Graphene–Bacteriophage Hybrid Nanomaterials for Specific and Rapid Electrochemical Detection of Pathogenic Bacteria
Abstract
1. Introduction

2. Search Strategy
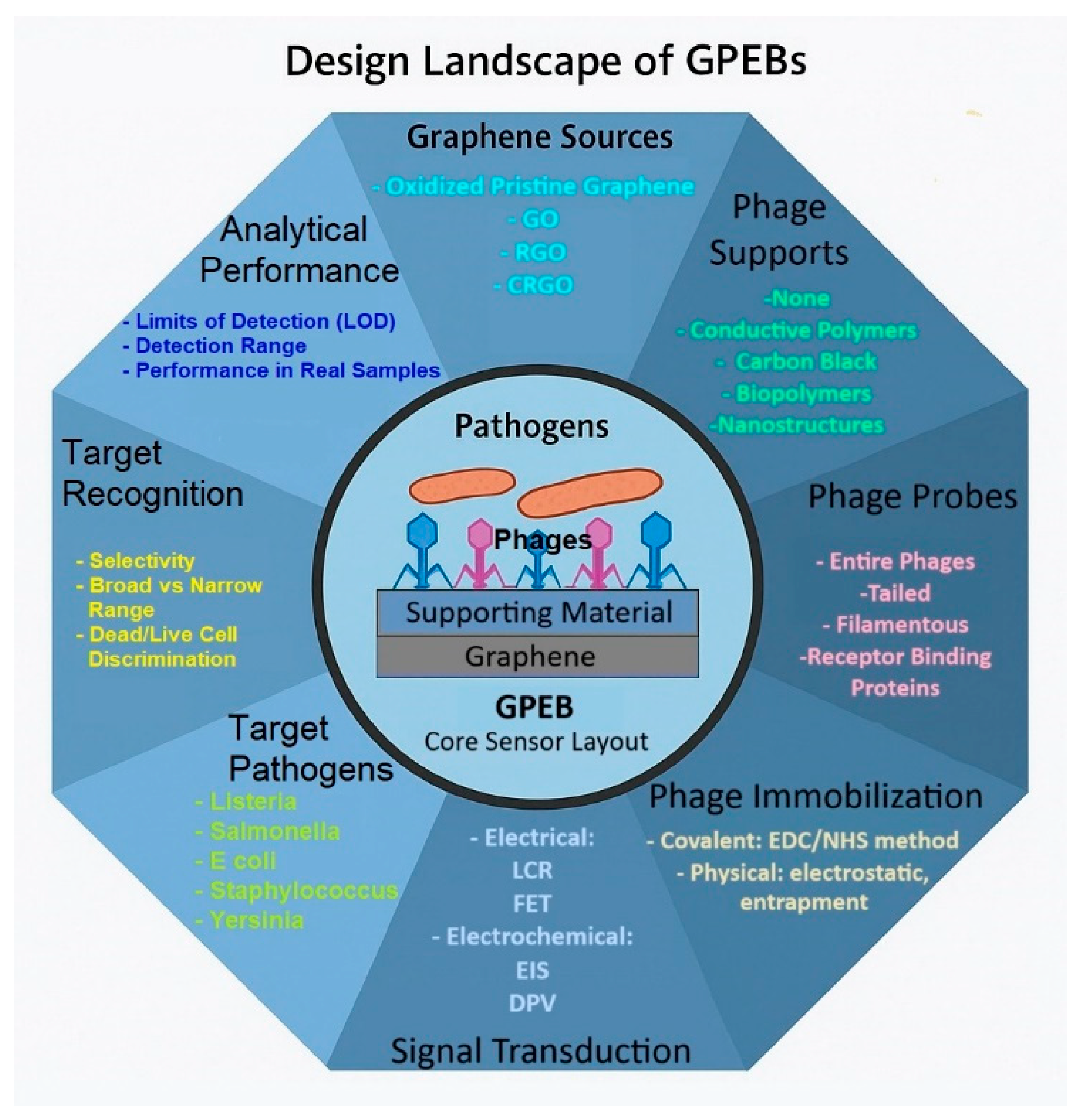
3. Results
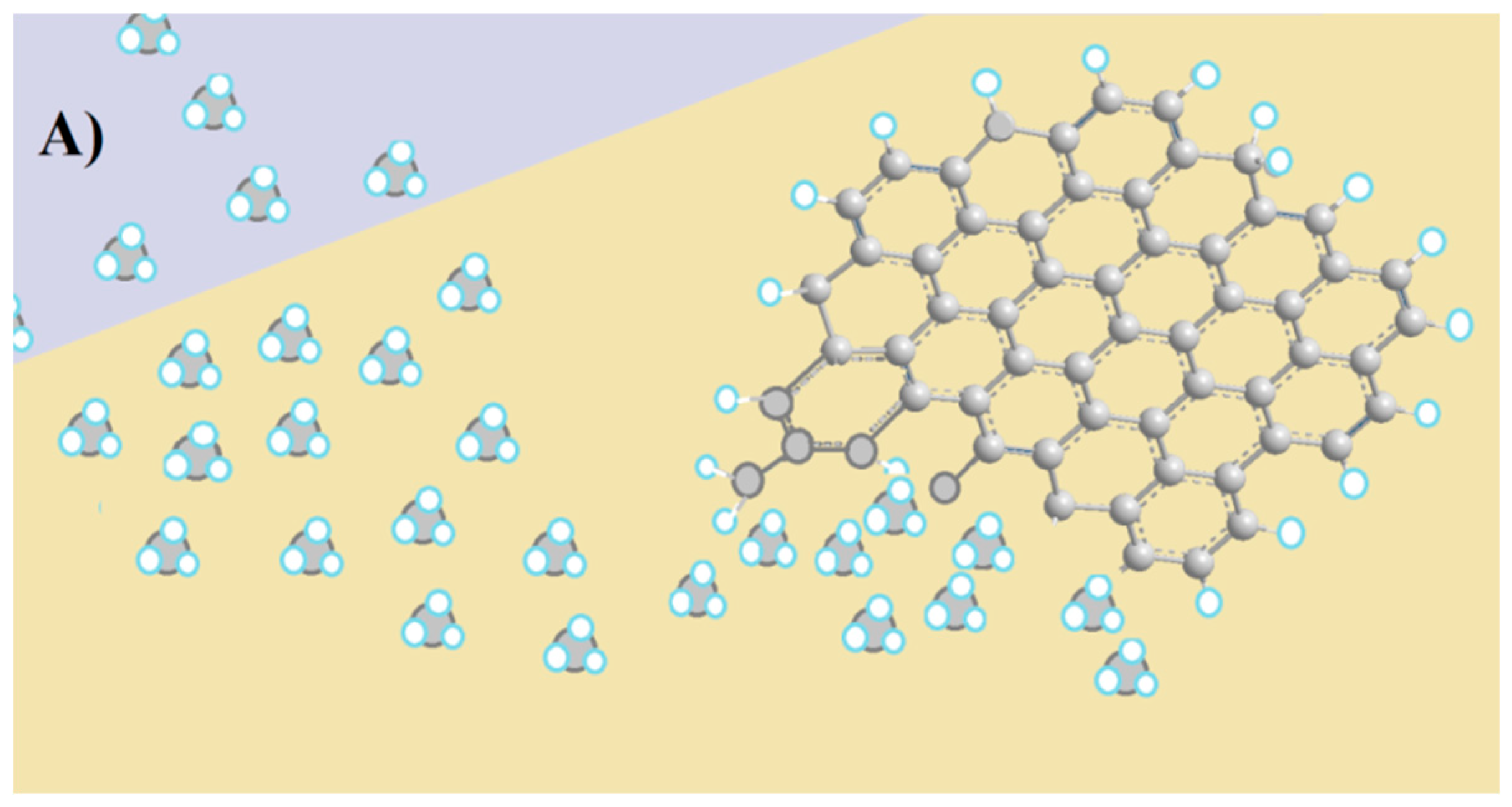
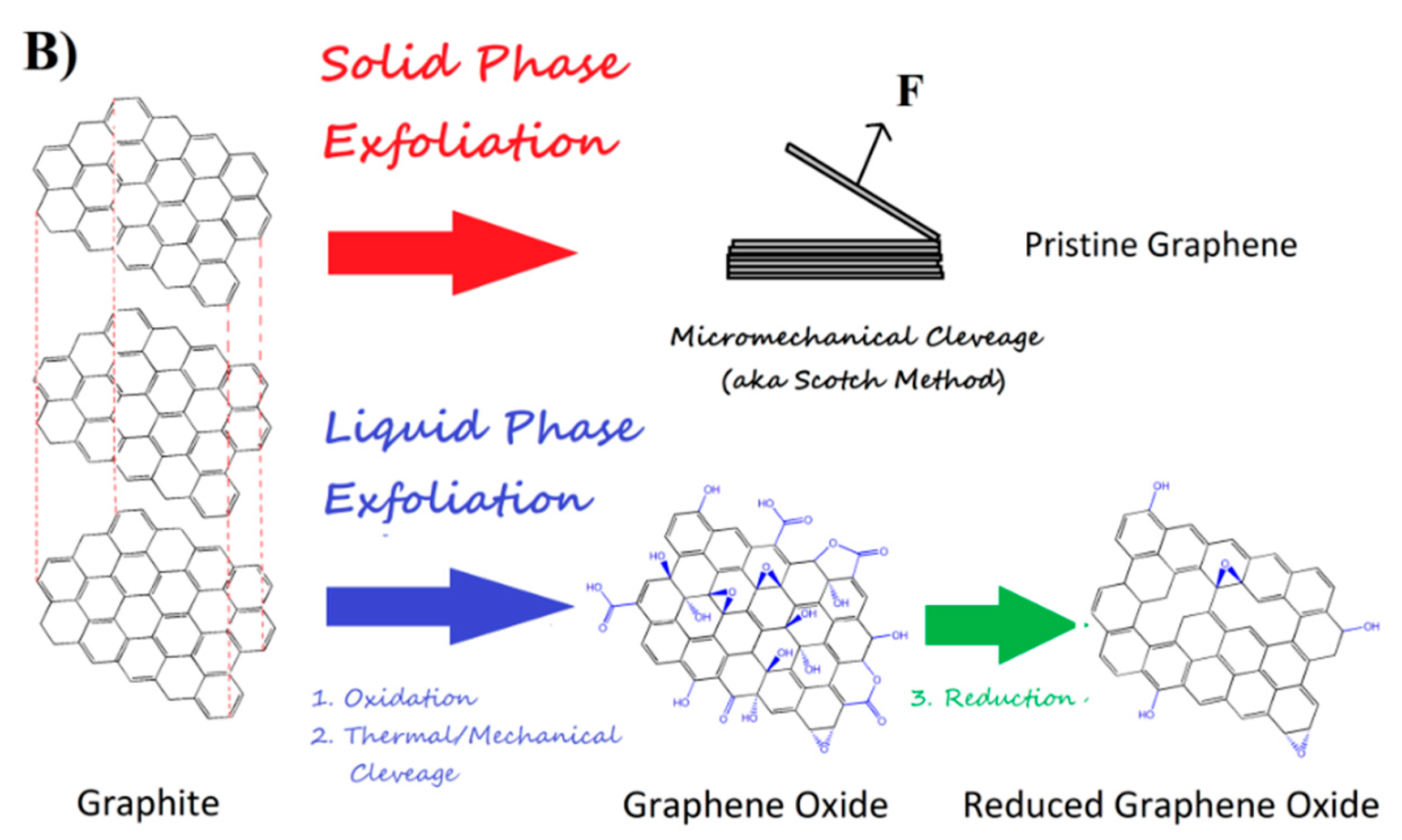
3.1. Graphene Sources and Sensor Preparation
3.2. Immobilization Methods
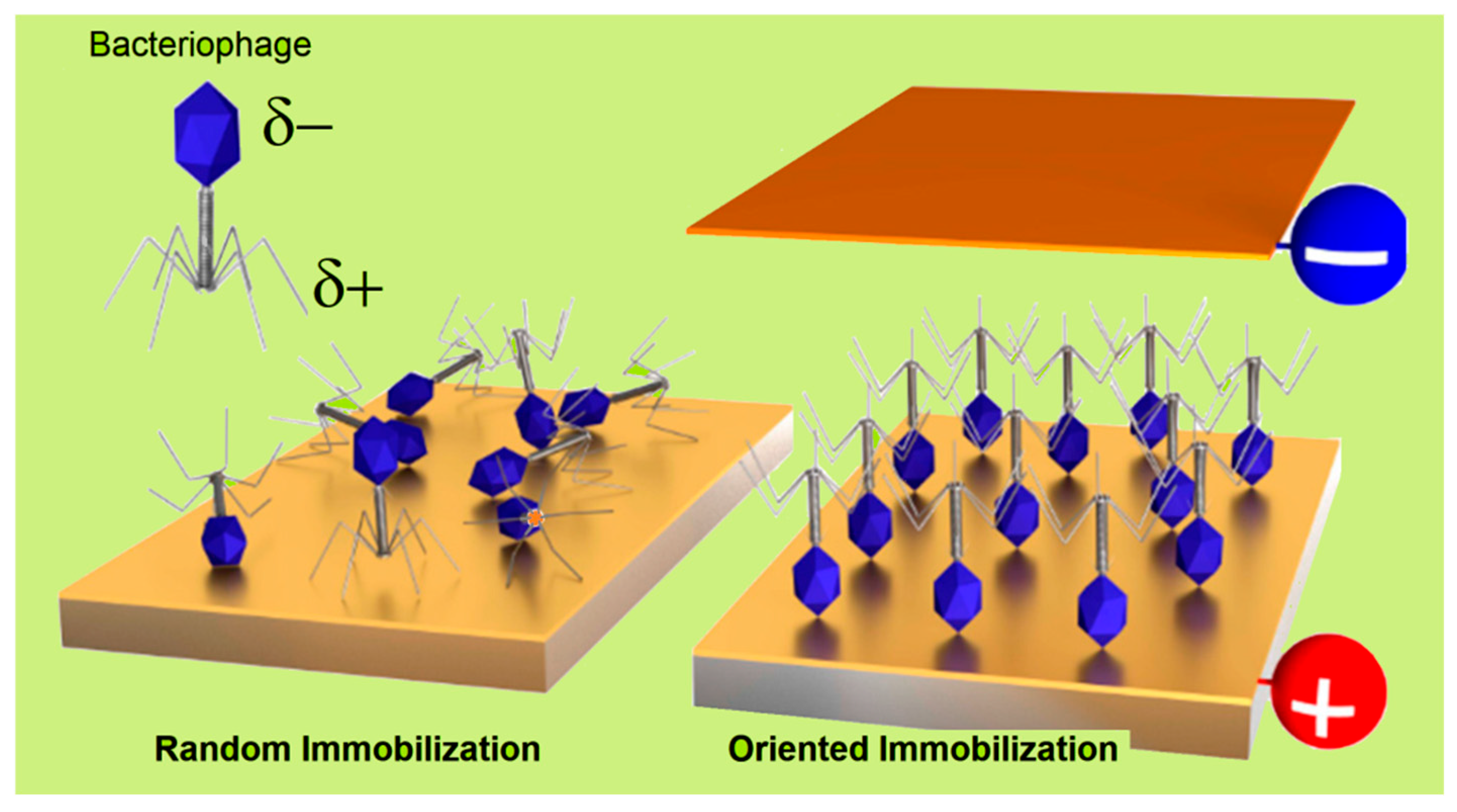
3.2.1. Covalent Bonding
3.2.2. Physisorption, Electrostatic Binding, and Affinity Binding
3.3. Signal Transduction and Detection Mechanisms
3.4. Electroanalytical Performance
4. Conclusions and Future Perspectives
- (a)
- Materials Engineering Aspects: The combination of phages with unexplored graphene derivatives—such as heteroatom-doped or graphene–nanoparticle conjugates—and the use of conductivity-enhancing agents (e.g., metal or semiconductor NPs, ionic liquids, or conducting polymers) could further amplify detection capabilities. Improvements in surface modification techniques could lead to enhanced probe immobilization efficiency and optimized phage orientation for better pathogen recognition. These innovations could potentially push GPEBs’ detection limits below 1 CFU·mL−1. While phage display has not yet been applied in reported GPEBs, it holds considerable potential for future integration [148]. By enabling genetically engineered phages with site-specific attachment tags, phage display could enhance immobilization stability, facilitate orientation control, and support modular receptor design for improved selectivity. These advantages may also contribute to improved reproducibility and cost-efficiency in complex sample conditions. For a more detailed overview of synthetic biology tools in phage-based biosensors, readers are referred to recent specialized reviews such as that reported by Wang et al. [91].
- (b)
- Cost and Scalability: Traditional detection methods, such as PCR and ELISA, face significant economic and scalability challenges. PCR, while highly sensitive, requires sophisticated laboratory infrastructure, expensive reagents, and trained personnel, making it impractical for widespread, on-site applications. Similarly, ELISA relies on monoclonal antibodies, which are not only expensive, but are also difficult to produce at scale, further limiting its accessibility. Electrochemical immunosensors and aptasensors offer improved detection limits and portability but also face cost-related drawbacks. Immunosensors rely on monoclonal antibodies, while custom aptamers, although highly specific and versatile, remain prohibitively expensive to synthesize [21,22], limiting their commercial adoption. In comparison, bacteriophages represent a simpler and less expensive alternative as recognition probes. Nonetheless, the labor-intensive and costly process of large-scale phage purification remains a significant bottleneck for commercialization. Improving the efficiency of this step is essential to enable scalable production in the medium term. Employing RBPs, which can be more easily produced through recombinant overexpression [149], also offers a promising solution to overcome this challenge. Additionally, developing strategies for recovering and reusing immobilized phages or RBPs in disposable devices could further reduce material costs and improve economic feasibility.
- (c)
- Sensor Stability and Durability: Long-term stability of immobilized phages under varying environmental conditions remains largely underexplored. Bacterial lysis following recognition and capture can destabilize the sensor signal, particularly in impedance-based platforms [150]. The rupture of large bacterial cells exposes substantial portions of the sensor surface, causing unpredictable signal fluctuations. Exploring phage’s binding proteins as alternative probes to whole phages could address lysis issues while enhancing overall sensor durability in practical applications (thanks to the greater physicochemical stability of RBPs under extreme conditions).
- (d)
- Expanding Multiplexing Capabilities: Early detection of multiple serovars in a single test could help contain outbreaks more rapidly by minimizing diagnostic delays. Except for Y. Ding et al.’s sensor [57], which responded to four S. enterica strains, current GPEBs typically focus on single-pathogen detection, limiting their practicality for broader applications. Future designs should enable simultaneous detection of various species or strains in a single test, significantly reducing time and resource requirements. Multiplexing could be achieved by co-immobilizing phages or RBPs targeting different genera or serovars. The smaller size of RBPs compared to whole phages may facilitate the assembly of a higher density and diversity of probes, further supporting multiplexing efforts. Co-immobilization with complementary recognition probes, such as antibodies or aptamers, is another possibility. Alternatively, multiple single-target GPEBs could be integrated into multichannel platforms.
- (e)
- Miniaturization and Integration: While miniaturized GPEB designs using SPEs have been demonstrated, further standardization is crucial to advance portable, cost-effective devices for real-time, on-site pathogen monitoring. Miniaturization not only facilitates on-site practical application, but also reduces maintenance requirements and enhances sensor durability, particularly in harsh environments.
- (f)
- Smart Integration: Linking GPEBs to cloud networks and integrating AI for real-time data analysis could enable continuous pathogen monitoring in food production, water systems, or healthcare applications. Integrated systems could provide predictive insights for early intervention during outbreaks but require substantial investments in infrastructure and standardization to ensure compatibility and reliability.
- (g)
- Matrix Effects in Real Samples: Matrix effects remain a significant challenge when applying GPEBs to complex food and environmental samples. High protein or fat content, dense tissue matrices, and interfering compounds can hinder pathogen accessibility to immobilized phages or RBPs, reducing detection capabilities and analytical accuracy [146,147]. Addressing these effects will likely require the implementation of sample pre-treatment strategies, involving processes such as dilution, filtration, emulsion, selective enrichment, or matrix cleanup, to reduce the presence of interfering species without compromising target detection [151]. However, such approaches must be carefully optimized, as sample processing could affect the concentration of bacterial analytes. Signal modulation approaches may help correct for background noise and variability in complex matrices. In addition to internal standards or matrix-specific calibration models, software-based techniques such as chemometric data processing, machine learning algorithms, and wavelet-based signal filtering have shown promise in enhancing signal-to-noise ratios without requiring hardware modification [152,153]. Although antifouling coatings (such as sol-gel and PEG-based or zwitterionic layers) could minimize nonspecific adsorption [154], they may also hinder bacterial access to the sensing surface or interfere with phage orientation, potentially introducing further disadvantages in whole-cell biosensing applications.

Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Au | Gold |
| AFM | Atomic Force Microscopy |
| APTES | (3-Aminopropyl)triethoxysilane |
| APTMS | (3-Aminopropyl)trimethoxysilane |
| BC | Bacterial Cellulose |
| BPs | Bacterial Pathogens |
| BSA | Bovine Serum Albumin |
| CB | Carbon Black |
| CFU | Colony-Forming Units |
| CPE | Carbon Paste Electrode |
| CRGO | Carboxyl-Rich Graphene Oxide |
| CV | Cyclic Voltammetry |
| DPV | Differential Pulse Voltammetry |
| EBs | Electrochemical Biosensors |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl) Carbodiimide |
| EFSA | European Food Safety Agency |
| EIS | Electrochemical Impedance Spectroscopy |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EOPG | Electro-Oxidized Pristine Graphene |
| FC | Flow Cytometry |
| FET | Field-Effect Transistor |
| GCE | Glassy Carbon Electrode |
| GEBs | Graphene-Based Electrochemical Biosensors |
| GFET | Graphene-Based Field-Effect Transistor |
| GNMs | Graphene Nanomaterials |
| GO | Graphene Oxide |
| GPEBs | Graphage-Based Electrochemical Biosensors |
| LOD | Limit of Detection |
| LCR | Low-Frequency Capacitance/Inductance |
| MES | 2-(N-Morpholino)ethanesulfonic Acid (MES buffer) |
| NCBI | National Center for Biotechnology Information |
| NHS | N-Hydroxysuccinimide |
| NPs | Nanoparticles |
| PCR | Polymerase Chain Reaction |
| PD | Phage Display |
| PEBs | Phage-Based Electrochemical Biosensors |
| PEI | Polyethylenimine |
| PI-5-CA | Polyindole-5-carboxylic acid |
| PLL | Poly-L-Lysine |
| PPy | Polypyrrole |
| PVA | Polyvinyl Alcohol |
| PDADMAC | Poly(Diallyldimethylammonium Chloride) |
| qPCR | Quantitative Polymerase Chain Reaction |
| RBPs | Receptor Binding Proteins |
| rGO | Reduced Graphene Oxide |
| SAMs | Self-Assembled Monolayers |
| SEM | Scanning Electron Microscopy |
| SPEs | Screen-Printed Electrodes |
References
- Oliver, H.F.; Wiedmann, M.; Boor, K.J. Environmental Reservoir and Transmission into the Mammalian Host. In Listeria Monocytogenes: Pathogenesis and Host Response, 1st ed.; Goldfine, H., Shen, H., Eds.; Springer: Boston, MA, USA, 2007; pp. 111–138. [Google Scholar] [CrossRef]
- European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; De Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Jaffee, S.; Henson, S.; Unnevehr, L.; Grace, D.; Cassou, E. The Safe Food Imperative: Accelerating Progress in Low- and Middle-Income Countries; Agriculture and Food Series; World Bank: Washington, DC, USA, 2019. [Google Scholar]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.L.; Fey, P.D.; Rupp, M.E. Coagulase-Negative Staphylococcal Infections. Infect. Dis. Clin. N. Am. 2009, 23, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.K.; Kass, P.H.; Soupir, M.L.; Biswas, S.; Singh, V.P. Contamination of Water Resources by Pathogenic Bacteria. AMB Express 2014, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, J.; Kramer, B.S.; Macdonald, H.M.; Schwartz, L.; Woloshin, S. Focusing on Overdiagnosis as a Driver of Too Much Medicine. BMJ 2018, 362, k3494. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, X.; Dong, Y.; Wang, B.; Li, D.; Shi, Y.; Wu, Y. Colorimetric Sensor Array Based on Gold Nanoparticles with Diverse Surface Charges for Microorganisms Identification. Anal. Chem. 2017, 89, 10639–10643. [Google Scholar] [CrossRef] [PubMed]
- Robinson-McCarthy, L.R.; Mijalis, A.J.; Filsinger, G.T.; de Puig, H.; Donghia, N.M.; Schaus, T.E.; Rasmusen, R.A.; Ferreira, R.; Lunshof, J.E.; Chao, G.; et al. Anomalous COVID-19 Tests Hinder Researchers. Science 2021, 371, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Kilic, T.; Erdem, A.; Ozsoz, M.; Carrara, S. MicroRNA Biosensors: Opportunities and Challenges among Conventional and Commercially Available Techniques. Biosens. Bioelectron. 2017, 99, 525–546. [Google Scholar] [CrossRef] [PubMed]
- Collison, M.E.; Stout, P.J.; Glushko, T.S.; Pokela, K.N.; Mullins-Hirte, D.J.; Racchini, J.R.; Walter, M.A.; Mecca, S.P.; Rundquist, J.; Allen, J.J.; et al. Analytical Characterization of Electrochemical Biosensor Test Strips for Measurement of Glucose in Low-Volume Interstitial Fluid Samples. Clin. Chem. 1999, 45, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, A.; Latta, D.; Laboria, N.; Von Germar, F.; Hansen-Hagge, T.E.; Kemmner, W.; Gärtner, C.; Klemm, R.; Drese, K.S.; O’Sullivan, C.K. Integrated Microfluidic Platform for the Electrochemical Detection of Breast Cancer Markers in Patient Serum Samples. Lab Chip 2010, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Ozin, G.A.; Arsenault, A.; Cademartiri, L. Nanochemistry: A Chemical Approach to Nanomaterials; Royal Society of Chemistry (RSC): Cambridge, UK, 2008. [Google Scholar] [CrossRef]
- Zheng, X.X.; Gao, S.; Wu, J.; Hu, X.S. Recent Advances in Aptamer-Based Biosensors for Detection of Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 605229. [Google Scholar] [CrossRef] [PubMed]
- Zambry, N.S.; Najib, M.A.; Awang, M.S.; Selvam, K.; Khalid, M.F.; Bustami, Y.; Hamzah, H.H.; Ozsoz, M.; Manaf, A.A.; Ismail, A. Aptamer-Based Electrochemical Biosensors for the Detection of Salmonella: A Scoping Review. Diagnostics 2022, 12, 3186. [Google Scholar] [CrossRef] [PubMed]
- Razmi, N.; Hasanzadeh, M.; Willander, M.; Nur, O. Electrochemical Genosensor Based on Gold Nanostars for the Detection of Escherichia coli O157:H7 DNA. Anal. Methods 2022, 14, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Dhiman, A.; Kapil, A.; Reddy, B.M.; Sharma, T. Aptamer-Mediated Colorimetric and Electrochemical Detection of Pseudomonas aeruginosa Utilizing Peroxidase-Mimic Activity of Gold NanoZyme. Anal. Bioanal. Chem. 2019, 411, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Low, K.; Chuenrangsikul, K.; Rijiravanich, P.; Surareungchai, W.; Chan, Y. Electrochemical genosensor for specific detection of the food-borne pathogen, Vibrio cholerae. World J. Microbiol. Biotechnol. 2012, 28, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y. Functional Nucleic Acid Based Biosensor for Microorganism Detection. In Functional Nucleic Acid Based Biosensors for Food Safety Detection; Springer: Singapore, 2018; pp. 15–79. [Google Scholar] [CrossRef]
- Yan, J.; Xiong, H.; Cai, S.; Wen, N.; He, Q.; Liu, Y.; Peng, D.; Liu, Z. Advances in Aptamer Screening Technologies. Talanta 2019, 200, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Ang Kian Meng, A. Custom Affinity Chromatrography: Development of a Novel Platform for Rapid Creation and Validation of Affinity Media Using DNA Aptamer Based Ligands. Master’s Thesis, University of British Columbia, Kelowna, BC, Canada, 2018. [Google Scholar]
- Walsh, G. Biopharmaceutical Benchmarks. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Garcia, Z.; Chen, D.; Liu, H.; Trelstad, P. Cost and Supply Considerations for Antibody Therapeutics. mAbs 2025, 17, 2451789. [Google Scholar] [CrossRef] [PubMed]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in Nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.M.; Liau, X.L.; Tang, S.S. Bacteriophages and Their Host Range in Multidrug-Resistant Bacterial Disease treatment. Pharmaceuticals 2023, 16, 1467. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi Silva, J.; Stroms, Z.; Sauvageau, D. Host Receptors for Bacteriophage Adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, F.L.; Vlot, M.; De Jonge, P.A.; Dreesens, L.L.; Beaumont, H.J.E.; Lavigne, R.; Dutilh, B.E.; Brouns, S.J.J. Targeting Mechanisms of Tailed Bacteriophages. Nat. Rev. Microbiol. 2018, 16, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Egido, J.E.; Costa, A.R.; Aparicio-Maldonado, C.; Haas, P.; Brouns, S.J.J. Mechanisms and Clinical Importance of Bacteriophage Resistance. FEMS Microbiol. Rev. 2021, 46, fuab048. [Google Scholar] [CrossRef] [PubMed]
- Anany, H.; Chou, Y.; Cucic, S.; Derda, R.; Evoy, S.; Griffiths, M. From Bits and Pieces to Whole Phage to Nanomachines: Pathogen Detection using Bacteriophages. Annu. Rev. Food Sci. Technol. 2017, 8, 305–329. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Poshtiban, S.; Evoy, S. Recent Advances in Bacteriophage Based Biosensors for Food-Borne Pathogen Detection. Sensors 2013, 13, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Janczuk-Richter, M.; Marinović, I.; Niedziółka-Jönsson, J.; Szot-Karpińska, K. Recent applications of bacteriophage-based electrodes: A mini-review. Electrochem. Comm. 2019, 99, 11–15. [Google Scholar] [CrossRef]
- Xu, J.; Chau, Y.; Lee, Y. Phage-based Electrochemical Sensors: A Review. Micromachines 2019, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Zolti, O.; Suganthan, B.; Maynard, R.K.; Asadi, H.; Locklin, J.; Ramasamy, R.P. Electrochemical Biosensor for Rapid Detection of Listeria monocytogenes. J. Electrochem. Soc. 2022, 169, 067510. [Google Scholar] [CrossRef]
- Fernandes, P.M.V.; Maciel, C.; Teixeira, P.; Pereira, C.M.; Campiña, J.M. Electrostatic Assembly of Anti-Listeria Bacteriophages on a Self-Assembled Monolayer of Aminoundecanethiol: Film Morphology, Charge Transfer Studies, and Infectivity Assays. Surfaces 2023, 6, 114–132. [Google Scholar] [CrossRef]
- Jijie, R.; Kahlouche, K.; Barras, A.; Yamakawa, N.; Bouckaert, J.; Gharbi, T.; Szunerits, S.; Boukherroub, R. Reduced graphene oxide/polyethylenimine based immunosensor for the selective and sensitive electrochemical detection of uropathogenic Escherichia coli. Sens. Actuators B Chem. 2018, 260, 255–263. [Google Scholar] [CrossRef]
- Niyomdecha, S.; Limbut, W.; Numnuam, A.; Kanatharana, P.; Charlermroj, R.; Karoonuthaisiri, N.; Thavarungkul, P. Phage-based capacitive biosensor for Salmonella detection. Talanta 2018, 188, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Richter, Ł.; Bielec, K.; Leśniewski, A.; Łoś, M.; Paczesny, J.; Hołyst, R. Dense Layer of Bacteriophages Ordered in Alternating Electric Field and Immobilized by Surface Chemical Modification as Sensing Element for Bacteria Detection. ACS Appl. Mater. Interfaces 2017, 9, 19622–19629. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, K.; Sikes, K.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H. Ultrahigh Electron Mobility in Suspended Graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Graphene in biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, Conductive Graphene Electrodes for Dye-Sensitized Solar Cells. Nano Lett. 2007, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H. Graphene Anchored with Co3O4 Nanoparticles as Anode of Lithium Ion Batteries with Enhanced Reversible Capacity and Cyclic Performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Boukherroub, R. Graphene-based Bioelectrochemistry and Bioelectronics: A Concept for the Future? Curr. Opin. Electrochem. 2018, 12, 141–147. [Google Scholar] [CrossRef]
- Chauhan, N.; Maekawa, T.; Kumar, D.S. Graphene Based Biosensors—Accelerating Medical Diagnostics to New-Dimensions. J. Mater. Res. 2017, 32, 2860–2882. [Google Scholar] [CrossRef]
- Park, C.B.; Yoon, H.; Kwon, O.J. Graphene-Based Nanoelectronic Biosensors. J. Ind. Eng. Chem. 2016, 38, 13–22. [Google Scholar] [CrossRef]
- Xu, L.; Wen, Y.; Pandit, S.; Mokkapati, V.R.S.S.; Mijakovic, I.; Li, Y.; Ding, M.; Ren, S.; Li, W.; Liu, G. Graphene-Based Biosensors for the Detection of Prostate Cancer Protein Biomarkers: A Review. BMC Chem. 2019, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Czarnik-Matusewicz, B.; Murayama, K.; Wu, A.Y.; Ozaki, Y. Two-Dimensional Attenuated Total Reflection/Infrared Correlation Spectroscopy of Adsorption-Induced and Concentration-Dependent Spectral Variations of Β-Lactoglobulin in Aqueous Solutions. J. Phys. Chem. B 2000, 104, 7803–7811. [Google Scholar] [CrossRef]
- Reddy, Y.V.M.; Shin, J.H.; Palakollu, V.N.; Sravani, B.; Choi, C.; Park, K.; Kim, S.; Madhavi, G.; Park, J.P.; Shetti, N.P. Strategies, Advances, and Challenges Associated with the Use of Graphene-Based Nanocomposites for Electrochemical Biosensors. Adv. Colloid Interface Sci. 2022, 304, 102664. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Kim, S.N.; Jones, S.E.; Wissler, L.L.; Naik, R.R.; McAlpine, M.C. Chemical Functionalization of Graphene Enabled by Phage Displayed Peptides. Nano Lett. 2010, 10, 4559–4565. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Bhardwaj, S.K.; Mehta, J.; Mohanta, G.C.; Deep, A. Bacteriophage Immobilized Graphene Electrodes for Impedimetric Sensing of Bacteria (Staphylococcus arlettae). Anal. Biochem. 2016, 505, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Passaretti, P.; Sun, Y.; Khan, I.; Chan, K.C.; Sabo, R.; White, H.; Dafforn, T.R.; Oppenheimer, P.G. Multifunctional Graphene Oxide-Bacteriophage Based Porous Three-Dimensional Micro-Nanocomposites. Nanoscale 2019, 11, 13318–13329. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; García, P.; Mullany, P.; Aminov, R. Editorial: Phage Therapy: Past, Present and Future. Front. Microbiol. 2017, 8, 981. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Sheath, P.; Martin, S.T.; Shinde, D.B.; Shaibani, M.; Banerjee, P.C.; Tkacz, R.; Bhattacharyya, D.; Majumder, M. Large-Area Graphene-Based Nanofiltration Membranes by Shear alignment of Discotic Nematic Liquid Crystals of Graphene Oxide. Nat. Commun. 2016, 7, 10891. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Huang, X.; Ma, Y.; Huang, Y.; Yang, R.; Duan, H.; Chen, Y. Multi-Functionalized Graphene Oxide Based Anticancer Drug-Carrier with Dual-Targeting Function and pH-sensitivity. J. Mat. Chem. 2010, 21, 3448–3454. [Google Scholar] [CrossRef]
- Quiton, P.A.; Carreon, B.M.; Cruz-Papa, D.M.D.; Bergantin, J., Jr. Bacteriophage-modified Graphene Oxide Screen-printed Electrodes for the Impedimetric Biosensing of Salmonella Enterica Serovar Typhimurium. Sens. Transducers 2018, 28, 38–42. Available online: https://sensorsportal.com/HTML/ST_JOURNAL/vol_28.html (accessed on 15 June 2024).
- Ding, Y.; Zhang, Y.; Huang, C.; Wang, J.; Li, H.; Wang, X. An Electrochemical Biosensor Based on Phage-Encoded Protein RBP 41 for Rapid and Sensitive Detection of Salmonella. Talanta 2024, 270, 125561. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Z.; Huang, J.; Wu, Y.; Mao, X.; Tan, Y.; Liu, H.; Ma, D.; Li, X.; Wang, X. Development of a Phage-Based Electrochemical Biosensor for Detection of Escherichia coli O157: H7 GXEC-N07. Bioelectrochemistry 2023, 150, 108345. [Google Scholar] [CrossRef] [PubMed]
- Nakama, K.; Sedki, M.; Mulchandani, A. Label-free Chemiresistor Biosensor Based on Reduced Graphene Oxide and M13 Bacteriophage for Detection of Coliforms. Anal. Chim. Acta 2021, 1150, 338232. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Deng, S.; Xu, J.; Farooq, U.; Yang, T.; Chen, W.; Zhou, L.; Gao, M.; Wang, S. Poly(indole-5-carboxylic acid)/Reduced Graphene Oxide/Gold Nanoparticles/Phage-based Electrochemical Biosensor for Highly Specific Detection of Yersinia pseudotuberculosis. Microchim. Acta 2021, 188, 107. [Google Scholar] [CrossRef] [PubMed]
- Keihan, A.H.; Hosseinzadeh, G.; Sajjadi, S.; Ashiani, D.; Dashtestani, F.; Eskandari, K. Bacteriophage-Based Biosensor for Detection of E. coli Bacteria on Graphene Modified Carbon Paste Electrode. Nanosci. Nanotechnol. Asia 2019, 9, 408–413. [Google Scholar] [CrossRef]
- Hussain, W.; Wang, H.; Yang, X.; Ullah, M.W.; Hussain, J.; Ullah, N.; Ul-Islam, M.; Awad, M.F.; Wang, S. Ultrasensitive Electrochemical Detection of Salmonella typhimurium in Food Matrices Using Surface-Modified Bacterial Cellulose with Immobilized Phage Particles. Biosensors 2024, 14, 500. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Feng, B.; Xu, J.; Qing, T.; Zhang, P.; Qing, Z. Graphene Biosensors for Bacterial and Viral Pathogens. Biosens. Bioelectron. 2020, 166, 112471. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Orozco, J. Hybrid Nanobioengineered Nanomaterial-Based Electrochemical Biosensors. Molecules 2022, 27, 3841. [Google Scholar] [CrossRef] [PubMed]
- Yusaf, T.; Mahamude, A.S.F.; Farhana, K.; Harun, W.S.W.; Kadirgama, K.; Ramasamy, D.; Kamarulzaman, M.K.; Subramonian, S.; Hall, S.; Dhahad, H.A. A Comprehensive Review on Graphene Nanoparticles: Preparation, Properties, and Applications. Sustainability 2022, 14, 12336. [Google Scholar] [CrossRef]
- Al-Dhahebi, A.M.; Gopinath, S.C.B.; Saheed, M.S.M. Graphene Impregnated Electrospun Nanofiber Sensing Materials: A Comprehensive Overview on Bridging Laboratory Set-Up to Industry. Nano Converg. 2020, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and Technology Roadmap for Graphene, Related Two-Dimensional Crystals, and Hybrid Systems. Nanoscale 2014, 7, 4598–4810. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Lombardo, A.; Hasan, T.; Sun, Z.; Colombo, L.; Ferrari, A.C. Production and Processing of Graphene and 2D Crystals. Mater. Today 2012, 15, 564–589. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable Production of Large Quantities of Defect-Free Few-Layer Graphene by Shear Exfoliation in Liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Shams, S.S.; Zhang, R.; Zhu, J. Graphene Synthesis: A Review. Mater. Sci.-Pol. 2015, 33, 566–578. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.; Huang, W.; Chen, P. Heteroatom-Doped Graphene Materials: Syntheses, Properties and Applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent Advances in Graphene-Based Biosensors. Biosen. Bioelec. 2011, 26, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.S.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, W.; Yan, L. Electrochemical Reduction of Bulk Graphene Oxide Materials. RSC Adv. 2016, 6, 80106–80113. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene Based Materials for Biomedical Applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Krishnan, S.; Singh, E.J.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A Review on Graphene-Based Nanocomposites for Electrochemical and Fluorescent Biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Luo, Y.; Zhu, C.; Li, H.; Du, D.; Lin, Y. Recent Advances in Electrochemical Biosensors Based on Graphene Two-Dimensional Nanomaterials. Biosens. Bioelectron. 2016, 76, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Campiña, J.M.; Fernandes, P.M.V.; Ventura, J.; Titus, E.; Silva, A.F. Reduced Graphene Oxide-Nickel Nanoparticles/Biopolymer Composite Films for the Sub-Millimolar Detection of Glucose. Analyst 2016, 141, 4151–4161. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.M.V.; Campiña, J.M.; Silva, A.F. A Layered Nanocomposite of Laccase, Chitosan, and Fe3O4 Nanoparticles-Reduced Graphene Oxide for the Nanomolar Electrochemical Detection of Bisphenol A. Mikrochim. Acta 2020, 187, 262. [Google Scholar] [CrossRef] [PubMed]
- Duffy, G.F.; Moore, E. Electrochemical Immunosensors for Food Analysis: A Review of Recent Developments. Anal. Lett. 2017, 50, 1–32. [Google Scholar] [CrossRef]
- Muniandy, S.; Dinshaw, I.J.; Teh, S.J.; Lai, C.W.; Ibrahim, F.; Thong, K.L.; Leo, B.F. Graphene-Based Label-Free Electrochemical Aptasensor for Rapid and Sensitive Detection of Foodborne Pathogen. Anal. Bioanal. Chem. 2017, 409, 6893–6905. [Google Scholar] [CrossRef] [PubMed]
- Crapnell, R.D.; Banks, C.E. The Handbook of Graphene Electrochemistry; Springer: London, UK, 2024. [Google Scholar] [CrossRef]
- Soares, R.R.A.; Milião, G.L.; Pola, C.C.; Jing, D.; Opare-Addo, J.; Smith, E.; Claussen, J.C.; Gomes, C.L. Insights into Solid-Contact Ion-Selective Electrodes Based on Laser-Induced Graphene: Key Performance Parameters for Long-Term and Continuous Measurements. Microchim. Acta 2024, 191, 615. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.-W. Bacteriophage Observations and Evolution. Res. Microbiol. 2003, 154, 245–251. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, L.A.; Marcoux, P.; Roupioz, Y. Strategies for Surface Immobilization of Whole Bacteriophages: A Review. ACS Biomater. Sci. Eng. 2021, 7, 1987–2014. [Google Scholar] [CrossRef] [PubMed]
- Carmody, C.M.; Goddard, J.M.; Nugen, S.R. Bacteriophage Capsid Modification by Genetic and Chemical Methods. Bioconjug. Chem. 2021, 32, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Y.; Chung, W.; Lee, D. Chemical Modulation of M13 Bacteriophage and its Functional Opportunities for Nanomedicine. Int. J. Nanomed. 2014, 9, 5825. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fokine, A.; Leiman, P.G.; Shneider, M.M.; Ahvazi, B.; Boeshans, K.M.; Steven, A.C.; Black, L.W.; Mesyanzhinov, V.V.; Rossmann, M.G. Structural and Functional Similarities Between the Capsid Proteins of Bacteriophages T4 and HK97 Point to a Common Ancestry. Proc. Natl. Acad. Sci. USA 2005, 102, 7163–7168. [Google Scholar] [CrossRef] [PubMed]
- Pande, J.; Szewczyk, M.M.; Grover, A.K. Phage Display: Concept, Innovations, Applications and Future. Biotechnol. Adv. 2010, 28, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Pang, S.; Zhang, H.; Yang, Z.; Liu, A. Phage Display Based Biosensing: Recent Advances and Challenges. TrAC Trends Anal. Chem. 2024, 173, 117629. [Google Scholar] [CrossRef]
- Machera, S.J.; Niedziółka-Jönsson, J.; Szot-Karpińska, K. Phage-Based Sensors in Medicine: A Review. Chemosensors 2020, 8, 61. [Google Scholar] [CrossRef]
- Tawil, N.; Sacher, E.; Mandeville, R.; Meunier, M. Strategies for the Immobilization of Bacteriophages on Gold Surfaces Monitored by Surface Plasmon Resonance and Surface Morphology. J. Phys. Chem. C 2013, 117, 6686–6691. [Google Scholar] [CrossRef]
- Handa, H.; Gurczynski, S.; Jackson, M.P.; Auner, G.; Walker, J.; Mao, G. Recognition of Salmonella Typhimurium by Immobilized Phage P22 Monolayers. Surf. Sci. 2008, 602, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Wu, X.; Fraser, K.; Zha, J.; Dordick, J.S. Flexible Peptide Linkers Enhance the Antimicrobial Activity of Surface-Immobilized Bacteriolytic Enzymes. ACS Appl. Mater. Interfaces 2018, 10, 36746–36756. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Jafari, M.R.; Derda, R. Bacteriophages and Viruses as a Support for Organic Synthesis and Combinatorial Chemistry. ACS Chem. Biol. 2011, 7, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Campiña, J.M.; Martins, A.; Silva, A.F. Immobilization of β-cyclodextrin on Gold Surfaces by Chemical Derivatization of an 11-Amino-1-Undecanthiol Self-Assembled Monolayer. Electrochim. Acta 2009, 55, 90–103. [Google Scholar] [CrossRef]
- Han, L.; Xia, H.; Yin, L.; Petrenko, V.A.; Liu, A. Selected Landscape Phage Probe as Selective Recognition Interface for Sensitive Total Prostate-Specific Antigen Immunosensor. Biosens. Bioelectron. 2018, 106, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sedki, M.; Chen, X.; Chen, C.; Ge, X.; Mulchandani, A. Non-lytic M13 Phage-Based Highly Sensitive ImpedimetricCytosensor for Detection of Coliforms. Biosens. Bioelectron. 2020, 148, 111794. [Google Scholar] [CrossRef] [PubMed]
- Vipin, S.; Pranay, J.; Swati, D. Bacteriophage Based Self-Assembled monolayer (SAM) on Gold Surface Used for Detection of Escherichia coli by Electrochemical Analysis. Afr. J. Microbiol. Res. 2015, 9, 1832–1839. [Google Scholar] [CrossRef]
- Shin, J.H.; Gul, A.R.; Hyun, M.S.; Choi, C.; Park, T.J.; Park, J.P. Electrochemical Detection of Caspase-3 Based on a Chemically Modified M13 Phage Virus. Bioelectrochemistry 2022, 145, 108090. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in Bio-Catalysts Design: A Useful Crosslinker and a Versatile Tool in Enzyme Immobilization. RSC Adv. 2013, 4, 1583–1600. [Google Scholar] [CrossRef]
- O’Connell, L.; Marcoux, P.R.; Perlemoine, P.; Roupioz, Y. Approaching the Geometric Limit of Bacteriophage Conjugation to Gold: Synergy of Purification with Covalent and Physisorption Strategies. ACS Biomater. Sci. Eng. 2023, 9, 2335–2346. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Xiang, M.; Mi, X. A New Bacteriophage-Modified Piezoelectric Sensor for Rapid and Specific detection of Mycobacterium. Anal. Lett. 2012, 45, 1242–1253. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhardwaj, S.K.; Mehta, J.; Nayak, M.K.; Deep, A. Bacteriophage Conjugated IRMOF-3 as a Novel Opto-Sensor for S. arlettae. New J. Chem. 2016, 40, 8068–8073. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhardwaj, S.K.; Mehta, J.; Kim, K.; Deep, A. MOF–Bacteriophage Biosensor for Highly Sensitive and Specific Detection of Staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 33589–33598. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Wang, X.; Tarabara, V.V.; Katayama, H. Virus Removal by Microfiltration: Effects of Electrostatic and Hydrophobic Interactions. Sep. Purif. Technol. 2024, 350, 127902. [Google Scholar] [CrossRef]
- Rosner, D.; Clark, J. Formulations for Bacteriophage Therapy and the Potential Uses of Immobilization. Pharmaceuticals 2021, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, M.; Maasilta, I.J.; Sundberg, L. Antibacterial Efficiency of Surface-Immobilized Flavobacterium-Infecting Bacteriophage. ACS Appl. Bio Mater. 2019, 2, 4720–4727. [Google Scholar] [CrossRef] [PubMed]
- Cademartiri, R.; Anany, H.; Gross, I.; Bhayani, R.; Griffiths, M.; Brook, M.A. Immobilization of Bacteriophages on Modified Silica Particles. Biomaterials 2010, 31, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Anany, H.; Chen, W.; Pelton, R.; Griffiths, M.W. Biocontrol of Listeria monocytogenes and Escherichia coli O157:H7 in Meat by Using Phages Immobilized on Modified Cellulose Membranes. Appl. Environ. Microbiol. 2011, 77, 6379–6387. [Google Scholar] [CrossRef] [PubMed]
- Lone, A.; Anany, H.; Hakeem, M.J.; Aguis, L.; Avdjian, A.; Bouget, M.; Atashi, A.; Brovko, L.; Rochefort, D.; Griffiths, M.W. Development of Prototypes of Bioactive Packaging Materials based on Immobilized Bacteriophages for Control of Growth of Bacterial Pathogens in Foods. Int. J. Food Microbiol. 2016, 217, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Vonasek, E.; Lu, P.; Hsieh, Y.; Nitin, N. Bacteriophages immobilized on electrospun cellulose microfibers by Non-specific Adsorption, Protein–Ligand Binding, and Electrostatic Interactions. Cellulose 2017, 24, 4581–4589. [Google Scholar] [CrossRef]
- Farooq, U.; Ullah, M.W.; Yang, Q.; Aziz, A.; Xu, J.; Zhou, L.; Wang, S. High-Density Phage Particles Immobilization in Surface-Modified Bacterial Cellulose for Ultra-Sensitive and Selective Electrochemical Detection of Staphylococcus aureus. Biosens. Bioelectron. 2020, 157, 112163. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Mine, K.; Tomonari, H.; Uchiyama, J.; Matuzaki, S.; Niko, Y.; Hadano, S.; Watanabe, S. Dark-Field Microscopic Detection of Bacteria using Bacteriophage-Immobilized SiO2@AuNP Core–Shell Nanoparticles. Anal. Chem. 2019, 91, 12352–12357. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, V.; Han, J.; Kim, C.; Kang, Y.; Oh, J.W. Self-Assembled Nanoporous Biofilms from Functionalized Nanofibrous M13 Bacteriophage. Viruses 2018, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Serwer, P. Agarose Gel Electrophoresis of Bacteriophages and Related Particles. J. Chromatogr. B Biomed. Sci. Appl. 1987, 418, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Fokine, A.; Mahalingam, M.; Zhang, Z.; Garcia-Doval, C.; Van Raaij, M.; Rossmann, M.G.; Rao, V.B. Molecular Anatomy of the Receptor Binding Module of a Bacteriophage Long Tail Fiber. PLoS Pathog. 2019, 15, e1008193. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P. Filamentous Fusion Phage: Novel Expression Vectors that Display Cloned Antigens on the Virion Surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Liu, G. Grand Challenges in Biosensors and Biomolecular Electronics. Front. Bioeng. Biotechnol. 2021, 9, 707615. [Google Scholar] [CrossRef] [PubMed]
- Prabowo, B.A.; Cabral, P.D.; Freitas, P.; Fernandes, E. The Challenges of Developing Biosensors for Clinical Assessment: A Review. Chemosensors 2021, 9, 299. [Google Scholar] [CrossRef]
- Yang, Z.; Albrow-Owen, T.; Cai, W.; Hasan, T. Miniaturization of Optical Spectrometers. Science 2021, 371, eabe0722. [Google Scholar] [CrossRef] [PubMed]
- Uniyal, A.; Srivastava, G.; Pal, A.; Taya, S.; Muduli, A. Recent Advances in Optical Biosensors for Sensing Applications: A Review. Plasmonics 2023, 18, 735–750. [Google Scholar] [CrossRef]
- Hameed, S.; Naz, I.; Badea, M.; Bano, N.; Andreescu, S.; Hayat, A.; Jubeen, F. Optical Fiber Mediated Biosensors for Multiplex and Onsite Food Safety Analysis: A Review. J. Electrochem. Soc. 2025, 172, 017522. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Q.; Xiang, T.; Chen, X. Recent Advances in Electrochemical Biosensors for Bacterial Detection. Nano Trans. Med. 2025, 4, 100078. [Google Scholar] [CrossRef]
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive Biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Randles, J.E.B. Kinetics of Rapid Electrode Reactions. Discuss. Faraday Soc. 1947, 1, 11. [Google Scholar] [CrossRef]
- Martinkova, P.; Kostelnik, A.; Valek, T.; Pohanka, M. Main Streams in the Construction of Biosensors and Their Applications. Int. J. Electrochem. Sci. 2017, 12, 7386–7403. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. A Review on Impedimetric Biosensors. Artif. Cells Nanomed. Biotechnol. 2014, 44, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.; Zhou, G.; Chang, J.; Pu, H.; Jin, B.; Sui, X.; Yuan, X.; Yang, C.; Magruder, M.; Chen, J. Rapid Detection of Single E. coli Bacteria using a Graphene-Based Field-Effect Transistor Device. Biosens. Bioelectron. 2018, 110, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, H.; Zhang, J.; Wang, J. Phage Long Tail Fiber Protein-Immobilized Magnetic Nanoparticles for Rapid and Ultrasensitive Detection of Salmonella. Talanta 2022, 248, 123627. [Google Scholar] [CrossRef] [PubMed]
- Denyes, J.M.; Dunne, M.; Steiner, S.; Mittelviefhaus, M.; Weiss, A.; Schmidt, H.; Klumpp, J.; Loessner, M.J. Modified Bacteriophage S16 Long Tail Fiber Proteins for Rapid and Specific Immobilization and Detection of Salmonella Cells. Appl. Environ. Microbiol. 2017, 83, 13. [Google Scholar] [CrossRef] [PubMed]
- Mohr, H.; Lambrecht, B.; Bayer, A.; Spengler, H.; Nicol, S.; Montag, T.; Müller, T.H. Basics of Flow Cytometry–Based Sterility Testing of Platelet Concentrates. Transfusion 2005, 46, 41–49. [Google Scholar] [CrossRef] [PubMed]
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration, and Serotyping of Salmonella—Part 1: Detection of Salmonella. International Society for Standarization: Geneva, Switzerland, 2017.
- Wang, K.; Lin, X.; Zhang, M.; Li, Y.; Luo, C.; Wu, J. Review of Electrochemical Biosensors for Food Safety Detection. Biosensors 2022, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, H.; Li, J.; Hu, J.; Lin, G.; Tan, B.K.; Lin, S. Current Perspectives on Viable but Non-Culturable Foodborne Pathogenic Bacteria: A Review. Foods 2023, 12, 1179. [Google Scholar] [CrossRef] [PubMed]
- Nath, S. Advancements in Food Quality Monitoring: Integrating Biosensors for Precision Detection. Sustain. Food Technol. 2024, 2, 976–992. [Google Scholar] [CrossRef]
- Emerson, J.B.; Adams, R.I.; Román, C.M.B.; Brooks, B.; Coil, D.A.; Dahlhausen, K.; Ganz, H.H.; Hartmann, E.M.; Hsu, T.; Justice, N.B.; et al. Schrödinger’s Microbes: Tools for Distinguishing the Living from the Dead in Microbial Ecosystems. Microbiome 2017, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Raji, H.; Kokabi, M.; Zou, D.; Chan, J.; Liu, Q.; Zhang, R.; Javanmard, M. Microfluidic Assays for CD4 T Lymphocyte Counting: A Review. Biosensors 2025, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Huang, C.; Zhu, W.; Li, Z.; Zhang, Y.; Wang, J.; Pan, H.; Li, H.; Wang, X. Characterization of a Novel Jerseyvirus Phage T102 and its Inhibition Effect on Biofilms of Multidrug-Resistant Salmonella. Virus Res. 2023, 326, 199054. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Huang, C.; Zhang, Y.; Wang, J.; Wang, X. Magnetic Microbead Enzyme-Linked Immunoassay Based on Phage Encoded Protein RBP 41-Mediated for Rapid and Sensitive Detection of Salmonella in Food Matrices. Food Res. Int. 2023, 163, 112212. [Google Scholar] [CrossRef] [PubMed]
- Masson, J. Consideration of Sample Matrix Effects and “Biological” Noise in Optimizing the Limit of Detection of Biosensors. ACS Sens. 2020, 5, 3290–3292. [Google Scholar] [CrossRef] [PubMed]
- Rizzotto, F.; Khalife, M.; Hou, Y.; Chaix, C.; Lagarde, F.; Scaramozzino, N.; Vidic, J. Recent Advances in Electrochemical Biosensors for Food Control. Micromachines 2023, 14, 1412. [Google Scholar] [CrossRef] [PubMed]
- Haiping, L.; Jiangyue, W.; Fanping, M.; Aifeng, L. Immunochromatographic Assay for the Detection of Antibiotics in Animal-Derived Foods: A Review. Food Control 2021, 130, 108356. [Google Scholar] [CrossRef]
- Bai, Y.; Shahed-Al-Mahmud, M.; Selvaprakash, K.; Lin, N.; Chen, Y. Tail Fiber Protein-Immobilized Magnetic Nanoparticle-Based Affinity Approaches for Detection of Acinetobacter baumannii. Anal. Chem. 2019, 91, 10335–10342. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.J.; Sacher, J.C.; Szymanski, C.M. Development of an Assay for the Identification of Receptor Binding Proteins from Bacteriophages. Viruses 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.P.; Nogueira, C.L.; Cunha, A.P.; Lisac, A.; Carvalho, C.M. Potential of Bacteriophage Proteins as Recognition Molecules for Pathogen Detection. Crit. Rev. Biotechnol. 2022, 43, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Temgoua, R.C.T.; Jiokeng, S.L.Z.; Tajeu, K.Y.; Deffo, G.; Vomo, L.A.; Dontsi, F.T.; Njanja, E.; Tonlé, I.K. Sample Preparation Techniques for Electrochemical Analysis of Pesticides and Heavy Metals in Environmental and Food Samples; IntechOpen eBooks: London, UK, 2023. [Google Scholar] [CrossRef]
- Jakubowska, M. Signal Processing in Electrochemistry. Electroanalysis 2011, 23, 553–572. [Google Scholar] [CrossRef]
- Ward, S.J.; Layouni, R.; Arshavsky-Graham, S.; Segal, E.; Weiss, S.M. Morlet Wavelet Filtering and Phase Analysis to Reduce the Limit of Detection for Thin Film Optical Biosensors. ACS Sens. 2021, 6, 2967–2978. [Google Scholar] [CrossRef] [PubMed]
- Jarosińska, E.; Zambrowska, Z.; Nery, E.W. Methods of Protection of Electrochemical Sensors against Biofouling in Cell Culture Applications. ACS Omega 2024, 9, 4572–4580. [Google Scholar] [CrossRef] [PubMed]


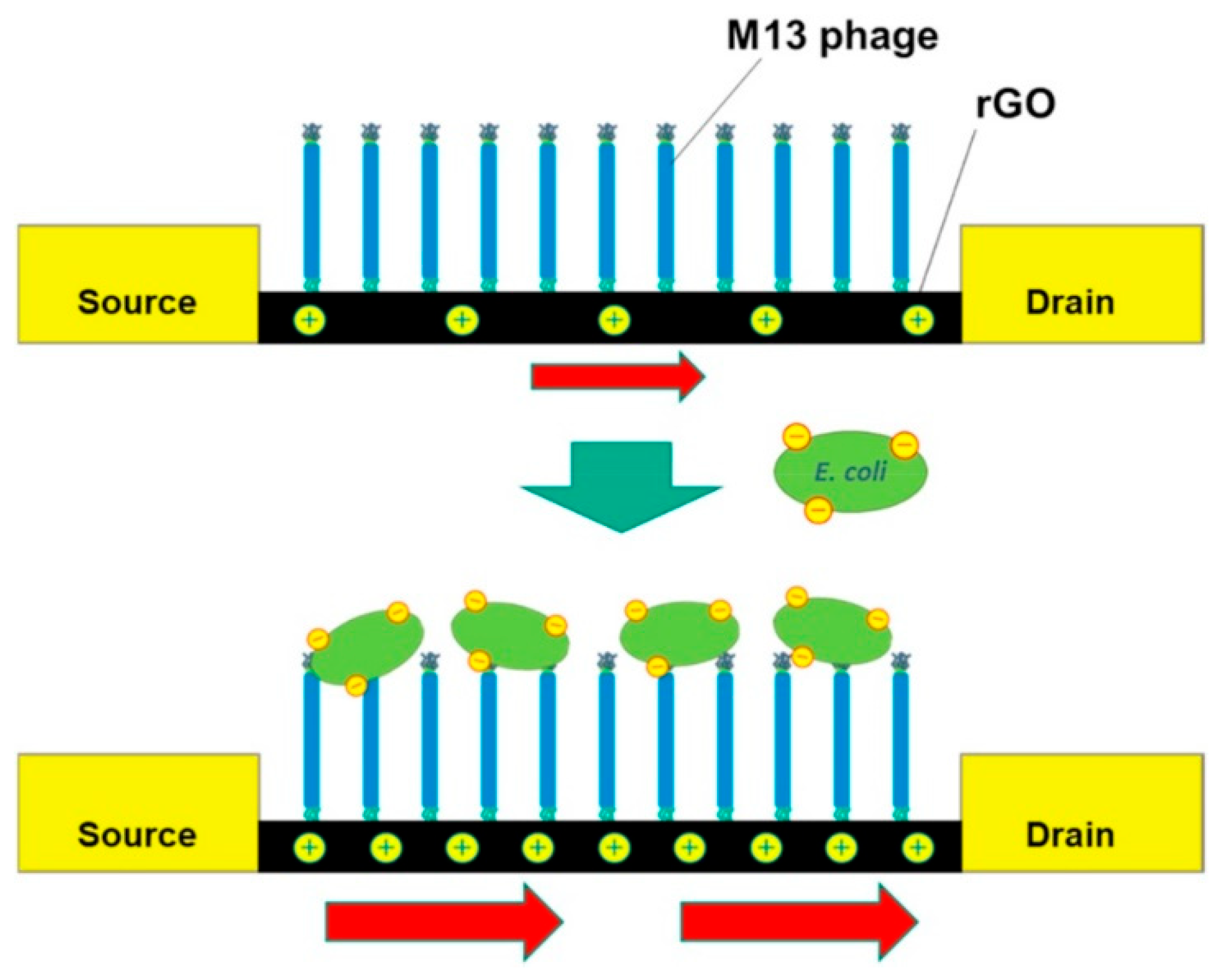
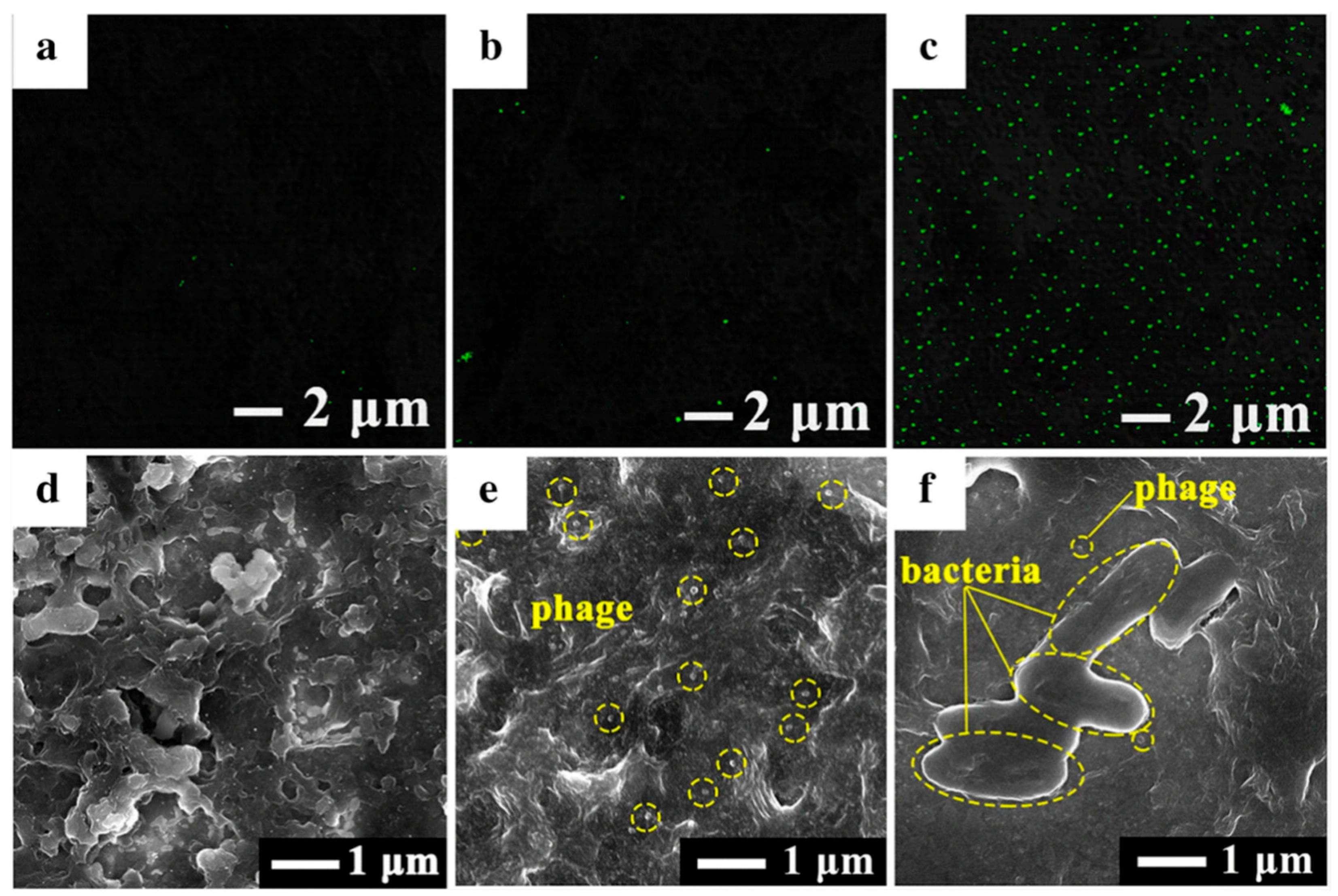

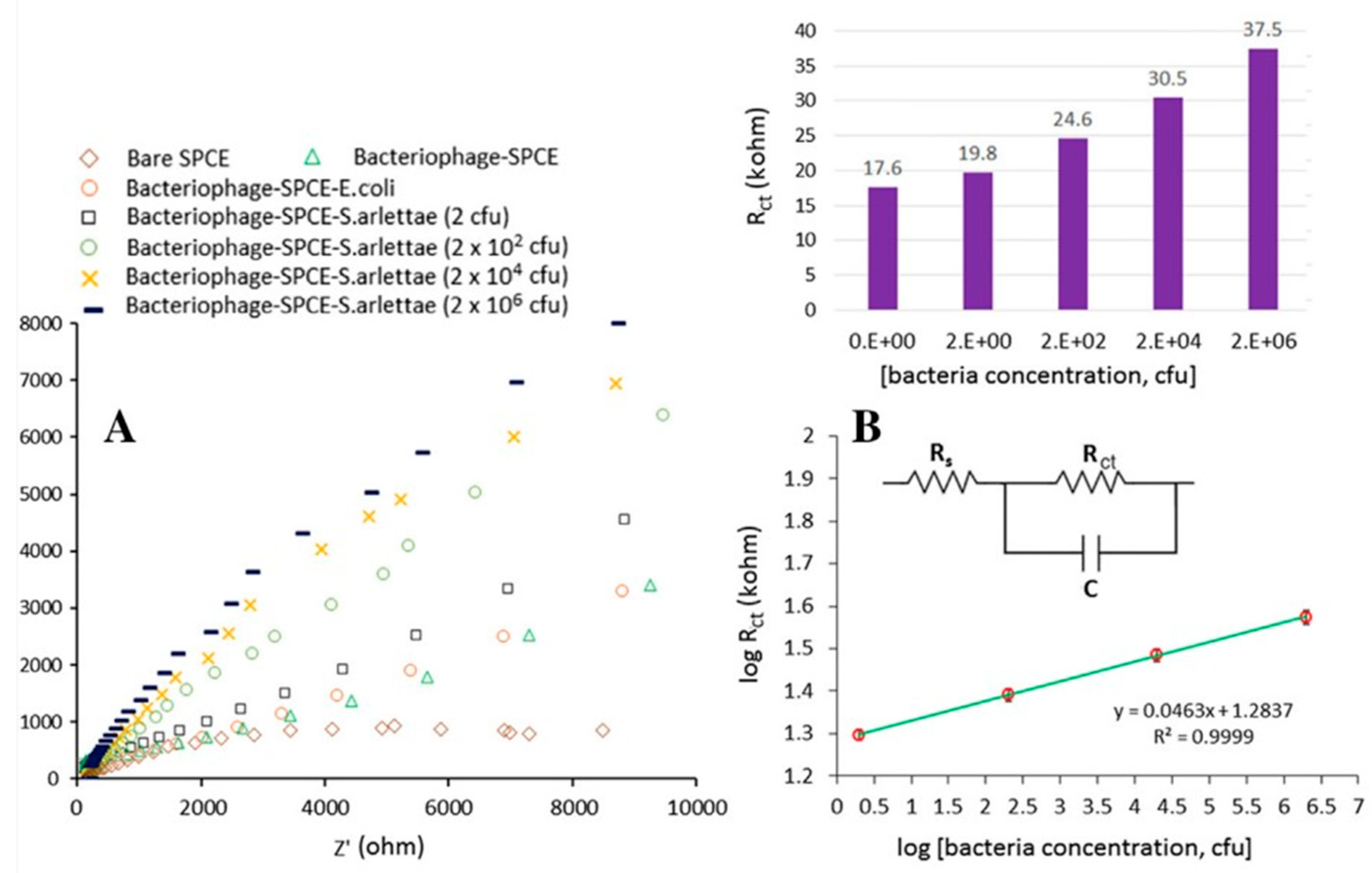
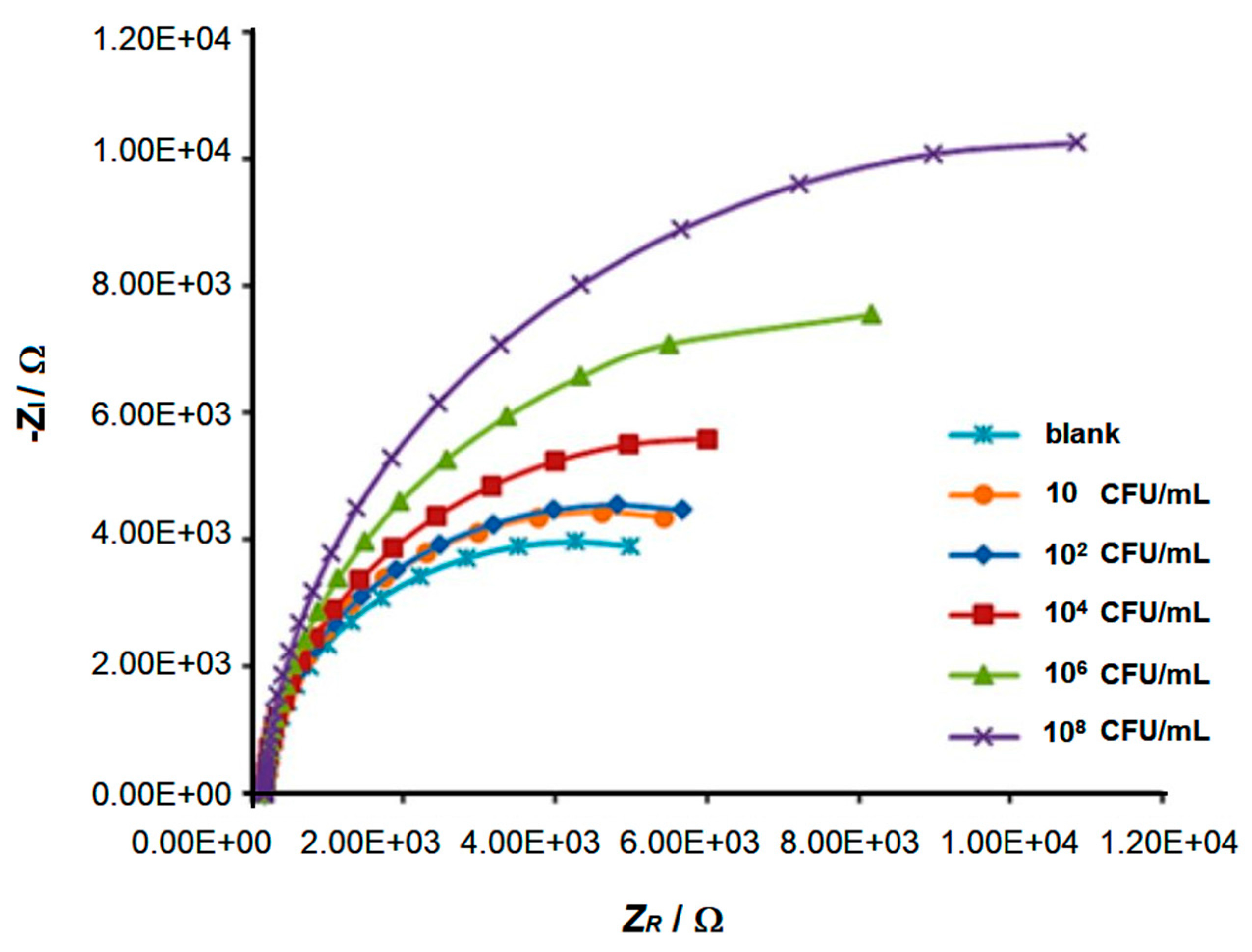
| GNM | Phage | Immobilization | Incubation Times | Biosensor Structure—Electrode | Real Samples | Transduction | Ref. |
|---|---|---|---|---|---|---|---|
| Electrochemically Oxidized Pristine Graphene (EOPG) | Anti-Staphylococcus arlettae | Covalent | 2 h | EOPG/phage—SPE | River Water and Apple Juice | Impedance | [51] |
| GO | Anti-Salmonella Typhimurium | Covalent | 6 h | GO/phage—SPE | None | Impedance | [56] |
| Carboxyl Rich GO (CRGO) | RBP 41 from Anti-Salmonella Typhimurium T102 | Covalent | 2 h | CRGO/AuNPs/phage—RBP—GCE | Milk and Lettuce | Voltammetry | [57] |
| CRGO | Anti-Escherichia coli EP01 | Covalent | Overnight | CRGO/CB/phage—GCE | Milk and Raw Pork | Impedance | [58] |
| rGO | Anti-E. coli M13 | Covalent | 3 h | rGO/phage—FET | Simulated River Water | Resistance | [59] |
| rGO | Anti-Yersinia pseudotuberculosis vB_YepM_ZN18 | Physical: Adsorption | 2 h | PI-5-CA/rGO/AuNPs/phage—GCE | River Water | Voltammetry | [60] |
| rGO | Anti-E. coli | Physical: Adsorption | Does not apply | rGO/phage—CPE | No | Capacitance | [61] |
| rGO | Anti-Salmonella Typhimurium | Physical: Adsorption | Overnight | BC/PPy/rGO/phage–SPE | Milk and Chicken | Voltammetry | [62] |
| Ref. | Target | Linear Range (CFU·mL−1) | Detection Method | Response Time | Verified Non-Interferent Species | LOD (CFU·mL−1) | Discerns Live/Dead Cells |
|---|---|---|---|---|---|---|---|
| [16] | Salmonella Typhimurium * | 1.0 × 10–1.0 × 108 | DPV | ~5 min | 5 S. entérica serovars 5 non-Salmonella bacteria | 10 | No |
| [17] | P. aeruginosa ATCC 27853 | 60.0–6.0 × 107 | Amperometry | <10 min | S. aureus * V. cholerae * | 60 | No |
| [33] | E. coli K12 | 1.0 × 102–1.0 × 108 | EIS | <30 min | S. Typhimurium DT108 | 104 | Non-tested |
| [34] | L. monocytogenes Scott A | 10.0–1.0 × 104 | EIS | <30 min | S. Typhimurium 291RH E. coli O157:H7 | 8 | Non-tested |
| [51] | S. arlettae | 2.0 × 102–2.0 × 108 | EIS | 2 min | S. aureus 96 S. lentus 2292 E. coli 614 | 200 | Non-tested |
| [56] | Salmonella Typhimurium | 1.0 × 10–1.0 × 108 | EIS | <40 min | None | 12 | Non-tested |
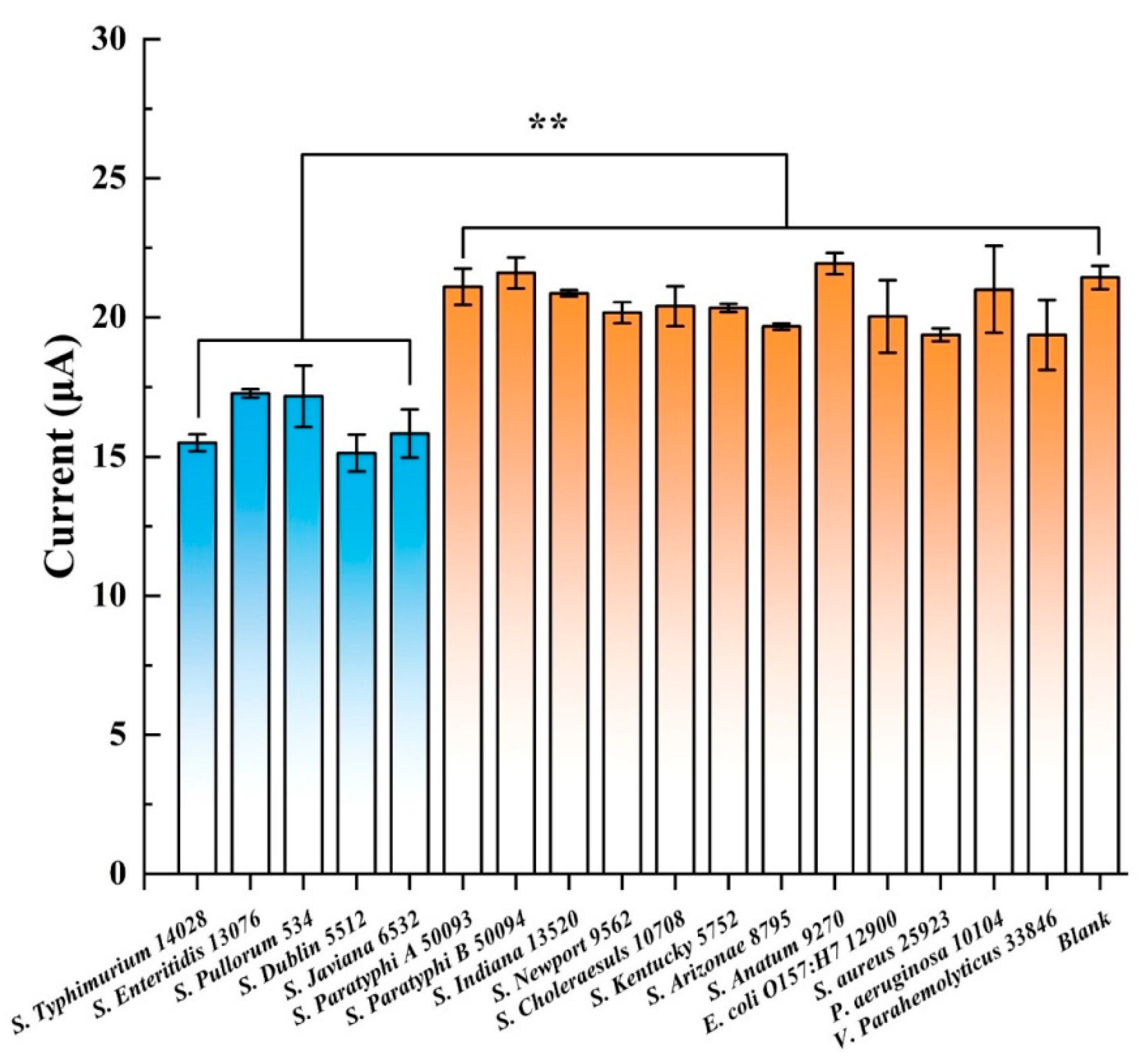
| [57] | Salmonella Typhimurium ATCC14028 | 3.0–1.0 × 106 | DPV | ~30 min | 8 Salmonella spp. 4 non-Salmonella bacteria | 2 | Non-tested |
| [58] | E. coli O157:H7GXEC-N07 | 1.0 × 102–1.0 × 107 | EIS | <30 min | P. aeruginosa PAI Klebsiella pneumoniae L30 S. enteritidis CVCC1806 | 12 | Non-tested |
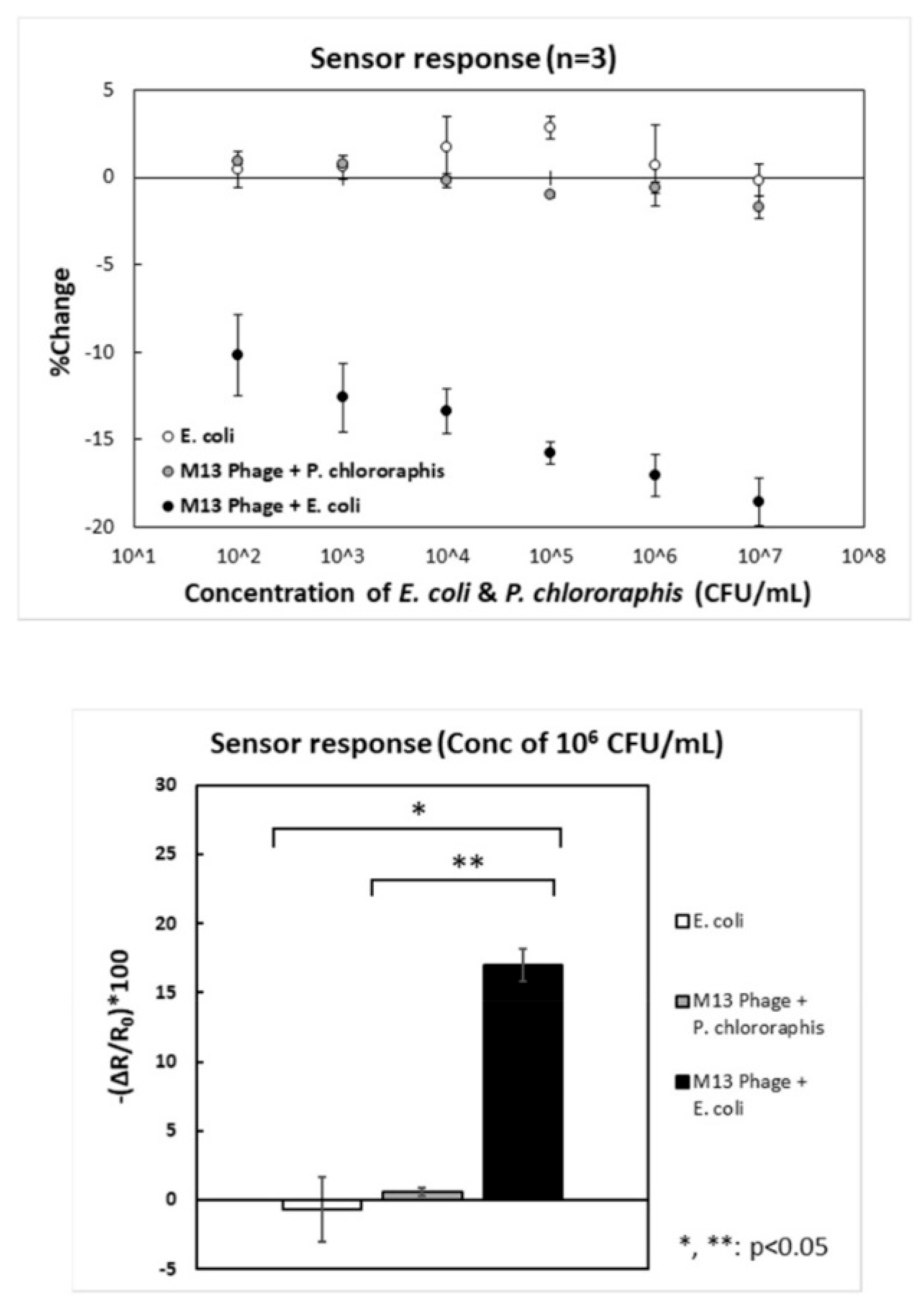
| [59] | E. coli XL1-blue | 1.0 × 102–1.0 × 107 | FET | 30 min | P. Chlororaphis | 45 | Non-tested |
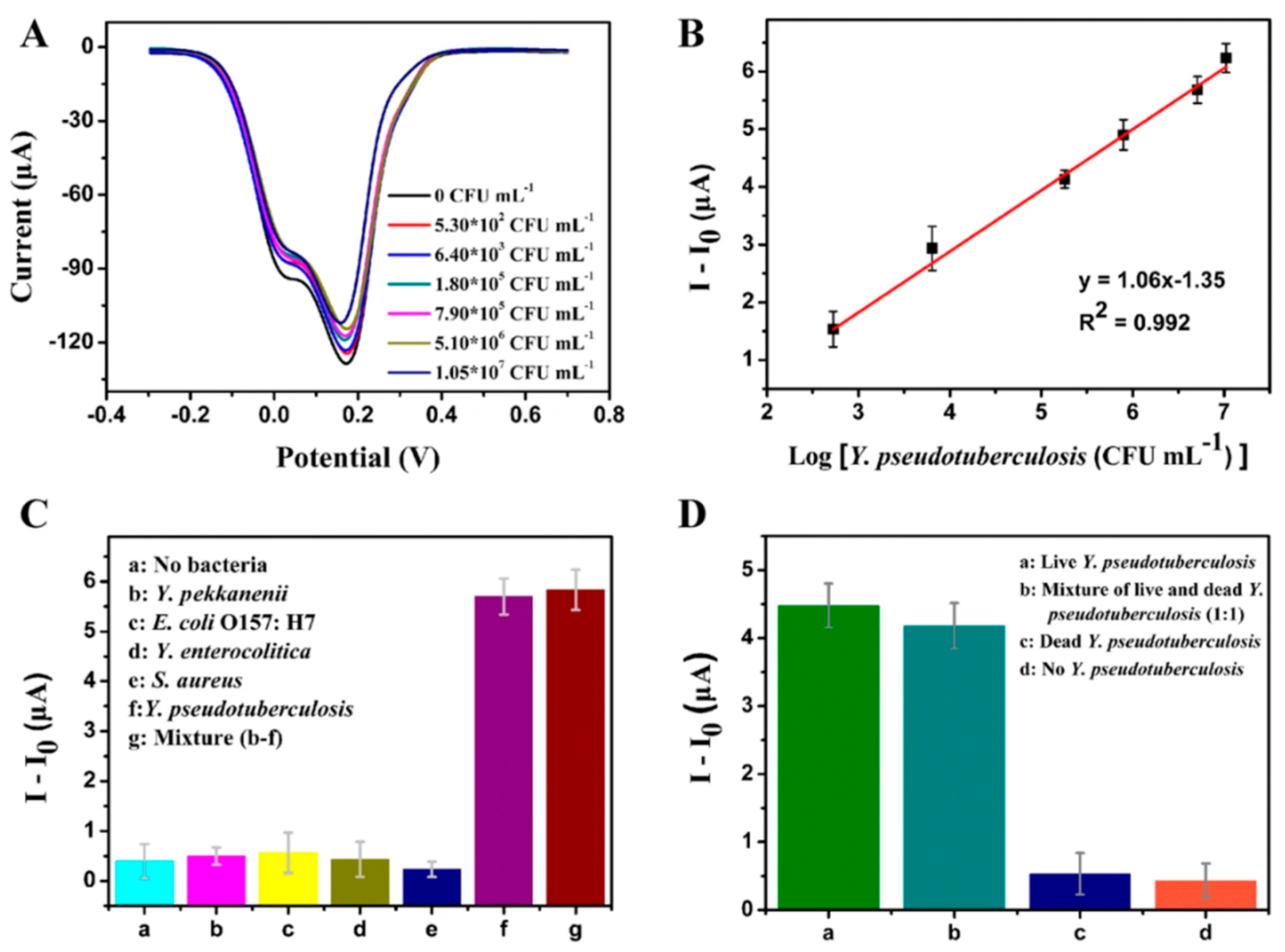
| [60] | Y. pseudotuberculosis | 5.3 × 102–1.1 × 107 | DPV | 35 min | Y. enterocolítica * Y. pekkanenii * S. aureus * E. coli O157:H7 | 3 | Yes |
| [61] | E. coli * | 3.3 × 10–3.3 × 102 | LCR | 5 s | S. aureus * Klebsiella * Shigella * V. cholerae * | 12 | Non-tested |
| [62] | Salmonella Typhimurium | 1.0–1.0 × 107 | DPV | 30 min | S. aureus* L. monocytogenes * E. coli * Bacillus subtilis * S. Typhimurium (heat-killed) | 1 | Yes |
| [133] | E. coli ATCC 25922 | 1.0 × 103–1.0 × 105 | FET | 50 s | Heat-killed: S. Typhimurium Streptococcus pneumonia | 103 | Non-tested |
| [134] | Salmonella Typhimurium ATCC14028 | 7.0–7.0 × 105 | qPCR | <3 h | 4 S. Typhimurium serovars 3 other Salmonella spp. 5 non-Salmonella bacteria | 7 | No |
| [135] | Salmonella Typhimurium DB7155 and 20 other Salmonella spp. | 1.0 × 105–1.0 × 107 | ELISA | 2 h | 10 non-Salmonella bacteria | 100 | No |
| [136] | E. coli and other 9 bacteria | - | Flow Cytometry | 21–64 h | - | 105 | No |
| [137] | Salmonella spp. | - | Bacterial Culture (ISO 6579) | 58–74 h | - | 1 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campiña, J.M.; Silva, A.F.; Pereira, C.M. Graphene–Bacteriophage Hybrid Nanomaterials for Specific and Rapid Electrochemical Detection of Pathogenic Bacteria. Biosensors 2025, 15, 467. https://doi.org/10.3390/bios15070467
Campiña JM, Silva AF, Pereira CM. Graphene–Bacteriophage Hybrid Nanomaterials for Specific and Rapid Electrochemical Detection of Pathogenic Bacteria. Biosensors. 2025; 15(7):467. https://doi.org/10.3390/bios15070467
Chicago/Turabian StyleCampiña, José M., António F. Silva, and Carlos M. Pereira. 2025. "Graphene–Bacteriophage Hybrid Nanomaterials for Specific and Rapid Electrochemical Detection of Pathogenic Bacteria" Biosensors 15, no. 7: 467. https://doi.org/10.3390/bios15070467
APA StyleCampiña, J. M., Silva, A. F., & Pereira, C. M. (2025). Graphene–Bacteriophage Hybrid Nanomaterials for Specific and Rapid Electrochemical Detection of Pathogenic Bacteria. Biosensors, 15(7), 467. https://doi.org/10.3390/bios15070467






