearity | LOD | Recovery % | Reference Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| AuE | Aptasensor based on Exo III and metal ion-dependent DNAzyme recycling and HCR | SWV | Tobramycin/milk | 5 nM~1 μM | 3.51 nM | 97.6–102.7 | - | [142] |
| AuE | Immunosensor based on Ce-MOF@AgAuNPs nanocomposite | DPV | MON/chicken liver, milk | 0.05–250 ng/mL | 0.008 ng/mL | 97.4–103.2 in chicken liver 96.0–104.7 In milk | - | [143] |
| GCE | Electrochemical sensor based on MnO2@ Zr-MOF | DPV | TC/milk, egg | 2–200 μM | 2.577 × 10−8 M | 106.26–115.01 | - | [144] |
| SPCE | Aptasensor based on AuNPs/ErGO/Cu-MOF | CV | OTC/milk, pork meat | 0.1–105 ng/mL | 0.03 ng/mL | 87.0–110.2 | LC–MS | [145] |
| SPCE | Immunosensor based on scMOF | CA | ERY/drinking water, pork, chicken | 1.0 fg/mL–1.0 ng/mL | 0.69 fg/mL | 91.0–124.0 in drinking water, 94.0–115.9 in pork meat 102.6–124.0 in chicken | - | [146] |
| GCE | Electrochemical sensor based on MIP/BPNS/AuNPs | LSV | PEF/milk, orange juice | 0.005–10 μM | 0.80 nM | 99.16–102.6 in milk 95.33–101.5 in orange juice | - | [147] |
| GCE | Electrochemical sensor including f-BN@ZnCuS nanocomposite | DPV | SDZ/milk water | 10–130 μM | 0.008 μM | 93.96–97.3 | - | [149] |
| Target Analyte and Samples | Recognition Strategy | Nanozyme Properties and Multifunctionality | Signal Generation and Measurement | LOD | Reported Limitations/Future Directions | Ref. |
|---|---|---|---|---|---|---|
| DON in spiked corn, wheat, flour and rice | Label-free electrochemical immunosensor (antibody–antigen interaction) | Ni-Fe PBA nanozymes with strong peroxidase-like activity | H2O2-driven oxidation of thionine catalyzed by nanozymes; signal inversely related to DON concentration | 0.005 ng/mL | No explicit limitations mentioned | [156] |
| D3G in wheat and barley | MIP-based electrochemical sensor using molecular imprinting for selective recognition | Mn-CeO2 nanozymes with implied peroxidase-like activity, used primarily for signal amplification | Electrochemical signal amplified by Mn-CeO2 nanozymes; mechanism not explicitly detailed but likely involves catalytic oxidation reactions | 0.003 ng/mL | Identifies need for evaluation of interference, stability, reusability, and reduction of sample prep complexity | [157] |
| AFB1 in peanut | Dual-signal EBFC system with aptamer-functionalized nanozymes | CoMn-CeO2 nanospheres with both glucose oxidase- and peroxidase-like activities | Signal 1: Reduced glucose oxidation due to aptamer-nanozyme release. Signal 2: Precipitate formation impeding electron transfer (via peroxidase activity); self-powered system | 5.8 pg/mL | No explicit drawbacks mentioned | [158] |
| Target Analytes and Applications | Nanozyme Composition and Design | Sensing Mechanism and Signal Transduction | LOD | References |
|---|---|---|---|---|
| Thiram in fruit and vegetable, samples (Pear, apple, broccoli, and cucumber) | MOF-derived electroactive nanozymes | Ratiometric electrochemical sensor with dual-signal response: THR suppresses catechol oxidation and enhances nanozyme conductivity, enabling self-calibrated detection | 0.15 nM | [163] |
| Trichlorfon in fruits and vegetables (plums, watermelon, lettuce and cucumber) | NiCoFeS/reduced graphene oxide (rGO) nanozyme | AChE inhibition modulates nanozyme peroxidase-like activity, altering electroactive product (e.g., OPDox) generation | 9.74 fg/mL | [164] |
| Paraoxon in Pak-Choi (Chinese cabbage) | Cobalt-doped Ti3C2 MXene nanosheets (CMNSs) | AChE inhibition affects peroxidase-like activity; in situ electroactive signal generation without external reagents | 0.02 ng/mL | [165] |
| Omethoate, methamidophos carbofuran, and carbosulfan in fruits and vegetables (broccoli, ginger, rape, celery, baby bok choy, Chinese cabbage, apple, and tomato) | Single-atom Ce-N-C nanozyme (SACe-N-C) | AChE inhibition affecting peroxidase-like activity of the nanozyme, changing electrochemical signals | Omethoate (55.83 ng/mL), methamidophos (71.51 ng/mL), carbofuran (81.81 ng/mL) carbosulfan (74.98 ng/mL) | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feroci, M.; Grasso, G.; Dragone, R.; Curulli, A. Electrochemical (Bio)Sensors for Toxins, Foodborne Pathogens, Pesticides, and Antibiotics Detection: Recent Advances and Challenges in Food Analysis. Biosensors 2025, 15, 468. https://doi.org/10.3390/bios15070468
Feroci M, Grasso G, Dragone R, Curulli A. Electrochemical (Bio)Sensors for Toxins, Foodborne Pathogens, Pesticides, and Antibiotics Detection: Recent Advances and Challenges in Food Analysis. Biosensors. 2025; 15(7):468. https://doi.org/10.3390/bios15070468
Chicago/Turabian StyleFeroci, Marta, Gerardo Grasso, Roberto Dragone, and Antonella Curulli. 2025. "Electrochemical (Bio)Sensors for Toxins, Foodborne Pathogens, Pesticides, and Antibiotics Detection: Recent Advances and Challenges in Food Analysis" Biosensors 15, no. 7: 468. https://doi.org/10.3390/bios15070468
APA StyleFeroci, M., Grasso, G., Dragone, R., & Curulli, A. (2025). Electrochemical (Bio)Sensors for Toxins, Foodborne Pathogens, Pesticides, and Antibiotics Detection: Recent Advances and Challenges in Food Analysis. Biosensors, 15(7), 468. https://doi.org/10.3390/bios15070468