Performance of Colorimetric Lateral Flow Immunoassays for Renal Function Evaluation with Human Serum Cystatin C
Abstract
1. Introduction
2. Materials and Methods
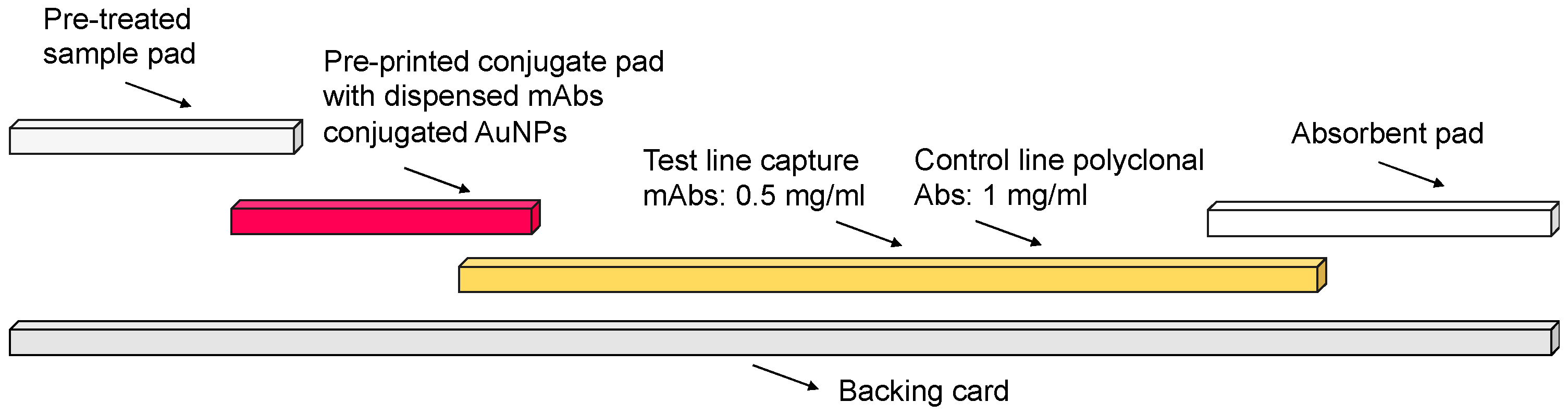
2.1. AuNPs’ Characterisation and LFIA Fabrication
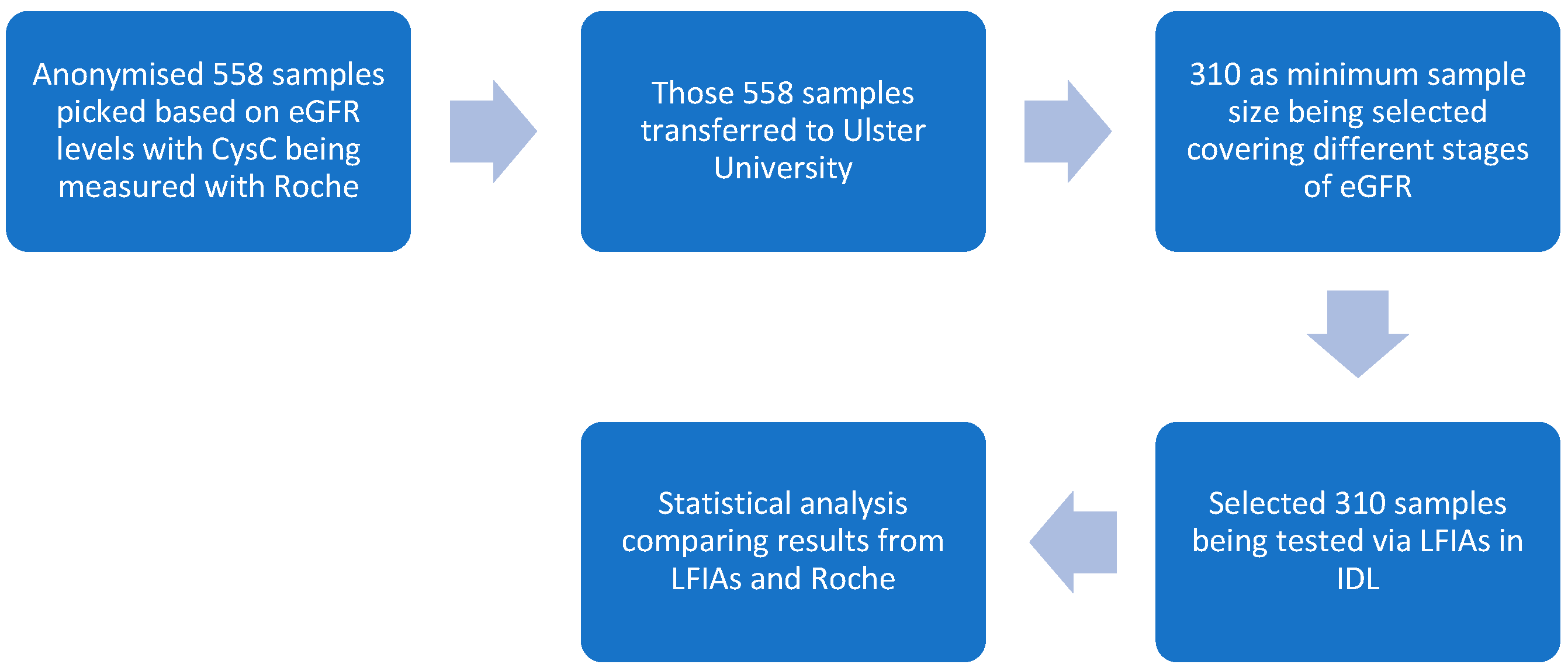
2.2. Clinical Study Phases and Sample Selection Criteria
2.2.1. Phase 1 Study
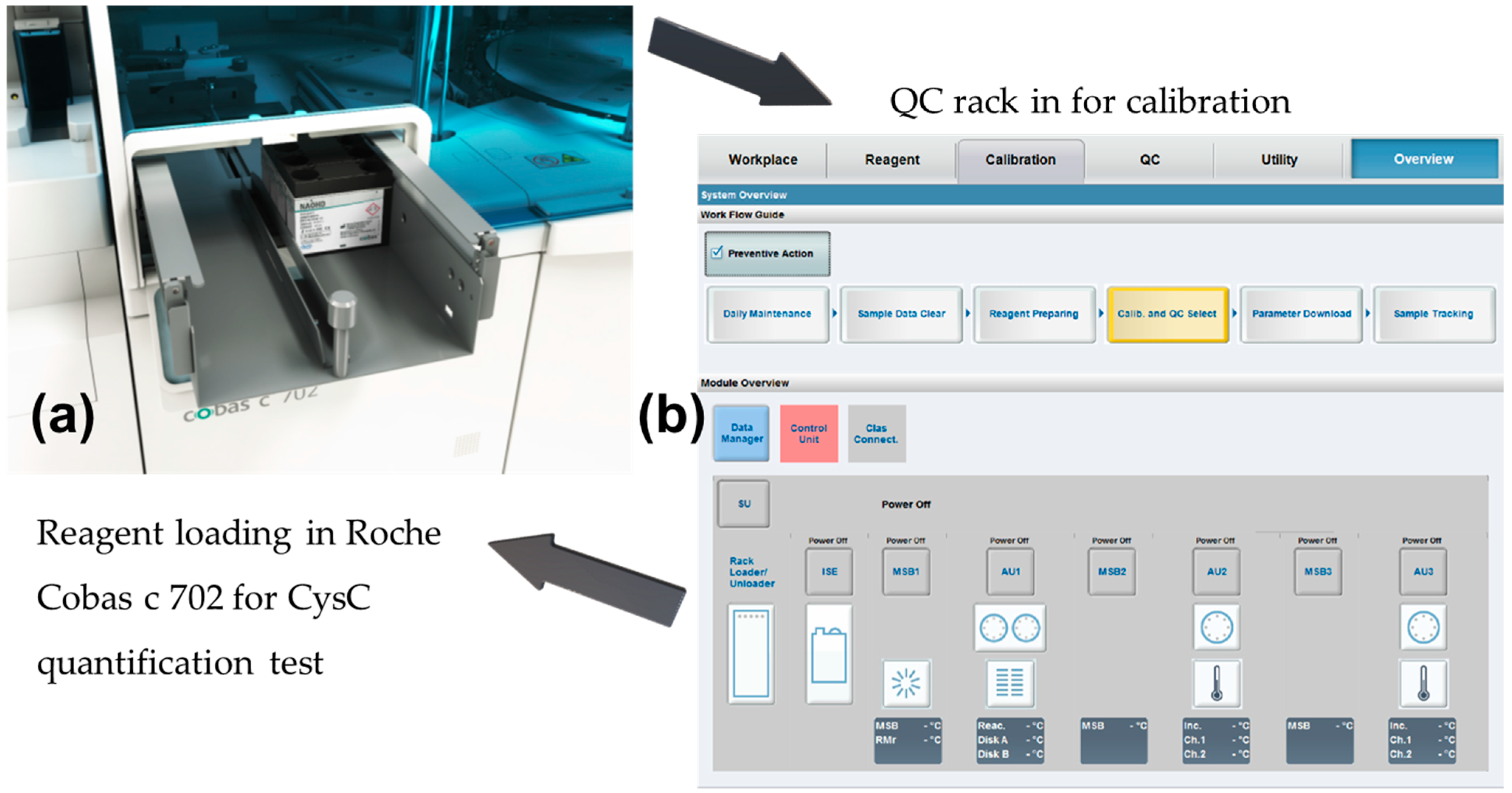
- As there was no difference in diagnostic values for serum CysC and plasma CysC, the level of serum CysC was also used to indicate the level of plasma CysC unless otherwise stated [38]. The 40 serum CysC samples were quantified with the standard via the Roche Cobas 8000 Modular Series (Cobas c 702 modules, Roche, Basel, Switzerland) to reveal CysC concentrations.
- For these 40 samples, a further measurement was conducted in Belfast Health & Social Care Trust, and standard deviations between the results were recorded. Details of the procedures and diagrams are shown in Figure 2.
2.2.2. Phase 2 Study
2.2.3. Phase 3 Study
- In total, 558 serum samples were tested via the Roche standard. (Note: Some of the previous 40 samples were selected and tested with the optimised LFIAs to reach the total sample amount of 310).
- In total, 310 samples were tested on LFIAs, and test and control line signal intensities were documented by the Lumos reader (Lumos Diagnostics, San Diego, CA, USA), with the optimised settings.
3. Results
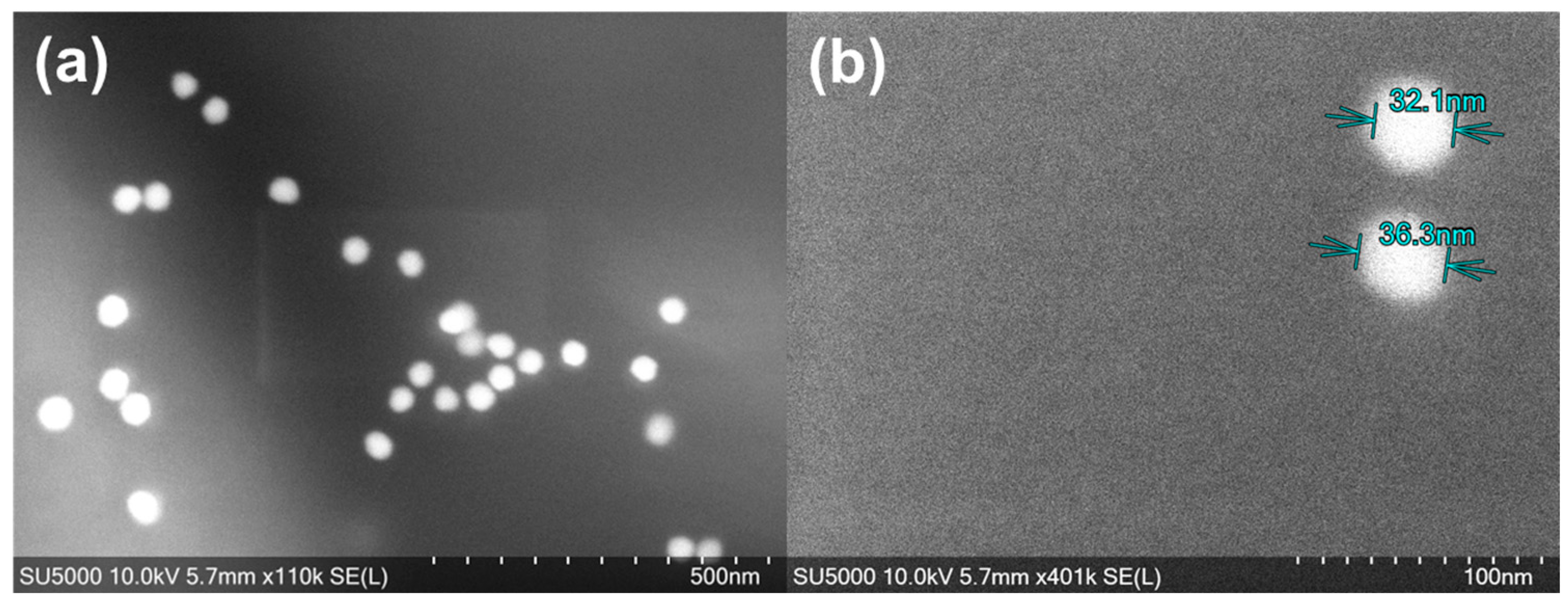
3.1. AuNPs’ Characterisation
3.2. Optimal OD Value and NC Membrane for AuNPs’ LFIAs
3.3. Optimisation of LFIAs’ Bed Volume and AuNPs’ Volume in LFD
3.4. Optimisation of Sample Dilution Factor and Diluted Sample Volume in LFD and LFIAs
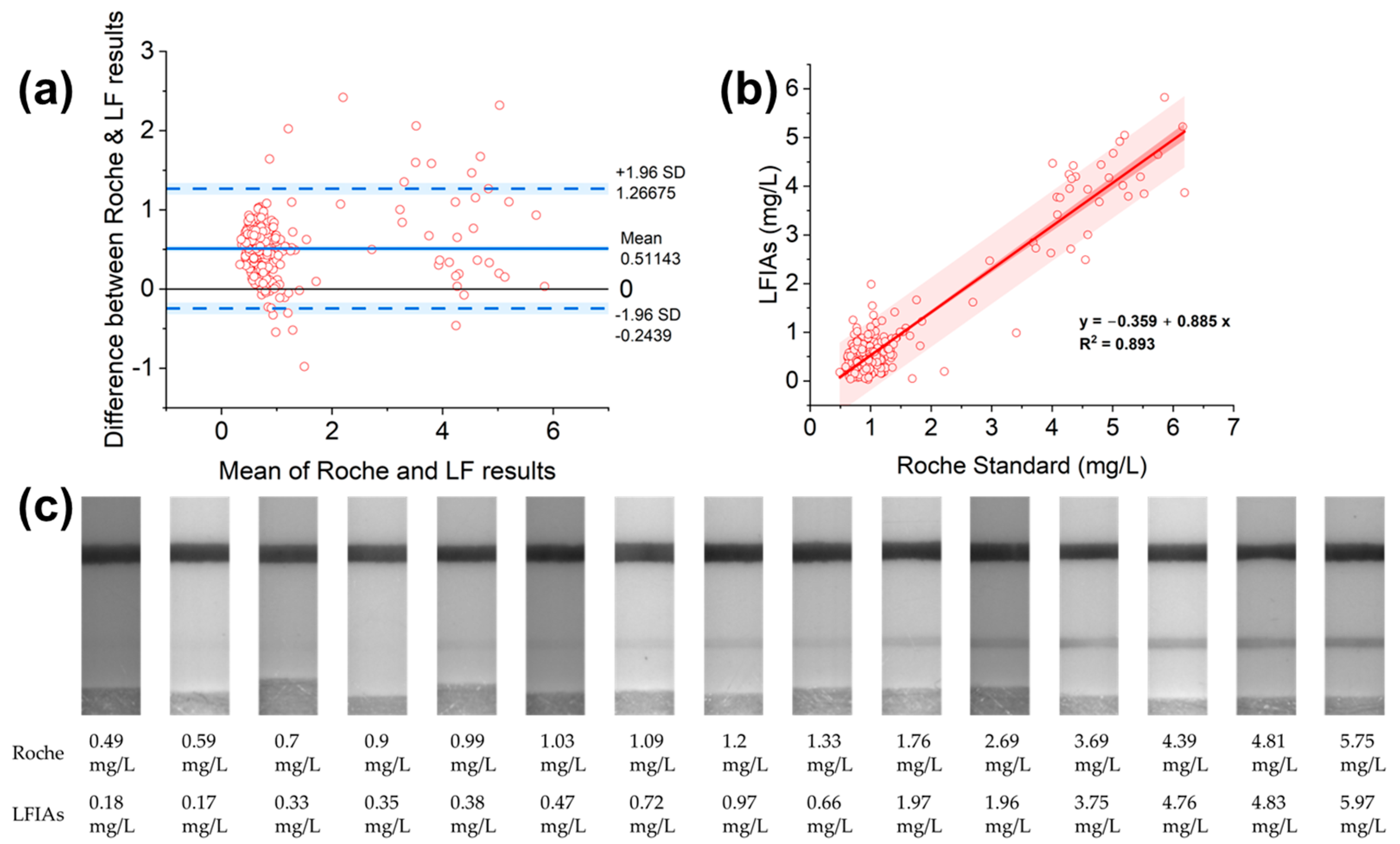
3.5. Evaluation of the LFIAs’ Performance with Roche Standard
4. Discussion
4.1. Optimisation of the LF System
4.2. Developed LFIAs’ Performance in Comparison with the Roche System
4.3. Developed LFIAs’ Sensitivity and Specificity Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| POC | Point-of-care |
| LFIA | Lateral flow immunoassay |
| LF | Lateral flow |
| NC | Nitrocellulose |
| NP | Nanoparticle |
| OD | Optical density |
| hCG | Human chorionic gonadotropin |
| eGFR | Estimated glomerular filtration rate |
| PBS | Phosphate buffered saline |
| BSA | Bovine serum albumin |
| LFD | Lateral flow dipstick |
| IRAS | Integrated Research Application System |
| REC | Research Ethics Committee |
| BMI | Body mass index |
| QC | Quality control |
| IDL | Integrated diagnostics lab |
| SD | Standard deviation |
| TSH | Thyroid-stimulating hormone |
| FESEM | Field-emission scanning electron microscope |
| mAbs | Monoclonal antibodies |
References
- Mak, W.C.; Beni, V.; Turner, A.P. Lateral-flow technology: From visual to instrumental. TrAC-Trends Anal. Chem. 2016, 79, 297–305. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.L. Home and Point-of-Care Pregnancy Tests: A Review of the Technology. Epidemiology 2002, 13, S15–S18. Available online: http://www.jstor.org/stable/3703541 (accessed on 17 November 2022). [CrossRef] [PubMed]
- Ross, G.M.S.; Filippini, D.; Nielen, M.W.F.; Salentijn, G.I. Unraveling the Hook Effect: A Comprehensive Study of High Antigen Concentration Effects in Sandwich Lateral Flow Immunoassays. Anal. Chem. 2020, 92, 15587–15595. [Google Scholar] [CrossRef]
- Andryukov, B.G. Six decades of lateral flow immunoassay: From determining metabolic markers to diagnosing covid-19. AIMS Microbiol. 2020, 6, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.S.; Ford, G.M.; Waikar, S.S.; Wang, Y.; Clement, M.B.; Ramirez, V.; Glaab, W.E.; Troth, S.P.; Sistare, F.D.; Prozialeck, W.C.; et al. A rapid urine test for early detection of kidney injury. Kidney Int. 2009, 76, 108–114. [Google Scholar] [CrossRef]
- Ji, Y.; Ren, M.; Li, Y.; Huang, Z.; Shu, M.; Yang, H.; Xiong, Y.; Xu, Y. Detection of aflatoxin B1 with immunochromatographic test strips: Enhanced signal sensitivity using gold nanoflowers. Talanta 2015, 142, 206–212. [Google Scholar] [CrossRef]
- Sohrabi, H.; Majidi, M.R.; Fakhraei, M.; Jahanban-Esfahlan, A.; Hejazi, M.; Oroojalian, F.; Baradaran, B.; Tohidast, M.; de la Guardia, M.; Mokhtarzadeh, A. Lateral flow assays (LFA) for detection of pathogenic bacteria: A small point-of-care platform for diagnosis of human infectious diseases. Talanta Int. 2022, 243, 123330. [Google Scholar] [CrossRef]
- Kim, M.J.; Haizan, I.; Ahn, M.J.; Park, D.H.; Choi, J.H. Recent Advances in Lateral Flow Assays for Viral Protein Detection with Nanomaterial-Based Optical Sensors. Biosensors 2024, 14, 197. [Google Scholar] [CrossRef]
- Raveendran, J.; Gangadharan, D.; Bayry, J.; Rasheed, P.A. Emerging trends in the cystatin C sensing technologies: Towards better chronic kidney disease management. RSC Adv. 2025, 15, 4926–4944. [Google Scholar] [CrossRef]
- Yang, M.; Xu, X.; Zhang, M.; Wang, J.; Wu, Y.; Wang, N.; Li, Z. Recent advances in lateral flow immunoassay based on sandwich format for whole-cell pathogen detection. Coord. Chem. Rev. 2025, 533, 216538. [Google Scholar] [CrossRef]
- Bikkarolla, S.K.; Venkatesan, K.; Revathy, Y.R.; Parameswaran, S.; Krishnakumar, S.; Dendukuri, D. The Quantitative Detection of Cystatin-C in Patient Samples Using a Colorimetric Lateral Flow Immunoassay. Biosensors 2024, 14, 30. [Google Scholar] [CrossRef]
- Zhang, X.; Fishlock, S.; Sharpe, P.; McLaughlin, J. Development of colorimetric lateral flow assays with gold nanostructures for Cystatin C detection. Sens. Actuators Rep. 2022, 4, 100121. [Google Scholar] [CrossRef]
- Bikkarolla, S.K.; McNamee, S.E.; Vance, P.; McLaughlin, J. High-Sensitive Detection and Quantitative Analysis of Thyroid-Stimulating Hormone Using Gold-Nanoshell-Based Lateral Flow Immunoassay Device. Biosensors 2022, 12, 182. [Google Scholar] [CrossRef]
- Huang, L.; Jin, J.; Ao, L.; Jiang, C.; Zhang, Y.; Wen, H.-M.; Wang, J.; Wang, H.; Hu, J. Hierarchical Plasmonic-Fluorescent Labels for Highly Sensitive Lateral Flow Immunoassay with Flexible Dual-Modal Switching. ACS Appl. Mater. Interfaces 2020, 12, 58149–58160. [Google Scholar] [CrossRef]
- Kong, D.Y.; Heo, N.S.; Kang, J.W.; Lee, J.B.; Kim, H.J.; Kim, M.I.l. Nanoceria-based lateral flow immunoassay for hydrogen peroxide-free colorimetric biosensing for C-reactive protein. Anal. Bioanal. Chem. 2022, 414, 3257–3265. [Google Scholar] [CrossRef]
- A Hirst, J.; Hill, N.; A O’cAllaghan, C.; Lasserson, D.; McManus, R.J.; Ogburn, E.; Mena, J.M.O.; Shine, B.; Taylor, C.J.; Vazquez-Montes, M.D.; et al. Prevalence of chronic kidney disease in the community using data from OxRen: A UK population-based cohort study. Br. J. Gen. Pract. 2020, 70, E285–E293. [Google Scholar] [CrossRef] [PubMed]
- Guidelines and Audit Implementation Network (GAIN). Northern Ireland Guidelines for the Management of Chronic Kidney Disease (CKD). 20151–27. Available online: https://www.rqia.org.uk/RQIA/files/07/071a66f7-e6f4-4b97-abba-10ace68dc0a4.pdf (accessed on 1 January 2023).
- Luis-Lima, S.; Escamilla-Cabrera, B.; Negrín-Mena, N.; Estupiñán, S.; Delgado-Mallén, P.; Marrero-Miranda, D.; González-Rinne, A.; Miquel-Rodríguez, R.; Cobo-Caso, M.Á.; Hernández-Guerra, M.; et al. Chronic kidney disease staging with cystatin C or creatinine-based formulas: Flipping the coin. Nephrol. Dial. Transplant. 2019, 34, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. In Renal Fibrosis: Mechanisms and Therapies; Liu, B.C., Lan, H.Y., Lv, L.L., Eds.; Springer: Singapore, 2019; pp. 3–15. [Google Scholar] [CrossRef]
- John, A.S.; Price, C. Existing and Emerging Technologies for Point-of-Care Testing. Clin. Biochem. Rev. 2014, 35, 155–167. [Google Scholar]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Waldum-Grevbo, B. What physicians need to know about renal function in outpatients with heart failure. Cardiology 2015, 131, 130–138. [Google Scholar] [CrossRef]
- Stern-Nezer, S. Chronic and End-Stage Kidney Disease in the Neurological Intensive Care Unit. J. Stroke Cerebrovasc. Dis. 2021, 30, 105819. [Google Scholar] [CrossRef] [PubMed]
- Shardlow, A.; McIntyre, N.J.; Fraser, S.D.S.; Roderick, P.; Raftery, J.; Fluck, R.J.; McIntyre, C.W.; Taal, M.W.; Remuzzi, G. The clinical utility and cost impact of cystatin C measurement in the diagnosis and management of chronic kidney disease: A primary care cohort study. PLoS Med. 2017, 14, e1002400. [Google Scholar] [CrossRef]
- Ge, C.; Ren, F.; Lu, S.; Ji, F.; Chen, X.; Wu, X. Clinical prognostic significance of plasma cystatin C levels among patients with acute coronary syndrome. Clin. Cardiol. 2009, 32, 644–648. [Google Scholar] [CrossRef]
- Urbonavicienea, G.; Shi, G.P.; Urbonaviciusa, S.; Henneberga, E.W.; Lindholta, J.S. Higher cystatin C level predicts long-term mortality in patients with peripheral arterial disease. Atherosclerosis 2011, 216, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Alhusseiny, A.H.; Al-Nimer, M.S.M.; Al-Neamy, S.I.A. Assessment of Serum Cystatin C Levels in Newly Diagnosed Acute Myocardial Infarction at the Onset and at the Time of Hospital Discharge. Cardiol. Res. 2015, 6, 226–231. [Google Scholar] [CrossRef]
- Matsue, Y.; Matsumura, A.; Abe, M.; Ono, M.; Seya, M.; Nakamura, T.; Iwatsuka, R.; Mizukami, A.; Setoguchi, M.; Nagahori, W.; et al. Prognostic implications of chronic kidney disease and anemia after percutaneous coronary intervention in acute myocardial infarction patients. Heart Vessels 2013, 28, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Guerchicoff, A.; Stone, G.W.; Mehran, R.; Xu, K.; Nichols, D.; Claessen, B.E.; Guagliumi, G.; Witzenbichler, B.; Henriques, J.P.; Dangas, G.D. Analysis of biomarkers for risk of acute kidney injury after primary angioplasty for acute st-segment elevation myocardial infarction: Results of the HORIZONS-AMI trial. Catheter. Cardiovasc. Interv. 2015, 85, 335–342. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.; Men, M.; Shen, G.; Ma, A. High cystatin C levels predict long-term mortality in patients with ST-segment elevation myocardial infarction undergoing late percutaneous coronary intervention: A retrospective study. Clin. Cardiol. 2019, 42, 572–578. [Google Scholar] [CrossRef]
- Gold Nanoparticles FAQZ-Nanopartz. Available online: https://www.nanopartz.com/faqz.asp (accessed on 1 April 2023).
- Robertson, L.J.; Moore, J.S.; Blighe, K.; Ng, K.Y.; Quinn, N.; Jennings, F.; Warnock, G.; Sharpe, P.; Clarke, M.; Maguire, K.; et al. Evaluation of the IgG antibody response to SARS CoV-2 infection and performance of a lateral flow immunoassay: Cross-sectional and longitudinal analysis over 11 months. BMJ Open 2021, 11, e048142. [Google Scholar] [CrossRef]
- Rai, A.J.; Gelfand, C.A.; Haywood, B.C.; Warunek, D.J.; Yi, J.; Schuchard, M.D.; Mehigh, R.J.; Cockrill, S.L.; Scott, G.B.I.; Tammen, H.; et al. HUPO Plasma Proteome Project specimen collection and handling: Towards the standardization of parameters for plasma proteome samples. Proteomics 2005, 5, 3262–3277. [Google Scholar] [CrossRef]
- Bujang, M.A.; Adnan, T.H. Requirements for minimum sample size for sensitivity and specificity analysis. J. Clin. Diagn. Res. 2016, 10, YE01–YE06. [Google Scholar] [CrossRef] [PubMed]
- Bargnoux, A.-S.; Piéroni, L.; Cristol, J.-P.; Kuster, N.; Delanaye, P.; Carlier, M.-C.; Fellahi, S.; Boutten, A.; Lombard, C.; González-Antuña, A.; et al. Multicenter evaluation of Cystatin C measurement after assay standardization. Clin. Chem. 2017, 63, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Daniel, W.W. Biostatistics: A Foundation for Analysis in the Health Sciences. Biometrics 1988, 44, 317. [Google Scholar] [CrossRef]
- Basu, D.; Kulkarni, R. Overview of blood components and their preparation. Indian. J. Anaesth. 2014, 58, 529–537. [Google Scholar] [CrossRef]
- Grubb, A.O. Cystatin C-Properties and use as diagnostic marker. Adv. Clin. Chem. 2001, 35, 63–99. [Google Scholar]
- Erlandsen, E.J.; Randers, E. Performance evaluation of the Roche Tina-quant Cystatin C assay and reference interval for cystatin C in healthy blood donors. Scand. J. Clin. Lab. Investig. 2010, 70, 300–304. [Google Scholar] [CrossRef]
- CYSC2 Tina-quant Cystatin C Gen.2 Cobas. 2023. Available online: https://elabdoc-prod.roche.com/eLD/api/downloads/3879362d-248b-ec11-1191-005056a71a5d?countryIsoCode=gb (accessed on 17 May 2025).
- Finney, H.; Newman, D.J.; Price, C.P. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann. Clin. Biochem. 2000, 37, 49–59. [Google Scholar] [CrossRef]
- Tian, S.; Kusano, E.; Ohara, T.; Tabei, K.; Itoh, Y.; Kawai, T.; Asano, Y. Cystatin C measurement and its practical use in patients with various renal diseases. Clin. Nephrol. 1997, 48, 104–108. [Google Scholar]
- Murty, M.S.N.; Sharma, U.K.; Pandey, V.B.; Kankare, S.B. Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J. Nephrol. 2013, 23, 180–183. [Google Scholar] [CrossRef]
- Haudek, V.J.; Slany, A.; Gundacker, N.C.; Wimmer, H.; Drach, J.; Gerner, C. Proteome maps of the main human peripheral blood constituents. J. Proteome Res. 2009, 8, 3834–3843. [Google Scholar] [CrossRef]
- Taglieri, N.; Koenig, W.; Kaski, J.C. Cystatin C and cardiovascular risk. Clin. Chem. 2009, 55, 1932–1943. [Google Scholar] [CrossRef] [PubMed]
- Newman, D. Rheumatoid factor can interfere with cystatin C measurement. Ann. Clin. Biochem. 2003, 40, 427. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Ishimitsu, T.; Uchida, M.; Hirao, J. Chronic kidney disease in the elderly population. Dokkyo J. Med. Sci. 2017, 44, 295–308. [Google Scholar]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Gold Nanoparticle Properties | Cytodiagnostics Inc. Available online: https://www.cytodiagnostics.com/pages/gold-nanoparticle-properties (accessed on 1 April 2023).
- Kapadia, C.H.; Melamed, J.R.; Day, E.S. Quantification of Gold Nanoparticles Using the NanoDrop One Microvolume UV-Vis Spectrophotometer; Thermo Fisher Scientific: Waltham, MA, USA, 2019; p. 3. [Google Scholar]
- Shin, J.H.; Park, J.K. Functional Packaging of Lateral Flow Strip Allows Simple Delivery of Multiple Reagents for Multistep Assays. Anal. Chem. 2016, 88, 10374–10378. [Google Scholar] [CrossRef]
- Natarajan, S.; Derosa, M.C.; Shah, M.I.; Jayaraj, J. Development and evaluation of a quantitative fluorescent lateral flow immunoassay for cystatin-c, a renal dysfunction biomarker. Sensors 2021, 21, 3178. [Google Scholar] [CrossRef]
- Millipore, M. Rapid Lateral Flow Test Strips: Considerations for Product Development; EMD Millipore Corporation: Billerica, MA, USA, 2013; Volume 29, pp. 702–707. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/535/793/tb500en00emm-mk.pdf (accessed on 17 June 2019).
- Parolo, C.; Sena-Torralba, A.; Bergua, J.F.; Calucho, E.; Fuentes-Chust, C.; Hu, L.; Rivas, L.; Álvarez-Diduk, R.; Nguyen, E.P.; Cinti, S.; et al. Tutorial: Design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 2020, 15, 3788–3816. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Tumskiy, R.S.; Burov, A.M.; Pylaev, T.E.; Khlebtsov, N.G. Quantifying the Numbers of Gold Nanoparticles in the Test Zone of Lateral Flow Immunoassay Strips. ACS Appl. Nano Mater. 2019, 2, 5020–5028. [Google Scholar] [CrossRef]
- Bunce, C. Correlation, Agreement, and Bland-Altman Analysis: Statistical Analysis of Method Comparison Studies. Am. J. Ophthalmol. 2009, 148, 4–6. [Google Scholar] [CrossRef]
- Xu, Q.F.; Xu, H.; Gu, H.; Li, J.B.; Wang, Y.; Wei, M. Development of lateral flow immunoassay system based on superparamagnetic nanobeads as labels for rapid quantitative detection of cardiac troponin I. Mater. Sci. Eng. C 2009, 29, 702–707. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, P.; Wang, S.; Melnykov, A.; Jiang, Q.; Seth, A.; Wang, Z.; Morrissey, J.J.; George, I.; Gandra, S.; et al. Ultrasensitive lateral-flow assays via plasmonically active antibody-conjugated fluorescent nanoparticles. Nat. Biomed. Eng. 2023, 7, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Kidney Test-Bloom Diagnostics. Available online: https://bloomdiagnostics.com/en-uk/tests/kidney-test/ (accessed on 2 July 2025).
- Villa, P.; Jiménez, M.; Soriano, M.C.; Manzanares, J.; Casasnovas, P. Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit. Care 2005, 9, 139–143. [Google Scholar] [CrossRef]
- Conti, M.; Moutereau, S.; Zater, M.; Lallali, K.; Durrbach, A.; Manivet, P.; Eschwège, P.; Loric, S. Urinary cystatin C as a specific marker of tubular dysfunction. Clin. Chem. Lab. Med. 2006, 44, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Séronie-Vivien, S.; Delanaye, P.; Piéroni, L.; Mariat, C.; Froissart, M.; Cristol, J.P. Cystatin C: Current position and future prospects. Clin. Chem. Lab. Med. 2008, 46, 1664–1686. [Google Scholar] [CrossRef]
- Croda-Todd, M.T.; Soto-Montano, X.J.; Hernández-Cancino, P.A.; Juárez-Aguilar, E. Adult cystatin C reference intervals determined by nephelometric immunoassay. Clin. Biochem. 2007, 40, 1084–1087. [Google Scholar] [CrossRef]
- Tang, D.; Sauceda, J.; Lin, Z.; Ott, S.; Basova, E.; Goryacheva, I.; Biselli, S.; Lin, J.; Niessner, R.; Knopp, D. Magnetic nanogold microspheres-based lateral-flow immunodipstick for rapid detection of aflatoxin B2 in food. Biosens. Bioelectron. 2009, 25, 514–518. [Google Scholar] [CrossRef]
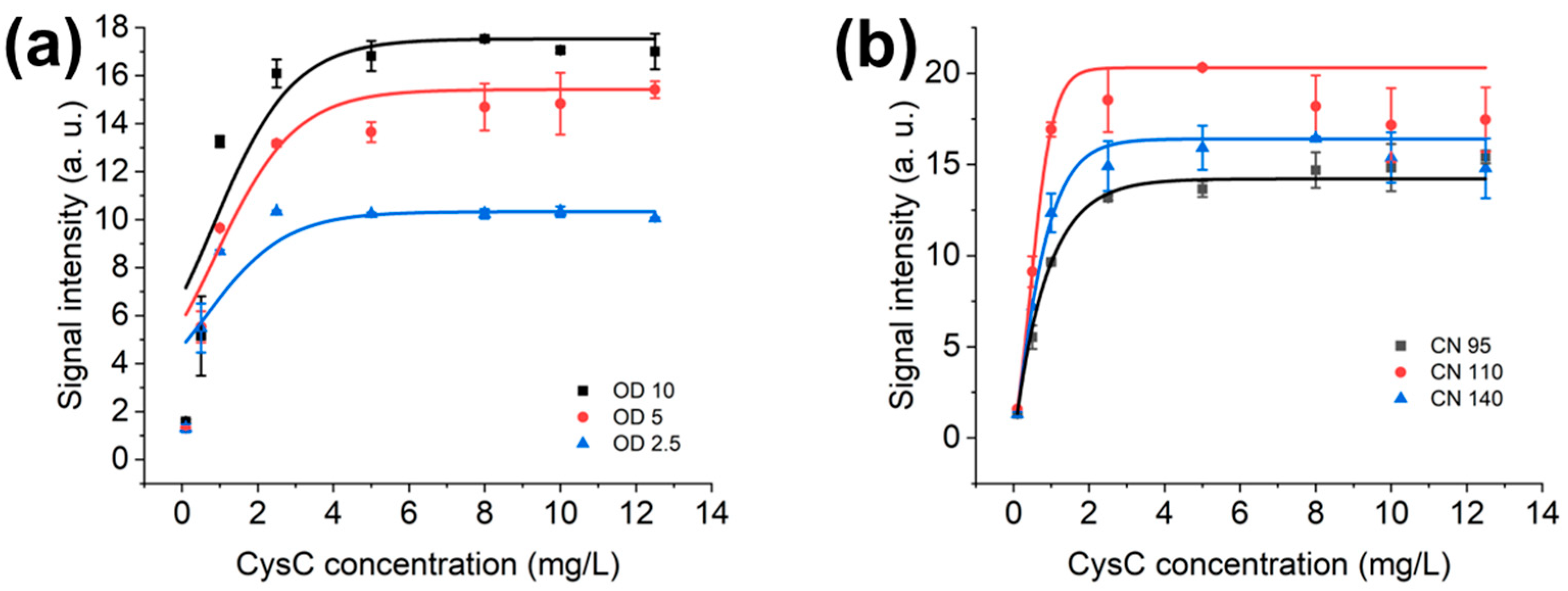
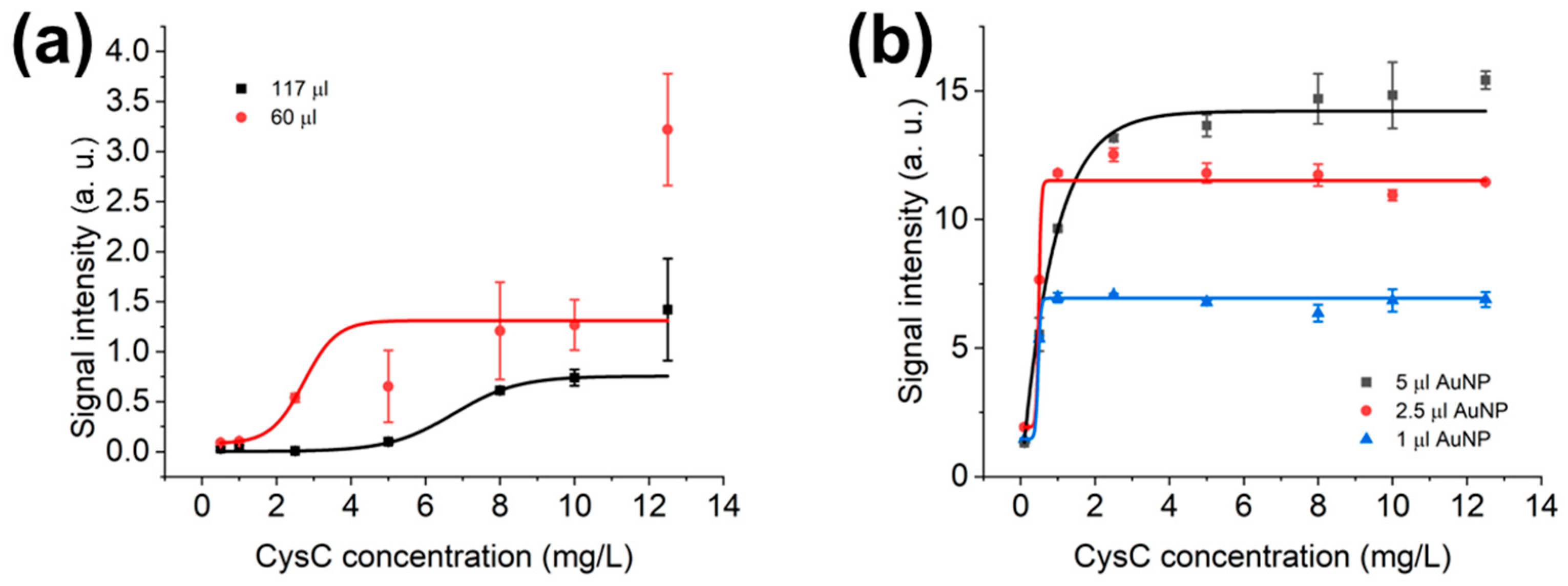

| Conc. mg/L | Average (Arb. Units) | Standard Deviation (SD) |
|---|---|---|
| 10 | 4.1492 | 0.511 |
| 8 | 3.5951 | 0.638 |
| 5 | 1.9450 | 0.807 |
| 2.5 | 1.5243 | 0.326 |
| 1 | 0.6039 | 0.171 |
| 0.5 | 0.3416 | 0.324 |
| 0.1 | 0.3370 | 0.225 |
| 0 | 0.1861 | 0.099 |
| Stages of CKD | |||
|---|---|---|---|
| Kidney Function | GFR (mL/min/1.73 m2) | Samples’ Distribution Measured by Roche | Samples Distribution Measured by LFIAs |
| 1 | ≥90 * | 279 | 279 |
| 2 | 60–89 * | ||
| 3a | 45–59 | 112 | 0 |
| 3b | 30–44 | ||
| 4 | 15–29 | 126 | 10 |
| 5 | <15/under dialysis | 41 | 21 |
| Parameter | Unit | Standard Error Formula | Standard Error (Se) | t Values for 309 Degrees of Freedom | Confidence (Se × t) | Confidence Intervals from | Confidence Intervals to |
|---|---|---|---|---|---|---|---|
| sample size (n) | 310 | ||||||
| degrees of freedom (n − 1) | 309 | ||||||
| difference mean (ū) | 0.51 | 0.02 | 1.97 | 0.04 | 0.47 | 0.55 | |
| standard deviation (SD) | 0.39 | ||||||
| ū − 1.96 s | −0.24 | 0.04 | 1.97 | 0.08 | −0.32 | −0.16 | |
| ū + 1.96 s | 1.27 | 0.04 | 1.97 | 0.08 | 1.19 | 1.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Fishlock, S.; Sharpe, P.; McLaughlin, J. Performance of Colorimetric Lateral Flow Immunoassays for Renal Function Evaluation with Human Serum Cystatin C. Biosensors 2025, 15, 445. https://doi.org/10.3390/bios15070445
Zhang X, Fishlock S, Sharpe P, McLaughlin J. Performance of Colorimetric Lateral Flow Immunoassays for Renal Function Evaluation with Human Serum Cystatin C. Biosensors. 2025; 15(7):445. https://doi.org/10.3390/bios15070445
Chicago/Turabian StyleZhang, Xushuo, Sam Fishlock, Peter Sharpe, and James McLaughlin. 2025. "Performance of Colorimetric Lateral Flow Immunoassays for Renal Function Evaluation with Human Serum Cystatin C" Biosensors 15, no. 7: 445. https://doi.org/10.3390/bios15070445
APA StyleZhang, X., Fishlock, S., Sharpe, P., & McLaughlin, J. (2025). Performance of Colorimetric Lateral Flow Immunoassays for Renal Function Evaluation with Human Serum Cystatin C. Biosensors, 15(7), 445. https://doi.org/10.3390/bios15070445






